Abstract
Aims
Beta‐lactam dose optimization in critical care is a current priority. We aimed to review the pharmacokinetics (PK) of three commonly used beta‐lactams (amoxicillin ± clavulanate, piperacillin–tazobactam and meropenem) to compare PK parameters reported in critically and noncritically ill neonates, children and adults, and to investigate whether allometric and maturation scaling principles could be applied to describe changes in PK parameters through life.
Methods
A systematic review of PK studies of the three drugs was undertaken using MEDLINE and EMBASE. PK parameters and summary statistics were extracted and scaled using allometric principles to 70 kg individual for comparison. Pooled data were used to model clearance maturation and decline using a sigmoidal (Hill) function.
Results
A total of 130 papers were identified. Age ranged from 29 weeks to 82 years and weight from 0.9–200 kg. PK parameters from critically ill populations were reported with wider confidence intervals than those in healthy volunteers, indicating greater PK variability in critical illness. The standard allometric size and sigmoidal maturation model adequately described increasing clearance in neonates, and a sigmoidal model was also used to describe decline in older age. Adult weight‐adjusted clearance was achieved at approximately 2 years postmenstrual age. Changes in volume of distribution were well described by the standard allometric model, although amoxicillin data suggested a relatively higher volume of distribution in neonates.
Conclusions
Critical illness is associated with greater PK variability than in healthy volunteers. The maturation models presented will be useful for optimizing beta‐lactam dosing, although a prospective, age‐inclusive study is warranted for external validation.
Keywords: antibiotics, critical care, paediatrics, pharmacokinetics, pharmacometrics
What is Already Known about this Subject
Antimicrobial resistance and high sepsis‐related mortality has led to increasing interest in the dose optimization of antibiotics
Pharmacokinetic data from paediatric and neonatal critically ill populations are lacking
Modern modelling approaches, using size and age maturation functions, may allow extrapolation of PK data from adults to children
What this Study Adds
To our knowledge, this is the first review of the pharmacokinetics of amoxicillin, clavulanic acid, meropenem and piperacillin–tazobactam across all ages.
The range of reported parameters has allowed comparison of values in critically ill and noncritically ill patients.
For the first time, parameters for a clearance maturation in young patients has been combined with a decline function in elderly patients, generating models that could be used for dose optimization in patients of all ages.
Introduction
Infection is a common reason for admission to intensive care, accounting for 25–30% of admissions to adult units 1, 2, 3 and 8–12% of admissions to paediatric units 4, 5. At any one time, half of the patients on an adult intensive care unit may be considered to have an infection 6 and up to 70% of intensive care patients will receive at least one course of antibiotics during their stay, regardless of age 6, 7. Mortality for those with severe infection remains as high as 25–30% 5, 8 and infection remains one of the most common causes of death in neonates in the UK and worldwide 9, 10, 11. Infection‐associated healthcare costs are considerable, with pneumonia and septicaemia accounting for over $30 billion (approximately 8%) of US healthcare spending 12.
The provision of prompt, targeted antimicrobial therapy is a key priority in the early stages of treatment of infection. While recent decades have seen the evolution of sepsis care bundles that tailor therapy for severe infection to the individual patient, antimicrobial dosing in critically ill patients remains largely identical to that in the noncritically ill 13. This is despite the fact that pharmacokinetics (PK) in critical illness, particularly in patients at the extremes of the age spectrum, may be radically different from that in health or noncritical illness 14.
In adults, antimicrobial PK in critical illness is increasingly an area of interest for researchers. Population approaches to PK data modelling have afforded the opportunity for the investigation of PK in specific patient groups, such as those with burns. However, studies are often small (n < 20) and reported PK parameters vary considerably. For example, Bourget et al. 15 reported a piperacillin clearance of 6.8 l h–1 70 kg–1, whereas Jeon et al. 16 reported a figure of 17.2 l h–1 70 kg–1 in critically ill patients with burns.
PK studies of antimicrobials in paediatric and neonatal populations are limited, with many dosing regimens still based on extrapolation from adults 17. Anderson and Holford 18 argue that the scaling with size of the majority of biological systems can be described using an allometric power model with fixed exponents (e.g. 0.75 for clearance). This theory is supported by other work – e.g. by Calvier et al. 19, who showed that 0.75 as a fixed scaling exponent provided a good explanation to the clearance of 12 620 hypothetical drugs in older children, with maturation meaning that this relationship breaks down with decreasing age. The age at which 0.75 scaling becomes inappropriate was found to be drug specific 19. Holford et al. 20 separately argue that clearance maturation in intrauterine, neonatal and early life can be described by a sigmoidal (Emax/Hill) function. Germovsek et al. 21 recently showed that combining these methods describes PK maturation well in neonates and children in a review of midazolam and gentamicin PK. Other examples of the success of this combined allometric and maturation approach include the busulfan model by McCune et al. 22 and a comparison of morphine models by Holford et al. 23. One criticism of the focus on paediatric patients in these studies is that a common standard adult mature value is assumed, whereas we know that drug clearance declines with age 24. To recommend beta‐lactam dosing for patients of all ages, it would seem sensible to develop a model based on data from the whole population.
We therefore undertook a review of PK studies of three commonly used beta‐lactam antibiotics: amoxicillin (± clavulanic acid), meropenem and piperacillin–tazobactam. Our aim was to compare the PK parameters reported in critically ill and noncritically ill neonates, children and adults, and to investigate whether allometric and maturation scaling principles could be applied to describe changes in PK parameters through life.
Methods
Data source and search strategy
The US national library of medicine PubMed search engine (including the MEDLINE database) and EMBASE electronic database (using the Wolters Kluwer OVID search engine) were used to search for human studies 25, 26, 27. Drug name (e.g. ‘amoxicillin’) and ‘pharmacokinetic*’ were the key words searched for. Results were taken up to the 30th week of 2017.
Eligibility criteria
English‐language studies were included that published PK parameters from original data or used data for which PK parameters had not been published previously, contained description of participant characteristics and the methods used for obtaining PK parameters, and included eight or more subjects (in order to exclude small case series or case reports).
Data extraction
We extracted the following data: number of participants, patient population and clinical setting, methods for estimating PK parameters and final structural model used (where compartmental methods were used), summary statistics of the age and weight of the study group, and PK parameters (clearance and volume of distribution). The 95% confidence intervals (CIs) for population mean values of PK parameters were recorded where published (including Bootstrap analyses) or were calculated, assuming a Student's t‐distribution where standard deviation or standard error were published.
Scaling of parameters
PK parameters were scaled to 70 kg, using mean participant weight (or median where the mean was not published). Volume of distribution was scaled linearly with weight and clearance was scaled with an allometric exponent of 0.75, as described previously 18 (Equations 1). For parameters that were already allometrically scaled to 70 kg, the typical PK values were used directly from the source paper.
-
Equations 1.Allometric scaling of volume (top) and clearance (bottom) parameters
Where V and CL are volume of distribution and clearance values identified from the study scaled to a 70 kg individual using the mean weight from the study participants (median used where the mean was not presented).
Data summary measures
Population mean/median PK parameters with CIs were plotted and compared in different populations using analysis of variance, where appropriate (adults/children/neonates, healthy/critically ill). Where comparisons between study groups were made (e.g. adult healthy and adult critically ill), unweighted mean parameter values were used. Unweighted means were used to avoid overinfluence of one or two larger studies in specific populations groups – e.g. the Udy et al. study (n = 48) of piperacillin in patients with augmented renal clearance 28. Neonates were less than 28 days’ corrected age, children were 28 days’ corrected age to 18 years and adults were aged over 18. Where corrected, age in prematurely delivered neonates is chronological age from birth minus the number of days of prematurity. Prematurity is defined as birth earlier than 36 weeks’ gestation.
Modelling the maturation–decline of PK through life
Pooled data (unweighted mean parameter estimates) were used to model the effect of ageing on PK parameters. A sigmoidal (Hill) function (Equation 2) was fitted to clearance values to model the maturation of drug clearance with age 21. Postmenstrual age was used (chronological age plus number of weeks’ gestation at birth) in these models. Similarly, a sigmoidal decline function was fitted to model decline in function in old age. An exponential error model was used as these parameters are commonly assumed to be log‐normally distributed. Studies in which the majority of participants were receiving some form of renal replacement therapy were excluded from this analysis. Parameters for these functions were estimated using NONMEM version 7.3 (ICON plc, Ellicott City, MD, USA) 29. Model fit was assessed using established statistical and graphical methods, including likelihood‐based diagnostics (via the NONMEM objective function value) and assessment of model simulation properties (visual predictive check). Model plots and graphical analysis were undertaken using R language and environment for statistical computing, with the ggplot2 package 30, 31.
-
Equation 2.Clearance maturation–decline function
Where: CL is model predicted clearance, CLSTD is a standardized clearance, PMA is postmenstrual age in weeks and PMA50 is the PMA age at which 50% of adult function is achieved; AGE is age in years and AGE50 is the AGE at which 50% of decline has occurred; θs are Hill coefficients. CLSTD, PMA50, AGE50 and θs are estimated in the model fitting process. Model is fitted to the observed (literature) values with parameters chosen to minimize ε.
Results
Study selection
A flowchart of study selection for each drug is provided in Figure 1. A total of 2082 articles were identified and screened, with 130 studies included in the final analysis 15, 16, 28, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158. Some studies provided PK parameters for two drugs (e.g. piperacillin and tazobactam) or several discrete groups (e.g. 0–1, 1–2 years etc.), meaning that 173 sets of PK parameters were available for analysis. A summary of the articles identified, the patient setting, the number of participants and scaled PK parameters with calculated confidence intervals is presented in Appendix 1. The range of methods used to calculate PK parameters included noncompartmental analyses and population approaches using parametric and nonparametric methodology.
Figure 1.
Flow diagram of studies identified in the review of antimicrobial pharmacokinetics (PK)
PK parameters
Table 1 summarizes the demographics and PK parameters from the identified studies, including the range of values identified. Plots of weight‐standardized clearance (Figure 2) and volume of distribution (Figure 3) with associated confidence intervals for population mean are shown. Clavulanic acid data are not presented as only a small number of studies (six) were identified. There were more adult models identified (129) than paediatric (28) and neonatal (16). The range of ages was 25 weeks to 82 years, and weight 0.9–200 kg. Mean drug clearance and volume of distribution were similar for the five drugs, with a range of 8.9–13.9 l h–1 70 k–1g and 23.6–28.9 l 70 kg–1, respectively. Mean clearance values for adults did not appear to differ between settings (healthy/hospital/critical illness), although CIs (Figure 2) appeared greater for studies in critical illness compared with healthy volunteers, perhaps suggesting greater PK variability in critically ill populations. Volume of distribution was significantly greater in critically ill adults compared with healthy volunteers administered piperacillin (25.4 vs. 13.4 l 70 kg–1; P < 0.001) and meropenem (26.2 vs. 16.1; P = 0.02). Comparison between settings for children and neonates was not possible as healthy volunteer data were not available.
Table 1.
Summary statistics from literature review of pharmacokinetic studies
| Drug | Amoxicillin | Piperacillin | Meropenem | Clavulanic acid | Tazobactam |
|---|---|---|---|---|---|
| Number of studies | 23 | 54 | 53 | 6 | 31 |
| By age (neonates/children/adults) | 7/4/13 | 3/8/47 | 3/8/43 | 0/3/3 | 3/5/23 |
| By setting (healthy/hospital/ITU) | 9/5/10 | 9/20/29 | 7/15/32 | 1/2/3 | 4/14/13 |
| Haemodialysis/filtration | 1 | 7 | 9 | 0 | 4 |
| Median number of participants | 13 | 14 | 15 | 13 | 12 |
| Age range (postmenstrual age) | 29 weeks to 82 years | 25 weeks to 71 years | 27 weeks to 76 years | 2.6–62 years | 30 weeks to 71 years |
| Weight range, kg | 1.1–79.4 | 0.9–164.0 | 0.9–200.4 | 14.4–75.0 | 1.4–161.0 |
| Mean drug clearance (all ages), l h –1 70 kg –1 (range) | 10.9 (1.3–22.4) | 10.6 (1.9–22.4) | 10.0 (1.0–24.1) | 13.9 (8.9–17.9) | 8.9 (2.1–25.2) |
| Mean clearance values (adults) by setting l h –1 70 kg –1 (standard deviation) | |||||
| Healthy volunteer | 13.5 (4.6) | 11.3 (3.8) | 11.8 (2.4) | 16.1 (−) | 10.0 (4.9) |
| Hospital inpatient | 11.3 (9.4) | 13.5 (4.2) | 10.8 (4.2) | – | 12.3 (6.8) |
| Critically ill | 10.7 (1.7) | 11.3 (5.3) | 11.0 (4.5) | 11.0 (3.0) | 10.5 (4.9) |
| Mean volume of distribution (all ages), l 70 kg –1 (range) | 28.9 (10.7–53.5) | 25.0 (9.8–203.7) | 23.8 (8.8–50.4) | 23.9 (21.0–30.4) | 23.6 (9.1–63.0) |
| Mean volume of distribution (adults) by setting l 70 kg –1 (standard deviation) | |||||
| Healthy volunteer | 22.3 (9.3) | 13.4 (4.8) | 16.1 (2.9) | 23.1 (−) | 23.9 (26.1) |
| Hospital inpatient | 18.3 (3.1) | 20.4 (4.7) P = 0.04 | 22.0 (10.0) | – | 21.0 (5.7) |
| Critically ill | 22.2 (4.7) | 25.3 (9.9) P < 0.001 | 26.2 (8.2) P = 0.02 | 21.1 (0.2) | 27.2 (9.6) |
| Summary of product characteristics 164, 169, 170 | |||||
| Clearance (l h –1 ) | 25 | 10–17 | 17 | Not published in SPC | |
| Volume of distribution (l) | 21–28 | 17 | 17.5 | ||
Note that some studies included multiple age groups or clinical settings. Analysis of variance undertaken on clearance and volume of distribution values by setting in adults. Healthy volunteer used as reference. Only P‐values <0.05 are shown. Comparison is not possible in other age groups as healthy volunteer studies were not found. Studies in haemodialysis settings were excluded from the analysis of clearance data. ITU, intensive care unit; SPC, summary of product characteristics
Figure 2.
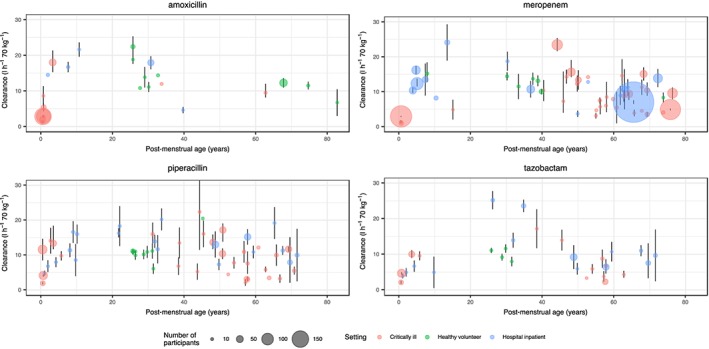
Weight‐standardized clearance values identified from the literature search, plotted against age. The mean clearance values (standardized to a 70‐kg individual) from each study are plotted with an associated confidence interval (where available). The size of the points is proportional to the number of participants. Colours are used to denote the setting of the study. There appears to be greater uncertainty in parameter estimates of studies in critically ill compared with healthy populations. As expected, there is a lower clearance in neonates and elderly populations, despite standardizing values allometrically
Figure 3.
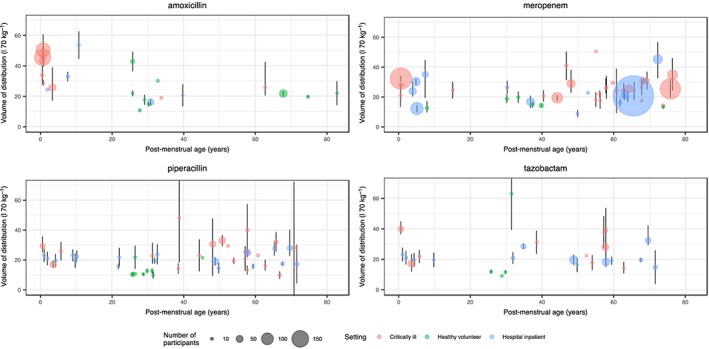
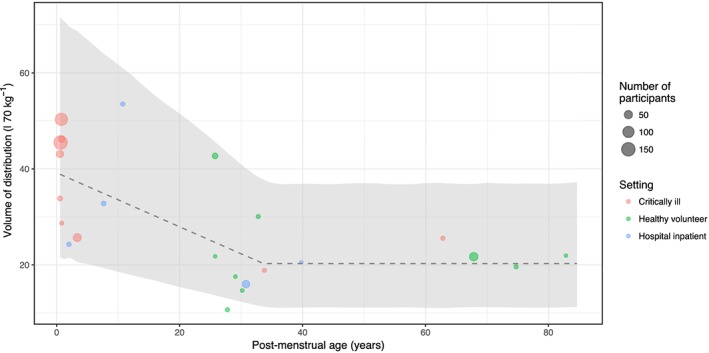
Weight‐standardized volume of distribution values identified from the literature search, plotted against age. Mean volume values (standardized to a 70‐kg individual) from each study are plotted with an associated confidence interval (where available). The size of the points is proportional to the number of participants. Colours are used to denote the setting of the study. There appears to be greater uncertainty in parameter estimates of studies in critically ill compared with healthy populations. Weight‐based allometric scaling appears to control for the effects of age, except for amoxicillin, where there appears to be a greater volume of distribution for neonates compared with adults
Maturation–decline functions
Parameters for the maturation–decline function for each drug are shown in Table 2. These were estimated using NONMEM from the PK parameters identified in the literature review. One study by Cohen‐Wolkowiez et al. 132 was excluded from the piperacillin model fit as it used a scavenged sampling technique and the parameter estimates from this study were distinctly different from others in similar participants, and uncertainty was large. Two ceftolozane–tazobactam studies 134, 135 were excluded from the tazobactam model fit as the clearance values from these studies deviated significantly from similar studies with piperacillin–tazobactam. Clavulanic acid was not modelled as the number of studies was small.
Table 2.
Parameter estimates for clearance maturation–decline function
| Model parameter | Amoxicillin | Piperacillin | Meropenem | Pooled data |
|---|---|---|---|---|
| CLSTD (l h –1 70 kg –1 ) | 17.0 (8) | 12.7 (9) | 34.6 (193) | 12.9 (6) |
| θ1 | 4.29 (34) | 1.8 (31) | 1.1 (26) | 3.45 (77) |
| PMA50 (weeks) | 49.0 (16) | 71.6 (23) | 398 (236) | 49.7 (32) |
| θ2 | 1.95 (41) | 13.8 (361) | 1.11 (65) | 4.0 (44) |
| AGE50 (years) | 79.0 (14) | 74.8 (27) | 31.3 (257) | 86.8 (9) |
| σ2 | 0.08 | 0.11 | 0.11 | 0.13 |
Where: CL is the model predicted clearance; CLSTD is a standardized clearance; PMA is the postmenstrual age in weeks and PMA50 is the PMA age at which 50% of adult function is achieved; AGE is the age in years and AGE50 is the AGE at which 50% of decline has occurred; θs are Hill coefficients. σ2 is the estimated variance of ε. Data presented above are mean parameter estimates (% relative standard error)
The pooled maturation model suggests that size‐standardized clearance approaches adult values at around 2 years postmenstrual age. Figure 4 shows a visual predictive check of the pooled model; it appears to describe age‐related changes at the extremes of life well. As the amoxicillin data suggested higher volume of distribution in neonates, a ‘hockey stick’ function was fitted to these data (Figure 5), with a pivot point at 34 years [relative standard error (RSE) 29%].
Figure 4.
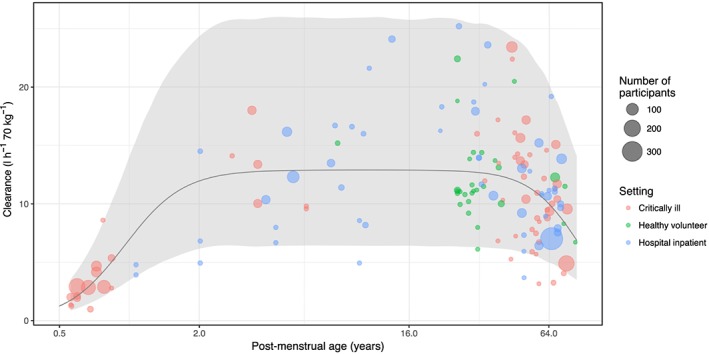
Visual predictive check of maturation–decline model for clearance using pooled data from amoxicillin, piperacillin, meropenem and tazobactam. The shaded area is the interval between the 2.5th and 97.5th centiles of clearance values, simulated using the maturation–decline function (solid black line). Simulations from the model encapsulate literature clearance values (coloured dots) relatively well, although some sit below the lower confidence level
Figure 5.
Visual predictive check of amoxicillin volume of distribution values using the ‘hockey‐stick’ function. Shaded area is the interval between 2.5th and 97.5th centiles of amoxicillin volume of distribution values, simulated using the hockey‐stick function (dashed line)
Discussion
We have, for the first time, presented a unified model to describe beta‐lactam PK throughout life. This was achieved by describing the changes in beta‐lactam PK in early life using the standard allometric scaling and organ maturation functions described by Holford et al. 20 and further extending this model by using a sigmoidal decline function to describe the decline in clearance associated with old age. Parameters from the pooled model suggest that adult values of clearance are achieved at approximately 2 years postmenstrual age, and at 87 years beta‐lactam clearance is half of that found in young adults. This quantification of the effect of age on beta‐lactam PK could be used in dose‐optimization studies.
The final parameter estimates for clearance maturation using pooled data were similar to values identified by Germovsek et al. 21 in their pooled analysis of gentamicin studies. These values are compared, along with the values suggested by Rhodin et al. 159 in their model of glomerular filtration maturation in Table 3, noting that these beta‐lactams undergo tubular secretion alongside filtration.
Table 3.
Maturation‐decline function parameters from this review compared with published values from similar studies
| Model | θ1 | PMA50 (weeks) |
|---|---|---|
| Germovsek et al. 21 | 4.19 (17) | 45.1 (7) |
| Rhodin et al. 159 | 3.40 | 47.7 |
| Pooled from this review | 3.45 (77) | 49.7 (32) |
PMA is the postmenstrual age in weeks and PMA50 is the PMA age at which 50% of adult function is achieved. θ1is the Hill parameter. PMA50 and θ1 are estimated in the model fitting process.
It is worth noting that Germovsek et al. 21, in common with other similar studies that estimate maturation, excluded results from older adults to avoid the confounding effects of age and the natural decline in renal function. In our analysis, we successfully described this decline using a sigmoidal function that mirrored that used to describe maturation. We used age as the covariate. It was not possible to use glomerular filtration to see if it explained all of the age effect, as studies were not consistent in the reporting of renal function. Some did not report it at all, some reported plasma creatinine, and those that did report glomerular filtration used a variety of methods. It is likely that there will remain some age effect, even after taking filtration into account, as active excretion plays a part in the elimination of these drugs. A further related limitation is that, for similar reasons, no account was taken in our model for studies that did include a creatinine clearance function in their clearance models. The AGE50 parameter associated with a decline in clearance with age was similar for amoxicillin and piperacillin at 79 years and 74.8 years, respectively (Table 2). The value of 31.3 years for meropenem suggests that the model may have been skewed by one higher clearance value in children from the study by Pettit et al. 79. The use of a decline function such as this therefore has merit as part of efforts toward dose optimization for all age groups, although investigating the validity of the decline function presented here requires pooled data across age groups with a consistent method of measuring renal function.
When comparing clearance parameters across these populations, it is perhaps interesting to note that mean clearance parameters were similar between critically and noncritically ill individuals (Table 1). Although the prevalence of acute kidney injury in critical illness might lead one to expect lower clearance values in this population, other physiological changes, including high cardiac output/low vascular resistance states, have been recognized to increase clearance for some patients 160, 161, 162. It is therefore not unexpected that mean clearance values in critically ill populations are similar to those in healthy populations. We think it is important to note that the CIs for the estimates of clearance were greater in critical illness studies compared with healthy volunteer studies, and suggest that this may indicate greater PK variability between critically ill individuals. Alternatively, this could be explained by greater systematic experimental error in critically ill studies. However, the observation arises from multiple studies (e.g. 25 critically ill and seven healthy volunteer datasets for piperacillin), and CIs for clearance in critically ill studies were also greater than in hospital inpatient studies (Appendix 1 and Figure 2), where the same systematic experimental errors would reasonably be expected. In addition, the number of participants (n) was greater in critically ill studies (piperacillin mean n of 26 in critically ill individuals vs. 13 in healthy volunteers), which one would ordinarily anticipate leading to greater certainty in parameter estimates. Increased PK variability might be clinically significant for drugs with concentration–time‐dependent killing. For example, the 95% CI for piperacillin clearance in the study by Shikuma et al. 89 had an almost threefold difference between lower and upper bounds (11.5–33.2 l h–1 70 kg–1). Indeed, Roberts et al., in an observational study of beta‐lactams, reported that 16% of patients failed to achieve PK–pharmacodynamic targets, and that this was associated with treatment failure 163. It was also interesting to note that the clearance values identified in the literature review for healthy individuals are lower than those published in the summary of product characteristics (Table 1). For example, the mean weight‐adjusted clearance for amoxicillin identified in healthy volunteer studies was 13.5 l h–1 70 kg–1, compared with 25 l h–1 published in the summary of product characteristics 164. It is not immediately clear why this should be the case. It may be that the summary of product characteristic values arise from unpublished data.
Changes in volume status are common in septic patients. Altered vascular tone and endothelial dysfunction lead to shifts in the distribution of fluid from the vascular to extravascular space 165. This is reflected in the significantly greater volume of distribution described in the patient groups who are likely to be the most unwell (critically ill patients receiving piperacillin–tazobactam and meropenem). The variability in volume of distribution was also marked in critical illness studies. For example, Jeon et al. 16 reported a 100‐fold variation between the lower and upper bounds of the CI of the mean for volume of distribution in a study of burns patients (10.2–1004 l 70 kg–1). This wide variation was all the more remarkable, given that this was a relatively large study, including 50 participants. The relatively larger volume of distribution of amoxicillin in neonates compared with adults reflects recognized physiological differences in this age group 20, and Eleveld et al. 166, 167 recently described similar volume of distribution changes for remifentanil and propofol. The absence of such an effect in meropenem and piperacillin is probably explained by the fact that adult distributions of body water are reached relatively early in life, and there were no studies of these drugs in the very young. Indeed, the pivot point of 34 years in the amoxicillin volume of distribution model is much later than one might expect. This probably reflects a lack of data in young children and adolescents to inform the pivot point in this empirical model fit.
The greatest limitation of the maturation–decline functions described is the degree of uncertainty associated with the parameter estimates. For example, the postmenstrual age at which 50% of adult function is achieved (PMA50) varied from 49 weeks to 398 weeks between drugs, with the large uncertainty for meropenem and tazobactam (relative standard error 236% and 151%, respectively), probably reflecting the lack of data in young children. These estimates are derived from what are, in general, small PK studies, with a median of 14 participants. Furthermore, the uncertainty of PK parameter estimates from these studies was not taken into account in the estimation of the parameters of the maturation–decline function. By using only mean (or median) values, information is clearly lost and each study contributes identically to the model fit, regardless of size of the study or uncertainty reported. However, it is worth noting that the median number of participants was similar across drugs (Table 1), and weighting for sample size might have led to overinfluence of larger studies in specific patient groups – e.g. Jeon et al. 16 n = 50 burns patients. Similarly, requesting raw data was impractical and unlikely to yield a significant response in the limited time available for the present study. Indeed, Germovsek et al. 168, in their review of gentamicin, obtained data from only two of eight authors. Such a low response rate was felt unlikely to improve the inferences that can be made from this retrospective review – particularly as some of the research dates back to the mid‐20th century, further decreasing the potential for obtaining raw data. A prospective age‐inclusive PK study could improve the accuracy of the parameter estimates.
Conclusions
Over the past decade, a standardized method has been developed to handle the maturation of clearance throughout childhood. Much less work has been undertaken to describe the effect of ageing on clearance, limiting the potential to fit models across all age groups. Antimicrobial resistance and high sepsis‐related mortality is a problem for patients of any age. The beta‐lactam model presented here could be used for dose optimization throughout life, although a prospective study to evaluate our model is warranted. We also foresee a number of other potential uses for our model by others. For example, for those conducting focused PK studies with narrow age ranges, the size and maturation parameters in our model could be fixed, thereby allowing for the exploration of other covariates after size and age are delineated. The parameters could potentially assist with the conduct of in vitro hollow‐fibre experiments seeking to mimic human concentration–time profiles in specific age groups. For secondary analysis of clinical trials where no PK are collected, our parameters could be used to predict typical exposure for given dose schemes.
Competing Interests
There are no competing interests to declare.
C.B. has received salary support from the National Institute for Health Research (NIHR ACF‐2016‐18‐016). J.F.S. and C.B. have been supported by the NIHR Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. J.F.S. was supported by a UK Medical Research Council fellowship (MR/M008665/1). No other support was received for this work from outside of the authors' affiliated institutions.
Appendix 1.
Summary of pharmacokinetic parameters identified in literature search
| Ref | Population (n) | Age (years unless other specified) | Weight (kg) | Structural model | Clearance (l h−1 70 kg−1) | Volume at steady state (l 70 kg−1) | Comments/modelling approach |
|---|---|---|---|---|---|---|---|
| Amoxicillin | |||||||
| 136 | Adults, critically ill (13) |
62 IQR (58–72) |
75 IQR (70–79) |
Two compartment |
9.5 (95% CI: 8.2–12.0) |
25.6 (95% CI: 20.4–42.4) |
Population approach |
| 137 | Adults, critically ill (haemorrhagic shock) (12) |
Med 33 range (18–51) |
Med 75 range (61–90) |
Two compartment |
12.0 (6.3–22.2) range only |
18.9 (7.7–28.7) range only |
Population approach + non‐compartmental analysis |
| 138 | Adults, hospitalised (57) |
67 sd (±16) |
78 sd (±20) |
One compartment |
12.3 (95% CI: 10.9–13.6) |
21.7 (95% CI: 20.4–23.0) |
Parameters derived from pre‐specified model using observed concentrations |
| 139 | Adults, long term dialysis (8) |
39 range (17–74) |
54.2 range (43–66) |
Two‐compartment linear model |
4.6 (95% CI: 3.8–5.4) |
20.5 (95% CI: 13.3–27.8) |
Predefined model |
| 140 | Adults, pregnant requiring amoxicillin (44) |
30 sd (±6.9) |
79.4 sd (±14.0) |
Three compartment |
17.9 (95% CI: 16.1–19.7) |
16.0 (95% CI: 13.7–18.4) |
Population approach |
| 137 | Adults, healthy volunteers (12) |
Med 32 Range (20–54) |
Med 74 range (53–89) |
Two compartment |
14.4 (12.7–18.4) range only |
30.1 (11.2–26.6) range only |
|
| 141 | Adults, healthy volunteers (9) |
28.3 range (21–45) |
66.4 range (46–88) |
Two compartment |
13.8 (95% CI: 11.0–16.7) |
17.6 (95% CI: 14.4–20.8) |
Pre‐specified model |
| 142 | Adults, healthy volunteers (24) | range (18–32) | range (57–98) | Two compartment |
22.4 (95% CI: 19.5–25.3) |
42.7 (95% CI: 36.3–49.1) |
Regression analysis with pre‐specified model. Mean weight not available. Assumed 70 kg |
| 143 | Adult, elderly (12) |
73.9 range (69–83) |
64.9 range (52–83) |
Two compartment |
11.5 (95% CI: 10.4–12.6) |
19.6 (95% CI: 18.7–20.5) |
Nonlinear least squares regression analysis, pre‐specified structural model |
| 144 | Adult, elderly (8) |
82 range (69–87) |
67 range (51–82) |
Non‐compartmental analysis |
6.7 (95% CI: 3.0–10.4) |
21.9 (95% CI: 14.1–29.8) |
Parameters calculated using trapezoid rule and log‐linear regression |
| 145 | Adults, healthy volunteers (9) |
29 range (21–38) |
75.0 range (63–94) |
Two compartment |
11.1 (95% CI: 9.7–12.5) |
14.7 (95% CI: 13.2–16.2) |
Nonlinear least squares regression analysis, pre‐specified structural model |
| 146 | Adults, healthy volunteers (8) | range (20–30) |
74.5 range (59–91) |
Two compartment |
18.8 (95% CI: 17.6–20.0) |
21.8 (95% CI: 20.0–23.6) |
Iterative least‐squares process, pre‐specified structural model |
| 147 | Adults, healthy volunteers (12) |
27 sd (±3.8) |
64.8 sd (± 5.1) |
Two compartment | 10.8* |
10.7 (95% CI: 10.4–11.0) |
Pre‐specified model. *Calculated from other parameters |
| 148 | Children, critically ill (50) |
2.6 range (1/12–15) |
14.4 range (4–65) |
Three compartment |
18.0 (95% CI: 15.3–21.3) |
25.7 (95% CI: 17.0–38.9) |
Population approach |
| 149 | Children, ‘seriously ill’ (15) |
6.9 range (2–14) |
Not recorded | Non‐compartmental |
16.7 (95% CI: 15.3–18.1) |
32.8 (95% CI: 29.8–35.8) |
Trapezoidal rule |
| 150 | Children, treated for viral infection or neurological disease (12) |
10 range (2–14.5) |
Not reported | Two‐compartment |
21.6 (95% CI: 19.6–23.6) |
53.5 (95% CI: 44.5–62.5) |
Regression/trapezoidal rule |
| 151 | Infants and Children treated for infection (14) |
14.6 months (mean only) |
Not reported |
14.5 (mean only reported) |
24.3 (mean only reported) |
Regression analysis using least mean squares | |
| 152 | Neonates, hypothermia (125) |
GA 40 weeks range (36–42) PNA 5 days (2–5) |
Median 3.3 (2.1–5.1) |
Two compartment |
2.9 (95% CI: 2.7–3.2) |
50.3 (95% CI: 40.6–60.5) |
Population approach; allometric scaling |
| 153 | Neonates, premature (40) |
GA 28.9 weeks range (24–32) PNA 1.1 days (1–3) |
1.1 (0.6–1.5) | One compartment |
2.0 (CV 6.6%) |
43.1 (CV 7.6%) |
Population approach |
| 154 | Neonates, premature (17) |
GA 29 weeks sd (±6/7) PNA 3 days |
1.2 (±0.3) | One compartment |
1.3 (95% CI: 1.0–1.5) |
33.8 (95% CI: 27.8–39.8) |
Visual inspection used to determine structural model |
| 155 | Neonates, premature (150) |
GA 34.6 weeks range (24.9–42.4) PNA 0.8 days range (0–9) |
2.3(±1.1) | One compartment |
2.9 (95% CI: 2.7–3.0) |
45.5 (95% CI: 44.0–47.0) |
Iterative two stage Bayesian fitting procedure, pre‐specified model |
| 156 | Neonates, premature (11) |
PNA 26 days range (1–63) |
3.4 range (2.9–3.8) |
One compartment |
2.8 (95% CI: 2.7–2.9) |
28.7 (95% CI: 26.8–30.6) |
Pre‐specified model |
| 157 | Neonates, PNA > 9 days (32) |
PNA 24.7 days range (10–52) |
2.3 range (0.8–4.3) | One compartment |
5.4 (95% CI: 4.3–6.4) |
46.2 (95% CI: 39.4–53.0) |
Iterative two stage Bayesian fitting procedure, pre‐specified model |
| 158 | Neonates (11) |
GA 38 weeks sd (±3) |
3 (±0.8) | Non‐compartmental |
8.6 (95% CI: 5.9–11.3) |
– | Continuous infusion study, steady state assumed |
| Clavulanic acid | |||||||
| 136 | Adults, critically ill (13) |
62 IQR (58–72) |
75 IQR (70–79) |
Two compartment |
8.9 (95% CI: 6.0–12.2) |
21.8 (95% CI: 14.2–68.1) |
Population approach |
| 137 | Adults, critically ill (haemorrhagic shock) (12) |
33 range (18–51) |
Med 75 range (61–90) | Two compartment |
13.1 range (6.6–22.8) |
21 range (13.5–32.3) |
Population approach + non‐compartmental analysis |
| 137 | Adults, well volunteers (12) |
32 range (20–54) |
74 range (53–89) |
Two compartment |
16.1 range (9.0–33.6) |
23.1 range (17.8–99.2) |
Population approach + non‐compartmental analysis |
| 148 | Children, critically ill (50) |
2.6 range (1/12–15) |
14.4 range (4–65) |
Two compartment |
12.2 (95% CI: 10.5–14.6) |
21.6 (95% CI: 14.2–68.1) |
Population approach |
| 149 | Children, ‘seriously ill’ (15) |
6.9 range (2–14) |
Not recorded | Non‐compartmental |
17.9 (95% CI: 13.3–22.5) |
30.4 (95% CI: 23.5–37.3) |
Trapezoidal rule |
| 150 | Children, with viral infection & neurological disease (12) |
10 range (2–14.5) |
Not reported | Two‐compartment |
15.2 (95% CI: 13.4–17.0) |
25.8 (95% CI: 23.6–28.0) |
Regression/trapezoidal rule |
Mean or median values presented, with associated range, interquartile range (IQR) or standard deviation (SD). The 95% confidence intervals (CIs) have been calculated, where SD or standard error data were available, assuming a Student's t‐distribution. GA, gestational age; PMA, postmenstrual age; CV, coefficient of variation, PNA is post‐natal age, CV is coefficient of variation. Where a ‘*’ appears, a corresponding explanatory comment is noted in the comments column
| Ref | Population (n) | Age (years unless other specified) | Weight (kg) | Structural model |
Clearance (l h−1 70 kg−1) |
Volume at steady state (l 70 kg−1) | Comments/modelling approach |
|---|---|---|---|---|---|---|---|
| Piperacillin | |||||||
| 85 | Adults, critically ill (15) |
62 IQR (58–72) |
78 IQR (70–79) |
Two compartment |
12.2 IQR (9.4–20.9) |
23.1 IQR (15.7–22.6) |
Population approach |
| 86 | Adults, critically ill, septic shock (high creatinine) (15) |
66 IQR (59, 79) |
80 IQR (70.2, 95) |
Two compartment |
3.3 (95% CI: 2.1–4.4) |
9.8 (95% CI: 7.4–12.2) |
Non‐linear mixed‐effects methods |
| 87 | Adults, critically ill (18) |
56 range (31.4–80.8) |
80.0 range (47–140) |
Two compartment (+ lung compartment) |
10.9 (95% CI: 7.9–14.0) |
*10.2 (95% CI: 8.1–12.4) |
Non‐parametric population approach. *central compartment only available |
| 88 | Adults, critically ill (22) |
65 range (22–89) |
70 range (38–120) |
One compartment |
10.0 (95% CI: 7.0–13.0) |
32.1 (95% CI: 25.7–38.5) |
Non‐linear mixed‐effects methods |
| 89 | Adults, critically ill surgical (11) |
43.6 sd (±15.9) |
76 sd (±11.0) |
Two compartment |
22.4 (95% CI: 11.5–33.2) |
23.0 (95% CI: 12.4–33.7) |
Non‐linear least‐squares regression analysis |
| 90 | Adults, critically ill with sepsis (16) |
30.5 range (22–65) |
76.5 range (64–86) |
Two compartment |
16.0 (95% CI: 13.5–19.3) |
22.9 (95% CI: 17.6–31.5) |
Non‐linear mixed‐effects methods |
| 91 | Adults, critically ill, indigenous Australian (9) |
43 sd (±11) |
76 sd (±11) |
Two compartment |
5.3 (95% CI: 3.0–7.6) |
*13.4 (95% CI: 8.7–18.0) |
P‐metrics compartmental analysis—parametric/non‐parametric not specified. *central compartment only published |
| 28 | Adults, critically ill with augmented renal clearance (48) |
47.3 sd (±17.9) |
88.4 sd (±24.2) |
Two compartment |
13.7 (95% CI: 11.8–15.9) |
30.6 (95% CI: 9.3–47.6) |
Non‐linear mixed‐effects methods |
| 15 | Adults, critically ill with burns and infection (10) |
37.7 range (22–50) |
77.8 range (45–105) |
Non‐compartmental |
6.8 (95% CI: 4.3–9.3) |
14.2 (95% CI: 10.8–17.7) |
Unspecified |
| 16 | Adults, critically ill with burns and infection (50) |
50.1 range (20–83) |
66.9 range (50–90) |
Two compartment |
17.2 (95% CI: 13.9–19.0) |
43.3 (95% CI: 10.2–1004.5) |
Non‐linear mixed‐effects methods |
| 92 | Adults, critically ill with burns and infection (9) |
38 range (20–58) |
80 range (55–96) |
Two compartment |
13.5 (95% CI: 9.1–17.9) |
48.1 (95% CI: 18.4–77.9) |
Nonlinear least‐squares regression |
| 93 | Adults, critically ill, obese and non‐obese (50) |
50 sd (±15) |
104 sd (±35) |
Two compartment |
10.4 (95% CI: 8.9–11.9) |
33.0* (95% CI: 29.3–36.6) |
Not specified. Presume population approach based on analysis of residuals. *central compartment only published |
| 94 | Adults, critically ill, obese (9) |
57 sd (+11) |
164 sd (±50) |
One compartment |
3.2 (95% CI: 2.5–3.8) |
13.2 (95% CI: 10.7–15.7) |
trapezoidal rule and log‐linear least squares |
| 95 | Adults, critically ill, hospital acquired pneumonia (50) |
68.4 sd (±7.1) |
66.7 sd (±8.6) |
Non‐compartmental |
11.7 (95% CI: 11.0–12.4) |
Volume not published | Log trapezoidal method |
| 96 | Adults, critically ill requiring haemofiltration (16) |
57 sd (±16) |
74 sd (±8) |
Two compartment |
7.6 (95% CI: 4.7–11.0) |
40.0 (95% CI: 26.7–57.3) |
Population approach |
| 97 | Adults, critically ill requiring haemofiltration (20) |
63 IQR (54–74.8) |
81.7 IQR (64.6, 93.2) |
Non‐compartmental |
3.9 IQR (2.9–5.5) |
Volume not published | Unspecified |
| 98 | Adults, critically ill, requiring haemofiltration (42) |
56.8 sd (±15.5) |
95.1 sd (±26.8) |
One compartment |
3.1 IQR (0.2–6.0) |
25.4 IQR (2.9–47.8) |
‘standard first‐order equations’ |
| 99 | Adults, critically ill, requiring haemofiltration (10) |
62 IQR (54.5–68.8) |
87.5 IQR (68.5–98.8) |
Two compartment |
5.8 (95% CI: 5.2–6.7) |
16.2 (95% CI: 13.0–20.2) |
Non‐linear mixed‐effects methods |
| 100 | Adults, critically ill, requiring haemofiltration (19) |
70 range (39–82) |
80 range (45–129) |
Two compartment |
5.5 (95% CI: 4.5–6.7) |
28.3 (95% CI: −6.8*–72.3) |
Non‐linear mixed‐effects methods. *Negative bootstrap estimate |
| 101 | Adults, critically ill, requiring haemofiltration (9) |
56.4 sd (±15.2) |
86.6 sd (±22.6) |
Two compartment |
2.1 (95% CI: 1.2–3.0) |
20.9 (95% CI: 12.9–29.0) |
Weighted non‐linear least‐square regression |
| 102 | Adults, critically ill, requiring haemofiltration (10) |
51.6 sd (±15.6) |
83.4 sd (±21.8) |
Non‐compartmental |
4.3 IQR (3.7–5.4) |
29.4 IQR (20.3–34.3) |
Log‐transformed concentration‐time plots |
| 103 | Adults, critically ill and hospitalised (13) |
53.2 sd (±13.2) |
79.6 sd (±13.8) |
Non‐compartmental |
7.8 (95% CI: 6.2–9.5) |
19.4 (95% CI: 27.3–21.6) |
Linear regression of log‐concentration plots and trapezoidal rule |
| 104 | Adults, hospitalised, nosocomial infections (50) |
57 sd (±16) |
61.1 sd (±10.1) |
One compartment |
15.2 (95% CI: 14.1–16.3) |
24.9 (95% CI: 21.9–27.8) |
Non‐linear mixed‐effects methods |
| 105 | Adults, obese, hospitalised, treated for infection (14) |
49 sd (±10) |
161 sd (±29) |
Unspecified |
7.3 (95% CI: 5.7–8.9) |
14.5 (95% CI: 11.0–18.0) |
Non‐linear least squares regression |
| 106 | Adults, hospitalised and critically ill, treated for infection (11) |
44.7 sd (±12.5) |
78 sd (±22.1) |
Two compartment |
16.1 (95% CI: 12.3–19.9) |
17.0* (95% CI: 11.4–22.5) |
Non‐parametric population approach. *central volume of distribution only published |
| 107 | Adults, hospitalised, treated for infection (33) |
68.8 sd (±11) |
58.2 sd (±10) |
Two compartment |
7.9 (95% CI: 2.1–15.1) |
28.0 (95% CI: 22.7–40.1) |
Non‐linear mixed‐effects methods |
| 108 | Adults, treated for intra‐abdominal infection (56) |
48 range (18–85) |
81.8 range (55–136) |
One compartment |
13.0 (95% CI: 9.3–16.7) |
19.1 (95% CI: 16.4–21.8) |
Non‐linear mixed‐effects methods |
| 109 | Adults, treated for intra‐abdominal infection (18) |
31.1 sd (±8.5) |
75.6 sd (±16.9) |
Non‐compartmental |
13.9 (95% CI: 12.1–15.8) |
19.4 (95% CI: 17.5–21.4) |
Unspecified, LAGRAN computer program |
| 110 | Adults, haematological malignancy, receiving chemotherapy (16) |
31.9 sd (±15.4) |
56.4 sd (±11.2) |
Non‐compartmental |
11.7 (95% CI: 7.6–15.7) |
23.8 (95% CI: 17.1–30.5) |
Calculated from time concentration plots |
| 111 | Adults, haematological malignancy, febrile neutropenic (12) |
64.5 IQR (60.5–71.0) |
75.0 IQR (63.7–93.2) |
Non‐compartmental |
19.2 (95% CI: 14.7–23.7) |
27.7 (95% CI: 23.0–32.5) |
PKSolver |
| 112 | Adults, cystic fibrosis with infection (9) |
33 sd (±12.6) |
53.6 sd (± 6.5) |
Two compartment |
20.2 (95% CI: 17.1–23.3) |
17.3* (95% CI: 9.4–25.2) |
Population approach, two compartment pre specified. *Central volume of distribution only published. |
| 113 | Adults, volunteers with cystic fibrosis (8) |
21 sd (±4) |
43.1 sd (±7.8) |
Two compartment |
16.3 (95% CI: 15.1–17.7) |
15.6 (95% CI: 14.0–17.2) |
Population approach |
| 114 | Adults, cystic fibrosis with infection (13) |
21.3 sd (±6.3) |
41.8 sd (±13) |
Non‐compartmental |
18.3 (95% CI: 12.6–24.0) |
21.7 (95% CI: 15.4–28.0) |
Least squares regression analysis of log‐linear plots and trapezoidal rule. *dose 450 mg kg−1 day−1 |
| 115 | Adults, undergoing elective surgery (18) |
66.8 sd (±12) |
72.3 sd (±11.4) |
Non‐compartmental |
11.3 (95% CI: 10.1–12.6) |
17.5 (95% CI: 16.1–18.9) |
Linear regression of log‐concentration plots and trapezoidal rule |
| 116 | Adults, undergoing prostate surgery (24) |
70.8 sd (±6.6) |
61.9 sd (±9.7) |
Three compartment |
10.0 (95% CI: 2.5–17.5) |
17.3 (95% CI: 4.3–30.3) |
Non‐linear mixed‐effects methods |
| 117 | Adults, hydrocephalus, treated for infection (9) |
58.6 sd (±9.6) |
81.2 sd (±10.3) |
Non‐compartmental |
10.9 (95% CI: 9.1–12.7) |
15.8 (95% CI: 14.1–17.4) |
Linear regression of log‐concentration and trapezoidal rule |
| 118 | Adults, healthy volunteers (11) |
29 sd (±8.9) |
69.8 sd (±15.7) |
Non‐compartmental |
10.9 (95% CI: 9.2–12.6) |
12.7 (95% CI: 11.3–14.2) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 119 | Adults, healthy volunteers (12) |
25 range (23–30) |
78.4 range (60.4–96.3) |
Non‐compartmental |
10.9 (95% CI: 10.2–11.6) |
10.6 (95% CI: 9.8–11.5) |
Non‐linear iterative least‐squares method |
| 120 | Adults, healthy volunteers (10) | range (25–64) |
70.9 sd (±13.9) |
Non‐compartmental |
20.5 No uncertainty reported |
30.7 No uncertainty reported |
Data fitted to regression lines |
| 113 | Adults, healthy volunteers (26) |
25 sd (±4) |
71.1 sd (±11.8) |
Two compartment |
11.2 (95% CI: 10.7–11.6) |
10.4 (95% CI: 9.7–10.8) |
Population approach |
| 121 | Adults, healthy volunteers (12) |
25.7 sd (±2.4) |
68.4 sd (±11.7) |
Two compartment |
10.0 (95% CI: 8.7–11.2) |
21.8 (95% CI: 14.1–29.5) |
Non‐linear least squares method |
| 122 | Adults, healthy volunteers (12) |
28 sd (±8) |
70 sd (±17) |
Non‐compartmental |
10.2 (95% CI: 8.9–11.5) |
10.5 (95% CI: 9.6–11.4) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 123 | Adults, healthy volunteer (10) |
25.7 sd (±3.1) |
69.6 sd (±9.7) |
Three compartment |
11.0 Intervals not disclosed |
10.9 Intervals not disclosed |
Non‐linear mixed‐effects methods |
| 124 | Adults, healthy volunteers (10) |
30.4 range (23–44) |
68.1 sd (±12.1) |
Two compartment |
11.2 (95% CI: 9.0–13.4) |
12.7 (95% CI: 11.0–14.3) |
Nonlinear regression analysis |
| 125 | Adults, healthy volunteers, high dose piperacillin (10) |
30.7 sd (±7.6) |
73.7 sd (±15.5) |
Non‐compartmental |
6.1 (95% CI: 4.7–7.6) |
9.9 (95% CI: 7.7–12.1) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 126 | Children, critically ill (13) |
2 range (0.75–6) |
14.5 sd (± 6) |
Two compartment |
14.1 (95% CI: 10.4–17.8) |
17.4* (95% CI: 8.5–26.4) |
Non‐parametric. *central compartment only published |
| 127 | Children, critically ill (47) |
2.8 range (0.17–15) |
14 range (3.4–45) |
Two compartment |
13.4 (95% CI: 11.7–18.4) |
17.0 (95% CI: 14.9–19.6) |
Non‐linear least squares method |
| 128 | Children, critically ill (12) |
5 IQR (1.75–6.5) |
17.8 IQR (11.4,20) |
One compartment |
9.8 (95% CI: 8.5–11.1) |
25.9 (95% CI: 19.8–31.9) |
Non‐linear least squares method |
| 129 | Children, oncology patients febrile neutropenia (21) |
7.4 sd (±2.1) |
28.5 sd (± 9.7) |
Two compartment |
11.4 (95% CI: 9.5–13.3) |
13.9* (95% CI: 10.5–17.3) |
Non‐parametric. *Central volume only published |
| 130 | Children with suspected infection (11) | Range (6–12) | Not reported | Non‐compartmental |
8.6 (95% CI: 7.9–9.2) |
19.6 (95% CI: 14.9–24.3) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 130 | Children with suspected infection (12) | Range (2–5) | Not reported | Non‐compartmental |
8.0 (95% CI: 6.6–9.4) |
19.6 (95% CI: 15.2–24.0) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 130 | Children with suspected infection (12) | Range (0.5–2) | Not reported | Non‐compartmental |
6.8 (95% CI: 5.1–8.5) |
21 (95% CI: 16.6–25.4) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 130 | Children with suspected infection (12) | Range (0.2–0.4) | Not reported | Non‐compartmental |
4.8 (95% CI: 4.1–5.5) |
23.1 (95% CI: 18.7–27.5) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 114 | Children, cystic fibrosis with infection (15)* |
9.4 sd (±1.8) |
23.4 sd (±7.2) |
Non‐compartmental |
16.0 (95% CI: 13.3–18.7) |
22.4 (95% CI: 18.5–26.3) |
Least squares regression analysis of log‐linear plots and trapezoidal rule. *900 mg kg−1 day−1 dose |
| 114 | Children, cystic fibrosis with infection (15)* |
8.3 sd (±3.3) |
20.8 sd (±6.3) |
Non‐compartmental |
16.6 (95% CI: 13.5–19.7) |
23.1 (95% CI: 19.2–27.0) |
Least squares regression analysis of log‐linear plots and trapezoidal rule. *600 mg kg−1 day−1 dose |
| 131 | Infants and neonates treated for infection (71) |
PMA 37.5 weeks sd (±5) |
2.76 sd (±1) |
Two compartment |
4.2 (95% CI: 3.9–4.5) |
18.8* (95% CI: 14.8–21.8) |
Non‐linear mixed‐effects. *Central compartment only published |
| 132 | Infants and neonates, treated for infection (77) | PMA 29 weeks range (23–40) |
0.9 range (0.4–2.5) |
One compartment |
11.6 (95% CI: 8.5–14.7) |
203.7 (95% CI: 114.8–393.1) |
Non‐linear mixed‐effects. Scavenged samples |
| 133 | Infantes and neonates treated for infection (32) | PMA 32 weeks range (25–48) |
1.4 range (0.4–4.0) |
One compartment |
1.9 (95% CI: 1.6–2.3) |
29.4 (95% CI: 25.2–35.7) |
Non‐linear mixed‐effects. Dried blood spot samples |
| Tazobactam | |||||||
| 87 | Adults, critically ill (18) |
56 range (31.4–80.8) |
80.0 range (47–140) |
Two compartment (+lung compartment) |
8.8 (95% CI: 6.3–11.3) |
*13.0 (95% CI: 9.8–16.1) |
Non‐parametric population approach. *central compartment only available |
| 96 | Adults, critically ill requiring haemofiltration |
57 sd (±16) |
74 sd (±8) |
Two compartment |
6.7 (95% CI: 4.6–8.6) |
39.4 (95% CI: 17.7–53.6) |
Population approach |
| 98 | Adults, critically ill, requiring haemofiltration (42) |
56.8 sd (±15.5) |
95.1 sd (±26.8) |
One compartment |
2.3 IQR (0.08–4.5) |
28.0 IQR (7.7–48.4) |
‘standard first‐order equations’ |
| 99 | Adults, critically ill, requiring haemofiltration (10) |
62 IQR (54.5, 68.8) |
87.5 IQR (68.5, 98.8) |
Two compartment |
4.3 (95% CI: 3.5–5.3) |
14.0 (95% CI: 10.8–18.2) |
Non‐linear mixed‐effects methods |
| 101 | Adults, critically ill, requiring haemofiltration (9) |
56.4 sd (±15.2) |
86.6 sd (±22.6) |
Two compartment |
3.8 (95% CI: 2.3–5.3) |
37.7 (95% CI: 27.1–48.2) |
Weighted non‐linear least‐square regression |
| 102 | Adults, critically ill, requiring haemofiltration (10) |
51.6 sd (±15.6) |
83.4 sd (±21.8) |
Non‐compartmental |
3.3 IQR (2.9–3.7) |
22.4 IQR (16.8–25.2) |
Log‐transformed concentration‐time plots |
| 15 | Adults, critically ill with burns and infection (10) |
37.7 range (22–50) |
77.8 range (45–105) |
Non‐compartmental |
17.2 (95% CI: 11.7–22.6) |
31.1 (95% CI: 23.6–38.7) |
Unspecified |
| 103 | Adults, critically ill and hospitalised (13) |
53.2 sd (±13.2) |
79.6 sd (±13.8) |
Non‐compartmental |
5.9 (95% CI: 4.6–7.2) |
17.9 (95% CI: 13.0–22.7) |
Linear regression of log‐concentration plots and trapezoidal rule |
| 104 | Adults, hospitalised, nosocomial infections (50) |
57 sd (±16) |
61.1 sd (±10.1) |
One compartment |
6.4 (95% CI: 4.3–7.6) |
18.3 (95% CI: 15.2–19.8) |
Non‐linear mixed‐effects methods |
| 105 | Adults, obese, hospitalised, treated for infection (10) |
49 sd (±10) |
161 sd (±29) |
Unspecified |
5.9 (95% CI: 4.3–7.6) |
16.3 (95% CI: 11.5–21.1) |
Non‐linear least squares regression. Note low parameter estimates after scaling in this obese cohort. |
| 106 | Adults, hospitalised and critically ill, treated for infection (11) |
44.7 sd (±12.5) |
78 sd (±22.1) |
Two compartment |
14.0 (95% CI: 11.1–16.8) |
19.0* (95% CI: 12.5–25.4) |
Non‐parametric population approach. *central volume of distribution only published |
| 107 | Adults, hospitalised, treated for infection (33) |
68.8 sd (±11) |
58.2 sd (±10) |
Two compartment |
7.5 (95% CI: 3.3–13.1) |
32.4 (95% CI: 29.3–42.2) |
Non‐linear mixed‐effects methods |
| 108 | Adults, treated for intra‐abdominal infection (56) |
48 range (18–85) |
81.8 range (55–136) |
One compartment |
9.2 (95% CI: 5.9–12.5) |
19.7 (95% CI: 16.4–23.0) |
Non‐linear mixed‐effects methods |
| 109 | Adults, treated for intra‐abdominal infection (18) |
31.1 sd (±8.5) |
75.6 sd (±16.9) |
Non‐compartmental |
14.0 (95% CI: 11.9–16.0) |
20.8 (95% CI: 17.0–24.6) |
Unspecified, LAGRAN computer program |
| 115 | Adults, undergoing elective surgery (18) |
66.8 sd (±12) |
72.3 sd (±11.4) |
Non‐compartmental |
11.0 (95% CI: 9.5–12.5) |
20.8 (95% CI: 18.2–21.0) |
Linear regression of log‐concentration plots and trapezoidal rule |
| 116 | Adults, undergoing prostate surgery (24) |
70.8 sd (±6.6) |
61.9 sd (±9.7) |
Three compartment |
9.7 (95% CI: 2.4–16.9) |
14.8 (95% CI: 3.7–25.9) |
Non‐linear mixed‐effects methods |
| 117 | Adults, hydrocephalus, treated for infection (9) |
58.6 sd (±9.6) |
81.2 sd (±10.3) |
Non‐compartmental |
10.7 (95% CI: 8.0–13.5) |
29.1 (95% CI: 16.4–21.7) |
Linear regression of log‐concentration and trapezoidal rule |
| 134 | Adults, cystic fibrosis (20) |
25.4 sd (±9.7) |
53.2 sd (±8.2) |
Two compartment |
25.2 (95% CI: 22.7–27.7) |
10.3* (95% CI: 8.7–12.0) |
Non‐parametric approach. Ceftolazone‐tazobactam study. *central compartment only published |
| 135 | Healthy adults, japanese/chinese/white (29) |
34 sd (±8.3) |
63.0 sd (±7.8) |
Non‐compartmental |
23.6 (95% CI: 21.9–25.3) |
28.4 (95% CI: 26.7–30.2) |
Log‐linear transformation, trapezoidal methods. Ceftolazone‐tazobactam study. |
| 118 | Adults, healthy volunteers (11) |
29 sd (±8.9) |
69.8 sd (±15.7) |
Non‐compartmental |
11.6 (95% CI: 10.5–12.8) |
11.6 (95% CI: 10.5–12.8) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 119 | Adults, healthy volunteers (12) | 25 range (23–30) |
78.4 range (60.4–96.3 |
Non‐compartmental |
11.1 (95% CI: 10.4–11.8) |
11.9 (95% CI: 10.7–13.1) |
Non‐linear iterative least‐squares method |
| 122 | Adults, healthy volunteers (12) |
28 sd (±8) |
70 sd (±17) |
Non‐compartmental |
9.2 (95% CI: 8.3–10.1) |
9.1 (95% CI: 8.9–9.3) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 125 | Adults, healthy volunteers, high dose tazobactam (10) |
30.7 sd (±7.6) |
73.7 sd (±15.5) |
Non‐compartmental |
8.0 (95% CI: 6.7–9.3) |
63 (95% CI: 39.3–86.7) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 127 | Children, critically ill (47) |
2.8 range (0.17–15) |
14 range (3.4–45) |
Two compartment |
10.0 (95% CI: 9.0–11.1) |
17.2 (95% CI: 11.9–23.3) |
Non‐linear least squares method |
| 128 | Children, critically ill (12) |
5 IQR (1.75, 6.5) |
17.8 IQR (11.4, 20) |
One compartment |
9.6 (95% CI: 8.3–10.8) |
21.8 (95% CI: 17.5–26.0) |
Non‐linear least squares method |
| 130 | Children with suspected infection (11) | 6–12 | Not reported | Non‐compartmental |
4.9 (95% CI: 0.5–9.3) |
19.6 (95% CI: 14.9–24.3) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 130 | Children with suspected infection (12) | 2–5 | Not reported | Non‐compartmental |
6.7 (95% CI: 5.1–8.2) |
19.6 (95% CI: 15.2–24.0) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 130 | Children with suspected infection (12) | 0.5–2 | Not reported | Non‐compartmental |
4.9 (95% CI: 3.7–6.1) |
21 (95% CI: 16.6–25.4) |
Least squares regression analysis of log‐linear plots and trapezoidal rule |
| 130 | Children with suspected infection (12) | 0.2–0.4 | Non‐compartmental |
3.9 (95% CI: 3.3–4.6) |
23.1 (95% CI: 18.7–27.5) |
Least squares regression analysis of log‐linear plots and trapezoidal rule | |
| 131 | Infants and neonates treated for infection (71) |
PMA 37.5 weeks sd (±5) |
2.76 sd (±1) |
Two compartment |
4.7 (95% CI: 4.3–5.0) |
30.3 (95% CI: 22.3–38.2) |
Non‐linear mixed‐effects |
| 133 | Infantes and neonates treated for infection (32) | PMA 32 weeks range (25–48) |
1.4 range (0.4–4.0) |
One compartment |
2.1 (95% CI: 1.7–2.5) |
39.9 (95% CI: 36.4–44.8) |
Non‐linear mixed‐effects. Dried blood spot samples |
Mean or median values presented. With associated range, interquartile range (IQR) or standard deviation (sd). 95% confidence intervals have been calculated, where standard deviation or standard error data was available, assuming a student's t‐distribution. PMA is post‐menstrual age. Where a ‘*’ appears, a corresponding explanatory comment is noted in the comments column
| Ref | Population (n) | Age (years unless other specified) | Weight (kg) | Structural model |
Clearance (l h−1 70 kg−1) |
Volume at steady state (l 70 kg−1)* | Comments/modelling approach |
|---|---|---|---|---|---|---|---|
| Meropenem | |||||||
| 32 | Adults, critically ill, (19) |
49 sd (±15.9) |
95 sd (±22) |
Two compartment |
12.3 (95% CI: 10.0–14.6) |
8.6* (95% CI: 6.6–10.6) |
Non‐parametric adaptive grid algorithm. *central volume only published |
| 33 | Adults, critically ill, requiring haemofiltration (15) |
59 range (33–85) |
82.3 range (45–128.5) |
Two compartment |
7.9 range (1.8–23.9) |
29.3 range (17.5–69.4) |
Non‐linear mixed‐effects methods |
| 34 | Adults, critically ill (55) |
63.4 sd (±15.1) |
78.4 sd (±18.4) |
Three compartment (one for lung) |
9.4 (95% CI: 7.6–10.0) |
25.5 (95% CI: 17.0–57.8) |
Non‐linear mixed‐effects methods |
| 35 | Adults, critically ill (15) |
73 IQR (52, 94) |
78 IQR (65.5, 90.5) |
Two compartment |
4.1 (95% CI: 2.7–7.2) |
14.1 (95% CI: 12.7–19.4) |
Non‐linear regression (WinNonlin) |
| 36 | Adults, critically ill (27) |
62 sd (±12) |
76.2 sd (±30.3) |
One compartment |
8.8 (95% CI: 7.1–10.5) |
24.1 (95% CI: 18.8–29.4) |
Non‐linear mixed‐effects methods |
| 37 | Adults, critically ill (10) |
67 sd (±19) |
72 sd (±15) |
Two compartment |
11.3 (95% CI: 9.1–13.4) |
26.3 (95% CI: 21.0–31.7) |
Extended least squares regression method, trapezoidal rule |
| 38 | Adults, critically ill (15) |
55.3 sd (±14.3) |
83.6 sd (±15.4) |
Not specified |
7.5 (95% CI: 6.7–8.3) |
21.9 (95% CI: 19.8–24.1) |
Kinetica program |
| 39 | Adults, critically ill with severe infection/septic shock (50) | 67.5 (±13.9) |
62.2 sd (±11.2) |
One compartment |
15.1 (95% CI: 13.2–16.9) |
30.7 (95% CI: 29.2–32.3) |
WinNonlin |
| 40 | Adults, critically ill, elderly (178) | 75 range (65–94) |
77 range (37–147) |
Two compartment |
4.9 (95% CI: 4.7–5.1) |
25.2 (95% CI: 19.5–31.2) |
Non‐linear mixed‐effects methods |
| 41 | Adults, critically ill, elderly (75) |
75.6 sd (±8.9) |
64.4 sd (±12.3) |
Two compartment |
9.6 (95% CI: 7.9–11.2) |
34.8 (95% CI: 24.2–45.8) |
Non‐linear mixed‐effects methods |
| 42 | Adults, critically ill, requiring haemofiltration (10) |
67 range (20–75) |
76 range (50–113) |
Non‐compartmental |
4.5 IQR (3.4, 14.3) |
17.4 IQR (14.1, 23.4) |
Log‐linear least squares regression and linear trapezoidal rule |
| 43 | Adults, critically ill with sepsis/septic shock (9) |
57.2 sd (±16.1) |
62.9 sd (±11.6) |
One compartment |
8.5 (95% CI: 4.2–12.8) |
26.4 (95% CI: 18.7–34.0) |
Non‐linear mixed‐effects methods |
| 44 | Adults, critically ill, obese (9) |
55.4 sd (±10.1) |
152.3 sd (±31) |
Two compartment |
5.7 (95% CI: 3.5–7.8) |
17.2 (95% CI: 12.0–22.4) |
Non‐linear least squares regression |
| 45 | Adults, critically ill with central nervous system infection (21) |
52 range (46–80) |
76 range (55–105) |
Three compartment |
14.2 range (7.6–29.9) |
12.7* range (5.1–15.0) |
Non parametric adaptive grid algorithm. *central volume only published |
| 46 | Adults, critically ill with central nervous system infection (9) |
45.1 sd (±17.6) |
70.3 sd (±12.6) |
Three compartment (two csf) |
7.2 (95% CI: 4.0–15.8) |
99.6* (95% CI: 43.1–162.8) |
Non‐linear mixed‐effects methods. *central volume only published. Unusually high volume. |
| 47 | Adults, critically ill with central nervous system infection (10) |
61.5 IQR (54.3, 68.3) |
80 IQR (70, 79.7) |
Two compartment (one csf) |
14.6 (95% CI: 9.9–19.3) |
10.7* (95% CI: 8.2–13.1) |
Non‐parametric adaptive‐grid Unusually high volume. *central volume only published |
| 48 | Adults, critically ill with central nervous system infection (82) |
43.4 sd (±13.1) |
65.2 sd (±11.6) |
Three compartment (one csf) |
23.4 (95% CI: 21.6–25.3) |
19.4 (95% CI: 17.4–21.0) |
Non‐linear mixed‐effects methods |
| 49 | Adults, critically ill requiring haemofiltration (10) |
57 IQR (49, 61) |
70 IQR (66, 103) |
Non‐compartmental |
6.0 IQR (5.2, 6.2) |
25.9 IQR (22.4, 32.2) |
Linear trapezoidal rule and log‐linear least squares regression |
| 50 | Adults, critically ill requiring haemofiltration (10) |
64.9 sd (±8.0) |
79.8 sd (±18.5) |
Two compartment |
3.9 (95% CI: 3.0–4.8) |
23.9 (95% CI: 17.8–30.0) |
Non‐linear regression |
| 51 | Adults, requiring haemofiltration (10) |
54.3 sd (±9.4) |
76.7 sd (±15.0) |
Non‐compartmental |
4.7 range (2.4–11.2) |
50.4 range (26.8–213.2) |
WinNonLin |
| 52 | Adults, anuric and requiring haemofiltration (9) |
54.2 sd (±19.7) |
69.4 sd (±9.7) |
Two compartment |
3.1 (95% CI: 2.3–3.9) |
18.2 (95% CI: 13.3–23.0) |
Weighted least squares regression. |
| 53 | Adults, critically ill requiring haemofiltration (15) |
60 sd (±8.3) |
71 sd (±16.3) |
Four compartment |
5.4 (95% CI: 1.0–9.9) |
24.0 (95% CI: 9.2–38.8) |
Compartmental approach. Kinetica program. |
| 54 | Adults, critically ill requiring haemofiltration (24) |
68.5 range (50–81) |
75 range (68–126) |
One compartment |
3.5 (95% CI: 2.8–4.2) |
30.8 (95% CI: 24.9–36.7) |
Non‐linear mixed‐effects methods |
| 55 | Adults, critically ill burn patients (59) |
47.3 range (19–86) |
65.9 range (42–95) |
Two compartment |
15.6 (95% CI: 14.2–19.0) |
28.8 (95% CI: 22.8–37.9) |
Non‐linear mixed‐effects methods |
| 56 | Adults, critically ill burns patients (12) |
46 sd (±16) |
82.9 sd (±17.5) |
Two compartment |
14.3 (95% CI: 12.3–16.3) |
40.7 (95% CI: 31.1–50.3) |
Non‐linear mixed‐effects methods |
| 57 | Adults, critically ill with ventilator associated pneumonia (9) |
39.6 sd (±15.8) |
54.2 sd (±11.6) |
One compartment |
10.3 (95% CI: 7.3–13.3) |
20.7 (95% CI: 17.0–24.3) |
Trapezoidal rule |
| 58 | Adults, critically ill with ventilator associated pneumonia (39) |
49.3 sd (±19.4) |
83.1 sd (±22.6) |
Three compartment (one lung) |
13.4 (95% CI: 10.6–16.1) |
10.6* (95% CI: 7.0–14.2) |
Non‐parametric adaptive‐grid. *central compartment only |
| 59 | Adults, critically ill surgical patients (32) |
68.5 IQR (62, 76) |
73.5 IQR (69, 89) |
Not reported |
10.4 (95% CI: 8.8–12.6) |
Not reported | Non‐linear mixed‐effects methods |
| 60 | Adults, surgical patients with moderate or severe infection (11) |
63.1 sd (±18.3) |
72 range (47.6–95) |
Two compartment |
11.2 (95% CI: 8.8–13.5) |
20.1 (95% CI: 16.6–23.7) |
Extended or least squares method |
| 61 | Adults, intra‐abdominal infection (12) |
29.5 sd (±13.1) |
70.95 sd (±7.7) |
Non‐compartmental |
18.7 (95% CI: 16.0–21.4) |
26.3 (95% CI: 22.0–30.6) |
LAGRAN program |
| 33 | Adults, haematological malignancy and infection (10) |
52 range (35–75) |
72 range (48–85) |
Two compartment |
12.8 range (10.5–20.7) |
22.7 range (15.0–37.0) |
Non‐linear mixed‐effects methods |
| 62 | Adults, haematological malignancy and febrile neutropenia (57) |
36 range (17–68) |
61.4 range (45–95.8) |
One compartment |
10.7 (95% CI: 8.3–13.0) |
16.6 (95% CI: 12.6–20.6) |
Non‐linear mixed‐effects methods |
| 63 | Adults, haematological malignancy and febrile neutropenia (12) |
61 range (36–82) |
Not published | Non‐compartmental |
8.94 (95% CI: 6.2–11.6) |
16.2 (95% CI: 13.7–18.7) |
Least square regression analysis, trapezoidal rule |
| 64 | Adults, hospitalised with infection (68) |
71.5 sd (±13.5) |
52.1 sd (±13.9) |
One compartment |
13.9 (95% CI: 11.4–16.5) |
45.1 (95% CI: 32.5–56.6) |
Non‐linear mixed‐effects methods |
| 65 | Adults, hospitalised with infection (42) |
62.2 sd (±19.6) |
56 sd (±10.4) |
Two compartment |
10.7 (95% CI: 5.0–16.4) |
21.1 (95% CI: 17.9–24.6) |
Non‐linear mixed‐effects methods |
| 66 | Adults, obese, hospitalised with infection (10) |
49.1 sd (±12.0) |
200.4 sd (±67.9) |
Two compartmetns |
3.7 (95% CI: 2.8–4.5) |
8.8 (95% CI: 6.5–11.0) |
Nonlinear least‐squares regression |
| 67 | Adults, obese, hospitalised with infection (375) |
64.7 sd (±13.4) |
95.3 sd (±18) |
One compartment |
7.0 (95% CI: 6.5–7.5) |
20.6 (95% CI: 20.5–20.7) |
ADAPT 5 program |
| 68 | Adults, healthy volunteers (12) |
29.4 sd (±6) |
80.3 sd (±7.2) |
Non‐compartmental/two compartment |
14.4 (95% CI: 13.3–15.5) |
18.6 (95% CI: 15.9–21.3) |
Log trapezoid rule, iterative least squares method |
| 69 | Adults, healthy volunteers (12) |
32.6 sd (±8.9) |
59.7 sd (±7.8) |
One compartment |
11.5 (95% CI: 7.9–15.1) |
19.7 (95% CI: 15.9–23.5) |
WinNonlin |
| 70 | Adults, healthy volunteers (9) |
36.6 range (23–59) |
79.0 range (67.9–89.7) |
Two compartment |
13.7 (95% CI: 12.0–15.4) |
14.8 (95% CI: 12.8–16.8) |
Least squares regression |
| 71 | Adults, healthy volunteers (18) |
38 sd (±10) |
74.4 sd (±9.1) |
Two compartment |
13.1 (95% CI: 11.6–14.7) |
9.7* (95% CI: 8.3–11.1) |
Nonparametric adaptive grid program. *Central volume only reported |
| 72 | Adults, healthy volunteers (25) |
39.0 sd (±10.6) |
80.3 sd (9.4) |
Non‐compartmental |
10.0 (95% CI: 9.2–10.8) |
14.2 (95% CI: 13.3–15.1) |
Linear trapezoidal method |
| 73 |
Adults, healthy elderly (8) Included as no other elderly studies |
73 sd (±4.6) |
68.9 sd (±8.3) |
Non‐compartmental |
8.3 (95% CI: 6.9–9.7) |
13.2 (95% CI: 12.0–14.4) |
Least‐squares regression, log‐trapezoidal rule |
| 74 | Children with malignancy and severe infection (14) |
7.1 sd (±2.4) |
22.7 sd (±9.7) |
Two compartment |
15.2 (95% CI: 12.8–18.4) |
12.5 (95% CI: 9.6–17.1) |
Non‐linear mixed‐effects methods |
| 75 | Children with infection following stem cell transplant (21) |
9.6 sd (±5.4) |
36.1 sd (±20.5) |
Non‐compartmental |
8.2 IQR (5.0, 15.6) |
Not calculated | Clearance at steady state calculated from infusion rate/concentration at steady state |
| 76 | Children with infection (99) |
4.3 sd (±3.8) |
16.8 sd (±11.6) |
Two compartment |
12.3 (95% CI: 11.5–13.7) |
12.2 (95% CI: 9.7–14.4) |
Non‐linear mixed‐effects methods |
| 77 | Children (40) |
6.6 sd (±4.4) |
23.2 sd (±13.5) |
Two compartment |
13.5 (95% CI: 8.9–18.0) |
35 (95% CI: 19.3–44.5) |
Non‐linear mixed‐effects methods. Pooled participant group. *pooled data from Japanese studies |
| 78 | Children, hospitalised with infection (50) |
3.1 sd (±3.2) |
14.8 sd (±8.1) |
Two compartment |
10.4 (95% CI: 9.7–11.2) |
23.8 (95% CI: 30.1–39.2) |
Non‐linear mixed‐effects methods |
| 79 | Children with cystic fibrosis and lung infection (30) |
12.7 sd (±2.9) |
40.9 sd (±12.2) |
Two compartment |
24.1 (95% CI: 18.9–29.3) |
21.0* (95% CI: 16.7–25.3) |
Non‐parametric adaptive grid. *central volume only published |
| 80 | Children, requiring haemofiltration (7) |
14.3 sd (±5.8) |
48.6 sd (±17.4) |
Not specified |
3.7 (95% CI: 2.1–7.7) |
24.5 (95% CI: 19.0–30.0) |
Bayesian estimation using MWPharm. *included as only study in children |
| 81 | Infants and children, hospitalised with infection (63) |
4 sd (±3.5) |
16.5 sd (±11) |
Non‐compartmental |
16.2 (95% CI: 14.9–17.4) |
30.1 (95% CI: 28.2–32.1) |
Linear trapezoidal rule and log‐linear least squares regression |
| 82 | Neonates (188) | PMA 33 weeks range (24–51) |
1.1 range (0.3–4.8) |
One compartment |
2.9 (95% CI: 2.8–3.1) |
32.2 (95% CI: 30.3–34.1) |
Non‐linear mixed‐effects methods |
| 83 | Neonates (22) | PNA 10 days sd (±15) GA 34 sd (±5) weeks |
2.4 sd (±1) |
One compartment |
1.0 range not published |
28 range not published |
Non‐linear mixed‐effects methods |
| 84 | Neonates (9) |
GA 26.9 weeks sd (±1.4) PNA 15.6 sd (±8.6) days |
0.9 sd (±0.2) |
Non‐compartmental |
1.4 (95% CI: 0.9–1.9) |
21.0 (95% CI: 13.3–28.7) |
WinNonlin |
Mean or median values presented. With associated range, interquartile range (IQR) or standard deviation (SD). The 95% confidence intervals have been calculated, where SD or standard error data were available, assuming a Student's t‐distribution. GA, gestational age; PMA, postmenstrual age, PNA is post‐natal age. Where a ‘*’ appears, a corresponding explanatory comment is noted in the comments column
Lonsdale, D. O. , Baker, E. H. , Kipper, K. , Barker, C. , Philips, B. , Rhodes, A. , Sharland, M. , and Standing, J. F. (2019) Scaling beta‐lactam antimicrobial pharmacokinetics from early life to old age. Br J Clin Pharmacol, 85: 316–346. 10.1111/bcp.13756.
References
- 1. Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 2003; 31: 2332–2338. [DOI] [PubMed] [Google Scholar]
- 2. Vincent J‐L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34: 344–353. [DOI] [PubMed] [Google Scholar]
- 3. Intensive Care National Audit and Research Centre (ICNARC): Case Mix Programme [online]. Available at https://www.icnarc.org/Our‐Audit/Audits/Cmp/Our‐National‐Analyses/Sepsis (last accessed 1 February 2018).
- 4. Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, et al Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicentre retrospective cohort study. Lancet Infect Dis 2015; 15: 46–54. [DOI] [PubMed] [Google Scholar]
- 5. Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo‐Bustamante JC, Salloo A, et al Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191: 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302: 2323–2329. [DOI] [PubMed] [Google Scholar]
- 7. Cantey JB, Wozniak PS, Sánchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Paediatr Infect Dis J 2015; 34: 267–272. [DOI] [PubMed] [Google Scholar]
- 8. Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta‐analysis. Crit Care Med 2014; 42: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Depani SJ, Ladhani S, Heath PT, Lamagni TL, Johnson AP, Pebody RG, et al The contribution of infections to neonatal deaths in England and Wales. Pediatr Infect Dis J 2011; 30: 345–347. [DOI] [PubMed] [Google Scholar]
- 10. Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 2015; 372: 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oza S, Lawn JE, Hogan DR, Mathers C, Cousens SN. Neonatal cause‐of‐death estimates for the early and late neonatal periods for 194 countries: 2000‐2013. Bull World Health Organ 2015; 93: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torio C, Andrews R. National Inpatient Hospital Costs: the most expensive conditions by payer, 2011: statistical brief #160, Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [online]. Rockville (MD): Agency for Healthcare Research and Quality (US). 2013. Available at https://www.ncbi.nlm.nih.gov/books/NBK169005/ (last accessed 1 February 2018).
- 13. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boucher BA, Wood GC, Swanson JM. Pharmacokinetic changes in critical illness. Crit Care Clin 2006; 22: 255–271. [DOI] [PubMed] [Google Scholar]
- 15. Bourget P, Lesne‐Hulin A, Le Reveille R, Le Bever H, Carsin H. Clinical pharmacokinetics of piperacillin‐tazobactam combination in patients with major burns and signs of infection. Antimicrob Agents Chemother 1996; 40: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeon S, Han S, Lee J, Hong T, Paek J, Woo H, et al Population pharmacokinetic analysis of piperacillin in burn patients. Antimicrob Agents Chemother 2014; 58: 3744–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed U, Spyridis N, Wong IC, Sharland M, Long PF, Network iCsAPUR . Dosing of oral penicillins in children: is big child=half an adult, small child=half a big child, baby=half a small child still the best we can do? BMJ 2011; 343: d7803. [DOI] [PubMed] [Google Scholar]
- 18. Anderson BJ, Holford NH. Mechanism‐based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 48: 303–332. [DOI] [PubMed] [Google Scholar]
- 19. Calvier EA, Krekels EH, Valitalo PA, Rostami‐Hodjegan A, Tibboel D, Danhof M, et al Allometric scaling of clearance in paediatric patients: when does the magic of 0.75 fade? Clin Pharmacokinet 2017; 56: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci 2013; 102: 2941–2952. [DOI] [PubMed] [Google Scholar]
- 21. Germovsek E, Barker CIS, Sharland M, Standing JF. Scaling clearance in paediatric pharmacokinetics: all models are wrong, which are useful? Br J Clin Pharmacol 2017; 83: 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH. Busulfan in infant to adult hematopoietic cell transplant recipients: a population pharmacokinetic model for initial and Bayesian dose personalization. Clin Cancer Res 2014; 20: 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holford NH, Ma SC, Anderson BJ. Prediction of morphine dose in humans. Paediatr Anaesth 2012; 22: 209–222. [DOI] [PubMed] [Google Scholar]
- 24. Lonsdale DO, Baker EH. Understanding and managing medication in elderly people. Best Pract Res Clin Obstet Gynaecol 2013; 27: 767–788. [DOI] [PubMed] [Google Scholar]
- 25. National Center for Biotechnology Information (NCBI) . National Library of Medicine (US), National Center for Biotechnology Information, Bethesda (MD) [online]. Available at https://www.ncbi.nlm.nih.gov (last accessed 1 February 2018).
- 26. Ovid . Wolters Kluwer Health [online]. Available at http://ovidsp.uk.ovid.com/ovidweb.cgi (last accessed 1 February 2018).
- 27. Embase . Elsevier [online]. Available at https://www.embase.com/login (last accessed 1 February 2018).
- 28. Udy AA, Lipman J, Jarrett P, Klein K, Wallis SC, Patel K, et al Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit Care 2015; 19: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM User's guides (1989–2011). Ellicott City, MD, USA: Icon Development Solutions, 2011. [Google Scholar]
- 30. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016.
- 31. Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer International Publishing, 2016. New York: ISBN:978‐0‐387‐98141‐3 [Google Scholar]
- 32. Alobaid AS, Wallis SC, Jarrett P, Starr T, Stuart J, Lassig‐Smith M, et al Effect of obesity on the population pharmacokinetics of meropenem in critically ill patients. Antimicrob Agents Chemother 2016; 60: 4577–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Binder L, Schworer H, Hoppe S, Streit F, Neumann S, Beckmann A, et al Pharmacokinetics of meropenem in critically ill patients with severe infections. Ther Drug Monit 2013; 35: 63–70. [DOI] [PubMed] [Google Scholar]
- 34. Frippiat F, Musuamba FT, Seidel L, Albert A, Denooz R, Charlier C, et al Modelled target attainment after meropenem infusion in patients with severe nosocomial pneumonia: the PROMESSE study. J Antimicrob Chemother 2015; 70: 207–216. [DOI] [PubMed] [Google Scholar]
- 35. Goncalves‐Pereira J, Silva NE, Mateus A, Pinho C, Povoa P. Assessment of pharmacokinetic changes of meropenem during therapy in septic critically ill patients. BMC Pharmacol Toxicol 2014; 15: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mattioli F, Fucile C, Del Bono V, Marini V, Parisini A, Molin A, et al Population pharmacokinetics and probability of target attainment of meropenem in critically ill patients. Eur J Clin Pharmacol 2016; 72: 839–848. [DOI] [PubMed] [Google Scholar]
- 37. Novelli A, Adembri C, Livi P, Fallani S, Mazzei T, De Gaudio AR. Pharmacokinetic evaluation of meropenem and imipenem in critically ill patients with sepsis. Clin Pharmacokinet 2005; 44: 539–549. [DOI] [PubMed] [Google Scholar]
- 38. Thalhammer F, Traunmuller F, El Menyawi I, Frass M, Hollenstein UM, Locker GJ, et al Continuous infusion versus intermittent administration of meropenem in critically ill patients. J Antimicrob Chemother 1999; 43: 523–527. [DOI] [PubMed] [Google Scholar]
- 39. Zhao HY, Gu J, Lyu J, Liu D, Wang YT, Liu F, et al Pharmacokinetic and pharmacodynamic efficacies of continuous versus intermittent administration of meropenem in patients with severe sepsis and septic shock: a prospective randomized pilot study. Chin Med J (Engl) 2017; 130: 1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Usman M, Frey OR, Hempel G. Population pharmacokinetics of meropenem in elderly patients: dosing simulations based on renal function. Eur J Clin Pharmacol 2017; 73: 333–342. [DOI] [PubMed] [Google Scholar]
- 41. Zhou QT, He B, Zhang C, Zhai SD, Liu ZY, Zhang J. Pharmacokinetics and pharmacodynamics of meropenem in elderly Chinese with lower respiratory tract infections: population pharmacokinetics analysis using nonlinear mixed‐effects modelling and clinical pharmacodynamics study. Drugs Aging 2011; 28: 903–912. [DOI] [PubMed] [Google Scholar]
- 42. Langan KM, Jacob J, Li J, Nation RL, Bellomo R, Howden B, et al Pharmacokinetics of short versus extended infusion meropenem dosing in critically ill patients: a pilot study. Crit Care Resusc 2014; 16: 190–196. [PubMed] [Google Scholar]
- 43. Jaruratanasirikul S, Thengyai S, Wongpoowarak W, Wattanavijitkul T, Tangkitwanitjaroen K, Sukarnjanaset W, et al Population pharmacokinetics and Monte Carlo dosing simulations of meropenem during the early phase of severe sepsis and septic shock in critically ill patients in intensive care units. Antimicrob Agents Chemother 2015; 59: 2995–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheatham SC, Fleming MR, Healy DP, Chung EK, Shea KM, Humphrey ML, et al Steady‐state pharmacokinetics and pharmacodynamics of meropenem in morbidly obese patients hospitalized in an intensive care unit. J Clin Pharmacol 2014; 54: 324–330. [DOI] [PubMed] [Google Scholar]
- 45. Blassmann U, Roehr AC, Frey OR, Vetter‐Kerkhoff C, Thon N, Hope W, et al Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: a prospective observational study. Crit Care 2016; 20: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li X, Sun S, Wang Q, Zhao Z. Population pharmacokinetics of combined intravenous and local intrathecal administration of meropenem in aneurysm patients with suspected intracranial infections after craniotomy. Eur J Drug Metab Pharmacokinet 2017; 1–9. 10.1007/s13318-017-0422-1. [DOI] [PubMed] [Google Scholar]
- 47. Lodise TP, Nau R, Kinzig M, Drusano GL, Jones RN, Sorgel F. Pharmacodynamics of ceftazidime and meropenem in cerebrospinal fluid: results of population pharmacokinetic modelling and Monte Carlo simulation. J Antimicrob Chemother 2007; 60: 1038–1044. [DOI] [PubMed] [Google Scholar]
- 48. Lu C, Zhang Y, Chen M, Zhong P, Chen Y, Yu J, et al Population pharmacokinetics and dosing regimen optimization of meropenem in cerebrospinal fluid and plasma in patients with meningitis after neurosurgery. Antimicrob Agents Chemother 2016; 60: 6619–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bilgrami I, Roberts JA, Wallis SC, Thomas J, Davis J, Fowler S, et al Meropenem dosing in critically ill patients with sepsis receiving high‐volume continuous venovenous hemofiltration. Antimicrob Agents Chemother 2010; 54: 2974–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Giles LJ, Jennings AC, Thomson AH, Creed G, Beale RJ, McLuckie A. Pharmacokinetics of meropenem in intensive care unit patients receiving continuous veno‐venous hemofiltration or hemodiafiltration. Crit Care Med 2000; 28: 632–637. [DOI] [PubMed] [Google Scholar]
- 51. Kielstein JT, Czock D, Schopke T, Hafer C, Bode‐Boger SM, Kuse E, et al Pharmacokinetics and total elimination of meropenem and vancomycin in intensive care unit patients undergoing extended daily dialysis. Crit Care Med 2006; 34: 51–56. [DOI] [PubMed] [Google Scholar]
- 52. Krueger WA, Schroeder TH, Hutchison M, Hoffmann E, Dieterich HJ, Heininger A, et al Pharmacokinetics of meropenem in critically ill patients with acute renal failure treated by continuous hemodiafiltration. Antimicrob Agents Chemother 1998; 42: 2421–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Robatel C, Decosterd LA, Biollaz J, Eckert P, Schaller MD, Buclin T. Pharmacokinetics and dosage adaptation of meropenem during continuous venovenous hemodiafiltration in critically ill patients. J Clin Pharmacol 2003; 43: 1329–1340. [DOI] [PubMed] [Google Scholar]
- 54. Ulldemolins M, Soy D, Llaurado‐Serra M, Vaquer S, Castro P, Rodriguez AH, et al Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother 2015; 59: 5520–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doh K, Woo H, Hur J, Yim H, Kim J, Chae H, et al Population pharmacokinetics of meropenem in burn patients. J Antimicrob Chemother 2010; 65: 2428–2435. [DOI] [PubMed] [Google Scholar]
- 56. Ramon‐Lopez A, Allen JM, Thomson AH, Dheansa BS, Elizabeth James S, Hanlon GW, et al Dosing regimen of meropenem for adults with severe burns: a population pharmacokinetic study with Monte Carlo simulations. J Antimicrob Chemother 2015; 70: 882–890. [DOI] [PubMed] [Google Scholar]
- 57. Jaruratanasirikul S, Sriwiriyajan S, Punyo J. Comparison of the pharmacodynamics of meropenem in patients with ventilator‐associated pneumonia following administration by 3‐hour infusion or bolus injection. Antimicrob Agents Chemother 2005; 49: 1337–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lodise TP, Sorgel F, Melnick D, Mason B, Kinzig M, Drusano GL. Penetration of meropenem into epithelial lining fluid of patients with ventilator‐associated pneumonia. Antimicrob Agents Chemother 2011; 55: 1606–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kees MG, Minichmayr IK, Moritz S, Beck S, Wicha SG, Kees F, et al Population pharmacokinetics of meropenem during continuous infusion in surgical ICU patients. J Clin Pharmacol 2016; 56: 307–315. [DOI] [PubMed] [Google Scholar]
- 60. Lovering AM, Vickery CJ, Watkin DS, Leaper D, McMullin CM, White LO, et al The pharmacokinetics of meropenem in surgical patients with moderate or severe infections. J Antimicrob Chemother 1995; 36: 165–172. [DOI] [PubMed] [Google Scholar]
- 61. Bedikian A, Okamoto MP, Nakahiro RK, Farino J, Heseltine PNR, Appleman MD, et al Pharmacokinetics of meropenem in patients with intra‐abdominal infections. Antimicrob Agents Chemother 1994; 38: 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee DG, Choi SM, Shin WS, Lah HO, Yim DS. Population pharmacokinetics of meropenem in febrile neutropenic patients in Korea. Int J Antimicrob Agents 2006; 28: 333–339. [DOI] [PubMed] [Google Scholar]
- 63. Nyhlen A, Ljungberg B, Nilsson‐Ehle I, Thuresson I, Glans S, Rodjer S, et al Pharmacokinetics of meropenem in febrile neutropenic patients. Eur J Clin Microbiol Infect Dis 1997; 16: 797–802. [DOI] [PubMed] [Google Scholar]
- 64. Muro T, Sasaki T, Hosaka N, Umeda Y, Takemoto S, Yamamoto H, et al Population pharmacokinetic analysis of meropenem in Japanese adult patients. J Clin Pharm Ther 2011; 36: 230–236. [DOI] [PubMed] [Google Scholar]
- 65. Ikawa K, Morikawa N, Ohge H, Ikeda K, Sueda T, Taniwaki M, et al Pharmacokinetic–pharmacodynamic target attainment analysis of meropenem in Japanese adult patients. J Infect Chemother 2010; 16: 25–32. [DOI] [PubMed] [Google Scholar]
- 66. Kays MB, Fleming MR, Cheatham SC, Chung EK, Juenke JM. Comparative pharmacokinetics and pharmacodynamics of doripenem and meropenem in obese patients. Ann Pharmacother 2014; 48: 178–186. [DOI] [PubMed] [Google Scholar]
- 67. Pai MP, Cojutti P, Pea F. Pharmacokinetics and pharmacodynamics of continuous infusion meropenem in overweight, obese, and morbidly obese patients with stable and unstable kidney function: a step toward dose optimization for the treatment of severe gram‐negative bacterial infections. Clin Pharmacokinet 2015; 54: 933–941. [DOI] [PubMed] [Google Scholar]
- 68. Dreetz M, Hamacher J, Eller J, Borner K, Koeppe P, Schaberg T, et al Serum bactericidal activities and comparative pharmacokinetics of meropenem and imipenem‐cilastatin. Antimicrob Agents Chemother 1996; 40: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jaruratanasirikul S, Sriwiriyajan S. Comparison of the pharmacodynamics of meropenem in healthy volunteers following administration by intermittent infusion or bolus injection. J Antimicrob Chemother 2003; 52: 518–521. [DOI] [PubMed] [Google Scholar]
- 70. Jones HK, Kelly HC, Hutchison M, Yates RA, Ross F, Lomax C, et al A comparison of the pharmacokinetics of meropenem after intravenous administration by injection over 2, 3 and 5 minutes. Eur J Drug Metab Pharmacokinet 1997; 22: 193–199. [DOI] [PubMed] [Google Scholar]
- 71. Lee LS, Kinzig‐Schippers M, Nafziger AN, Ma L, Sorgel F, Jones RN, et al Comparison of 30‐min and 3‐h infusion regimens for imipenem/cilastatin and for meropenem evaluated by Monte Carlo simulation. Diagn Microbiol Infect Dis 2010; 68: 251–258. [DOI] [PubMed] [Google Scholar]
- 72. Wenzler E, Gotfried MH, Loutit JS, Durso S, Griffith DC, Dudley MN, et al Meropenem‐RPX7009 concentrations in plasma, epithelial lining fluid, and alveolar macrophages of healthy adult subjects. Antimicrob Agents Chemother 2015; 59: 7232–7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ljungberg B, Nilsson‐Ehle I. Pharmacokinetics of meropenem and its metabolite in young and elderly healthy men. Antimicrob Agents Chemother 1992; 36: 1437–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kongthavonsakul K, Lucksiri A, Eakanunkul S, Roongjang S, Issaranggoon Na Ayuthaya S, Oberdorfer P. Pharmacokinetics and pharmacodynamics of meropenem in children with severe infection. Int J Antimicrob Agents 2016; 48: 151–157. 10.1016/j.ijantimicag.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 75. Cojutti P, Maximova N, Pea F. Pharmacokinetics and pharmacodynamics of continuous‐infusion meropenem in pediatric hematopoietic stem cell transplant patients. Antimicrob Agents Chemother 2015; 59: 5535–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Du X, Li C, Kuti JL, Nightingale CH, Nicolau DP. Population pharmacokinetics and pharmacodynamics of meropenem in pediatric patients. J Clin Pharmacol 2006; 46: 69–75. [DOI] [PubMed] [Google Scholar]
- 77. Ikawa K, Morikawa N, Ikeda K, Miki M, Kobayashi M. Population pharmacokinetics and pharmacodynamics of meropenem in Japanese pediatric patients. J Infect Chemother 2010; 16: 139–143. [DOI] [PubMed] [Google Scholar]
- 78. Ohata Y, Tomita Y, Nakayama M, Kozuki T, Sunakawa K, Tanigawara Y. Optimal dosage regimen of meropenem for pediatric patients based on pharmacokinetic/pharmacodynamic considerations. Drug Metab Pharmacokinet 2011; 26: 523–531. [DOI] [PubMed] [Google Scholar]
- 79. Pettit RS, Neu N, Cies JJ, Lapin C, Muhlebach MS, Novak KJ, et al Population pharmacokinetics of meropenem administered as a prolonged infusion in children with cystic fibrosis. J Antimicrob Chemother 2016; 71: 189–195. [DOI] [PubMed] [Google Scholar]
- 80. Nehus EJ, Mizuno T, Cox S, Goldstein SL, Vinks AA. Pharmacokinetics of meropenem in children receiving continuous renal replacement therapy: validation of clinical trial simulations. J Clin Pharmacol 2016; 56: 291–297. [DOI] [PubMed] [Google Scholar]
- 81. Blumer JL, Reed MD, Kearns GL, Jacobs RF, Gooch IWM, Yogev R, et al Sequential, single‐dose pharmacokinetic evaluation of meropenem in hospitalized infants and children. Antimicrob Agents Chemother 1995; 39: 1721–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Smith PB, Cohen‐Wolkowiez M, Castro LM, Poindexter B, Bidegain M, Weitkamp JH, et al Population pharmacokinetics of meropenem in plasma and cerebrospinal fluid of infants with suspected or complicated intra‐abdominal infections. Pediatr Infect Dis J 2011; 30: 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bradley JS, Sauberan JB, Ambrose PG, Bhavnani SM, Rasmussen MR, Capparelli EV. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J 2008; 27: 794–799. [DOI] [PubMed] [Google Scholar]
- 84. Padari H, Metsvaht T, Korgvee LT, Germovsek E, Ilmoja ML, Kipper K, et al Short versus long infusion of meropenem in very‐low‐birth‐weight neonates. Antimicrob Agents Chemother 2012; 56: 4760–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. De Waele J, Carlier M, Hoste E, Depuydt P, Decruyenaere J, Wallis SC, et al Extended versus bolus infusion of meropenem and piperacillin: a pharmacokinetic analysis. Minerva Anestesiol 2014; 80: 1302–1309. [PubMed] [Google Scholar]
- 86. Obrink‐Hansen K, Juul RV, Storgaard M, Thomsen MK, Hardlei TF, Brock B, et al Population pharmacokinetics of piperacillin in the early phase of septic shock: does standard dosing result in therapeutic plasma concentrations? Antimicrob Agents Chemother 2015; 59: 7018–7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Felton TW, McCalman K, Malagon I, Isalska B, Whalley S, Goodwin J, et al Pulmonary penetration of piperacillin and tazobactam in critically ill patients. Clin Pharmacol Ther 2014; 13 10.1038/clpt.2014.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Delattre IK, Musuamba FT, Jacqmin P, Taccone FS, Laterre PF, Verbeeck RK, et al Population pharmacokinetics of four beta‐lactams in critically ill septic patients comedicated with amikacin. Clin Biochem 2012; 45: 780–786. [DOI] [PubMed] [Google Scholar]
- 89. Shikuma LR, Ackerman BH, Weaver RH, Solem LD, Strate RG, Cerra FB, et al Effects of treatment and the metabolic response to injury on drug clearance: a prospective study with piperacillin. Crit Care Med 1990; 18: 37–41. [DOI] [PubMed] [Google Scholar]
- 90. Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J. First‐dose and steady‐state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents 2010; 35: 156–163. [DOI] [PubMed] [Google Scholar]
- 91. Tsai D, Stewart P, Goud R, Gourley S, Hewagama S, Krishnaswamy S, et al Pharmacokinetics of piperacillin in critically ill Australian indigenous patients with severe sepsis. Antimicrob Agents Chemother 2016; 60: 7402–7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shikuma LR, Ackerman BH, Weaver RH, Solem LD, Strate RG, Cerra FB, et al Thermal injury effects on drug disposition: a prospective study with piperacillin. J Clin Pharmacol 1990; 30: 632–637. [DOI] [PubMed] [Google Scholar]
- 93. Alobaid AS, Wallis SC, Jarrett P, Starr T, Stuart J, Lassig‐Smith M, et al Population pharmacokinetics of piperacillin in nonobese, obese, and morbidly obese critically ill patients. Antimicrob Agents Chemother 2017; 61: pii:: e01276–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sturm AW, Allen N, Rafferty KD, Fish DN, Toschlog E, Newell M, et al Pharmacokinetic analysis of piperacillin administered with tazobactam in critically ill, morbidly obese surgical patients. Pharmacotherapy 2014; 34: 28–35. [DOI] [PubMed] [Google Scholar]
- 95. Bao H, Lv Y, Wang D, Xue J, Yan Z. Clinical outcomes of extended versus intermittent administration of piperacillin/tazobactam for the treatment of hospital‐acquired pneumonia: a randomized controlled trial. Eur J Clin Microbiol Infect Dis 2017; 36: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Asin‐Prieto E, Rodriguez‐Gascon A, Troconiz IF, Soraluce A, Maynar J, Sanchez‐Izquierdo JA, et al Population pharmacokinetics of piperacillin and tazobactam in critically ill patients undergoing continuous renal replacement therapy: application to pharmacokinetic/pharmacodynamic analysis. J Antimicrob Chemother 2014; 69: 180–189. [DOI] [PubMed] [Google Scholar]
- 97. Awissi DK, Beauchamp A, Hebert E, Lavigne V, Munoz DL, Lebrun G, et al Pharmacokinetics of an extended 4‐hour infusion of piperacillin‐tazobactam in critically ill patients undergoing continuous renal replacement therapy. Pharmacotherapy 2015; 35: 600–607. [DOI] [PubMed] [Google Scholar]
- 98. Bauer SR, Salem C, Connor MJ Jr, Groszek J, Taylor ME, Wei P, et al Pharmacokinetics and pharmacodynamics of piperacillin‐tazobactam in 42 patients treated with concomitant CRRT. Clin J Am Soc Nephrol 2012; 7: 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tamme K, Oselin K, Kipper K, Tasa T, Metsvaht T, Karjagin J, et al Pharmacokinetics and pharmacodynamics of piperacillin/tazobactam during high volume haemodiafiltration in patients with septic shock. Acta Anaesthesiol Scand 2016; 60: 230–240. [DOI] [PubMed] [Google Scholar]
- 100. Ulldemolins M, Martin‐Loeches I, Llaurado‐Serra M, Fernandez J, Vaquer S, Rodriguez A, et al Piperacillin population pharmacokinetics in critically ill patients with multiple organ dysfunction syndrome receiving continuous venovenous haemodiafiltration: effect of type of dialysis membrane on dosing requirements. J Antimicrob Chemother 2016; 71: 1651–1659. [DOI] [PubMed] [Google Scholar]
- 101. van der Werf TS, Mulder PO, Zijlstra JG, Uges DR, Stegeman CA. Pharmacokinetics of piperacillin and tazobactam in critically ill patients with renal failure, treated with continuous veno‐venous hemofiltration (CVVH). Intensive Care Med 1997; 23: 873–877. [DOI] [PubMed] [Google Scholar]
- 102. Varghese JM, Jarrett P, Boots RJ, Kirkpatrick CMJ, Lipman J, Roberts JA. Pharmacokinetics of piperacillin and tazobactam in plasma and subcutaneous interstitial fluid in critically ill patients receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents 2014; 43: 343–348. [DOI] [PubMed] [Google Scholar]
- 103. Shea KM, Cheatham SC, Wack MF, Smith DW, Sowinski KM, Kays MB. Steady‐state pharmacokinetics and pharmacodynamics of piperacillin/tazobactam administered by prolonged infusion in hospitalised patients. Int J Antimicrob Agents 2009; 34: 429–433. [DOI] [PubMed] [Google Scholar]
- 104. Chen R, Qian Q, Sun MR, Qian CY, Zou SL, Wang ML, et al Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with nosocomial infections. Eur J Drug Metab Pharmacokinet 2016; 41: 363–372. [DOI] [PubMed] [Google Scholar]
- 105. Cheatham SC, Fleming MR, Healy DP, Chung CE, Shea KM, Humphrey ML, et al Steady‐state pharmacokinetics and pharmacodynamics of piperacillin and tazobactam administered by prolonged infusion in obese patients. Int J Antimicrob Agents 2013; 41: 52–56. [DOI] [PubMed] [Google Scholar]
- 106. Felton TW, Hope WW, Lomaestro BM, Butterfield JM, Kwa AL, Drusano GL, et al Population pharmacokinetics of extended‐infusion piperacillin‐tazobactam in hospitalized patients with nosocomial infections. Antimicrob Agents Chemother 2012; 56: 4087–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kim YK, Jung JA, Choi HK, Bae IG, Choi WS, Hur J, et al Population pharmacokinetic analysis of piperacillin/tazobactam in Korean patients with acute infections. Infect Chemother 2016; 48: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li C, Kuti JL, Nightingale CH, Mansfield DL, Dana A, Nicolau DP. Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with complicated intra‐abdominal infection. J Antimicrob Chemother 2005; 56: 388–395. [DOI] [PubMed] [Google Scholar]
- 109. Jhee SS, Kern JW, Burm JP, Yellin AE, Gill MA. Piperacillin‐tazobactam pharmacokinetics in patients with intraabdominal infections. Pharmacotherapy 1995; 15: 472–478. [PubMed] [Google Scholar]
- 110. Alvarez JC, Cuervo SI, Garzon JR, Gomez JC, Diaz JA, Silva E, et al Pharmacokinetics of piperacillin/tazobactam in cancer patients with hematological malignancies and febrile neutropenia after chemotherapy. BMC Pharmacol Toxicol 2013; 14: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sime FB, Roberts MS, Warner MS, Hahn U, Robertson TA, Yeend S, et al Altered pharmacokinetics of piperacillin in febrile neutropenic patients with hematological malignancy. Antimicrob Agents Chemother 2014; 58: 3533–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Butterfield JM, Lodise TP, Beegle S, Rosen J, Farkas J, Pai MP. Pharmacokinetics and pharmacodynamics of extended‐infusion piperacillin/tazobactam in adult patients with cystic fibrosis‐related acute pulmonary exacerbations. J Antimicrob Chemother 2014; 69: 176–179. [DOI] [PubMed] [Google Scholar]
- 113. Bulitta JB, Duffull SB, Kinzig‐Schippers M, Holzgrabe U, Stephan U, Drusano GL, et al Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother 2007; 51: 2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Reed MD, Stern RC, Myers CM, Klinger JD, Yamashita TS, Blumer JL. Therapeutic evaluation of piperacillin for acute pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol 1987; 3: 101–109. [DOI] [PubMed] [Google Scholar]
- 115. Kinzig M, Sorgel F, Brismar B, Nord CE. Pharmacokinetics and tissue penetration of tazobactam and piperacillin in patients undergoing colorectal surgery. Antimicrob Agents Chemother 1992; 36: 1997–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kobayashi I, Ikawa K, Nakamura K, Nishikawa G, Kajikawa K, Yoshizawa T, et al Penetration of piperacillin‐tazobactam into human prostate tissue and dosing considerations for prostatitis based on site‐specific pharmacokinetics and pharmacodynamics. J Infect Chemother 2015; 21: 575–580. [DOI] [PubMed] [Google Scholar]
- 117. Nau R, Kinzig‐Schippers M, Sorgel F, Schinschke S, Rossing R, Muller C, et al Kinetics of piperacillin and tazobactam in ventricular cerebrospinal fluid of hydrocephalic patients. Antimicrob Agents Chemother 1997; 41: 987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mattoes HM, Capitano B, Kim MK, Xuan D, Quintiliani R, Nightingale CH, et al Comparative pharmacokinetic and pharmacodynamic profile of piperacillin/tazobactam 3.375g q4h and 4.5g q6h. Chemotherapy 2002; 48: 59–63. [DOI] [PubMed] [Google Scholar]
- 119. Occhipinti DJ, Pendland SL, Schoonover LL, Rypins EB, Danziger LH, Rodvold KA. Pharmacokinetics and pharmacodynamics of two multiple‐dose piperacillin‐ tazobactam regimens. Antimicrob Agents Chemother 1997; 41: 2511–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Meyers BR, Hirschman SZ, Strougo L, Srulevitch E. Comparative study of piperacillin, ticarcillin, and carbenicillin pharmacokinetics. Antimicrob Agents Chemother 1980; 17: 608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Colaizzi PA, Polk RE, Poynor WJ. Comparative pharmacokinetics of azlocillin and piperacillin in normal adults. Antimicrob Agents Chemother 1986; 29: 938–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kim MK, Capitano B, Mattoes HM, Xuan D, Quintiliani R, Nightingale CH, et al Pharmacokinetic and pharmacodynamic evaluation of two dosing regimens for piperacillin‐tazobactam. Pharmacotherapy 2002; 22: 569–577. [DOI] [PubMed] [Google Scholar]
- 123. Landersdorfer CB, Bulitta JB, Kirkpatrick CM, Kinzig M, Holzgrabe U, Drusano GL, et al Population pharmacokinetics of piperacillin at two dose levels: influence of nonlinear pharmacokinetics on the pharmacodynamic profile. Antimicrob Agents Chemother 2012; 56: 5715–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lode H, Elvers A, Koeppe P, Borner K. Comparative pharmacokinetics of apalcillin and piperacillin. Antimicrob Agents Chemother 1984; 25: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kim MK, Xuan D, Quintiliani R, Nightingale CH, Nicolau DP. Pharmacokinetic and pharmacodynamic profile of high dose extended interval piperacillin‐tazobactam. J Antimicrob Chemother 2001; 48: 259–267. [DOI] [PubMed] [Google Scholar]
- 126. Cies JJ, Shankar V, Schlichting C, Kuti JL. Population pharmacokinetics of piperacillin/tazobactam in critically ill young children. Pediatr Infect Dis J 2014; 33: 168–173. [DOI] [PubMed] [Google Scholar]
- 127. De Cock PAJG, van Dijkman SC, de Jaeger A, Willems J, Carlier M, Verstraete AG, et al Dose optimization of piperacillin/tazobactam in critically ill children. J Antimicrob Chemother 2017; 72: 2002–2011. [DOI] [PubMed] [Google Scholar]
- 128. Nichols K, Chung EK, Knoderer CA, Buenger LE, Healy DP, Dees J, et al Population pharmacokinetics and pharmacodynamics of extended‐infusion piperacillin and tazobactam in critically ill children. Antimicrob Agents Chemother 2016; 60: 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cies JJ, Jain J, Kuti JL. Population pharmacokinetics of the piperacillin component of piperacillin/tazobactam in pediatric oncology patients with fever and neutropenia. Pediatr Blood Cancer 2015; 62: 477–482. [DOI] [PubMed] [Google Scholar]
- 130. Reed MD, Goldfarb J, Yamashita TS, Lemon E, Blumer JL. Single‐dose pharmacokinetics of piperacillin and tazobactam in infants and children. Antimicrob Agents Chemother 1994; 38: 2817–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Li Z, Chen Y, Li Q, Cao D, Shi W, Cao Y, et al Population pharmacokinetics of piperacillin/tazobactam in neonates and young infants. Eur J Clin Pharmacol 2013; 69: 1223–1233. [DOI] [PubMed] [Google Scholar]
- 132. Cohen‐Wolkowiez M, Benjamin DK Jr, Ross A, James LP, Sullivan JE, Walsh MC, et al Population pharmacokinetics of piperacillin using scavenged samples from preterm infants. Ther Drug Monit 2012; 34: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cohen‐Wolkowiez M, Watt KM, Zhou C, Bloom BT, Poindexter B, Castro L, et al Developmental pharmacokinetics of piperacillin and tazobactam using plasma and dried blood spots from infants. Antimicrob Agents Chemother 2014; 58: 2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Monogue ML, Pettit RS, Muhlebach M, Cies JJ, Nicolau DP, Kuti JL. Population pharmacokinetics and safety of ceftolozane‐tazobactam in adult cystic fibrosis patients admitted with acute pulmonary exacerbation. Antimicrob Agents Chemother 2016; 60: 6578–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Aiudi A, Miller B, Krishna G, Adedoyin A, Xiao A. Pharmacokinetics, safety, and tolerability of ceftolozane/tazobactam in healthy Japanese, Chinese, and white subjects. Fundam Clin Pharmacol 2016; 30: 625–633. [DOI] [PubMed] [Google Scholar]
- 136. Carlier M, Noe M, De Waele JJ, Stove V, Verstraete AG, Lipman J, et al Population pharmacokinetics and dosing simulations of amoxicillin/clavulanic acid in critically ill patients. J Antimicrob Chemother 2013; 68: 2600–2608. [DOI] [PubMed] [Google Scholar]
- 137. Mimoz O, Schaeffer V, Incagnoli P, Louchahi K, Edouard A, Petitjean O, et al Co‐amoxiclav pharmacokinetics during posttraumatic hemorrhagic shock. Crit Care Med 2001; 29: 1350–1355. [DOI] [PubMed] [Google Scholar]
- 138. Haeseker M, Havenith T, Stolk L, Neef C, Bruggeman C, Verbon A. Is the standard dose of amoxicillin‐clavulanic acid sufficient? BMC Pharmacol Toxicol 2014; 15: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Francke EL, Appel GB, Neu HC. Kinetics of intravenous amoxicillin in patients on long‐term dialysis. Clin Pharmacol Ther 1979; 26: 31–35. [DOI] [PubMed] [Google Scholar]
- 140. Muller AE, Oostvogel PM, DeJongh J, Mouton JW, Steegers EAP, Dorr PJ, et al Pharmacokinetics of amoxicillin in maternal, umbilical cord, and neonatal sera. Antimicrob Agents Chemother 2009; 53: 1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Arancibia A, Guttmann J, Gonzalez G, Gonzalez C. Absorption and disposition kinetics of amoxicillin in normal human subjects. Antimicrob Agents Chemother 1980; 17: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Spyker DA, Rugloski RJ, Vann RL, O'Brien WM. Pharmacokinetics of amoxicillin: dose dependence after intravenous, oral, and intramuscular administration. Antimicrob Agents Chemother 1977; 11: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sjövall J, Alván G, Huitfeldt B. Intra‐ and inter‐individual variation in pharmacokinetics of intravenously infused amoxycillin and ampicillin to elderly volunteers. Br J Clin Pharmacol 1986; 21: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Janknegt R, Boogaard‐Van den Born J, Hameleers BA, Hooymans PM, Rang J, Smits CA, et al Pharmacokinetics of amoxycillin in elderly in‐patients. Pharm Weekbl Sci 1992; 14: 27–29. [DOI] [PubMed] [Google Scholar]
- 145. Sjovall J, Westerlund D, Alvan G. Renal excretion of intravenously infused amoxycillin and ampicillin. Br J Clin Pharmacol 1985; 19: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zarowny D, Ogilvie R, Tamblyn D, Macleod C, Ruedy J. Pharmacokinetics of amoxicillin. Clin Pharmacol Ther 1974; 16: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 147. Adam D, Koeppe P, Heilmann HD. Pharmacokinetics of amoxicillin and flucloxacillin following the simultaneous intravenous administration of 4 g and 1 g, respectively. Infection 1983; 11: 150–154. [DOI] [PubMed] [Google Scholar]
- 148. De Cock PA, Standing JF, Barker CI, de Jaeger A, Dhont E, Carlier M, et al Augmented renal clearance implies a need for increased amoxicillin‐clavulanic acid dosing in critically ill children. Antimicrob Agents Chemother 2015; 59: 7027–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jones AE, Barnes ND, Tasker TC, Horton R. Pharmacokinetics of intravenous amoxycillin and potassium clavulanate in seriously ill children. J Antimicrob Chemother 1990; 25: 269–274. [DOI] [PubMed] [Google Scholar]
- 150. Schaad UB, Casey PA, Cooper DL. Single‐dose pharmacokinetics of intravenous clavulanic acid with amoxicillin in pediatric patients. Antimicrob Agents Chemother 1983; 23: 252–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rudoy RC, Goto N, Pettit D, Uemura H. Pharmacokinetics of intravenous amoxicillin in pediatric patients. Antimicrob Agents Chemother 1979; 15: 628–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Bijleveld YA, Mathôt RAA, van der Lee JH, Groenendaal F, Dijk PH, van Heijst A, et al Population pharmacokinetics of amoxicillin in term neonates undergoing moderate hypothermia. Clin Pharmacol Ther 2018; 103: 458–67. [DOI] [PubMed] [Google Scholar]
- 153. Charles BG, Preechagoon Y, Lee TC, Steer PA, Flenady VJ, Debuse N. Population pharmacokinetics of intravenous amoxicillin in very low birth weight infants. J Pharm Sci 1997; 86: 1288–1292. [DOI] [PubMed] [Google Scholar]
- 154. Huisman‐de Boer JJ, van den Anker JN, Vogel M, Goessens WH, Schoemaker RC, de Groot R. Amoxicillin pharmacokinetics in preterm infants with gestational ages of less than 32 weeks. Antimicrob Agents Chemother 1995; 39: 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Pullen J, Stolk LM, Nieman FH, Degraeuwe PL, van Tiel FH, Zimmermann LJ. Population pharmacokinetics and dosing of amoxicillin in (pre) term neonates. Ther Drug Monit 2006; 28: 226–231. [DOI] [PubMed] [Google Scholar]
- 156. Adrianzen Vargas MR, Danton MH, Javaid SM, Gray J, Tobin C, Brawn WJ, et al Pharmacokinetics of intravenous flucloxacillin and amoxicillin in neonatal and infant cardiopulmonary bypass surgery. Eur J Cardiothorac Surg 2004; 25: 256–260. [DOI] [PubMed] [Google Scholar]
- 157. Pullen J, Driessen M, Stolk LM, Degraeuwe PL, van Tiel FH, Neef C, et al Amoxicillin pharmacokinetics in (preterm) infants aged 10 to 52 days: effect of postnatal age. Ther Drug Monit 2007; 29: 376–380. [DOI] [PubMed] [Google Scholar]
- 158. van Boekholt A, Fleuren H, Mouton J, Kramers C, Sprong T, Gerrits P, et al Serum concentrations of amoxicillin in neonates during continuous intravenous infusion. Eur J Clin Microbiol Infect Dis 2016; 35: 1007–1012. [DOI] [PubMed] [Google Scholar]
- 159. Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 2009; 24: 67–76. [DOI] [PubMed] [Google Scholar]
- 160. Felton TW, Hope WW, Roberts JA. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol Infect Dis 2014; 79: 441–447. [DOI] [PubMed] [Google Scholar]
- 161. Baptista JP, Udy AA, Sousa E, Pimentel J, Wang L, Roberts JA, et al A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care 2011; 15: R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents 2012; 39: 420–423. [DOI] [PubMed] [Google Scholar]
- 163. Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al DALI: defining antibiotic levels in intensive care unit patients: are current β‐lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014; 58: 1072–1083. [DOI] [PubMed] [Google Scholar]
- 164. Amoxicillin sodium for injection – summary of product characteristics (SPC) – (eMC) [online]. Available at https://www.medicines.org.uk/emc/medicine/5359#PHARMACODYNAMIC_PROPS. last accessed 1 February 2018.
- 165. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369: 840–851. [DOI] [PubMed] [Google Scholar]
- 166. Eleveld DJ, Proost JH, Vereecke H, Absalom AR, Olofsen E, Vuyk J, et al An allometric model of remifentanil pharmacokinetics and pharmacodynamics. Anesthesiology 2017; 126: 1005–1018. [DOI] [PubMed] [Google Scholar]
- 167. Eleveld DJ, Proost JH, Cortinez LI, Absalom AR, Struys MM. A general purpose pharmacokinetic model for propofol. Anesth Analg 2014; 118: 1221–1237. [DOI] [PubMed] [Google Scholar]
- 168. Germovsek E, Kent A, Metsvaht T, Lutsar I, Klein N, Turner MA, et al Development and evaluation of a gentamicin pharmacokinetic model that facilitates opportunistic gentamicin therapeutic drug monitoring in neonates and infants. Antimicrob Agents Chemother 2016; 60: 4869–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Piperacillin/tazobactam 4 g/0.5 g powder for solution for infusion – summary of product characteristics (SPC) – (eMC) [online]. Available at https://www.medicines.org.uk/emc/medicine/30564#PHARMACOLOGICAL_PROPS (last accessed 1 February 2018).
- 170. Meropenem 1 g powder for solution for injection or infusion – summary of product characteristics (SPC) – (eMC) [online]. Available at https://www.medicines.org.uk/emc/medicine/31234#PHARMACOLOGICAL_PROPS (last accessed 1 February 2018).


