Abstract
Aims
To characterize ticagrelor exposure‐response relationship for platelet inhibition in patients with stable coronary artery disease (CAD) and a history of myocardial infarction (MI), using nonlinear mixed effects modelling and simulation.
Methods
Platelet function data were integrated with plasma concentration data of ticagrelor and its active metabolite AR‐C1249010XX in a population pharmacokinetic (PK) and pharmacodynamic (PD) model, based on two clinical studies. In the ONSET/OFFSET study, PK and platelet function were assessed in 123 CAD patients receiving placebo, ticagrelor (180 mg followed by 90 mg twice daily) or clopidogrel (600 mg followed by 75 mg once daily). In the PEGASUS‐TIMI 54 platelet function substudy, PK and platelet function were assessed during maintenance dosing in 180 prior MI patients receiving placebo, ticagrelor 60 mg or ticagrelor 90 mg twice daily.
Results
Platelet inhibition by ticagrelor was described by a sigmoidal Emax model. On average, half maximal inhibition was reached at ticagrelor concentrations of 116 (RSE: 5.3%) nmol l–1. Simulations showed that near maximal platelet inhibition is achieved with both ticagrelor 60 and 90 mg twice daily. At simulated lower doses, platelet inhibition is overall reduced, more variable between patients, and show greater peak‐to‐trough variability. Ticagrelor antiplatelet response was similar between the studied patient populations.
Conclusions
In patients with stable CAD or a history of MI, near maximal platelet inhibition is achieved with both ticagrelor 60 and 90 mg twice daily. At modelled doses <60 mg, the response is reduced overall, more variable between patients, and patients will display greater peak‐to‐trough variability.
Keywords: inhibition of platelet aggregation, NONMEM, pharmacodynamic, pharmacokinetic, ticagrelor
What is Already Known about this Subject
Ticagrelor is an oral P2Y12 receptor antagonist for prevention of cardiovascular events in acute coronary syndrome or prior myocardial infarction (MI) patients.
High platelet inhibition may explain superior efficacy of ticagrelor vs. clopidogrel in acute coronary syndrome patients, and similar efficacy of ticagrelor 60 and 90 mg in prior MI patients.
What this Study Adds
We describe the platelet inhibition time course and ticagrelor exposure relationship and predict the dose–response relationship in stable coronary artery and prior MI patients.
Reductions of the currently used ticagrelor doses 60 and 90 mg twice daily to 45 mg or lower may pose a risk of high platelet reactivity.
Introduction
Ticagrelor (180 mg loading dose followed by 90 mg twice daily), in combination with low dose acetylsalicylic acid (ASA), is used for the prevention of cardiovascular (CV) death, myocardial infarction (MI) and stroke in patients with acute coronary syndromes (ACS), based on the results of the PLATelet inhibition and patient Outcomes (PLATO) study 1. Following the PEGASUS‐TIMI 54 study, ticagrelor 60 mg twice daily, in combination with ASA, is also used for the long‐term prevention of adverse CV events in patients with a history of MI 2.
Dose‐dependent platelet inhibition has been demonstrated in healthy subjects receiving single doses of ticagrelor 30 to 400 mg 3, and 50 to 300 mg twice daily provides a consistently higher and more sustained platelet inhibition compared with ticagrelor 100 to 600 mg once daily 4. A rapid onset and high degree of platelet inhibition was seen with ticagrelor in patients with stable coronary artery disease (CAD) in the ONSET/OFFSET study 5. The inhibition was sustained during the maintenance phase and normalized platelet function was achieved within 120 h after last dose.
The pharmacokinetic (PK) profile of ticagrelor and its active, and similar potent 3, metabolite AR‐C124910XX have been evaluated in healthy subjects as well as in patients with CAD, including stable atherosclerotic disease 6, 7, ACS 8 and patients with a history of MI 9. Although the platelet response to ticagrelor has been measured in several studies, a detailed quantitative model‐based description of the longitudinal relationship between systemic ticagrelor exposure and platelet inhibition has not been previously published. The objective of this population PK/PD analysis was to mathematically characterize the ticagrelor dose‐concentration–response relationship by nonlinear mixed effects modelling, using data from the ONSET/OFFSET study and the PEGASUS‐TIMI 54 platelet function substudy.
Methods
Clinical studies and data
The present work is based on clinical data from the ONSET/OFFSET (Funded by AstraZeneca, NCT00528411) 5 and the PEGASUS‐TIMI 54 study (Funded by AstraZeneca; NCT01225562) 2. All subjects in both studies provided written informed consent, and the studies were approved by an Independent Ethics Committee and performed in accordance with guidelines established by the Declaration of Helsinki.
ONSET/OFFSET study
In the ONSET/OFFSET study 5, a total of 123 mainly white (88%) stable CAD patients were randomized to receive ticagrelor 90 mg twice daily following a 180 mg loading dose (n = 57), clopidogrel 75 mg once daily following a 600 mg loading dose (n = 54) or placebo (n = 12) for approximately 6 weeks. All patients received ASA 75–100 mg once daily. Ticagrelor and AR‐C124910XX plasma concentrations were measured at predose and 0.5, 1, 2, 4, 8 and 24 h after the first dose as well as at predose and 2, 4, 8, 24 and 48 h after the last dose. Platelet function was measured at predose and 2, 8 and 24 h after the first dose as well as at predose and 8, 24, 48 h, 5 and 10 days after the last dose. In total, there were 648 ticagrelor and 648 AR‐C124910XX plasma concentration measurements (including measurements below LOQ) and 608 platelet function measurements (including 103 from placebo‐treated patients) from the ONSET/OFFSET study included in the model‐based PK/PD analysis, see Table 1. The observed clopidogrel platelet function data (487 measurements) were summarized and compared with the model‐based ticagrelor predictions.
Table 1.
Number of pharmacokinetic (PK) and P2Y12 reaction units (PRU) measurements included in modelling
| Study | Visit | Data | Sampling time (hour) | Number of measurements |
|---|---|---|---|---|
| ONSET/OFFSET | After first dose | PK | 0.5,1,2,4,8,24 | 338 + 338(5 + 26) |
| PRU | 0,2,8,24 | 251 | ||
| After last dose | PK | 0,2,4,8,24,48 | 310 + 310(1 + 3) | |
| PRU | 0,8,24,48 120 240 | 357 | ||
| PEGASUS | After last dose | PK | 0,2 | 223 + 224 (0 + 0) |
| PRU | 0,2 | 346 |
Numbers for PK are ticagrelor + AR‐C124910XX and numbers within parenthesis are for data below limit of quantification Numbers for PRU data include data from placebo treated patients
PEGASUS‐TIMI 54 platelet function substudy
The PEGASUS‐TIMI 54 study 2 included 1161 patients with a history of MI at 1–3 years prior to enrolment and at high risk of an atherothrombotic event. A total of 180 patients from the PEGASUS‐TIMI 54 study, mainly white (93%) took part in a platelet function substudy 10 conducted at four centres: three in the UK (Sheffield, Rotherham and Nottingham) and one in the USA (Jacksonville, Florida). Patients were randomized to receive placebo (n = 64), ticagrelor 60 mg twice daily (n = 58) or ticagrelor 90 mg twice daily (n = 58), and all patients received acetylsalicylic acid (ASA) 75–150 mg once daily. Ticagrelor and AR‐C124910XX plasma concentrations and platelet function were measured at predose and 2 h postdose after at least 4 months of study treatment. In total, 113 ticagrelor‐ and 64 placebo‐treated patients had observed data on both platelet inhibition and ticagrelor plasma concentrations. In total, there were 223 ticagrelor and 224 AR‐C124910XX plasma concentration measurements and 346 platelet function measurements (including 127 from placebo‐treated patients) from the PEGASUS‐TIMI 54 platelet function substudy included in the model‐based PK/PD analyses, see Table 1.
PK/PD measurements assays
Samples for determination of ticagrelor and AR‐C124910XX plasma concentrations were analysed by York Bioanalytical Solutions (York Bioanalytical Solutions, York, UK – ONSET/OFFSET study) and Covance (Covance Bioanalytical Services, Indianapolis, Indiana, USA – PEGASUS‐TIMI 54 study). Ticagrelor, AR‐C124910XX and the deuterated internal standard D7−ZD6140 were isolated from human plasma using protein precipitation. After reversed‐phase liquid chromatography of the extract, ticagrelor, AR‐C124910XX and the internal standard were measured by atmospheric pressure chemical ionization (APCI) mass spectrometry in negative mode. The analytical methods have a validated calibration range of 1.9 to 3820 nmol l–1 for ticagrelor and 5.2 to 2080 nmol l–1 for AR‐C124910XX, utilizing a 100‐μl sample aliquot with a validated dilution with human plasma. All samples were analysed within the known stability period 11.
Platelet function was measured as P2Y12 reaction units (PRU) using the VerifyNow™ assay (formerly Accumetrics, now Accriva Diagnostics, San Diego, CA, USA) shown not to be influenced by aspirin treatment 12. In addition to PRU, VerifyNow™ also reports a BASE channel value where platelets are activated via thrombin receptors (using protease activated receptor‐1 and receptor‐4 peptides). The BASE channel has been shown in vitro to be insensitive to high level of P2Y12 inhibition motivating using the BASE channel measurement as a baseline PRU measurement in absence of a true baseline PRU measurement 13. Moreover, BASE channel values in PEGASUS‐TIMI 54 study were overall similar in the placebo (mean = 264), clopidogrel (mean = 256) and ticagrelor (mean = 262) treated patients. As no baseline measurements were included in the PEGASUS‐TIMI 54 study, graphical display of % inhibition from baseline were reported for each group based on the BASE channel value.
Population PK/PD model development
A population PK/PD model describing the relationship between ticagrelor dose, plasma concentrations and platelet function was developed in NONMEM version 7.3.0, using first‐order conditional estimation with interaction 14. R version 3.2.0 15, PsN version 4.4.0 16 and nonmem2R version 0.1.8 17 were used for model execution, data management and presentation of results.
Firstly, the population PK model was developed based on data from the ONSET/OFFSET study and the PEGASUS‐TIMI 54 platelet function substudy. The plasma concentration–time profiles of ticagrelor and AR‐C124910XX were jointly modelled, both described using two‐compartment disposition models with first‐order absorption/formation and elimination. The fraction of ticagrelor metabolized to AR‐C124910XX (Fm) was fixed to 0.22. PK parameters were tested for difference between stable CAD and prior MI patients if suggested by the data and or model diagnostic. Ticagrelor (<1%) and AR‐C124910XX (3.3%) measurements after first dose below the respective lower limit of quantification were included in the analysis and modelled using the M3 method 18. However, all PK measurements prior to the first dose in the ONSET/OFFSET study were below the lower limit of quantification and were excluded from the analysis.
Subsequently, the PK/PD model was developed to characterize the concentration‐response relationship between ticagrelor and platelet function based on data from the ONSET/OFFSET study and PEGASUS‐TIMI 54 platelet function substudy. The PK/PD model was developed with fixed population PK parameters from the final PK but with individual parameters estimated simultaneously based on both PK and PD data using the PPP&D method 19. Models using the model predicted ticagrelor or the sum of ticagrelor and AR‐C124910XX (both have similar potency in inhibiting the P2Y12 receptor 3) concentrations as the driver of the drug response were tested. Direct or indirect‐response relationships with linear or nonlinear concentration–response functions were tested if suggested by the data. The absolute PRU values were used in the model development and hence no BASE channel data were used for modelling. The model‐predicted PRU change from baseline was calculated to describe the extent of platelet inhibition. PRU data from the placebo patients were included in the model to enable description of the PRU baseline levels. It was tested if PRU baseline and the response to ticagrelor (EC50 and/or Emax parameters) were different between stable CAD and prior MI patients.
Between‐subject variability (BSV) was estimated for some of the PK and PD parameters using additive or exponential models. The residual errors for ticagrelor, AR‐C124910XX and PRU were tested to be described by additive, proportional, or combined additive and proportional models.
Model evaluation
Model development was guided by precision of parameter estimates, graphical goodness‐of‐fit assessments and changes in the NONMEM objective function value (OFV). The difference in OFV between two nested models is approximately χ2‐distributed, with degrees of freedom (df) equal to the difference in the number of model parameters. Based on this, the statistical significance for inclusion/exclusion of a model parameter can be judged. A significance level of 0.01, corresponding to a 6.64 change in OFV for 1 df, was used for discrimination among nested models and was used in the final backward elimination of different model parameters between stable CAD and prior MI patients. Visual predictive checks (VPC) were performed for key models. The observed data were graphically compared to model predictions based on 1000 simulated study datasets using the final model.
Illustration of the model‐predicted dose–response relationship
The model‐predicted dose–response relationship was illustrated based on simulations with the final population PK/PD model. The expected platelet inhibition from baseline was simulated at steady‐state after repeated administration of placebo or ticagrelor 15, 30, 45, 60 or 90 mg twice daily in 1000 patients at predose and 2 h postdose.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 20 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 21.
Results
A total of 871 ticagrelor and 872 AR‐C124910XX plasma concentration measurements and 954 platelet measurements from the ONSET/OFFSET study and PEGASUS‐TIMI 54 platelet function substudy were used in building the above described models. Further details on number of measurements and sampling times of measurements are presented in Table 1.
Ticagrelor population PK model
The ticagrelor and the AR‐C124910XX plasma concentration–time data were adequately described by the two‐compartment models. Describing the ticagrelor absorption by Erlang‐type absorption models 22 significantly lowered the OFV compared to a first‐order absorption model. Absorption from the dosing compartment to the central compartment is in Erlang models linked via one or more transit compartments. In the present modelling, the same transit rate (KTR) was used from the dose compartment to the first transit compartment, between transit compartments, and from the last transit compartment to the central compartment. Eight transit compartments provided the lowest OFV among Erlang‐type absorption models with 4–10 transit compartments. Introducing a lag‐time to the first‐order absorption model did not provide a better model fit than the Erlang‐type absorption model. Parameter estimates of the final PK model are presented in Table 1. Ticagrelor CL/F was estimated at 16.6 l h–1 with no statistically significant difference identified between the CAD and prior MI patients. However, adding a lag‐time to the Erlang‐type absorption model for the prior MI patients significantly lowered the OFV and provided better data to model agreement in VPC's. BSV was included for ticagrelor CL/F, KTR, the relative bioavailability (Frel) and inter‐compartment clearance (Q/F). For AR‐C124910XX, BSV was included on CLm/F and the peripheral volume of distribution (Vpm/F). Separate proportional residual error models were used for ticagrelor and AR‐C124910XX. There was good agreement between observed and predicted percentiles of the PK data (Figure 1; Supporting Information Figure S1). For a single loading dose of ticagrelor 180 mg, median Cmax was predicted at 2096 nmol l–1. The average steady‐state concentration (Css,av) was predicted at 576 nmol l–1 for ticagrelor 60 mg and at 864 nmol l–1 for ticagrelor 90 mg.
Figure 1.
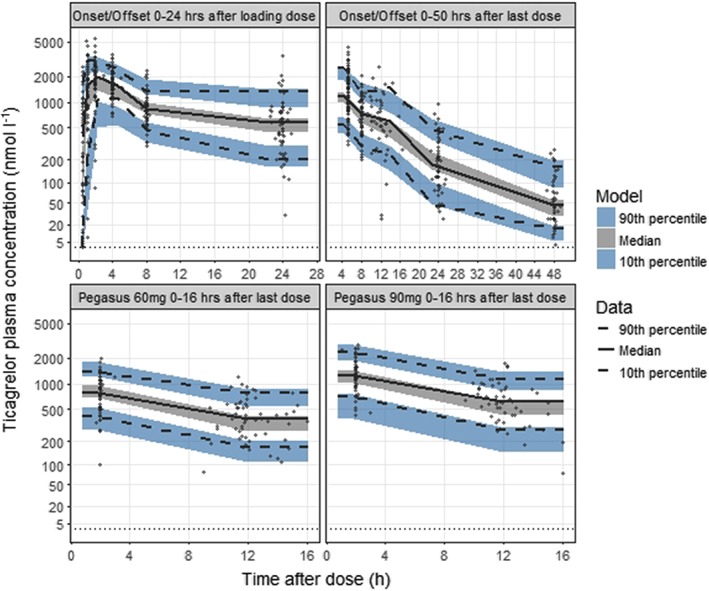
Visual predictive check of observed and predicted ticagrelor plasma concentrations 0–24 h after the first 180 mg dose (upper left panel) and 0–50 h after the last 90 mg steady‐state dose (upper right panel) vs. time after dose in the ONSET/OFFSET study and at predose and 2 h postdose after 60 mg (lower left panel) and 90 mg steady‐state dosing (lower right panel) in the PEGASUS‐TIMI 54 platelet function substudy. The solid and dashed lines represent the median and the 10th and 90th percentiles of the observations. The shaded areas represent 95% confidence intervals of the median and the 10th and 90th percentiles predicted by the model. The symbols represent the observed ticagrelor concentrations. Dotted line indicates the lower limit of quantification
Ticagrelor population PK/PD model for platelet function
The platelet inhibition was seen to fluctuate over time with the varying ticagrelor plasma concentrations. The ticagrelor antiplatelet response was direct with no delay between plasma concentrations and platelet inhibition. The inhibitory effect of ticagrelor on platelets was best described by a direct‐effects sigmoidal Emax model with a proportional drug response relative to the platelet function at baseline:
Parameter estimates of the final PK/PD model are presented in Table 2. The baseline PRU (PRUbaseline) was estimated lower in the PEGASUS‐TIMI 54 study compared to the baseline in ONSET/OFFSET, estimated at 261 and 311 respectively. The drug potency parameter describing the ticagrelor concentration at half the maximal effect (EC50) was estimated at 116 nmol l–1 and the maximal drug effect (Emax) parameter was estimated at 98.5%. The exponent γ describing the steepness of the exposure–response relationship was estimated at 1.59. None of the EC50, Emax or γ differed between the stable CAD and prior MI patient populations. Using the sum of ticagrelor and AR‐C124910XX plasma concentration (opposed to only ticagrelor) did not provide a significant improvement although with difference in OFV of 3.6 in favour of using the sum of concentrations. For simplicity and greater usability, the model utilizing only ticagrelor for the drug effect was chosen. To provide adequate description of the variability, the additive residual error was described as a nonlinear function increasing with increasing PRU levels. BSV was included for PRUbaseline, Emax and EC50, and covariance between PRUbaseline and EC50 was estimated. PRUbaseline was assumed normally distributed, Emax was assumed to be normally distributed on the logit transformed scale whereas EC50 was assumed to be log‐normally distributed. There was good agreement between observed and predicted percentiles of the PRU data (Figure 2). The PK/PD model predicted that 80% median platelet inhibition [interquartile range (IQR): 66–86%], near the maximal response plateau, was on average reached at ticagrelor concentrations of 344 nmol l–1 (Figure 3), which is well below the herein predicted median ticagrelor Css,av for both ticagrelor 60 mg and ticagrelor 90 mg twice daily.
Table 2.
Final pharmacokinetic and pharmacodynamic model parameter estimates
| Parameter | Estimate | (RSE%) | BSV (%) | (RSE%) |
|---|---|---|---|---|
| CL/F (l h –1 ) | 16.6 | (3.6) | 24 | (8.1) |
| Q/F (l h –1 ) | 10.4 | (4.7) | 95 | (45) |
| Vc/F (l) | 156 | (4.2) | ||
| Vp/F (l) | 55.8 | (8.3) | ||
| KTR (h −1 ) | 10.1 | (5.8) | 54 | (7.9) |
| Absorption lag time prior MI (h) | 0.48 | (11) | ||
| F rel | 1 FIX | 32 | (6.6) | |
| Proportional residual error ticagrelor (%) | 32 | (4.5) | ||
| CL m /F (l h –1 ) | 10.2 | (3.0) | 28 | (9.2) |
| F m | 0.22 FIX | |||
| Q m /F (l h –1 ) | 4.41 | (4.7) | ||
| Vc m /F (l) | 7.04 | (6.7) | ||
| Vp m /F (l) | 42.3 | (6.1) | 37 | (29) |
| Proportional residual error metabolite (%) | 26 | (4.9) | ||
| PRU baseline ONSET/OFFSET |
311 261 |
(1.9) (1.7) |
35 (add*) |
(8.9) |
| PRU baseline PEGASUS | ||||
| EC 50 (nmol l –1 ) | 116 | (5.3) | 66 | (7.1) |
| PRU baseline ‐EC 50 correlation | 0.33 | (27) | ||
| E max (%) | 98.5 | (0.1) |
2.2 (logit*) |
(6.1) |
| Steepness of exposure‐response (γ) | 1.59 | (3.4) | ||
| Additive residual error PRU (at PRU = 300) | 47.6 | (5.6) | ||
| Exponent for PRU error (α) | 0.48 | (2.8) |
CL/F, ticagrelor apparent clearance; Q/F, ticagrelor inter‐compartment clearance; Vc/F, ticagrelor central volume of distribution; Vp/F, ticagrelor peripheral volume of distribution; KTR, absorption transfer rate constant; Frel, ticagrelor relative bioavailability; CLm/F, metabolite; apparent clearance; Fm, fraction of ticagrelor metabolised to AR‐C124910XX; Qm/F, intercompartment clearance for the metabolite; Vcm/F, central volume of distribution for the metabolite; Vpm, peripheral volume of distribution for the metabolite; PRUbaseline, baseline P2Y12 reaction units (PRU); Emax, maximal platelet inhibition; EC50, ticagrelor concentration at half Emax; γ, steepness of the concentration‐response curve; α, Exponent for PRU residual error. PRU residual error was described by a power function, (PRU/300)α × SDat PRU 300; BSV, between subject variability (*standard deviation for PRUbaseline and Emax); RSE%, relative standard error (100*SE/mean); the RSE for BSV are reported on the approximate standard deviation scale (SE/variance estimate)/2; Emax is derived from the estimated parameter logit (Emax) and the RSE is based on numerical integration from the normal distribution of logit (Emax)
Figure 2.
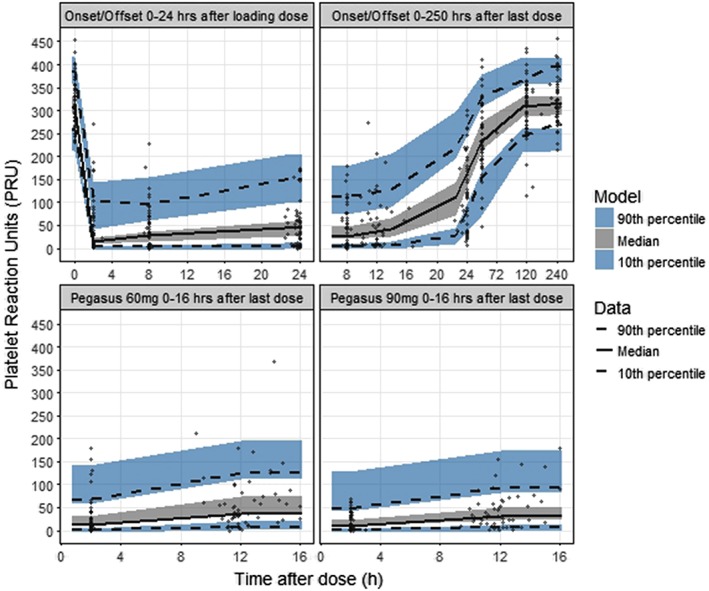
Visual predictive check of observed and predicted absolute P2Y12 reaction units (PRU) vs. 0–24 h after the first ticagrelor 180 mg loading dose (upper left panel) and 0–250 h after the last ticagrelor 90 mg steady‐state dose (upper right panel) in the ONSET/OFFSET study and at predose and 2 h postdose after 60 mg (lower left panel) and 90 mg (lower right panel) steady‐state dosing in the PEGASUS‐TIMI 54 platelet function substudy. The solid and dashed lines represent the median and the 10th and 90th percentiles of the observations. The shaded areas represent 95% confidence intervals of the median and the 10th and 90th percentiles predicted by the model. The symbols represent observed data
Figure 3.
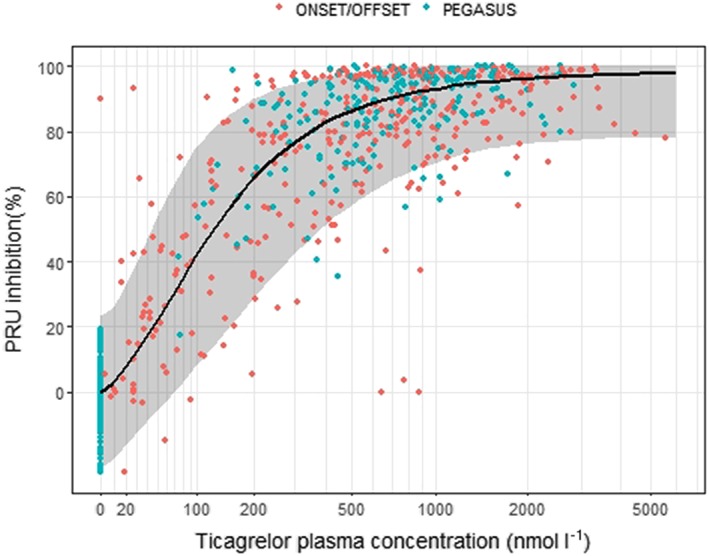
Observed P2Y12 reaction unit (PRU) inhibition in ONSET/OFFSET study (red) and PEGASUS‐TIMI 54 platelet function substudy (green), and model predicted median PRU inhibition (black line) with 95% prediction interval (shaded area)
Ticagrelor dose–response simulation for platelet inhibition
The predicted dose–response relationship for ticagrelor‐induced platelet inhibition is illustrated in Figure 4. Near maximal inhibition was achieved with both ticagrelor 60 mg and 90 mg twice daily. The median maximal predicted PRU inhibition was 96% (IQR: 91–98%) and on average predicted to occur at 1.8 h following a loading dose of ticagrelor 180 mg in CAD patients and at 2.4 h in prior MI patients. For steady‐state twice‐daily drug‐administration, the median predicted PRU inhibition 2 h post dose was 94% (IQR: 87–97%) for ticagrelor 90 mg (92% for 60 mg, IQR: 83–96%) and trough PRU median inhibition 12 h post dose was 87% (IQR: 73–93%) for ticagrelor 90 mg (80% for 60 mg, IQR: 62–90%). The trough PRU inhibition variability was 28% for ticagrelor 60 mg and 22% for ticagrelor 90 mg, and the expected proportion of patients with inhibition below 70% 12 h after dose was 34% and 22% for ticagrelor 60 mg and 90 mg, respectively. At lower doses the simulations showed decreased platelet inhibition but also more variable platelet inhibition between patients and greater peak‐to‐trough variability. The trough PRU inhibition variability was 33% for ticagrelor 45 mg twice daily and 42% for ticagrelor 30 mg twice daily. The median inhibition ranged between 73% (IQR: 53–86%) and 89% (IQR: 78–95%) during a dosing interval with ticagrelor 45 mg twice daily and between 60% (IQR: 39–77%) and 83% (IQR: 68–91%) with ticagrelor 30 mg twice daily. The expected proportion of patients with inhibition below 70% 12 h after dose was of 46% and 63% for ticagrelor 45 mg and 30 mg twice daily respectively.
Figure 4.
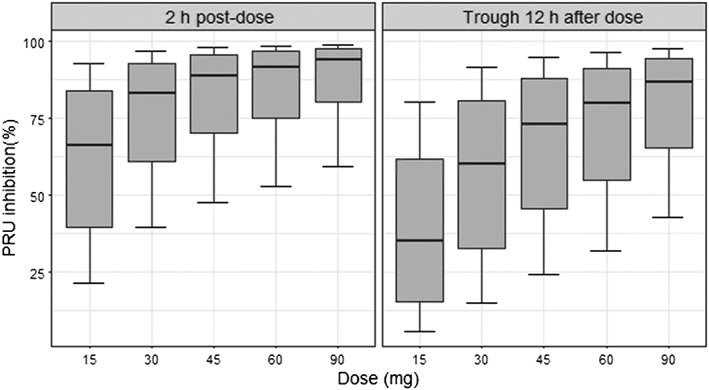
Predicted P2Y12 reaction unit (PRU) inhibition dose response at steady state for twice daily dosing at 2 h postdose (left) and at trough 12 h after dose (right) for Ticagrelor 15–90 mg twice daily dosing. Whiskers extend from the 5% to the 95% quantile
Discussion
In this work, we have quantified the nonlinear but direct relationship between systemic ticagrelor exposure and platelet inhibition in patients with stable CAD and patients with a history of MI. Using a model‐based approach, the PK and PD data from the ONSET/OFFSET study 5 and PEGASUS‐TIMI 54 platelet function substudy 10 could be integrated and jointly analysed.
Platelet inhibition increased with increasing ticagrelor plasma concentrations and fluctuated with the ticagrelor levels over the dosing interval. There was no delay in the onset and offset of response, consistent with the reversible binding of ticagrelor to the P2Y12 receptor. A ticagrelor concentration of 344 nmol l–1 was predicted to translate into 80% median platelet inhibition (IQR: 66–86%), close to the maximal response plateau. As the median Css,av was higher, near maximal platelet inhibition was predicted to be consistently achieved with both ticagrelor 60 mg and 90 mg twice daily for both patient populations. There were relatively small fluctuations in platelet inhibition over the dosing interval, and even trough concentrations at premaintenance dosing with ticagrelor 60 mg were predicted to be high enough to generate close to full inhibition. This contrasts with the lower and more variable inhibition, in the steeper portion of the exposure–response curve, seen at therapeutic doses with clopidogrel or with lower modelled doses of ticagrelor. In the ONSET/OFFSET study the median observed platelet inhibition at 8 h after multiple doses (close to the expected time of maximal inhibition for clopidogrel), was 49% with clopidogrel 75 mg.
The PEGASUS‐TIMI 54 platelet function substudy data were in agreement with the ONSET/OFFSET data. The mean PRU baseline value was lower in the prior MI compared with the stable CAD patients, but the relative treatment response appeared consistent regardless of a patient's baseline level; there were overlapping exposure–response relationships between the populations. The PK/PD model predictions were in line with the observed data in the PEGASUS‐TIMI 54 platelet function study where the platelet inhibition was similar between ticagrelor 60 mg and 90 mg, close to the maximal response, despite 37% lower ticagrelor concentrations for ticagrelor 60 mg both at predose and 2 h postdose 10. The PK/PD model predictions based on PEGASUS‐TIMI 54 and ONSET/OFFSET data were further supported by the PLATO platelet function substudy 23. The study showed high platelet inhibition both at predose and 2–4 h postdose. The inhibition was slightly higher 2–4 h postdose than at predose with ticagrelor 90 mg, in accordance with the PK/PD simulations.
The population PK of ticagrelor in patients with stable CAD was similar to that previously reported in ACS and prior MI patients. In this analysis, the mean ticagrelor CL/F was 16.6 l h–1 in both CAD patients and patients with prior MI. In previous population PK analyses, the mean ticagrelor CL/F was 14 l h–1 for ACS patients in the PLATO study and 15.4 l h–1 for patients on ticagrelor 90 mg with a history of MI in the PEGASUS‐TIMI 54 study 8, 9. In the PEGASUS‐TIMI 54 population PK analysis, CL/F of both ticagrelor and AR‐C124910XX were approximately 10% lower with ticagrelor 90 mg than with ticagrelor 60 mg 9. In the current work, studying a smaller subset of the PEGASUS PK population, we could not identify any statistically‐significant difference between the two doses. Based on less samples and fewer patients, the herein modelled Css,av was generally similar to what was observed in the prior MI patients in the main PEGASUS PK population and in the stable CAD patients. In the main PEGASUS‐TIMI 54 study, the median Css,av was 606 nmol l–1 with the 60 mg dose and 998 nmol l–1 with the 90 mg dose 9. In PLATO the median Css,av was 999 nmol l–1 .with ticagrelor 90 mg 8. Even if AR‐C124910XX and ticagrelor have similar potency in inhibiting the P2Y12 receptor 3, including the contribution of active metabolite exposure did not provide a better description of the data. This may potentially be explained by the fact that the metabolite exposure was approximately one third of the ticagrelor exposure and there was high correlation between ticagrelor and metabolite concentration time profiles (half‐life for the elimination phase was 10.2 h for ticagrelor and 9.6 h for the metabolite). The current PK/PD evaluation showing high platelet inhibition with both ticagrelor 60 mg and 90 mg supports the similar primary efficacy outcome observed for the two dosing regimens in PEGASUS‐TIMI 54 2. Whereas ticagrelor 60 mg on average gives close to maximal inhibition, lower doses are expected to translate into platelet inhibition in the steeper part of the exposure‐response curve, with a higher proportion of patients having low inhibition. At lower doses, platelet inhibition is predicted to be overall reduced and more variable between patients and show greater peak‐to‐trough variability in platelet inhibition. The variability in the platelet response due to variability in ticagrelor exposure and in the sensitivity to ticagrelor treatment is thus more manifested at trough 12 h after dose than at 2 h postdose. The PK and platelet inhibition of ticagrelor was assessed in 36 Chinese patients with stable CAD 24. Patients were randomized 1:1:1 to ticagrelor 45, 60 or 90 mg (all with low dose ASA). PK and platelet function was measured as PRU using the VerifyNow™ assay at baseline, after single dose, and after 4 days of twice daily dosing. The PRU mean inhibition from baseline over the dosing interval were 64–82% (45 mg), 69–87% (60 mg), and 89–94% (90 mg) after 4 days of twice daily dosing. The model predicted [mean and 95% prediction interval (CI)] observed mean PRU inhibition over the dosing interval for the Chinese study is 73% (CI:56–84) to 89% (CI:79–94) for 45 mg, 80% (CI:65–88) to 92% (CI:83–95) for 60 mg and 87% (CI:75–93) to 94% (CI:88–97) for 90 mg. Even though in the low range for 45 and 60 mg, the observed data were within the range of the model predicted PRU inhibition. Although the optimal degree of platelet inhibition is not fully known, our present results indicate that reducing the ticagrelor dose below the currently‐used 60 mg and 90 mg twice‐daily dosing to doses 45 mg or lower, may pose a risk of high platelet reactivity in patients with demography similar to those patients included in the present analysis.
In conclusion, we have characterized the dose‐concentration–response relationship between ticagrelor and platelet function in patients with a prior MI and stable CAD. Near maximal platelet inhibition was consistently seen with both ticagrelor 60 and 90 mg twice‐daily dosing. At lower doses, platelet inhibition is predicted to be subsequently lower and more variable between patients. Similarly, the within‐patient fluctuations in platelet inhibition over a dosing interval are predicted to increase at lower doses.
Competing Interests
M.Å., C.A., A.H., B.H. are employees of AstraZeneca. D.R. is a former employee of AstraZeneca. D.J.A. reports receiving payments as an individual for: a) Consulting fee or honorarium from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Bristol‐Myers Squibb, Chiesi, Daiichi‐Sankyo, Eli Lilly, Janssen, Merck, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; b) Participation in review activities from CeloNova and St. Jude Medical. Institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi‐Sankyo, Eisai, Eli‐Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions; in addition, is recipient of a funding from the Scott R. MacKenzie Foundation and the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH/NHGRI U01 HG007269, outside the submitted work. R.F.S. reports research grants, consultancy fees and honoraria from AstraZeneca, research grants and consultancy fees from PlaqueTec, consultancy fees and honoraria from Bayer, and consultancy fees from Actelion, Bristol Myers Squibb/Pfizer, Idorsia, Novartis and The Medicines Company. P.A.G. reports receiving grants from the National Institutes of Health, Bayer, Medicure, Instrumentation labs, Haemonetics, Amgen, Idorsia, Ionis, Haemonetics, Janssen, and Merck; receiving honoraria and payment for lectures, consultations, including service on speakers' bureaus from Bayer, Janssen, Merck, and Medicure; and holding patents in the area of personalized antiplatelet therapy and interventional cardiology. M.P.B. has received grant support to the TIMI Study Group from AstraZeneca and Merck; and has been a consultant to Aralez, AstraZeneca, Bayer, and Merck.
Principal investigator of the ONSET/OFFSET study was Dr Paul Gurbel. Principal Investigator of the PEGASUS‐TIMI 54 study was Dr Marc Sabatine. Dr Sabatine was contacted for co‐authorship of the present manuscript however instead suggested asking Dr Marc Bonaca for co‐authorship. Dr Bonaca was the Co‐Principal Investigator of the PEGASUS‐TIMI 54.
Supporting information
Figure S1 Visual predictive check of observed and predicted AR‐C124910XX plasma concentrations 0–24 h after the first 180 mg dose (upper left panel) and 0–50 h after the last 90 mg steady‐state dose (upper right panel) vs. time after dose in the ONSET/OFFSET study and at predose and 2 h postdose after 60 mg (lower left panel) and 90 mg steady‐state dosing (lower right panel) in the PEGASUS‐TIMI 54 platelet function substudy. The solid and dashed lines represent the median and the 10th and 90th percentiles of the observations. The shaded areas represent 95% confidence intervals of the median and the 10th and 90th percentiles predicted by the model. The symbols represent the observed AR‐C124910XX concentrations. The dotted line indicates the lower limit of quantification
Åstrand, M. , Amilon, C. , Röshammar, D. , Himmelmann, A. , Angiolillo, D. J. , Storey, R. F. , Gurbel, P. A. , Bonaca, M. P. , and Hamrén, B. (2019) Pharmacokinetic–pharmacodynamic modelling of platelet response to ticagrelor in stable coronary artery disease and prior myocardial infarction patients. Br J Clin Pharmacol, 85: 413–421. 10.1111/bcp.13812.
References
- 1. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 2. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–1800. [DOI] [PubMed] [Google Scholar]
- 3. Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability and safety of single ascending doses of ticagrelor, a reversibly binding oral P2Y12 receptor antagonist, in healthy subjects. Eur J Clin Pharmacol 2010; 66: 487–489. [DOI] [PubMed] [Google Scholar]
- 4. Butler K, Teng R. Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol 2010; 70: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al Randomized double‐blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009. Dec 22; 120: 2577–2585. [DOI] [PubMed] [Google Scholar]
- 6. Teng R. Ticagrelor: pharmacokinetic, pharmacodynamic and pharmacogenetic profile: an update. Clin Pharmacokinet 2015; 54: 1125–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Husted SE, Storey RF, Bliden K, Tantry US, Høimark L, Butler K, et al Pharmacokinetics and pharmacodynamics of ticagrelor in patients with stable coronary artery disease: results from the ONSET‐OFFSET and RESPOND studies. Clin Pharmacokinet 2012; 51: 397–409. [DOI] [PubMed] [Google Scholar]
- 8. Li J, Tang W, Storey RF, Husted S, Teng R. Population pharmacokinetics of ticagrelor in patients with acute coronary syndromes. Int J Clin Pharmacol Ther 2016; 54: 666–674. [DOI] [PubMed] [Google Scholar]
- 9. Röshammar D, Bergstrand M, Andersson T, Storey RF, Hamrén B. Population pharmacokinetics of ticagrelor and AR‐C124910XX in patients with prior myocardial infarction. Int J Clin Pharmacol Ther 2017; 55: 416–424. [DOI] [PubMed] [Google Scholar]
- 10. Storey RF, Angiolillo DJ, Bonaca MP, Thomas MR, Judge HM, Rollini F, et al Platelet inhibition with ticagrelor 60 mg versus 90 mg twice daily in the PEGASUS‐TIMI 54 study. J Am Coll Cardiol 2016; 67: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 11. Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 2299–2306. [DOI] [PubMed] [Google Scholar]
- 12. Bagoly Z, Sarkady F, Magyar T, Kappelmayer J, Pongrácz E, Csiba L, et al Comparison of a new P2Y12 receptor specific platelet aggregation test with other laboratory methods in stroke patients on clopidogrel monotherapy. PLoS One 2013; 8: e69417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jakubowski JA, Zhou C, Egan B, Wells M, Kotob‐Yahfoufi M, Sugidachi A, et al Modification of the VerifyNow® P2Y12 test BASE channel to accommodate high levels of P2Y12 antagonism. Platelets 2011; 22: 619–625. [DOI] [PubMed] [Google Scholar]
- 14. Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM Users Guides 2011. Ellicott City, Maryland, USA: Icon Development Solutions. [Google Scholar]
- 15. Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2007. ISBN 3–900051–07‐0. Available at http://www.R‐project.org.
- 16. Lindbom L, Ribbing J, Jonsson EN. Perl‐speaks‐NONMEM (PsN)–a Perl module for NONMEM related programming. Comput Methods Programs Biomed 2004; 75: 85–94. [DOI] [PubMed] [Google Scholar]
- 17. Åstrand M. nonmem2R: Loading NONMEM Output Files and Simulate with Parameter Uncertainty. R package version 0.1.8 (2018). Available at http://CRAN.R‐project.org/package=nonmem2R.
- 18. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001. Oct; 28: 481–504. [DOI] [PubMed] [Google Scholar]
- 19. Zhang L, Beal SL, Sheiner LB. Simultaneous vs. sequential analysis for population PK/PD data I: best case performance. J Pharmacokinet Pharmacodyn 2003; 30: 387–403. [DOI] [PubMed] [Google Scholar]
- 20. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alexander SP, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 2017; 174 (Suppl. 1): S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rousseau A, Leger F, Le Meur Y, Saint‐Marcoux F, Paintaud G, Buchler M, et al Population pharmacokinetic modeling of oral cyclosporin using NONMEM ‐ comparison of absorption pharmacokinetic models and design of a Bayesian estimator. Drug Monit 2004; 26: 23–30. [DOI] [PubMed] [Google Scholar]
- 23. Storey RF, Angiolillo DJ, Patil SB, Desai B, Ecob R, Husted S, et al Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient outcomes) PLATELET substudy. J Am Coll Cardiol 2010; 56: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 24. Li H, Guo J, Carlson GF, Teng R. Pharmacodynamics, pharmacokinetics, and safety of ticagrelor in Chinese patients with stable coronary artery disease. Br J Clin Pharmacol 2016; 82: 352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Visual predictive check of observed and predicted AR‐C124910XX plasma concentrations 0–24 h after the first 180 mg dose (upper left panel) and 0–50 h after the last 90 mg steady‐state dose (upper right panel) vs. time after dose in the ONSET/OFFSET study and at predose and 2 h postdose after 60 mg (lower left panel) and 90 mg steady‐state dosing (lower right panel) in the PEGASUS‐TIMI 54 platelet function substudy. The solid and dashed lines represent the median and the 10th and 90th percentiles of the observations. The shaded areas represent 95% confidence intervals of the median and the 10th and 90th percentiles predicted by the model. The symbols represent the observed AR‐C124910XX concentrations. The dotted line indicates the lower limit of quantification


