Abstract
Aims
Gemcitabine has been associated with thrombotic microangiopathy (TMA). We conducted a national retrospective study of gemcitabine‐associated TMA (G‐TMA).
Methods
From 1998 to 2015, all cases of G‐TMA reported to the French Pharmacovigilance Network and the French TMA Reference Center, and cases explored for complement alternative pathway abnormalities, were analysed.
Results
G‐TMA was diagnosed in 120 patients (median age 61.5 years), after a median of 210 days of treatment, and a cumulative dose of 12 941 mg m–2. Gemcitabine indications were: pancreatic (52.9%), pulmonary (12.6%) and breast (7.6%) cancers, metastatic in 34.2% of cases. Main symptoms were oedema (56.7%) and new‐onset or exacerbated hypertension (62.2%). Most patients presented with haemolytic anaemia (95.6%) and thrombocytopenia (74.6%). Acute kidney injury was reported in 97.4% and dialysis was required in 27.8% of patients. Treatment consisted of: plasma exchange (PE; 39.8%), fresh frozen plasma (21.4%), corticosteroids (15.3%) and eculizumab (5.1%). A complete remission of TMA was obtained in 42.1% of patients and haematological remission in 23.1%, while 34.7% did not improve. The survival status was known for 52 patients, with 29 deaths (54.7%). Patients treated with PE, despite a more severe acute kidney injury, requiring dialysis more frequently, displayed comparable rates of remission, but with more adverse events. No abnormality in complement alternative pathway was documented in patients explored.
Conclusion
This large cohort confirms the severity of G‐TMA, associated with severe renal failure and death. Oedema and hypertension could be monitored in patients treated with gemcitabine to detect early TMA. The benefit of PE or eculizumab deserves further investigation.
Keywords: medication safety, pharmacovigilance, adverse drug reactions, chemotherapy, acute kidney injury
What is Already Known about this Subject
Thrombotic microangiopathy (TMA) can be secondary to cancer or chemotherapies, including gemcitabine.
The occurrence of TMA exposes patients to a risk of acute renal failure and death, and can jeopardize the continuation of cancer therapy.
Little is known about the optimal therapeutic strategy in secondary TMA in general, and in gemcitabine‐associated TMA (G‐TMA) in particular.
What this Study Adds
This study provides the clinical description of G‐TMA in the largest cohort published to date, from the national databases of French Pharmacovigilance network and reference centres.
The systematic screening for hypertension and/or oedema in patients treated with gemcitabine could allow the early detection of TMA and thus prevent further damage.
The benefit of plasma exchanges in G‐TMA remains uncertain: patients treated with plasma exchange show similar outcomes despite a more severe clinical presentation.
Introduction
Gemcitabine was first approved for the treatment of unresectable pancreatic carcinoma more than 25 years ago. Its use has expanded a lot to include the treatment of a wide variety of malignancies, including non‐small‐cell lung carcinoma, hepatobiliary, urothelial, mammary and ovarian cancers, soft tissue sarcomas, and lymphomas. Primary side effects are myelosuppression (thrombocytopenia, neutropenia), mild liver function abnormalities, flu‐like symptoms and oedema 1.
Thrombotic microangiopathy (TMA) is characterized by more or less generalized microvascular occlusion by platelet thrombi, leading to variable end‐organ damage, especially in the brain, kidneys and heart. The classical symptoms are the association of mechanical haemolytic anaemia (HA), thrombocytopenia, fever, renal insufficiency and neurological symptoms. TMA can present either as thrombocytopenic thrombotic purpura, with predominant neurological involvement and the severe deficiency or autoantibody‐mediated inhibition of the von Willebrand factor‐cleaving metalloproteinase ADAMTS13, or as haemolytic and uraemic syndrome (HUS), with predominant renal involvement. The different types of renal TMA are: typical HUS, caused by infections by shiga toxin‐secreting bacteria; atypical HUS, associated with complement alternative pathway (CAP) dysregulation; and secondary HUS, related to autoimmune diseases, infections, cancers or drug toxicities 2. Several antitumour agents have been associated with TMA, including mitomycin C, cisplatin, bleomycin, 5‐fluorouracil and anti‐vascular endothelial growth factor therapies 3, 4, 5, 6. The causal relationship between a specific anticancer therapy and TMA is difficult to establish, given that malignancy itself can induce TMA and that patients are often treated with several drugs.
The first case of gemcitabine‐associated TMA (G‐TMA) was reported in 1994 7, during a phase II trial in pancreatic cancer. Since then, many case reports have been published 2, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, with an incidence estimated between 0.015% 24 and 1,4% 13. Although rare, G‐TMA is a severe complication, associated with an important mortality and renal damage possibly leading to end‐stage renal disease 23.
Plasma exchange (PE) is used in secondary forms of TMA, even if its benefit has not been clearly documented in chemotherapy‐related TMA 25. The humanized monoclonal anti‐C5 antibody, eculizumab, which inhibits terminal complement, is indicated in the treatment of atypical HUS 26, 27, with great success in patients with CAP dysregulation. Its efficacy has been reported in a few cases of patients with G‐TMA 28, 29, 30.
Considering the increasing number of patients treated with gemcitabine worldwide, a precise description and a deeper understanding of this rare and serious complication are warranted. Thus, we conducted a large national retrospective study of the French cases of G‐TMA, to describe the clinical and biological patterns of the disease, the therapeutic management and the patients' outcomes. We compared the outcomes of patients treated with PE and other modalities, and described the outcomes of patients treated with eculizumab.
Methods
Data sources
We collected retrospectively the cases of G‐TMA that occurred in France from August 1998 to July 2015, from three complementary sources:
The French Pharmacovigilance Network, which comprises 31 Regional Pharmacovigilance Centers that collect and analyse spontaneous reports of adverse drug reactions from health care professionals and patients. All validated cases are anonymously stored in a common computerized database
The French TMA Reference Center
The French registry of patients explored for Complement Alternative Pathway abnormalities (Laboratory of Immunology, AP‐HP HEGP, Paris)
Data collection
All data were collected in accordance with the French legislation on retrospective clinical studies, in accordance with the precepts established by the Helsinki declaration. Cases of patients identified through the French TMA Reference Center and of patients explored for Complement Alternative Pathway abnormalities were reviewed through a standardized questionnaire after studying the patients' medical files. Original medical files were not available in cases from the French Pharmacovigilance Network database, which were anonymous (only date of birth and initials were available), but commentaries and data recorded were collected. Cases declared in more than one source were identified and merged using initials, birthdate and date of onset.
The data collected were: age at the time of G‐TMA diagnosis, sex, date of diagnosis, medical background, type of cancer and treatments, clinical and biological characteristics of TMA, TMA treatments, and patient outcomes. Results of ADAMTS13 activity and CAP exploration were recorded when available.
Definitions
G‐TMA was defined, in patients treated with gemcitabine, by: (i) at least two of the three following criteria: HA, thrombocytopenia (platelet count <150 109 l–1) and acute kidney injury (AKI); or (ii) biopsy‐proven renal TMA.
HA was defined by anaemia with elevated lactate dehydrogenase and low or absent haptoglobin with negative direct Coombs test. Schizocytes were considered present when superior to 1% on a peripheral blood smear 31.
AKI was defined by an increase in serum creatinine by ≥0.3 mg dl–1 within 48 h, an increase to ≥1.5 × baseline serum creatinine within 7 days, or a diuresis <0.5 ml kg–1 h–1 for 6 h 32.
In patients who underwent a renal biopsy, the pathological lesions confirming the diagnosis of renal TMA were: acute lesions, characterized by intravascular fibrin thrombi with mucoid changes, accompanied by mesangiolysis, endothelial swelling, corrugation of the glomerular basement membrane and a bloodless appearance in segments of glomeruli uninvolved by thrombi; and/or more chronic lesions, characterized by the presence of double contours of the glomerular basement membranes with variable endocapillary hypercellularity, with no immune complexes and intimal proliferation of arterioles 33.
We also divided patients between those with a definite diagnosis of TMA (combination of Coombs negative HA with the presence of schizocytes, of thrombocytopenia, and of AKI or biopsy‐proven renal TMA), those with a probable diagnosis of TMA (for whom one criterion could be missing), and those with a renal‐limited biopsy‐proven TMA (without peripheral haematological signs). All cases were reviewed by TMA experts.
Haematological remission of TMA was defined by the normalization of platelet count and lactate dehydrogenase levels in two consecutive measurements over a 4 week‐period. Renal remission, in case of AKI, was defined by the improvement of serum creatinine level of more than 25% compared to the worst value. Complete remission of TMA was defined by haematological and renal remission.
The severity of TMA was graded according to the National Cancer Institute Common Toxicity Criteria (CTCAE v4.0 33) 34: grade 1 = evidence of red blood cell destruction without clinical consequences; grade 3 = laboratory findings with clinical consequences (e.g. renal insufficiency, petechiae); grade 4 = life‐threatening consequences, (e.g. central nervous system haemorrhage or thrombosis/embolism or renal failure); grade 5 = death.
Statistical analysis
Comparison of variables was performed, for continuous variables, by Student t test or Mann–Whitney test when the number of patients in each group was <30 and by the χ2 test or Fisher's exact test in the case of qualitative variables. P values <0.05 were considered statistically significant. All reported P values are two‐sided.
Percentages were determined for all qualitative variables and continuous variables were expressed as median with interquartile ranges.
Overall survival was analysed with the use of the Kaplan–Meier method; the log‐rank test was used to compare patients treated with or without PE.
Results
Patient characteristics
During the 17‐year study period, after merging of duplicate cases, 120 patients with G‐TMA were included in this study: 23 (19.1%) from 1998 to 2005, 50 (41.7%) from 2006 to 2010 and 47 (39.2%) from 2011 to 2015.
The main characteristics of patients at baseline are shown in Table 1.
Table 1.
Characteristics of the 120 patients at the time of diagnosis of gemcitabine‐associated thrombotic microangiopathy
| Patients (n = 120) | |||
|---|---|---|---|
| Age | 61.5 (54–68.25) | ||
| Sex, male (%) | 68 (56.7%) | ||
| Medical background (n = 88) | |||
| Cardiovascular disease | 10.2% | ||
| Hypertension | 31.8% | ||
| Diabetes | 11.3% | ||
| Dyslipidaemia | 11.3% | ||
| COPD | 7.9% | ||
| Tobacco use | 15.9% | ||
| Alcoholism | 7.9% | ||
| CKD | 7.9% | ||
| Other cancer | 9.1% | ||
| Autoimmune disease | 4.5% | ||
| Type of cancer treated with gemcitabine (n = 119) | |||
| Pancreas | 52.9% | ||
| Lung | 12.6% | ||
| Breast | 7.6% | ||
| Cholangiocarcinoma and biliary tract cancers | 6.7% | ||
| Bladder | 4.2% | ||
| Ovarian cancer | 3.4% | ||
| Mesothelioma | pleural | 2.5% | |
| peritoneal | 0.8% | ||
| Lymphoma | Non‐Hodgkin | 2.5% | |
| Hodgkin | 2.5% | ||
| Uterus | 1.7% | ||
| Mycosis fungoid | 0.8% | ||
| Testicle | 0.8% | ||
| Liver | 0.8% | ||
| Metastatic cancer | 34.2% | ||
| Chemotherapy prior to gemcitabine (n = 104) | 45.2% | ||
| Concomitant treatments | |||
| Chemotherapy (n = 80) | 41.2% | ||
| Hormonotherapy (n = 80) | 2.5% | ||
| Anticoagulants or antiaggregants (n = 79) | 19% | ||
| clopidrogel, ticlodipin | 2.5% | ||
Age is expressed as median with interquartile ranges. All qualitative variables are expressed as percentages calculated among patients for whom the information was available, therefore excluding the missing data. COPD, chronic obstructive pulmonary disease, CKD: chronic kidney disease
Clinical presentation of TMA
The exposure of patients to gemcitabine, the clinical symptoms of G‐TMA and the renal and haematological presentations, are detailed in Table 2.
Table 2.
Clinical presentation of gemcitabine‐associated thrombotic microangiopathy in the 120 patients
| Patients (n = 120) | ||
|---|---|---|
| Duration of treatment (days; n = 109) | 210 (120–256) | |
| Cumulative dose (mg m –2 ; n = 32) | 12 941 (6303–17 647) | |
| Clinical symptoms | (n = 111) | |
| Oedema | 56.7% | |
| Hypertension | 62.2% | |
| Neurological signs | 9.9% | |
| Digestive symptoms | 9% | |
| Fever | 7.2% | |
| Sepsis concomitant | 2.7% | |
| Arthralgia | 0.9% | |
| Purpura | 6.3% | |
| Haematological characteristics | Haemolytic anaemia | 95.6% (110/115) |
| Thrombocytopenia | 74.6% (85/114) | |
| Platelet (109 l–1; n = 74) | 65.5 (35–104) | |
| Schizocytes | 55.3% (57/103) | |
| Renal characteristics | AKI | 97.4% (113/116) |
| Creatinine at the time of diagnosis (n = 86) | 204.5 (135–325) | |
| Need for renal replacement therapy | 27.8% (27/97) | |
| Haematuria | 22.8% (26/114) | |
| Proteinuria | 34.2% (39/114) | |
| Proteinuria (g/24 h; n = 28) | 1.4 (0,6‐2,86) | |
| Kidney biopsy | 20% (23/115) | |
Quantitative variables are expressed as median with interquartile ranges. All qualitative variables are expressed as percentages calculated among patients for whom the information was available, therefore excluding the missing data. AKI, acute kidney injury
Among the 120 patients, 49 had a definite diagnosis of TMA, 70 had a probable diagnosis of TMA, and one had a biopsy‐proven renal TMA with hypertension, oedema and AKI but without peripheral haematological abnormalities. Hypertension was more frequent in patients with definite than probable TMA (76.6% vs. 52.4%, P = 0.009), and their median platelet count was lower (61 vs. 121 109 l–1, P = 0.01).
Severity of G‐TMA, evaluated with CTCAE v4.0 (34), was as follows: grade 3 in 51.7% of patients, grade 4 in 32.5% and grade 5 in 15.8% of patients.
Treatment of G‐TMA and outcome
Treatment of G‐TMA, and patients' outcome, are detailed in Table 3. Gemcitabine was discontinued in all patients for whom the information was available. It was reintroduced in only one patient who was in remission of TMA after treatment interruption, who relapsed after reintroduction and reached remission again after definitive gemcitabine withdrawal. Thirty‐nine patients (23 with a definite diagnosis and 16 with a probable diagnosis of TMA) were treated with plasma exchange (PE). PE were performed with plasma replacement [with a median of 10 exchanges (interquartile range 5–20)]. Five patients received eculizumab (four with a definite diagnosis and one with a probable diagnosis of TMA). A third of patients had no improvement of TMA. Haemorrhage and infections each occurred in 11.5% of patients with G‐TMA. The survival status after the treatment of the TMA was known for 52 patients (43% of the cohort), with the report of 29 deaths, but a lot of patients were lost during the follow‐up. The Kaplan–Meier survival estimate of the cohort is shown in Figure 1A. The causes of death were G‐TMA itself (particularly in the early phase of TMA) and the progression of cancer. One patient, who was re‐exposed to gemcitabine, had recurrent G‐TMA.
Table 3.
Treatments of gemcitabine‐associated thrombotic microangiopathy (TMA) and patient outcome
| Patients (n = 120) | ||
|---|---|---|
| Type of treatment | Cessation of gemcitabine | 100% (52/52) |
| Anti‐hypertensive treatment | 57.4% (54/94) | |
| Plasma exchange | 39.8% (39/98) | |
| FFP infusion | 21.4% (21/98) | |
| Steroids | 15.3% (15/98) | |
| Eculizumab | 5.1% (5/98) | |
| Response to treatment (n = 95) | Complete remission | 42.1% |
| Haematological remission only | 23.1% | |
| Absence of remission | 34.7% | |
| Non‐lethal serious adverse events | Haemorrhage | 11.5% (9/77) |
| Infection | 11.5% (9/77) | |
| Death | 54.7% (29/52) | |
| Main cause of death (n = 29) | Cancer evolution | 34.5% |
| TMA | 65.5% |
All qualitative variables are expressed as percentages calculated among patients for whom the information was available, therefore excluding the missing data. FFP, fresh frozen plasma
Figure 1.
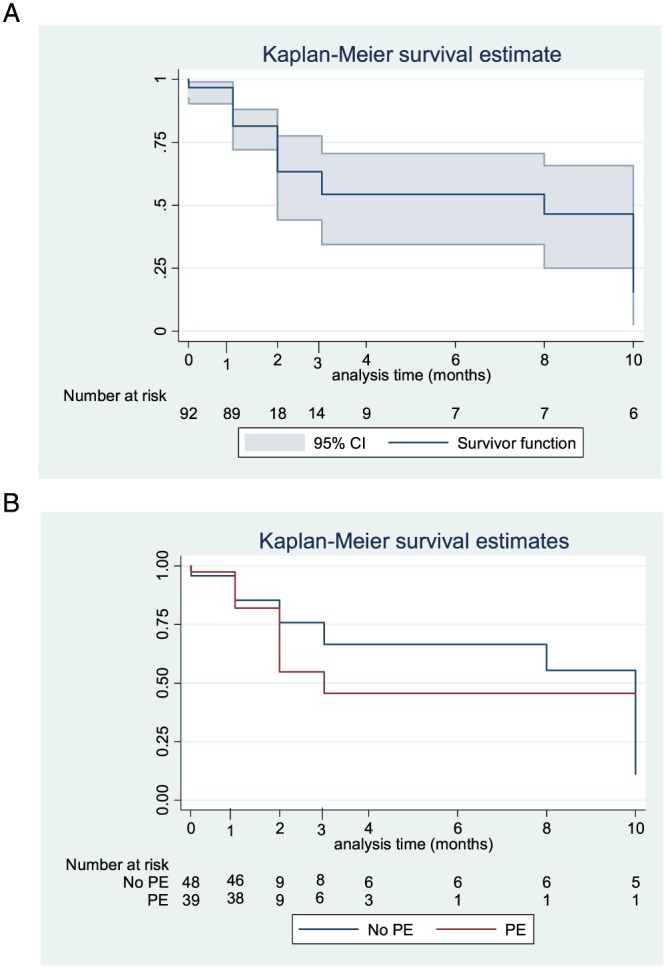
Kaplan–Meier survival curves of (A) the 92 patients with available follow‐up data and (B) the 87 patients with available data concerning treatment of gemcitabine‐associated thrombotic microangiopathy and follow‐up. CI, confidence interval; PE, plasma exchange
Comparison between patients treated with plasma exchange and others
As shown in Table 4, there was no significant difference in the baseline characteristics of patients treated with PE and others. Concerning clinical symptoms, patients treated with PE had more frequent hypertension and reported presence of schizocytes, higher serum creatinine levels and more frequent need for renal replacement therapy. Overall, patients treated with PE had a more severe renal presentation of G‐TMA.
Table 4.
Comparison of patients' characteristics at baseline and clinical presentation of thrombotic microangiopathy between patients treated with plasma exchange (PE) and others
| PE (n = 39) | No PE (n = 59) | P | |||
|---|---|---|---|---|---|
| Age (years) | 59 (53.5–67) | 63 (54–68.5) | 0.98 | ||
| Sex, male (%) | 51.3% | 54.2% | 0.77 | ||
| Medical background | NS | ||||
| Chemotherapy prior to gemcitabine | 36.4% (12/33) | 48.1% (25/52) | 0.29 | ||
| Type of cancer causing the treatment | NS | ||||
| Metastatic cancer | 33.3% (13/39) | 40.7% (24/59) | 0.46 | ||
| Associated treatments | NS | ||||
| Duration of treatment (days) | 180 (90–240) | 210 (136–304) | 0.11 | ||
| Missing | 4 (10.3%) | 5 (8.5%) | |||
| Clinical symptoms | |||||
| Oedema | 65.8% (25/38) | 58.9% (33/56) | 0.50 | ||
| Hypertension | 81.6% (31/38) | 62.5% (35/56) | 0.047 | ||
| Neurological signs | 18.4% (7/38) | 5.3% (3/56) | 0.08 | ||
| Digestive symptoms | 18.4% (7/38) | 5.3% (3/56) | 0.08 | ||
| Fever | 10.5% (4/38) | 5.3% (3/56) | 0.43 | ||
| Sepsis | 2.6% (1/38) | 1.8% (1/56) | 1 | ||
| Arthralgia | 2.6% (1/38) | 0 | 0.40 | ||
| Purpura | 7.9% (3/38) | 5.3% (3/56) | 0.68 | ||
| Haematological characteristics | |||||
| Haemolytic anaemia | 100% (59/59) | 92.8% (52/56) | 0.15 | ||
| Thrombocytopenia | 76.3% (29/38) | 78.2% (43/55) | 0.83 | ||
| Platelets (109 l–1) | 60 (31–87) | 70 (415–113) | 0.31 | ||
| Missing | 10 (25.6%) | 21 (35.6%) | |||
| Schizocytes | 84.4% (27/32) | 49% (25/51) | <0.001 | ||
| Renal characteristics | |||||
| AKI | 100% (39/39) | 96.5% (56/58) | 0.51 | ||
| Creatinine at the time of diagnosis | 297.5 (192.5–410) | 162 (135–300) | 0.0017 | ||
| Missing | 9 (23.1%) | 14 (23.7%) | |||
| Need for RRT | 45.9% (17/37) | 11.9% (7/59) | <0.001 | ||
| Haematuria | 30.8% (12/39) | 24.1% (14/58) | 0.55 | ||
| Proteinuria | 51.3% (20/39) | 31% (18/58) | 0.096 | ||
| Proteinuria (g/24 h) | 1.4 (0.6–3) | 1.4 (0.77–2.25) | 0.60 | ||
| Missing | 22 (56,4%) | 48 (81,3%) | |||
| Kidney biopsy | 24.3% (9/37) | 21% (12/57) | 0.71 | ||
Age is expressed as median with interquartile ranges. Quantitative variables are expressed as median with interquartile ranges. All qualitative variables are expressed as percentages calculated among patients for whom the information was available, therefore excluding the missing data. All reported P values are two sided. AKI, acute kidney injury; PE, plasma exchange; RRT, renal replacement therapy
As shown in Table 5, the other treatments of G‐TMA did not differ between patients treated with PE and others. Similar rates of complete of haematological remission were observed between the groups. Haemorrhagic and infectious adverse events were more frequent in the PE group, and one case of transfusion‐related acute lung injury was reported in the PE group. Although many follow‐up data were missing, there was no evident difference in the survival of patients treated or not with PE (Figure 1B).
Table 5.
Comparison of the outcome of the gemcitabine‐associated thrombotic microangiopathy between patients treated with plasma exchange (PE) and others
| PE (n = 39) | No PE (n = 59) | P | ||
|---|---|---|---|---|
| Type of treatment | ||||
| Cessation of gemcitabine | 100% (16/16) | 100% (35/35) | 0.49 | |
| Antihypertensive treatment | 61.1% (22/36) | 54.2% (32/59) | 0.57 | |
| FFP infusion | 28.2% (11/39) | 16.9% (10/59) | 0.18 | |
| Steroids | 20.5% (8/39) | 11.9% (7/59) | 0.24 | |
| Eculizumab | 7.7% (3/39) | 3.4% (2/59) | 0.38 | |
| Response to treatment | ||||
| Complete remission | 39.5% (15/38) | 45.8% (22/48) | 0.55 | |
| Haematological remission only | 21% (8/38) | 29.2% (14/48) | 0.39 | |
| Absence of remission | 39.5% (15/38) | 25% (12/48) | 0.15 | |
| Non lethal serious adverse events | ||||
| Haemorrhage | 28.6% (8/28) | 2.1% (1/48) | 0.001 | |
| Infection | 25% (7/28) | 4.2% (2/48) | 0.01 | |
| Death | 40% (10/25) | 62.5% (15/24) | 0.115 | |
| Cause of Death | Cancer | 20% (2/10) | 46.7% (7/15) | 0.229 |
| TMA | 80% (8/10) | 53.3% (8/15) | 0.174 | |
All qualitative variables are expressed as percentages calculated among patients for whom the information was available, therefore excluding the missing data. All reported P values are two sided. FFP, fresh frozen plasma; PE, plasma exchange
Among patients treated with PE with available follow‐up data (n = 38), nonresponders (n = 15) were more frequently exposed to other chemotherapies prior to gemcitabine than responders (n = 23; 61.5% vs. 20%, P = 0.027), with a shorter duration of gemcitabine before the occurrence of TMA (124 vs. 224 days, P = 0.0144). Nonresponders tended to be treated more frequently for pulmonary cancer (20% vs. 0%, P = 0.054). They displayed less frequent hypertension (64.3 vs 91.3%, P = 0.042), and tended to have more frequent thrombocytopenia (92.8 vs. 55.2%, P = 0.057).
Outcome of patients treated with eculizumab
Five patients were treated with eculizumab: two women and three men, age 53–68 years. Three had pancreatic cancer and two had metastatic lung cancer. Gemcitabine was used alone in four patients and in association with platinum salts in one patient. Eculizumab was initiated after the failure of PE in four patients (no improvement after three to five PE or recurrence of TMA after a transient response to treatment), while it was used as a first line therapy in the last patient who presented with severe AKI requiring immediate dialysis. Complete remission of TMA was achieved in one patient, and another reached haematological remission with stabilization of renal function. One patient reached haematological remission but remained on dialysis and died after a decision of therapeutic limitation. One patient died of cancer progression, while he had a haematological remission but remained dialysis‐dependent. The last patient, who had experienced no improvement of the TMA, died of septicaemia.
Complement exploration and ADAMTS 13 activity
ADAMTS13 activity was measured in 25 patients and was undetectable (< 5% of activity) in two (8%) patients, without detectable anti‐ADAMTS13 antibodies.
Complement fractions C3 and C4 and CH50 were measured in 25 patients, among whom five (20%) had signs of complement consumption (low C3 in one, low C4 in two, and low C3, C4 and CH50 in two). A functional exploration of complement alternative pathway was performed in eight patients, of whom none had a significant abnormality.
Discussion
We report the largest case series of G‐TMA published to date, with 120 patients collected from complimentary sources. Indeed, previous data relied mostly on isolated case reports and limited case series. This work allows a more precise description of G‐TMA presentation, which involves mainly the kidney. We highlight the importance of oedema and new‐onset or exacerbated hypertension as early signs of G‐TMA, requiring a close monitoring of renal function and haematological abnormalities in patients treated with gemcitabine. The benefit of PE in G‐TMA was uncertain in this retrospective cohort: patients treated with PE, despite a more severe presentation, had comparable outcomes as those not treated with PE, but with more serious adverse events.
New‐onset hypertension or exacerbation of a known hypertension are key signs of G‐TMA, that can appear long before haemolysis, thrombocytopenia and AKI. Systematic screening for these early signs of G‐TMA, and physician awareness of their value of red flag, could permit an earlier detection and prevention of G‐TMA 2, 12, 13, 23, 35 . Haematological and renal features at the time of diagnosis were consistent with the common symptoms of HUS with HA, AKI and thrombocytopenia. As described before, the absence of thrombocytopenia did not exclude the diagnosis of HUS 36, 37, and pathological lesions of renal TMA could be documented in the absence of schizocytes or HA.
The median duration of gemcitabine treatment before G‐TMA, reported here at 7 months, is consistent with other reports 12 (from 5.3 to 13.3 months 35). Cumulative doses of gemcitabine were lower in the present cohort (12 941 mg m–2) than in previous reports (23 000 2, 19 052 24 and 23 200 mg m–2 13). Although the risk of TMA has been considered higher above 20 000 mg m–2, this study shows that TMA can occur earlier, especially if gemcitabine is associated with other drugs such as pegylated liposomal doxorubicine 15, tegafur 22 and oxaliplatine 8, or if several lines of chemotherapies have preceded gemcitabine 9, 11, 18, which was the case for many patients from this cohort.
In this cohort, patients treated with PE had a more severe renal presentation, with a more frequent report of hypertension, more severe AKI, requiring renal replacement therapy more often, as well as a more frequent presence of schizocytes. These patients, who had a more evident renal TMA, may have been taken in charge more frequently by nephrologists, who may have been prone to treat them as patients with atypical HUS. Despite a more severe renal presentation in patients treated with PE group, their prognosis was comparable to other patients. This, which suggests a benefit of PE in this setting, but should be balanced by the occurrence of more frequent serious adverse events. Data on the survival of patients after their initial care is too scarce in this cohort to conclude on the benefit of PE, and no data are available on long‐term renal outcome. Cases of spontaneous remissions with symptomatic treatment only 10, 16 have been reported previously in G‐TMA. In a retrospective cohort of 44 patients with G‐TMA, Gore et al. 38 reported remission rates of 36% in patients treated with PE vs. 56% in other patients. Like in our study, patients in the PE group presented with more severe renal disease (61% vs. 38% of patients requiring renal replacement therapy).
The exact pathophysiology of G‐TMA is still unknown. The different forms of TMA share a common feature of endothelial cell injury, of diverse origin. In typical HUS, endothelial injury is caused by the Shiga toxin. In atypical HUS, a default in complement alternative pathway activation leads to glomerular endothelial damage and thrombosis. The origin of endothelial damage in secondary HUS, and especially in drug‐related HUS, could be linked to a direct endothelial toxicity, such as in anti‐vascular endothelial growth factor therapy 5, or to a secondary immune aggression 35. Only few cases of G‐TMA occurring in patients treated for lymphomas are reported in the present cohort. This could be due to a smaller number of patients treated in haematology compared to oncology, to the use of lower cumulative doses or to the frequent concomitant use of rituximab in haematology 9, 39, 40, which may have controlled this immune part. No patient from the present cohort was treated with rituximab in association with gemcitabine before G‐TMA, and no patient was treated with rituximab for the G‐TMA. Renal TMA features can precede haemolysis and thrombocytopenia, and be documented by renal pathology 41. The role of CAP in the secondary forms of HUS is still unknown, and should be assessed in future studies. Here, none of the eight patients who were explored displayed a CAP abnormality. The anti‐C5 complement inhibitor eculizumab has been prescribed in some patients with G‐TMA, based on a possible role of CAP dysregulation in this setting. In the present cohort, five patients were treated with eculizumab, with one complete remission and three haematological remissions. Previous case series, including one from our group 30 reported good outcomes after eculizumab in the setting of G‐TMA, with cases of complete remission 28, 29. Although none of these patients had a documented abnormality of CAP, deposits of C5b9 and C4d were documented on some renal biopsies from patients with G‐TMA, suggesting an implication of complement activation. The possible benefit of eculizumab in patients with G‐TMA and other forms of secondary HUS should be questioned in a dedicated study. In this respect, a broader search for CAP abnormalities in patients with secondary HUS could allow the identification of patients at higher risk of G‐TMA, and/or more likely to respond to complement blockade.
Our study has some limitations. First, it was based on registry data, with missing data. The anonymity of cases from the French Pharmacovigilance network database did not allow the retrieval of initial case report data, or long‐term follow‐up data. Second, the benefit of G‐TMA treatments could not be assessed due to the observational and retrospective design of the study. Third, the imputability of gemcitabine in the occurrence of TMA, in patients with progressive cancers and frequent concomitant chemotherapies, is difficult to ascertain. In the context of cancer, even more so when metastatic, the differential diagnosis between G‐TMA and TMA secondary to cancer is difficult to make. Last, the prevalence of G‐TMA could not be evaluated, because the information on the number of patients exposed to gemcitabine during the same period was not available, and because the registries were not comprehensive. This lack of exhaustiveness can be explained both by a default of diagnosis, because symptoms of TMA can be mistaken with those of the underlying cancer, and by a default of systematic reporting of drug‐related adverse events to the pharmacovigilance networks and reference centres.
The strength of this work relies on a robust methodological approach, with well‐established definition criteria for G‐TMA and outcomes, and on the collaboration of different specialties (haematologists, oncologists, nephrologists and pharmacovigilance practitioners) leading to complementary visions of the pathology.
Conclusion
We show, from the largest cohort published to date, that G‐TMA is associated with severe renal damage and risk of death. The systematic screening for hypertension and/or oedema in patients treated with gemcitabine could allow the early detection of TMA. Control of hypertension and close monitoring to determine the optimal timing for gemcitabine cessation could prevent full‐blown G‐TMA and severe outcome. The benefit of PE or eculizumab in this setting deserves further investigation.
Competing Interests
There are no competing interests to declare.
Daviet, F. , Rouby, F. , Poullin, P. , Moussi‐Francès, J. , Sallée, M. , Burtey, S. , Mancini, J. , Duffaud, F. , Sabatier, R. , Pourroy, B. , Grandvuillemin, A. , Grange, S. , Frémeaux‐Bacchi, V. , Coppo, P. , Micallef, J. , and Jourde‐Chiche, N. (2019) Thrombotic microangiopathy associated with gemcitabine use: Presentation and outcome in a national French retrospective cohort. Br J Clin Pharmacol, 85: 403–412. 10.1111/bcp.13808.
Presentations: Preliminary results from this study were presented as a poster at the congress of the American Society of Nephrology in San Diego, 2016. The results were presented at the annual meeting of the French Reference Center of Thrombotic Microangiopathy in Paris in October 2017.
References
- 1. Green MR. Gemcitabine safety overview. Semin Oncol 1996; 23 (5 Suppl. 10): 32–35. [PubMed] [Google Scholar]
- 2. Humphreys BD, Sharman JP, Henderson JM, Clark JW, Marks PW, Rennke HG, et al Gemcitabine‐associated thrombotic microangiopathy. Cancer 2004; 100: 2664–2670. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug‐induced thrombotic microangiopathy: a systematic review of published reports. Blood 2015; 125: 616–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Govind Babu K, Bhat GR. Cancer‐associated thrombotic microangiopathy. Ecancermedicalscience 2016; 10: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Izzedine H, Perazella MA. Thrombotic microangiopathy, cancer, and cancer drugs. Am J Kidney Dis 2015; 66: 857–868. [DOI] [PubMed] [Google Scholar]
- 6. Blake‐Haskins JA, Lechleider RJ, Kreitman RJ. Thrombotic microangiopathy with targeted cancer agents. Clin Cancer Res 2011; 17: 5858–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casper ES, Green MR, Kelsen DP, Heelan RT, Brown TD, Flombaum CD, et al Phase II trial of gemcitabine (2,2′‐difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs 1994; 12: 29–34. [DOI] [PubMed] [Google Scholar]
- 8. Crouzet L, Edeline J, Du FL, Boucher E, Audrain O, Raoul JL. Haemolytic uremic syndrome and gemcitabine: jaundice is not always progression in cholangiocarcinoma. Acta Oncol 2012; 51: 687–688. [DOI] [PubMed] [Google Scholar]
- 9. Willemsen AECAB, van Herpen CML, Wesseling P, Bult P, van Laarhoven HWM. Fatal thrombotic microangiopathy after a single dose of gemcitabine as fourth‐line palliative treatment for metastasized ductal breast carcinoma. Acta Oncol 2011; 50: 462–465. [DOI] [PubMed] [Google Scholar]
- 10. Flombaum CD, Mouradian JA, Casper ES, Erlandson RA, Benedetti F. Thrombotic microangiopathy as a complication of long‐term therapy with gemcitabine. Am J Kidney Dis 1999; 33: 555–562. [DOI] [PubMed] [Google Scholar]
- 11. Thomas JG, Sethi S, Norby SM. Chronic thrombotic microangiopathy secondary to chemotherapy for urothelial carcinoma in a patient with a history of Wegener granulomatosis. Am J Kidney Dis 2011; 57: 799–802. [DOI] [PubMed] [Google Scholar]
- 12. Walter RB, Joerger M, Pestalozzi BC. Gemcitabine‐associated hemolytic‐uremic syndrome. Am J Kidney Dis 2002; 40: E16. [DOI] [PubMed] [Google Scholar]
- 13. Müller S, Schütt P, Bojko P, Nowrousian MR, Hense J, Seeber S, et al Hemolytic uremic syndrome following prolonged gemcitabine therapy: report of four cases from a single institution. Ann Hematol 2005; 84: 110–114. [DOI] [PubMed] [Google Scholar]
- 14. Lee HW, Chung MJ, Kang H, Choi H, Choi YJ, Lee KJ, et al Gemcitabine‐induced hemolytic uremic syndrome in pancreatic cancer: a case report and review of the literature. Gut Liver 2014; 8: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lewin SN, Mutch DG, Whitcomb BP, Liapis H, Herzog TJ. Three cases of hemolytic uremic syndrome in ovarian cancer patients treated with combination gemcitabine and pegylated liposomal doxorubicin. Gynecol Oncol 2005; 97: 228–233. [DOI] [PubMed] [Google Scholar]
- 16. Richmond J, Gilbar P, Abro E. Gemcitabine‐induced thrombotic microangiopathy. Intern Med J 2013; 43: 1240–1242. [DOI] [PubMed] [Google Scholar]
- 17. Saif MW, McGee PJ. Hemolytic‐uremic syndrome associated with gemcitabine: a case report and review of literature. JOP 2005; 6: 369–374. [PubMed] [Google Scholar]
- 18. Leal F, Macedo LT, Carvalheira JBC. Gemcitabine‐related thrombotic microangiopathy: a single‐centre retrospective series. J Chemother 2014; 26: 169–172. [DOI] [PubMed] [Google Scholar]
- 19. Lesesne JB, Rothschild N, Erickson B, Korec S, Sisk R, Keller J, et al Cancer‐associated hemolytic‐uremic syndrome: analysis of 85 cases from a national registry. J Clin Oncol 1989; 7: 781–789. [DOI] [PubMed] [Google Scholar]
- 20. Brodowicz T, Breiteneder S, Wiltschke C, Zielinski CC. Gemcitabine‐induced hemolytic uremic syndrome: a case report. J Natl Cancer Inst 1997; 89: 1895–1896. [DOI] [PubMed] [Google Scholar]
- 21. Choi M, Woywodt A, Göbel U, Schneider W, Kettritz R. Haemolytic uraemic syndrome after gemcitabine treatment for pancreatic carcinoma. Nephrol Dial Transplant 1999; 14: 2523–2524. [DOI] [PubMed] [Google Scholar]
- 22. Ruiz I, Del Valle J, Gómez A. Gemcitabine and haemolytic‐uraemic syndrome. Ann Oncol 2004; 15: 1575–1576. [DOI] [PubMed] [Google Scholar]
- 23. Glezerman I, Kris MG, Miller V, Seshan S, Flombaum CD. Gemcitabine nephrotoxicity and hemolytic uremic syndrome: report of 29 cases from a single institution. Clin Nephrol 2009; 71: 130–139. [DOI] [PubMed] [Google Scholar]
- 24. Fung MC, Storniolo AM, Nguyen B, Arning M, Brookfield W, Vigil J. A review of hemolytic uremic syndrome in patients treated with gemcitabine therapy. Cancer 1999; 85: 2023–2032. [DOI] [PubMed] [Google Scholar]
- 25. Szczepiorkowski ZM, Winters JL, Bandarenko N, Kim HC, Linenberger ML, Marques MB, et al Guidelines on the use of therapeutic apheresis in clinical practice—evidence‐based approach from the apheresis applications committee of the American Society for Apheresis. J Clin Apheresis 2010; 25: 83–177. [DOI] [PubMed] [Google Scholar]
- 26. Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al Terminal complement inhibitor Eculizumab in atypical hemolytic–uremic syndrome. N Engl J Med 2013; 368: 2169–2181. [DOI] [PubMed] [Google Scholar]
- 27. Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, et al Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2‐year extensions of phase 2 studies. Kidney Int 2015; 87: 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rogier T, Gerfaud‐Valentin M, Pouteil‐Noble C, Taleb A, Guillet M, Noel A, et al Clinical efficacy of eculizumab as treatment of gemcitabine‐induced thrombotic microangiopathy: a case report. Rev Med Interne 2016; 37: 701–704. [DOI] [PubMed] [Google Scholar]
- 29. Turner JL, Reardon J, Bekaii‐Saab T, Cataland SR, Arango MJ. Gemcitabine‐associated thrombotic microangiopathy: response to complement inhibition and reinitiation of gemcitabine. Clin Colorectal Cancer 2017; 16: e119–e122. [DOI] [PubMed] [Google Scholar]
- 30. Grall M, Provôt F, Coindre JP, Pouteil‐Noble C, Guerrot D, Benhamou Y, et al Efficacy of eculizumab in gemcitabine‐induced thrombotic microangiopathy: experience of the French Thrombotic Microangiopathies Reference Centre. Blood 2016; 128: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zini G, d'Onofrio G, Briggs C, Erber W, Jou JM, Lee SH, et al ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes. Int J Lab Hematol 2012; 34: 107–116. [DOI] [PubMed] [Google Scholar]
- 32. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group . KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9 (Suppl. 3): S1–155. [DOI] [PubMed] [Google Scholar]
- 33. Lusco MA, Fogo AB, Najafian B, Alpers CE. AJKD atlas of renal pathology: thrombotic microangiopathy. Am J Kidney Dis 2016; 68: e33–e34. [DOI] [PubMed] [Google Scholar]
- 34.Available at https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 (last accessed 11 December 2018)
- 35. Zupancic M, Shah PC, Shah‐Khan F, Nagendra S. Gemcitabine‐associated thrombotic thrombocytopenic purpura. Lancet Oncol 2007; 8: 634–641. [DOI] [PubMed] [Google Scholar]
- 36. Sallée M, Ismail K, Fakhouri F, Vacher‐Coponat H, Moussi‐Francés J, Frémaux‐Bacchi V, et al Thrombocytopenia is not mandatory to diagnose haemolytic and uremic syndrome. BMC Nephrol 2013; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Serres D, A S, Isenring P. Athrombocytopenic thrombotic microangiopathy, a condition that could be overlooked based on current diagnostic criteria. Nephrol Dial Transplant 2009; 24: 1048–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gore EM, Jones BS, Marques MB. Is therapeutic plasma exchange indicated for patients with gemcitabine‐induced hemolytic uremic syndrome? J Clin Apheresis 2009; 24: 209–214. [DOI] [PubMed] [Google Scholar]
- 39. Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Bello CM, et al Hodgkin lymphoma, version 2.2015. J Natl Compr Cancer Netw JNCCN 2015; 13: 554–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bharthuar A, Egloff L, Becker J, George M, Lohr JW, Deeb G, et al Rituximab‐based therapy for gemcitabine‐induced hemolytic uremic syndrome in a patient with metastatic pancreatic adenocarcinoma: a case report. Cancer Chemother Pharmacol 2009; 64: 177–181. [DOI] [PubMed] [Google Scholar]
- 41. Izzedine H, Escudier B, Lhomme C, Pautier P, Rouvier P, Gueutin V, et al Kidney diseases associated with anti‐vascular endothelial growth factor (VEGF): an 8‐year observational study at a single center. Medicine (Baltimore) 2014; 93: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]


