Abstract
Aims
We compared the 1‐year safety and effectiveness of dabigatran 110 mg (D110) or 150 mg (D150) twice daily to vitamin K antagonists (VKA) in patients with nonvalvular atrial fibrillation.
Methods
New user cohort study of patients dispensed D110 or D150 vs. VKA in 2013 for nonvalvular atrial fibrillation, followed 1 year in the French Système National des Données de Santé (66 million persons). D110 and D150 users were matched 1:1 with VKA users on sex, age, date of first drug dispensing and high‐dimensional propensity score. Hazard ratios [HR (95% confidence intervals)] for stroke and systemic embolism (SSE), major bleeding (MB) and death were computed using Cox proportional hazards or Fine and Gray models during exposure.
Results
In 14 442 matched D110 and VKA patients, mean age 79, 49% male, 91% with CHA2DS2‐VASc ≥2 and 8% with HAS‐BLED score >3, incidence rates of SSE were 1.9% and 2.6% person‐years [HR 0.69 (0.56–0.84)], MB 1.8% and 2.9% [0.62 (0.51–0.76)], death 7.2% and 8.6% [0.84 (0.76–0.94)]. In 8389 matched D150 and VKA patients, mean age 67, 67% male, 65% with CHA2DS2‐VASC ≥2; < 5% HAS‐BLED >3, incidence rates were for SSE 1.4% and 1.9% [0.76 (0.56–1.04)], MB 0.6% and 1.9% [0.30 (0.20–0.46)], death 1.6% and 3.6% [0.46 (0.35–0.59)]. Numbers needed to treat to observe one fewer death were 78 for D110, 88 for D150.
Conclusion
In real life D110 and D150 were at least as effective, and safer than VKA.
Keywords: comparative effectiveness, dabigatran, dose‐effect, pharmacoepidemiology, vitamin K antagonists
Introduction
Atrial fibrillation (AF) is associated with a 5‐fold increased risk of ischaemic stroke 1. Vitamin K antagonists (VKA) such as warfarin have long been the reference treatment for stroke prevention, at the cost of a risk of serious bleeding 2, 3, 4, 5, 6. Among the direct oral anticoagulants (DOAC), dabigatran was approved for stroke prevention in nonvalvular AF (NVAF) in 2012 7. In the open‐label RE‐LY pivotal trial, compared to patients randomized to VKA, patients randomized to dabigatran 150 mg twice daily (D150) had fewer strokes and systemic embolisms (SSE) without significant difference in clinically relevant bleeding (CRB) or major bleeding (MB). Patients randomized to dabigatran 110 mg twice daily (D110) had the same rate of SSE but fewer bleeds. In addition, there was an excess of acute coronary syndrome (ACS) and upper gastrointestinal bleeding (UGIB) with D150 vs. VKA.
The translation of clinical trial results to actual practice is uncertain, since physicians, patients, drug prescriptions and usage may not be the same 8, 9, 10, 11. These differences may be especially relevant here for dose related effects. In the clinical trial, D110 and D150 were randomized in the same patient population. In contrast, in real life, D150 is the standard dose and D110 is a reduced dose indicated in patients with renal impairment, a higher risk of bleeding, or in older patient, i.e. in different patient populations. To estimate the real‐life benefits and risks of D110 and D150 as used in the French population, a high‐dimensional propensity score (hdPS)‐matched cohort study of D110 or D150 compared to VKA was undertaken, using the Systeme National des Données de Santé (SNDS) nationwide claims and hospitalization database 12.
Research question and objectives
To compare the 1‐year event rates of SSE, MB and all‐cause death, as well as CRB, ACS and UGIB (and other bleeding sites), in new users of D110 or D150 vs. VKA for NVAF.
Methods
Study design
This was an hdPS‐matched cohort study of all new users of D110 or D150 vs. VKA for NVAF in 2013, followed for one year.
Further information on methods can be found in supplementary data Appendix S1.
Setting
SNDS is the national healthcare data system in France. It links the nationwide mandatory public health insurance system claims database SNIIRAM to the national hospital discharge database PMSI and to the national death registry CépiDC. It includes more than 99% of the French population (66 million persons in 2013) from birth (or immigration) to death (or emigration), even if a subject changes occupation or retires, and irrespective of socioeconomic status. The SNDS contains individual anonymized information on all medical and paramedical encounters, drugs claims, hospital admissions and procedures, and date of death, which are linked to create a longitudinal record of outpatient health encounters, hospital diagnoses and drug dispensing 12.
SNDS includes the hospital discharge summaries database, which includes all hospital discharge summaries from all private or public hospitals with main, associated and secondary diagnoses. Procedures are also recorded, as are the more expensive drugs or implantable devices.
Diagnoses may be identified from:
Registration for long‐term diseases [LTD, >4000 International Classification of Diseases (ICD)‐10 codes] that warrant full healthcare coverage with no copayment. This registration is requested by the patient's physicians (outpatient or hospital), and diagnoses are verified by the healthcare insurance system. Registration is not mandatory. It may be missing for instance if the medical expenses are already covered by another chronic disease or the treatment is not expensive. LTD concern background diseases and are not directly related to any individual medical encounter. They are therefore not used for outcomes, only for baseline patient description parameters.
Hospital discharge diagnoses (main, associated, secondary), which will inform on the reason for admission (main), and on background risk modifiers (e.g., diabetes, renal failure, coronary heart disease) that modify the hospital costs. Associate diagnoses inform on reasons for procedures as main diagnosis.
Drug, laboratory, imaging or other outpatient or inpatient procedures, combinations of which may be diagnostic 13. A patient with regular cardiology consultations, Holter recordings, use of rate‐limiting anti‐arrhythmic drugs and anticoagulants might be suspect of AF 4.
A patient with a registration for LTD for AF, or a hospital diagnosis of AF, would be considered as definite AF. Patients without definite AF but with a combination of specialist visit, procedure and medication would be suspected of having probable AF. Only definite AF patients were included in this study.
Subjects
All adults with a first dispensing of any oral anticoagulant in 2013 (cohort entry date, or index date) were identified. Patients had to have a 3‐year database history without any dispensing of an anticoagulant before index date. Patients with valvular heart disease, valve replacement or repair, patients with another indication for anticoagulation (deep vein thrombosis or pulmonary embolism, orthopaedic surgery), as well as patients with erroneous or incomplete data were excluded (Table 1). Only patients with definite NVAF, and complete datasets (3‐year look‐back, 1‐year follow‐up were retained in the cohort study (103 101 patients). Of these, 44 653 patients had been prescribed VKA and 27 060 dabigatran, of whom 15 532 received D110 and 10 847 D150. New D110 and D150 users were separately 1:1 matched with new VKA users on sex, age at cohort entry date (± 1 year), cohort entry date (± 2 weeks), CHA2DS2‐VASc and HAS‐BLED scores, and a 500 variable hdPS using the Greedy method with a calliper of 0.05.
Table 1.
Patient disposition
| Study populations N | |
|---|---|
| Selection criteria | 371 539 |
|
‐ First dispensing of DOAC or VKA between 1
st
January 2013 and 31
st
December 2013
‐ With a 3‐year history without DOAC or VKA dispensing |
|
| Exclusion criteria | 227 319 |
| ‐ Missing or incorrect data (age, death date) | 701 |
| ‐ Younger than 18 years at index date | 888 |
| ‐ At least two treatment groups at index date | 151 |
| ‐ Death at index date | 98 |
| ‐ Uncertain identification (several beneficiaries, e.g., twins) | 732 |
| ‐ Less than 3 years history in the SNDS before index date | 12 610 |
| ‐ Alive at 1 year with incomplete data after index date | 284 |
| ‐ Other probable indications | 86 857 |
| ‐ Valvular disease history before index date | 25 509 |
| ‐ No definite atrial fibrillation identified | 99 489 |
| Possible NVAF without other probable indication | 41 119 |
| Study population | 103 101 |
| Definite NVAF without other probable indication | |
| ‐ Dabigatran | 27 060 |
| D110 | 15 532 |
| D150 | 10 847 |
| ‐ VKA | 44 653 |
| ‐ Other anticoagulants | 31 388 |
| Matched populations | |
| ‐ D110 vs. VKA (per group) | 14 442 (93% of D110) |
| ‐ D150 vs. VKA (per group) | 8389 (77% of D150) |
DOAC, direct acting anticoagulants; VKA, vitamin K antagonists; NVAF, nonvalvular atrial fibrillation; D110, dabigatran 110 mg twice daily; D150, dabigatran 150 mg twice daily
Variables
The index date or cohort entry date was the date of first dispensing of the anticoagulant, between 1 January 2013 and 31 December 2013, the first full year of marketing of dabigatran for NVAF in France.
Baseline covariates
Chronic medical conditions, cardiovascular risk factors, previous and concomitant drug dispensings, and hospital admission diagnoses were collected over the 3 years prior to the index date. From these, the CHA2DS2‐VASc and HAS‐BLED scores were calculated, indicative of the stroke risk in AF, and the bleeding risk in patients treated with VKA 4, 14. HAS‐BLED was adapted to the database information and, for example, labile INR, which is not relevant to patients without previous anticoagulant use or to DOAC, was not included. We found this score predictive of bleeding in a previous study 4.
The Charlson comorbidity score, adapted to the data source 15, was computed and used for further adjustment. We also included the total medical expenses over the previous year and the previous month as an indicator of overall disease burden 16.
The ICD‐10 codes used to identify the elements of the CHA2DS2‐VASc and HAS‐BLED scores are indicated in Appendix S2 in supplementary data.
Exposure
The drug exposure period started at the index date and ended 30 days after the last dispensing, or at dispensing of a different anticoagulant (switch). Last dispensing was defined as a dispensing that was not followed by another dispensing of the same drug within 60 days 4.
Follow‐up
Follow‐up began on index date and continued until patient death, treatment discontinuation, occurrence of an outcome of interest (for that outcome only), or the end of the study period (1 year), whichever came first. There was no loss to follow‐up.
Study outcomes
The primary outcomes were hospitalizations with a main diagnosis of ischaemic stroke or systemic embolism, major bleeding, or all‐cause death, individually and combined.
Secondary outcomes included ACS (myocardial infarction or unstable angina), CRB and specific bleeding sites.
Major bleeding was intracerebral haemorrhage, critical organ bleeding, any CRB with blood transfusion or acute posthaemorrhagic anaemia, or death during hospital stay (ISTH definition) 17. CRB were all hospitalizations with a main diagnosis of bleeding. Specific bleeding sites were intracerebral haemorrhage, gastrointestinal (GI) bleeding, urogenital bleeding, other critical organ or site bleeding, and other bleeding.
The ICD‐10 codes used to identify these outcomes are given in supplementary material Appendix S3. The same codes have been used for other studies of outcomes in anticoagulant users and NVAF 4, 14 and for studies of outcomes after myocardial infarction. 11, 18 Stroke has been validated in a specific study, with a PPV >90% 19.
Statistical methods
To reduce confounding due to imbalance in study covariates, hdPS matching was used 20. The hdPS is a measure of the probability of being treated by one of the anticoagulants studied related to the outcome (D110 vs. VKA, D150 vs. VKA). Unconditional binary logistic regression was used to estimate the association of the available variables with the outcomes, deriving separately predicted probability of patients initiating D110 or D150 rather than VKA 21, 22. HdPS considered all the information in the database, with multiple data dimensions from patient data and healthcare reimbursements during the 3‐year period before index date. The variables included demographic variables at inclusion (age, sex), individual stroke and bleeding risk factors from the CHA2DS2‐VASc and HAS‐BLED scores, hospitalization other than cardioversion or catheter ablation in the month before index date, chronic obstructive pulmonary disease, coronary heart disease, diabetes, and 500 other variables 21, 22, 23, 24 selected from four dimensions:
Chronic disease registration ICD‐10 codes;
ICD‐10 hospitalization codes during the 3 years before index date;
Medical and paramedical visits and laboratory tests;
Drugs dispensed during the previous year.
The codes and variables concerned are listed in appendix S4.
D110 vs. VKA and D150 vs. VKA patients were matched separately on the hdPS score.
HdPS calculation used the routines developed within the Division of Pharmacoepidemiology and Pharmacoeconomics and Brigham and Women's Hospital and Harvard Medical School (www.drugepi.org) 25.
Standardized mean differences were used to measure goodness of matching. An absolute standardized mean difference of 0.1 (10%) or less indicates a negligible difference between groups 26 (see supplementary data Figures S2B and S3B).
The main analysis was performed in the matched patients during exposure as defined above (on treatment). A complementary analysis added further adjustment on Charlson's index and medical costs during the year and during the month previous to index date (Supplementary Table S7).
Incidence rates were measured using event counts and exposed patient‐time, and represented with Kaplan–Meier plots. Hazard ratio and 95% confidence intervals (CI) of D110 vs. VKA and D150 vs. VKA were estimated using Cox proportional hazards model for death, and Fine and Gray's model for competing risks for nonfatal events 27.
In addition, the number needed to observe one fewer event in one group compared to the other (NNT) was computed using 1/[p(A) – p (B)] × 100 where p(A) and p(B) are event rates with treatment A or B.
Sensitivity analyses were performed on the whole population using adjustment on sex, age at index date and hdPS (in deciles), rather than matching, to check the generalizability of the matched results to the full populations. (See Supplementary Figure S4A,B).
Statistical analysis was conducted by Bordeaux PharmacoEpi, a research platform of University of Bordeaux, using SAS® software (SAS Institute, Version 9.4, Cary, NC, USA) and the Harvard routines for hdPS (SAS pharmacoepi toolbox, www.drugepi.org).
Ethics and registration
This study was done in an anonymized claims database. For such secondary use of previously collected data, individual patient consent was not required by law. This study was requested by national healthcare authorities. It was authorized by the National Consultative Committee for the treatment of healthcare data (CCTIRS), and by the National Commission on Informatics and Liberties (CNIL) on 22 October 2015 under N°1 858 904. It is registered with the EUPAS registry as EUPAS13017 (www.encepp.eu), and in Clinicaltrials.gov as NCT02785354.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 28.
Results
Of 371 539 incident anticoagulant users in 2013, 103 101 had NVAF; 44 653 received VKA and 27 060 dabigatran, of whom 15 532 were dispensed D110 and 10 847 D150 (Table 1). Overall, D110 patients were older and at higher risk of stroke and bleeding than D150 patients (see Supplementary Tables S1 and S2).
In total, 14 442 D110 patients (93% of D110 patients) were matched to the same number of VKA patients, and 8389 D150 patients (77% of D150 patients) were matched to the same numbers of VKA patients. (Tables 1 and 2). VKA patients had the same characteristics as their respective matched dabigatran populations. HdPS were identically distributed in matched D110 and VKA or D150 and VKA patients (Supplementary Figure S1A,B). Standardized mean differences in matched patients were < 5% for all parameters, and generally <2%. (Supplementary Figure S2A,B for D110 vs. VKA, supplementary Figure S3A,B for D150 vs. VKA).
Table 2.
Baseline patient characteristics of the patients on dabigatran 110 mg twice daily (D110) or dabigatran 150 mg twice daily (D150), matched to vitamin K antagonists (VKA). All standardized differences were < 5% (see Supplementary Figures 2B and 3B for distribution of standardized differences)
| Matched populations | ||||
|---|---|---|---|---|
| D110 n = 14 442 | Matched VKA n = 14 442 | D150 n = 8389 | Matched VKA n = 8389 | |
| Sex, n (%) | ||||
| Male | 7077 (49.0) | 7077 (49.0) | 5634 (67.2) | 5634 (67.2) |
| Age at index date (in years) | ||||
| Mean (± SD) | 78.6 (9.1) | 78.6 (9.1) | 67.3 (9.0) | 67.3 (9.1) |
| Age at index date (in categories), n (%) | ||||
| <60 years | 553 (3.8) | 545 (3.8) | 1509 (18.0) | 1502 (17.9) |
| 60–69 years | 1672 (11.6) | 1710 (11.8) | 3239 (38.6) | 3229 (38.5) |
| 70–79 years | 4420 (30.6) | 4425 (30.6) | 3173 (37.8) | 3168 (37.8) |
| ≥ 80 years | 7797 (54.0) | 7762 (53.7) | 468 (5.6) | 490 (5.8) |
| Stroke risk factors (score), n (%) | ||||
| Congestive heart failure | 2966 (20.5) | 3030 (21.0) | 1083 (12.9) | 1132 (13.5) |
| Hypertension | 6651 (46.1) | 6681 (46.3) | 2793 (33.3) | 2962 (35.3) |
| Age > 65 years | 13 056 (90.4) | 13 048 (90.3) | 5123 (61.1) | 5147 (61.4) |
| Age 65–74 years | 2635 (18.2) | 2636 (18.3) | 3611 (43.0) | 3583 (42.7) |
| Age ≥ 75 years | 10 622 (73.5) | 10 609 (73.5) | 1881 (22.4) | 1903 (22.7) |
| Diabetes mellitus | 3016 (20.9) | 3171 (22.0) | 1811 (21.6) | 2001 (23.9) |
| Stroke or transient ischaemic attack | 1980 (13.7) | 1969 (13.6) | 801 (9.5) | 865 (10.3) |
| Stroke | 1691 (11.7) | 1702 (11.8) | 676 (8.1) | 762 (9.1) |
| Vascular disease | 2165 (15.0) | 2022 (14.0) | 843 (10.0) | 1028 (12.3) |
| Abnormal renal function | 705 (4.9) | 776 (5.4) | 137 (1.6) | 175 (2.1) |
| Abnormal liver function | 245 (1.7) | 207 (1.4) | 115 (1.4) | 166 (2.0) |
| Bleeding history | 342 (2.4) | 328 (2.3) | 104 (1.2) | 163 (1.9) |
| Medication usage predisposing to bleeding | 8596 (59.5) | 8413 (58.3) | 4479 (53.4) | 4821 (57.5) |
| CHA 2 DS 2 ‐VASc score, n (%) | ||||
| 0–1 | 1290 (8.9) | 1331 (9.2)) | 3024 (36.0) | 2882 (34.3) |
| 2 | 2562 (17.7) | 2472 (17.1) | 2196 (26.2) | 2120 (25.3) |
| >2 | 10 590 (73.4) | 10 639 (73.7) | 3169 (37.8) | 3387 (40.4) |
| HAS‐BLED score, n (%) | ||||
| 0 | 442 (3.0) | 426 (3.0) | 1187 (14.1) | 987 (11.8) |
| 1 | 3172 (22.0) | 3165 (21.9) | 2842 (33.9) | 2681 (32.0) |
| 2 | 5727 (39.7) | 5856 (40.5) | 2786 (33.2) | 2996 (35.7) |
| 3 | 3894 (27.0) | 3832 (26.5) | 1299 (15.5) | 1406 (16.8) |
| >3 | 1207 (8.3) | 1163 (8.1) | 275 (3.3) | 319 (3,8) |
| First drug exposure duration per patient (in days) | ||||
| Median | 205.0 | 251.0 | 246.0 | 206.0 |
| Interquartile range | 66.0–365.0 | 110.0–365.0 | 87.0–365.0 | 90.0–365.0 |
Median duration of treatment exposure was 205 days for D110 vs. 251 days for matched VKA, and 246 days for D150 vs. 206 days for their matched VKA.
Outcomes
In the D110 population, all primary outcomes: SSE, MB and all‐cause death were significantly less common than in their matched VKA population (Figure 1). Among secondary outcomes, CRB, including intracerebral haemorrhage and other critical organ or site bleeding were also less common in D110 users. There was no difference between D110 and VKA for ACS or UGIB.
Figure 1.
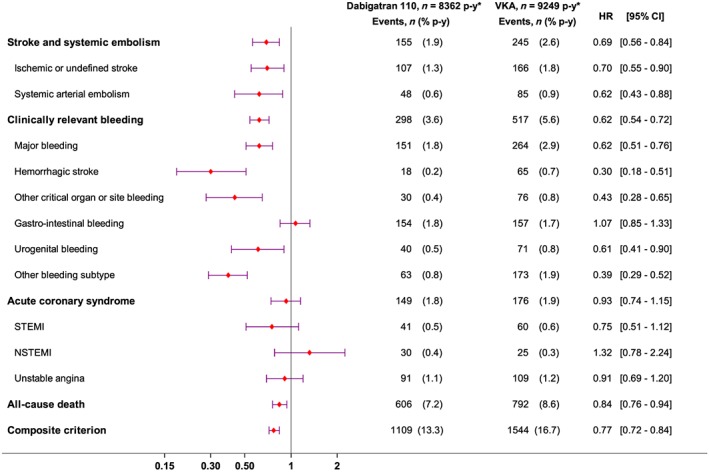
Effectiveness and safety outcomes in dabigatran 110 mg twice daily vs. vitamin K antagonist (VKA) patients: forest plots. STEMI, ST‐elevation myocardial infarction; NSTEMI, non‐ST‐elevation myocardial infarction
The NNT with D110 compared to matched VKA users was 78 patients to observe one fewer death and 33 for any one of the primary endpoints (composite endpoint).
Compared to matched VKA patients, D150 patients had significantly fewer MB and deaths. The hazard ratio for SSE (0.75 [0.56–1.04)] did not quite reach statistical significance, due to the small number of events (Figure 2).
Figure 2.
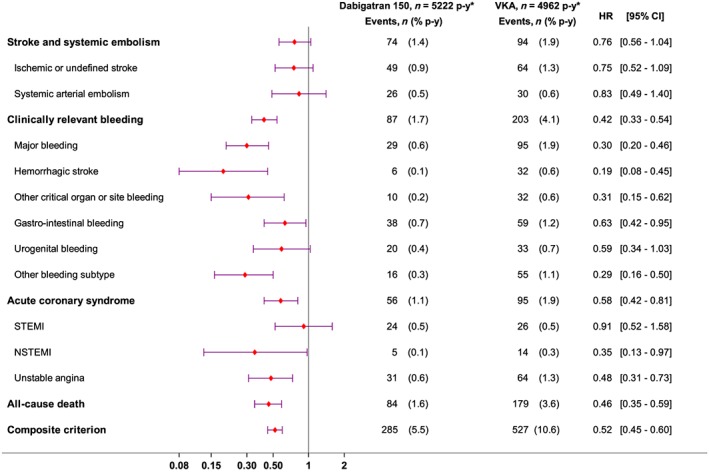
Effectiveness and safety outcomes in dabigatran 150 mg twice daily vs. vitamin K antagonist (VKA) patients: forest plots. STEMI, ST‐elevation myocardial infarction; NSTEMI, non‐ST‐elevation myocardial infarction
Among secondary outcomes, CRB, including intracerebral haemorrhage, other critical organ or site bleeding, UGIB and ACS were less common in D150 users than in matched VKA users (Figure 2). The relevant NNT were 88 patients treated with D150 rather than VKA to observe one fewer death, and 35 to observe one fewer of any primary event.
Results were similar when analyses were done in the whole population, adjusted on the same parameters used for matching (Supplementary Figures S4 and S5), with similar NNT.
Adding the Charlson comorbidity index as adapted to SNDS, a predictor of 1‐year mortality, did not modify the results (Supplementary Table S7).
Events occurred regularly throughout the study period (Supplementary Figures S6 and S7).
Discussion
In this countrywide new users cohort study of the real‐life experience with dabigatran 110 mg or 150 mg twice daily compared to hdPS‐matched VKA‐treated patients with definite NVAF, dabigatran at either dose was associated with better safety than VKA, and lower mortality. D110 also showed better effectiveness. We did not find the excess UGIB and ACS reported in the RE‐LY clinical trial 29, 30, 31.
The populations were different from those included in RE‐LY (Supplementary Tables S3 and S4), with lower overall bleeding and death in RE‐LY (Supplementary Tables S5 and S6). In RE‐LY, the attribution of the drugs in the three treatment groups was random, so that the patient characteristics for D110, D150 and VKA were identical. In real life, D110 is indicated in older patients at higher risk of stroke or bleeding than D150. In our populations, D110 was indeed used in older patients than D150, with more previous history of or concomitant diseases, with higher CHAD2VA2SC and HAS‐BLED scores, and correspondingly higher event rates (Supplementary Tables S5 and S6). In addition, in RE‐LY over 50% of the patients had had an experience of VKA use before randomization, whereas all our patients were new users. Previous experience of VKA might select a population of users at lower risk of events. For these reasons and because of the usual exclusion of patients with poor prognosis from clinical trials 11 it was not unexpected to find real life results different from RE‐LY.
Differences in bleeding rates between D110 and D150 relate to the choice of the drug dosage in real life, adapted to the patients' individual bleeding risk as perceived by the prescriber. Since D110 is indicated in patients with a higher risk of bleeding, old age or organ failures, it is unsurprising that D110 patients would have higher bleeding rates than the younger, lower‐risk D150 patients. This was not found in RE‐LY, where the choice of dosage was imposed by randomization. This difference in event rates was also noted between VKA patients matched to D110 or D150, compared to the RE‐LY VKA arm (Figure S4A,B).
The lower risk of bleeding with D110 or D150 than matched VKA was not at the expense of effectiveness. The signals of increased GI bleeding and increased coronary artery events with D150 in RE‐LY were not confirmed (Supplementary Table S6) 32, 33.
Our results are globally consistent with other epidemiological studies done in different settings 33 with some differences: we did not find more GI bleeding compared to VKA with either dose, and in fact less GI bleeding with D150 than VKA, in contrast for instance with RE‐LY, Graham 31 or Hernandez 30. This might be related to excess GI bleeding in France with VKA, maybe because of environmental or sociocultural factors, poorer control of bleeding risks with warfarin or other regional differences as shown with Rocket‐AF 34. It might also be due to higher relative VKA dosing, which would fit with the combination of no difference for SSE and more bleeding, relative to D150 instead of more SSE and less bleeding with VKA in these other studies.
For D110 (Supplementary Table S6), event rates were much higher here than in RE‐LY, as expected from the age and risk differences. In this elderly high‐risk population, SSE, bleeding and death rates were lower with D110 than with VKA. This cannot be compared to the RE‐LY trial, where the patients on D110 were irrelevant to real‐life use, or to the pharmacoepidemiological studies done in the USA, where D110 is not marketed 35, 36.
In Denmark (in a healthcare system similar to ours), Larsen et al. 37 included only D150, with a median age of 67 vs. 72 years for warfarin. They also found lower rates of all bleeding with dabigatran compared to warfarin but did not specifically study GI bleeding or D110 37.
Staerk et al.'s study did not include warfarin 38.
In Taiwan, Chan et al. 39 found an HR for GI bleeding of 0.77 [0.59–1.02], dabigatran vs. warfarin. These results were reasonably similar to ours (HR < 1, but wide confidence intervals because of fewer patients and events), for both doses of dabigatran 39.
These studies provide globally coherent results 33. Results could differ in other populations, depending on healthcare systems, or social and cultural differences. We did not directly compare D110 with D150, which were used in very different populations. Our results are observations of the drugs as they were used during the timeframe of the study. We cannot presume what the results might be if patients on VKA were switched to dabigatran, although there are indications that the results might be the same 40.
Study limits and biases
This was not a randomized clinical trial, so that the results, despite our best efforts, might still suffer from unmeasured confounding.
The matched cohorts were identical to within <5% of standardized mean differences in 500 variables in four dimensions, including all measurable bleeding and embolism risk factors as well as common fatality risks, and all previous medical history and drug dispensings in the previous 3 years. The differences between crude and matched results indicate the degree to which biases were corrected. Adding the Charlson comorbidity index, a predictor of 1‐year mortality in the database 15, did not change the results, especially on mortality. The hdPS distribution curves overlapped over the whole population range, so that results in the matched population might safely be extrapolated to the whole population, as indicated by the similarity between results in matched populations and adjusted results in whole populations.
HdPS matching has already been used in many studies in the field of pharmacoepidemiology and drug safety. Unmeasured confounders such as smoking, obesity etc. may be approached by various combinations of claims such as visits, prescriptions, procedures, tests and hospitalizations, which may collectively be a proxy for risk factors that are not present as such in the database 22, 23.
The data in the national claims database are collected prospectively for healthcare insurance purposes, independently from this or any other study, a priori excluding information bias 12. Data are present for the whole population, and there is no selection of patients according to social status, employer, age or pre‐existing conditions as might exist in other population databases 12, 31. All outpatients dispensed an anticoagulant during the study inclusion period were identified. Drugs started in hospital would be rapidly relayed by an outpatient prescription, which is captured. There are no sampling issues, since essentially the whole population is captured.
There is little or no unrecorded use (e.g. internet pharmacies) of these expensive reimbursed drugs. Drugs are dispensed as fixed quantity preparations (e.g. dabigatran, 150 mg per capsule, 60 capsules) that are individually identified in the system, providing the exact quantity and dosage dispensed over time.
Diagnoses were based on hospital discharge summaries and on registration for chronic diseases, and any other available data such as drug dispensing or procedures. The same methods and diagnostic algorithms were used in similar studies of VKA and DOAC in NVAF, 4, 14, 40 or of the same outcomes in other circumstances 11, 18. The identification of all‐cause death is exhaustive.
Undocumented bleeding resulting in death before the patient reaches the hospital would have been captured in the overall all‐cause death rates, which was also lower with dabigatran at both dosages.
Conclusions
This countrywide hdPS‐matched new users cohort study found in real‐life that dabigatran as used appears to be at either dose at least as effective and safer than VKA for the prevention of thromboembolic events in NVAF, with a lower overall mortality and an NNT around 80 for death. Considering the annual number of users of anticoagulants for NVAF, these results could translate into hundreds fewer deaths and thousands fewer events yearly in a country such as France.
Competing Interests
N.M. declares fees from Sanofi, Merck‐Serono, Merck & Co, IPSEN, Novartis, Vifor Pharma and Servier for training, data safety monitoring boards, and consulting activities, none in the field of this study. Y.C. declares consulting fees from Bayer, Servier, Astra‐Zeneca, Boehringer, Vifor Pharma. P.M. declares consulting fees with Bristol‐Myers Squibb, Pfizer and Bayer. The other authors have no competing interests to declare.
The authors wish to thank Association pour le Developpement de l'Enseignement et la Recherche en Aquitaine (ADERA) for the administrative, legal and human resources support without which this project could not have been done.
This study was funded by an unrestricted grant from Boehringer Ingelheim. The company had an observer role, and had no part in study design, implementation or analysis, in the decision to publish this paper or its content as specified in the ENCEPP code of conduct (see www.ENCEPP.eu).
Supporting information
Table S1
Description of dabigatran 110 mg twice daily, dabigatran 150 mg twice daily and vitamin K antagonist populations before matching
Table S2 Crude event rates during drug exposure
Table S3 Baseline patient characteristics in dabigatran 110 mg twice daily users: comparison with RE‐LY
Table S4 Baseline patient characteristics in dabigatran 150 mg twice daily users: comparison with RE‐LY
Table S5 Outcomes in matched dabigatran 110 mg twice daily users: comparison with RE‐LY
Table S6 Outcomes in matched dabigatran 150 mg twice daily users: comparison with RE‐LY
Table S7 matched analyses of main outcomes with Charlson score and cost before index date as additional adjustment variables
Figure S1 High‐dimensional propensity score distributions for unmatched (left panels) and matched (right panels) cohorts, of dabigatran 110 mg twice daily vs. vitamin K antagonist (A) and dabigatran 150 mg twice daily vs. vitamin K antagonist (B)
Figure S2 (A) Standardized mean differences in characteristics included in the high‐dimensional propensity score, before matching, dabigatran 110 twice daily vs. vitamin K antagonist. Each line is a different parameter (490 shown). (B) Standardized mean differences in characteristics included in the high‐dimensional propensity score, after matching, dabigatran 110 vs. vitamin K antagonist. Each line is a different parameter (490 shown)
Figure S3 (B) Standardized mean differences in characteristics included in the high‐dimensional propensity score, before matching, dabigatran 150 twice daily vs. vitamin K antagonist. Each line is a different parameter (490 shown). (B) Standardized mean differences in characteristics included in the high‐dimensional propensity score, after matching, dabigatran 150 vs. vitamin K antagonist. Each line is a different parameter (490 shown)
Figure S4 Forest plots for crude, adjusted and matched analyses of main outcomes, dabigatran 110 mg twice daily vs. vitamin K antagonist
Figure S5 Forest plots for crude, adjusted and matched analyses of main outcomes, dabigatran 150 mg twice daily vs. vitamin K antagonist
Figure S6 One‐year cumulative incidence (Kaplan–Meier estimate) of outcomes during the first drug exposure period for matched dabigatran 110 mg twice daily and vitamin K antagonist patients. Upper panels, from left to right: acute thrombotic events, clinically relevant bleeding, major bleeding. Lower panels, from left to right acute coronary syndromes, death, composite outcomes
Figure S7 One‐year cumulative incidence (Kaplan–Meier estimate) of outcomes during the first drug exposure period for matched dabigatran 150 mg twice daily and vitamin K antagonist patients. Upper panels, from left to right: acute thrombotic events, clinically relevant bleeding, major bleeding. Lower panels, from left to right acute coronary syndromes, death, composite outcomes
Appendix S1 Methods
Appendix S2 Codes used for CHA2DS2‐VASc and HAS‐BLED scores
Appendix S3 International Classification of Diseases codes for outcomes
Appendix S4 Variables included in the high‐dimensional propensity score
Blin, P. , Dureau‐Pournin, C. , Cottin, Y. , Bénichou, J. , Mismetti, P. , Abouelfath, A. , Lassalle, R. , Droz, C. , and Moore, N. (2019) Effectiveness and safety of 110 or 150 mg dabigatran vs. vitamin K antagonists in nonvalvular atrial fibrillation. Br J Clin Pharmacol, 85: 432–441. 10.1111/bcp.13815.
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study Arch Intern Med 1987; 147: 1561–1564. [PubMed] [Google Scholar]
- 2. Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non‐valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev 2005; CD001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goto S, Bhatt DL, Röther J, Alberts M, Hill MD, Ikeda Y, et al Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J 2008; 156: 855, 63 e2–863. [DOI] [PubMed] [Google Scholar]
- 4. Blin P, Dureau‐Pournin C, Lassalle R, Abouelfath A, Droz‐Perroteau C, Moore N. A population database study of outcomes associated with vitamin K antagonists in atrial fibrillation before DOAC. Br J Clin Pharmacol 2016; 81: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004; 329: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pouyanne P, Haramburu F, Imbs JL, Bégaud B. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. Fr Pharmacovigilance Centres BMJ 2000; 320: 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014; 383: 955–962. [DOI] [PubMed] [Google Scholar]
- 8. Steg PG, Lopez‐Sendon J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA Jr, et al External validity of clinical trials in acute myocardial infarction. Arch Intern Med 2007; 167: 68–73. [DOI] [PubMed] [Google Scholar]
- 9. Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet 2005; 365: 82–93. [DOI] [PubMed] [Google Scholar]
- 10. Beyer‐Westendorf J, Buller H. External and internal validity of open label or double‐blind trials in oral anticoagulation: better, worse or just different? J Thromb Haemost 2011; 9: 2153–2158. [DOI] [PubMed] [Google Scholar]
- 11. Blin P, Dureau‐Pournin C, Lassalle R, Jové J, Thomas‐Delecourt F, Droz‐Perroteau C, et al Outcomes in patients after myocardial infarction similar to those of the PEGASUS‐TIMI 54 trial: A cohort study in the French national claims database. Br J Clin Pharmacol 2017; 83: 2056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bezin J, Duong M, Lassalle R, Droz C, Pariente A, Blin P, et al The national healthcare system claims databases in France, SNIIRAM and EGB: Powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2017; 26: 954–962. [DOI] [PubMed] [Google Scholar]
- 13. Bernard MA, Benichou J, Blin P, Weill A, Bégaud B, Abouelfath A, et al Use of health insurance claim patterns to identify patients using nonsteroidal anti‐inflammatory drugs for rheumatoid arthritis. Pharmacoepidemiol Drug Saf 2012; 21: 573–583. [DOI] [PubMed] [Google Scholar]
- 14. Maura G, Blotiere PO, Bouillon K, Billionnet C, Ricordeau P, Alla F, et al Comparison of the short‐term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity‐matched cohort study. Circulation 2015; 132: 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bannay A, Chaignot C, Blotiere PO, Basson M, Weill A, Ricordeau P, et al The best use of the Charlson comorbidity index with electronic health care database to predict mortality. Med Care 2016; 54: 188–194. [DOI] [PubMed] [Google Scholar]
- 16. Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001; 154: 854–864. [DOI] [PubMed] [Google Scholar]
- 17. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost 2005; 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 18. Bezin J, Groenwold RH, Ali MS, Lassalle R, Robinson P, de Boer A, et al Comparative effectiveness of recommended versus less intensive drug combinations in secondary prevention of acute coronary syndrome. Pharmacoepidemiol Drug Saf 2017; 26: 285–293. [DOI] [PubMed] [Google Scholar]
- 19. Giroud M, Hommel M, Benzenine E, Fauconnier J, Béjot Y, Quantin C, et al Positive predictive value of French hospitalization discharge codes for stroke and transient ischemic attack. Eur Neurol 2015; 74: 92–99. [DOI] [PubMed] [Google Scholar]
- 20. Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci 2010; 25: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High‐dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009; 20: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schneeweiss S, Eddings W, Glynn RJ, Patorno E, Rassen J, Franklin JM. Variable selection for confounding adjustment in high‐dimensional covariate spaces when analyzing healthcare databases. Epidemiology 2017; 28: 237–248. [DOI] [PubMed] [Google Scholar]
- 23. Garbe E, Kloss S, Suling M, Pigeot I, Schneeweiss S. High‐dimensional versus conventional propensity scores in a comparative effectiveness study of coxibs and reduced upper gastrointestinal complications. Eur J Clin Pharmacol 2013; 69: 549–557. [DOI] [PubMed] [Google Scholar]
- 24. Rassen JA, Schneeweiss S. Using high‐dimensional propensity scores to automate confounding control in a distributed medical product safety surveillance system. Pharmacoepidemiol Drug Saf 2012; 21 (Suppl. 1): 41–49. [DOI] [PubMed] [Google Scholar]
- 25. Rassen JA, Glynn RJ, Brookhart MA, Schneeweiss S. Covariate selection in high‐dimensional propensity score analyses of treatment effects in small samples. Am J Epidemiol 2011; 173: 1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med 2009; 28: 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 28. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Zureik S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 30. Hernandez I, Baik SH, Pinera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med 2015; 175: 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, et al Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015; 131: 157–164. [DOI] [PubMed] [Google Scholar]
- 32. Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, et al Stroke, bleeding, and mortality risks in elderly medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med 2016; 176: 1662–1671. [DOI] [PubMed] [Google Scholar]
- 33. Carmo J, Moscoso Costa F, Ferreira J, Mendes M. Dabigatran in real‐world atrial fibrillation. Meta‐analysis of observational comparison studies with vitamin K antagonists. Thromb Haemost 2016; 116: 754–763. [DOI] [PubMed] [Google Scholar]
- 34. Singer DE, Hellkamp AS, Piccini JP, Mahaffey KW, Lokhnygina Y, Pan G, et al Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc 2013; 2: e000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang SV, Franklin JM, Glynn RJ, Schneeweiss S, Eddings W, Gagne JJ. Prediction of rates of thromboembolic and major bleeding outcomes with dabigatran or warfarin among patients with atrial fibrillation: new initiator cohort study. BMJ 2016; 353: i2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martinez BK, Sood NA, Bunz TJ, Coleman CI Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in frail patients with nonvalvular atrial fibrillation. J Am Heart Assoc 2018; 7 pii: e008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Larsen TB, Skjoth F, Nielsen PB, Kjældgaard JN, Lip GYH. Comparative effectiveness and safety of non‐vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ 2016; 353: i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Staerk L, Gerds TA, Lip GYH, Ozenne B, Bonde AN, Lamberts M, et al Standard and reduced doses of dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation: a nationwide cohort study. J Intern Med 2018; 283: 45–55. [DOI] [PubMed] [Google Scholar]
- 39. Chan YH, See LC, Tu HT, Yeh YH, Chang SH, Wu LS, et al Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in Asians with nonvalvular atrial fibrillation. J Am Heart Assoc 2018; 7 pii: e008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bouillon K, Bertrand M, Maura G, Blotière PO, Ricordeau P, Zureik M. Risk of bleeding and arterial thromboembolism in patients with non‐valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non‐vitamin K‐antagonist oral anticoagulant: a retrospective, matched‐cohort study. Lancet Haematol 2015; 2: e150–e159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Description of dabigatran 110 mg twice daily, dabigatran 150 mg twice daily and vitamin K antagonist populations before matching
Table S2 Crude event rates during drug exposure
Table S3 Baseline patient characteristics in dabigatran 110 mg twice daily users: comparison with RE‐LY
Table S4 Baseline patient characteristics in dabigatran 150 mg twice daily users: comparison with RE‐LY
Table S5 Outcomes in matched dabigatran 110 mg twice daily users: comparison with RE‐LY
Table S6 Outcomes in matched dabigatran 150 mg twice daily users: comparison with RE‐LY
Table S7 matched analyses of main outcomes with Charlson score and cost before index date as additional adjustment variables
Figure S1 High‐dimensional propensity score distributions for unmatched (left panels) and matched (right panels) cohorts, of dabigatran 110 mg twice daily vs. vitamin K antagonist (A) and dabigatran 150 mg twice daily vs. vitamin K antagonist (B)
Figure S2 (A) Standardized mean differences in characteristics included in the high‐dimensional propensity score, before matching, dabigatran 110 twice daily vs. vitamin K antagonist. Each line is a different parameter (490 shown). (B) Standardized mean differences in characteristics included in the high‐dimensional propensity score, after matching, dabigatran 110 vs. vitamin K antagonist. Each line is a different parameter (490 shown)
Figure S3 (B) Standardized mean differences in characteristics included in the high‐dimensional propensity score, before matching, dabigatran 150 twice daily vs. vitamin K antagonist. Each line is a different parameter (490 shown). (B) Standardized mean differences in characteristics included in the high‐dimensional propensity score, after matching, dabigatran 150 vs. vitamin K antagonist. Each line is a different parameter (490 shown)
Figure S4 Forest plots for crude, adjusted and matched analyses of main outcomes, dabigatran 110 mg twice daily vs. vitamin K antagonist
Figure S5 Forest plots for crude, adjusted and matched analyses of main outcomes, dabigatran 150 mg twice daily vs. vitamin K antagonist
Figure S6 One‐year cumulative incidence (Kaplan–Meier estimate) of outcomes during the first drug exposure period for matched dabigatran 110 mg twice daily and vitamin K antagonist patients. Upper panels, from left to right: acute thrombotic events, clinically relevant bleeding, major bleeding. Lower panels, from left to right acute coronary syndromes, death, composite outcomes
Figure S7 One‐year cumulative incidence (Kaplan–Meier estimate) of outcomes during the first drug exposure period for matched dabigatran 150 mg twice daily and vitamin K antagonist patients. Upper panels, from left to right: acute thrombotic events, clinically relevant bleeding, major bleeding. Lower panels, from left to right acute coronary syndromes, death, composite outcomes
Appendix S1 Methods
Appendix S2 Codes used for CHA2DS2‐VASc and HAS‐BLED scores
Appendix S3 International Classification of Diseases codes for outcomes
Appendix S4 Variables included in the high‐dimensional propensity score


