Abstract
Background: The objective of this study is to assess if infusion of osteoblasts can temporarily reverse osteoporosis in rats.
Methods: Osteoporosis was induced in 20 female Sprague-Dawley rats by performing ovariectomy (OVX) that was carried out at 4 weeks of age. At 3 months a biopsy of the iliac crest was made to assess the bone quality and the same site bone marrow was harvested. From the bone marrow aspirate, MSCs were separated. Osteoblasts were then generated and were characterized using Alizarin red staining. Osteoblasts were injected in the tail vein of 10 rats. Two weeks after the injection of osteoblasts, a second biopsy was done. Animals were euthanized after 8 weeks of osteoblasts infusion by overdose of ketamine mixed with xylazine. The whole femurs and lumbar spine were dissected and the specimens were stored in 2% formalin. The specimens were analyzed using HRpQCT (High-resolution peripheral quantitative computerized tomography (μCT 100, SCANCO Medical AG, Brüttisellen, Switzerland).
Results: In all the 10 animals from which bone aspiration was performed, osteoblasts were cultured and transplanted. Analysis showed that there was significant bone formation at bone sites of distal femur and lumbar spine (<0.001), with increased number of trabeculae and thickness (P<0.001). Further analysis revealed that there was robust bone formation in the animals that had osteoblasts injection.
Conclusions: This preliminary study indicates that osteoblasts infusion can lead to new bone formation in osteoporosis induced by ovariectomy in rats.
Keywords: Osteoporosis, Ovariectomy, Osteoblasts, Mesenchymal Stimulating Cells (MSCs)
Introduction
Osteoporosis is a disease in which the net loss of bone exceeds bone formation and it occurs in women after estrogen loss in postmenopausal age[2-4]. Postmenopausal osteoporosis (PMO) is a major public health epidemic world over. The importance of PMO is very clear as with expected increased aging of the world population the complications such as the hip fractures will treble to over six million a year by 2050[5]. Drug therapy for osteoporosis is effective but comes with side effects like any other drug, the effects ranging from simple gastric irritation, myalgias, arthralgias, hypocalcemia, osteonecrosis of jaw to serious infections[6-12]. At present MSCs are the most widely used stem cells in the research but in vitro expanded MSCs have a short lifetime after in vivo administration. Moreover, the adverse effects of MSCs, especially. in the context of tumor modulation and spontaneous malignant transformation makes it difficult to use them routinely[13]. Inspite of this, many trials are ongoing using MSCs in various conditions.
Previously, we successfully used osteoblasts in the treatment of avascular necrosis of femur in humans[14] and in non-union femurs in animals[15] and this encouraged us to use osteoblasts in an attempt to treat osteoporosis in ovariectomised (OVX) rats, as they present an ideal preclinical animal model that shows changes due to estrogen deficit, very similar to human skeleton[16].
Methods
Osteoporosis was induced in 20 female Sprague-Dawley rats by performing ovarectomy at 4 weeks of age. After obtaining the ethical approval from the Institutional Review Board of Imam AbdulRahman Bin Faisal University, Dammam, Saudi Arabia (Vide number 2015115/2015), 20 Sprague-Dawley female rats were procured and kept for three days before the study was started. All animals were housed and handled in accordance with the guidelines. Animals were kept in large cages with free mobility and fed with standard diet. They were provided with food, water ad libitum and maintained at 25-28 degrees Centigrade. At 3 months, a biopsy of the iliac crest was made to assess the bone quality and from the same site bone marrow was harvested later. From the bone marrow aspirate, MSCs were separated as described by Piao et al. (2005) [1].
The cell suspension was mixed together and centrifuged at 1 100Å~g for 4 minutes at 37°C. The supernatant and adipose tissue was removed. The cell suspension was transferred to a 15-ml centrifuge tube containing 5 ml of Percoll (1.073 g/ml, Sigma Corp., St. Louis, Missouri, USA). Cells were dispersed by pipetting again and centrifuged at 1 500Å~g for 30 minutes. The mononuclear cells in the middle layer were obtained, washed three times with phosphate buffered saline (PBS) and then suspended in low-glucose Dulbecco’s modified Eagle’s medium (L-DMEM; Invitrogen, UK) with 20% heat-inactivated fetal bovine serum (FBS; Gibco BRL, Gaithersburg, MD, USA), 100 U/ml penicillin G, and 100 μg/ml streptomycin. We opted for acceptable methods of marker analysis like Reverse transcriptase PCR (RT-PCR). We analyzed the hMSC cell populations for the expression of CD44, CD90 and CD45 using RT-PCR. Our hMSC populations showed positive amplification for CD44 (+); CD90 (+) and expected negative expression of CD45 (-). These results confirmed that our cell population was MSC. To confirm the osteogenic potential of the MSCs used, BALB/c MSCs (2 x 104 cells/cm2) cells were incubated in CEM until a confluent layer was achieved and then osteogenic medium was added, containing IMDM supplemented with 9 % FBS, 9 % HS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μ g/mL streptomycin, 50 ng/mL L-thyroxine (Sigma Aldrich), 20 mM β -glycerol phosphate, (Sigma Aldrich), 100 nM dexamethasone (Sigma Aldrich) and 50 μ M ascorbic acid (Sigma Aldrich). Medium was changed every 5th day. The osteogenic differentiation process was followed as per the recommendations of the commercial media manufacturers. The MSC cells were incubated in osteogenic medium for 14 days. Two numbers of 3.5 mm cell culture dish were also seeded with MSC and were subjected to the same process of osteogenic differentiation parellelly. At the end of the differentiation one of the 3.5 mm dish was used for total RNA harvesting (which was later used for RT PCR confirmation of expression of osteopontin marker which confirms the final maturation into osteoblasts. Second dish was used for alizarin red staining which stains into dark orange color when calcium mineralization is seen around the cells (confirms the osteogenic differentiation). The calcium mineralization intensity and distribution under the field of microscope was used roughly to estimate the percentage of osteogenic differentiation which was estimated to be upto 80%. After 15th day, osteogeneic differentiated cells from the MSCs were available for transplantation. Once cells were differentiated, and were characterized using Alizarin red staining. Cells after differentiation have been immediately used for the treatment after being suspended in physiological saline and a backup of the batch of the cells had been frozen in -80 degree celcius before being transferred to liquid nitrogen. As many as 25,000 osteoblasts were calculated and suspended in 500 ul of NSS and infused in the tail vein of 10 rats. Animals were euthanized after 8 weeks of osteoblasts infusion by overdose of ketamine mixed with xylazine. The whole femurs and lumbar spine were dissected out and the specimens were stored in 2% formalin. The specimens were shipped to b-cube AG Bio-Technopark, Wagistrasse 13CH-8952 Schlieren-Zurich, Switzerland. The samples were measured with High-Resolution Peripheral Quantitative Computed Tomography (HR-pQCT) with a commercially available cabinet cone-beam, (μCT 100, SCANCO Medical AG, Brüttisellen, Switzerland). MicroCT examinations were captured by the photons and detected by a CCD-based area detector and the projection data was computer-reconstructed into a 3072 x 3072 image matrix. Individual assessment of the Total volume (TV) [mm^3], Bone volume (BV) [mm^3], Relative bone volume BV/TV%, Connectivity density (Conn.D), Trabecular number (DT-Tb.N) mm, Trabecular thickness (DT-Tb.Th) mm and DT-Tb.Sp: Trabecular separation = marrow thickness (DT-Tb.Sp) mm.Quality Control Calibration tests were carried out every day for the two techniques using an external phantom to detect any potential drift of the instrumentation.
There was no drift during the period of the protocol. The same observer analyzed all examinations HR-pQCT.
Statistical Analysis
For each sample in the reproducibility study, a coefficient of variation (CV) was calculated as the standard deviation of the three repeated measurements divided by the subject mean. Furthermore, the short-term precision errors were then calculated as root-mean-square (RMS) averages of the precision errors for each of the sample. The statistical level of significance was <0.05.
Results
There were no complications of infection or deaths in either the group. In all the 10 animals that the bone aspirate was done, osteoblasts were cultured and were transplanted. Figure 1A shows the HRpQCT picture of the distal femur of the study group. The figure shows large number of trabecular pattern quality and quantity as compared to the control group (Figure 1B). Figure 1C shows the magnification of the distal femur in the study group, which highlights the presence of trabecular pattern. In the control group the section shows large empty spaces and reduced number of trabeculae (Figure 1D). Figures 1E study group and sample six of the control Group showing similar changes. Figures 1F is the Sagittal section of the distal femur in the study group showing the condyles filled with new bone with plenty of trabeculae while Figure 1G is the study group’s saggital section of the distal femur showing the condyles filled with new bone with plenty of trabeculae compared to control group (Figure 1H) showing wide spaces of no bone to minimal bone in the areas examined when compared to the study group. Figures 9 and 10 are that of HRpQCT scans of the lumbar spine in the study and the control groups show decisively more bone in the study group as compared to the control group. Figures 2 A-D shows the HRPQCT reconstruction of Trabecular Pattern of Vertebral Body in study group. Figures 3A and B shows the histological sections in the study and the control group. Table I and Table II gives the various indices of the Femur and the spine.
Figure 1:
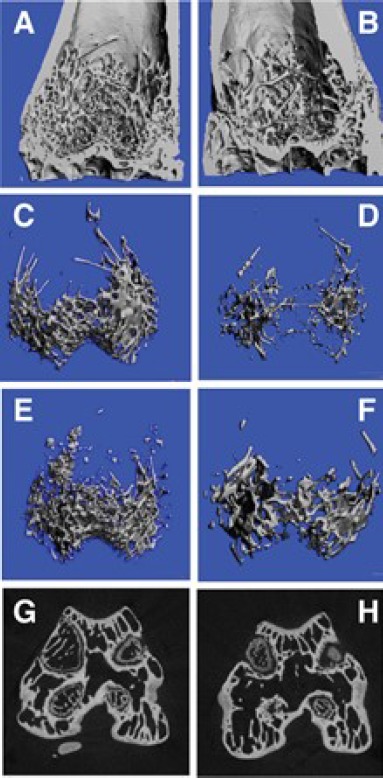
A - Section of Distal Femur of the Study Group showing dense trabeculae; B-Section of the Distal Femur of the Control Group sparse trabecular pattern; C-HRPQCT reconstruction of Trabecular Pattern of Distal Femur in Study Group indicating dense bony structure;D-HRPQCT reconstruction of Trabecular Pattern of Distal Femur in Control Group reveals wide trabecular spaces and empty areas; E-HRPQCT reconstruction of Trabecular Pattern of Distal Femur in Study Group of another animal showing high quantity and quality of the areas; F-HRPQCT reconstruction of Trabecular Pattern of Distal Femur in Control Group of another animal showing sparse bone; G - Saggital section of the distal femur in the study group showing the condyles filled with new bone with plenty of trabeculae; H - Saggital section of the distal femur in the control group showing wide spaces of no bone to minimal bone in the areas examined when compared to the study group.
Discussion
Our study shows that the culture-expanded osteoblasts were effective in increasing bone formation in OVX rats. Both (HRpQCT and doubled labeled tetracycline biopsy) objective assessment of osteoporosis in the two groups gave a promising picture. Secondly our result also shows and we speculate that the effect observed was due to homing of the infused osteoblasts on to the bone surface rather in the other tissues of the body to produce the desired results.Wang et al [17] used MSCs and reported that these cells increased the strength in the osteoporotic bone, while Ocarino et al (2010)[18] injected BMMSCs in the bone marrow of osteoporotic rats and concluded that osteoporosis can be treated by injection of BMMSCs. Our strategy was to study the effect of osteoblasts rather MSCs because in an earlier study we found that direct osteoblast infusion at the fracture site gave robust fracture healing[16]. It is accepted now MSCs act via multifaceted pathways, which is not completely understood to increase bone regeneration and homing of the infused cells at the site of intended location[19]. Moreover, difficulty of homing of the MSCs onto the bone surface thereby, low bone forming effect[20] . Recently, Kiernen et al[21] used minimally expanded exogenous MSCs in a mouse model showed enhanced bone formation. With use of osteoblasts although we observed enhanced new bone formation in the study group of animals, we believe we need to replicate the same in a larger animal to further confirm our findings.
Figure 2:
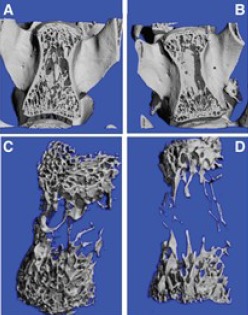
A-Section of Vertebral body of the Study Group showing the area examined with good quantity of the trabecular pattern; B - Section of Vertebral body of the Control Group with empty areas and thinned out trabeculae. C - HRPQCT reconstruction of Trabecular Pattern of Vertebral Body in Study Group depicting the dense trabeculae. D - HRPQCT reconstruction of Trabecular Pattern of Vertebral Body in Control Group with empty spaces when compared to the study group.
Figure 3:
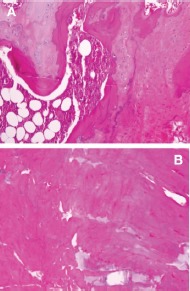
A - H & E X400 Section of Distal Femur in Pre injection of Osteoblasts, showing wide area of immature bone and cartilage; B - H & E X400 Section of Distal Femur in Post injection of Osteoblasts after 8 weeks showing formation of new bone in the entire area of the field.
Table I: Structural Indices of Distal Femurs.
| Parameter | Study Group | Control Group | P Value |
| VOX-TV | 45.047±4.54 | 38.611±1.02 | <0.001 |
| VOX-BV | 2.16±0.46 | 1.098±0.22 | <0.001 |
| Connectivity Density | 16.57±4.5 | 4.92±0.31 | <0.001 |
| Trabecular Number | 0.7±0.1 | 0.54±0.11 | <0.001 |
| Trabecular Thickness | 0.07±0.01 | 0.06±0.01 | <0.01 |
| Trabecular Spacing | 1.40±1.4 | 1.59±0.17 | <0.006 |
| Mean1 mg/HA/ccm | 60.69±35.55 | 45.77±20.15 | <0.05 |
| Mean 2 mg/HA/ccm | 1088.17±8.99 | 1048.10±4.8 | <0.001 |
| TRI –Total Volume | 42.86±2.70 | 38.21±1.02 | <0.001 |
| TRI –Bone Volume | 1.74±0.80 | 1.048±0.22 | <0.001 |
| TRI Bone Surface | 62.89±5.77 | 36.58±5.71 | <0.001 |
| TRI-Trabecular Number | 0.75±0.33 | 0.48±0.08 | <0.001 |
| TRI-Trabecular Thickness | 0.054±0.01 | 0.043±0.01 | <0.007 |
| TRI-Trabecular Spacing | 1.65±0.93 | 2.08±0.39 | <0.006 |
VOX= Based on counting voxels, TRI= based on triangularization of surface . Mean 1= mean voxel values of everything within volume of interest (Bone and Background) Mean 2= of segmented region only what was considered bone
Table 2: Structural Indices of Vertebral Bodies.
| Parameter | Study Group | Control Group | P Value |
| VOX-Total Volume | 38.79±8.03 | 27.9±1.03 | <0.001 |
| VOX-Bone Volume | 5.33±1.82 | 2.32±0.14 | <0.001 |
| Connectivity Density | 36.92±4.86 | 15.84±0.72 | <0.001 |
| Trabecular Number | 1.69±1.31 | 1.14±0.04 | <0.001 |
| Trabecular Thickness | 0.073±0.01 | 0.05±0.02 | <0.005 |
| Trabecular Spacing | 0.65±0.05 | 0.82±0.04 | <0.001 |
| Mean 1 mg/HA/ccm | 196.81±4.65 | 145.68±2.07 | <0.001 |
| Mean 2 mg/HA/ccm | 1070.32±7.89 | 1032.53±8.99 | <0.001 |
| TRI –Total Volume | 38.18±7.94 | 26.46±2.94 | <0.001 |
| TRI –Bone Volume | 5.30±1.84 | 2.22±0.16 | <0.001 |
| TRI-BS | 171.14±3.18 | 84.42±0.97 | <0.001 |
| TRI-Trabecular Number | 2.19±0.23 | 1.49±0.04 | <0.001 |
| TRI-Trabecular Thickness | 0.06±0.01 | 0.051±0.01 | <0.01 |
| TRI-Trabecular Spacing | 0.39±0.05 | 0.62±0.02 | <0.001 |
VOX= Based on counting voxels, TRI= based on triangularization of surface . Mean 1= mean voxel values of everything within volume of interest (Bone and Background) Mean 2= of segmented region only what was considered bone
There are two issues, which need to be addressed. How long can we use bisphosphonates for treatment of osteoporosis is still unknown? The long-term effects of drug therapy of osteoporosis are serious and detrimental to the bone itself. Atypical femoral fractures, osteonecrosis of the jaw and safety concerns in patients with renal and gastro-intestinal diseases are well known. The newer drugs like denosumab might ameliorate some of the complications but cannot eradicate them completely. Hence, a search of alternate treatment modality to treat patients with osteoporosis for years with no complications to prevent fractures remains essential. Secondly life expectancy is on the rise and passed over 100 years[22] in some countries and is expected to continue to rise even in the developing countries, making morbidity to rise many fold in patients suffering with osteoporosis.
Our study has some limitations and one such is that we did not perform the homing of the osteoblasts on to the bone surface which could have give strength to the study and differentiate from the MSCs which are suppose to be trapped in the pulmonary capillaries.
This study opens wide array of questions and possibilities. Even though our study shows that in experimental animals osteoporosis can be treated by osteoblasts infusion and it will require more studies to confirm this for a routine use. If the studies do confirm the ability to treat osteoporosis by osteoblasts infusion then how it could be made available for general use. It makes more questions than answers to the issue of alternate treatment of osteoporosis, but stem cell therapy of osteoporosis is a potential treatment modality, which should not be ignored.
Acknowledgments
The authors acknowledge the help of Dr Fawaz Mazin Alanii and Mohammed Khaled Baroudi for their help during the study.
Glossary
Abbreviations
- OVX
Ovariectomy
- MSCs
Mesenchymal stimulating cells
- HRpQCT
High resolution peripheral quantitative computerized tomography
- PMO
Postmenopausal osteoporosis
- TV
Total volume
- BV
Bone volume
- RBV
Relative bone volume
- Conn. D
Connectivity density
- DT-Tb.N
Trabecular number
- DT-Tb.Th
Trabecular thickness
- DT-Tb.Sp
Trabecular separation = marrow thickness
Potential Conflicts of Interests
None
Prior Presentations
This paper was presented at the “Translational Opportunities in Stem Cell Research. Basel, Switzerland, 27 February -1 March 2017.
References
- 1.Piao H, Youn TJ, Kwon JS, Kim YH, Bae JW, Bora-Sohn, Kim DW, Cho MC, Lee MM, Park YB. Effects of bone marrow derived mesenchymal stem cells transplantation in acutely infarcting myocardium. Eur. J. Heart Fail. 2005;7(5):730–8. doi: 10.1016/j.ejheart.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Rosen CJ. Restoring aging bones. Sci Am. 2003;288(3):70–7. doi: 10.1038/scientificamerican0303-70. [DOI] [PubMed] [Google Scholar]
- 3.Marcus R. Post-menopausal osteoporosis. Best Prac Res Clin Obstet Gynaecol. 2002;16(3):309–27. doi: 10.1053/beog.2002.0284. [DOI] [PubMed] [Google Scholar]
- 4.Heaney RP. Pathophysiology of Osteoporosis. Endocrinology and Metabolism Clinics of North America. 1998;27(2):255–65. doi: 10.1016/s0889-8529(05)70004-9. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Camplon G, Melton LJ. Hip fractures in the elderly: A world-wide projection. Osteoporosis International. 1992;2:285–9. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 6.Graham DY. What the gastroenterologists should know about the gastrointestinal safety profiles of bisphosphonates. Dig Dis Sci. 2002 Aug;47:1665–78. doi: 10.1023/a:1016495221567. [DOI] [PubMed] [Google Scholar]
- 7.de Groen PC, Lubbe DF, Hirsch LJ, Daifotis A, Stephenson W, Freedholm D, Pryor-Tillotson S, Seleznick MJ, Pinkas H, Wang KK. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335(1016):21. doi: 10.1056/NEJM199610033351403. [DOI] [PubMed] [Google Scholar]
- 8.Rupel K, Ottaviani G, Gobbo M, Contardo L, Tirelli G, Vescovi P, Di Lenarda R, Biasotto M. A systematic review of therapeutical approaches in bisphosphonates-related osteonecrosis of the jaw (BRONJ). Oral Oncol. 2014;50(11):1049–57. doi: 10.1016/j.oraloncology.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Holzinger D, Seemann R, Matoni N, Ewers R, Millesi W, Wutzl A. Effect of dental implants on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2014;72(10):1937.e1–8. doi: 10.1016/j.joms.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Kharazmi M, Hallberg P. Bisphosphonate-associated atypical femoral fractures and one-year mortality. Ups J Med Sci. 2014;18:1–2. doi: 10.3109/03009734.2014.959213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhadada SK, Sridhar S, Muthukrishnan J, Mithal A, Sharma DC, Bhansali A, Dhiman V. Predictors of atypical femoral fractures during long term bisphosphonate therapy: A case series and review of literature. Indian J Med Res. 2014;140(1):46–12. [PMC free article] [PubMed] [Google Scholar]
- 12.May FE. Novel drugs that target the estrogen-related receptor alpha: their therapeutic potential in breast cancer. Cancer Manag Res. 2014;6:225–52. doi: 10.2147/CMAR.S35024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong RS. Mesenchymal stem cells: angels or demons?. J Biomed Biotechnol. 2011;2011:459510. doi: 10.1155/2011/459510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadat-Ali M, Azam QM, Al-Dakheel DA, Acharya S. Healing of Experimentally Created Non-Union of Femur in Rats Using Bone Precursor Cells from Mesenchymal Stem Cells (MSCs). Journal of Stem Cells. 2015;10(2):91–96. [PubMed] [Google Scholar]
- 15.Sadat-Ali M, Azam MQ, Elshabouri EM, Tantawy AM, Acharya SK. Stem Cell Therapy for Avascular necrosis of Femoral Head in Sickle cell disease: Report of 11 cases and Review of Literature. Int J Stem Cells. 2017;10(2):179–183. doi: 10.15283/ijsc17019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimmel DB. Animal models for in vivo experimentation in osteoporosis research. In: In: Marcus R, Feldman D, Kelsey J, editors. (eds) Osteoporosis. Academic Press, San Diego: 1996. pp. 671–690. [Google Scholar]
- 17.Wang Z, Goh J, Das De S, Ge Z, Ouyang H, Chong JS, Low SL, Lee EH. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12(1753):61. doi: 10.1089/ten.2006.12.1753. [DOI] [PubMed] [Google Scholar]
- 18.Ocarino Nde M, Boeloni JN, Jorgetti V, Gomes DA, Goes AM, Serakides R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect Tissue Res. 2010;51(426):33. doi: 10.3109/03008201003597049. [DOI] [PubMed] [Google Scholar]
- 19.Hernigou P, Trousselier M, Roubineau F, Bouthors C, Chevallier N, Rouard H. Flouzat-Lachaniette CH.Stem Cell Therapy for the Treatment of Hip Osteonecrosis: A 30-Year Review of Progress Clinics in Orthopedic Surgery. 2016;8:1–8. doi: 10.4055/cios.2016.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckland J. Bone: Homing MSCs to the surface of bones. Nat Rev Rheumatol. 2012;8(4):185. doi: 10.1038/nrrheum.2012.22. [DOI] [PubMed] [Google Scholar]
- 21.Kiernan J, Hu S, Grynpas MD, Davies JE, Stanford WL. Systemic Mesenchymal Stromal Cell Transplantation Prevents Functional Bone Loss in a Mouse Model of Age-Related Osteoporosis. Stem Cells Translational Medicine. 2016;5:683–93. doi: 10.5966/sctm.2015-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodward A, Blakely T. The Healthy Country? A History of Life and Death in New Zealand (Auckland University Press.) 2014.


