Data suggest that HIV+ individuals unable to control infection fail to do so due to impaired cytokine production and/cytotoxic effector cell function. Consequently, the success of cure agendas such as the shock-and-kill strategy will probably depend on enhancing patient effector cell function. In this regard, NK cells are of particular interest since they complement the function of CD8+ T cells. Here, we demonstrate the ability of short-course alpha interferon (IFN-α) treatments to effectively enhance such effector functions in chronic progressor NK cells without inhibiting their general CD8+ T cell function. These results point to the possibility of exploring such short-course IFN-α treatments for the enhancement of effector cell function in HIV+ patients in future cure strategies.
KEYWORDS: CD cells, NK cells, HIV-1, interferon alpha
ABSTRACT
Current shock-and-kill strategies for the eradication of the HIV-1 reservoir have resulted in blips of viremia but not in a decrease in the size of the latent reservoir in patients on suppressive antiretroviral therapy (ART). This discrepancy could potentially be explained by an inability of the immune system to kill HIV-1-infected cells following the reversal of latency. Furthermore, some studies have suggested that certain latency-reversing agents (LRAs) may inhibit CD8+ T cell and natural killer (NK) cell responses. In this study, we tested the hypothesis that alpha interferon (IFN-α) could improve the function of NK cells from chronic progressors (CP) on ART. We show here that IFN-α treatment enhanced cytokine secretion, polyfunctionality, degranulation, and the cytotoxic potential of NK cells from healthy donors (HD) and CP. We also show that this cytokine enhanced the viral suppressive capacity of NK cells from HD and elite controllers or suppressors. Furthermore, IFN-α enhanced global CP CD8+ T cell cytokine responses and the suppressive capacity of ES CD8+ T cells. Our data suggest that IFN-α treatment may potentially be used as an immunomodulatory agent in HIV-1 cure strategies.
IMPORTANCE Data suggest that HIV+ individuals unable to control infection fail to do so due to impaired cytokine production and/cytotoxic effector cell function. Consequently, the success of cure agendas such as the shock-and-kill strategy will probably depend on enhancing patient effector cell function. In this regard, NK cells are of particular interest since they complement the function of CD8+ T cells. Here, we demonstrate the ability of short-course alpha interferon (IFN-α) treatments to effectively enhance such effector functions in chronic progressor NK cells without inhibiting their general CD8+ T cell function. These results point to the possibility of exploring such short-course IFN-α treatments for the enhancement of effector cell function in HIV+ patients in future cure strategies.
INTRODUCTION
Attempts to cure HIV-1 have been unsuccessful due to the presence of latent HIV reservoirs (1, 2). Several approaches have thus far been proposed to meet this challenge, including the “shock-and-kill” strategy, where latency-reversing agents (LRAs) are used to pharmacologically reverse latency in order to render formerly quiescent reservoirs susceptible to both antiretroviral therapy (ART) and the immune system (3–5). However, while treatments with certain LRAs have resulted in viral blips, no trial has demonstrated changes in the size of the latent reservoir (6–12), owing perhaps to the dysfunctional state of potential effector cells. This awareness has drawn attention to the need to potentiate immune responses in order to efficiently eliminate reactivated reservoirs (13, 14).
As part of these efforts, there has been a lot of attention focused on CD8+ T cells. However, while these cells play a critical role in the control of HIV-1 replication, they also possess limitations. They are slower to respond to viral infections due to the timing of the adaptive immune response, they are susceptible to viral escape strategies such as HLA downregulation (15), and they are typically excluded from B cell follicles in lymph nodes (16–18), which can then serve as sanctuaries for sustained productive infection (1). For these reasons, it is important to also analyze other immune effector cells capable of complementing CD8+ T cell effector function. Natural killer (NK) cells are ideal candidates for this role. They respond to viral infection without a need for clonal expansion, are thought to home to and control viral replication in lymph node sanctuaries (19), and are equipped to kill infected cells that evade CD8+ T cell elimination by HLA downregulation (20).
NK cell function can be augmented by various cytokines, including interleukin-15 (IL-15), IL-18, and IL-21, as well as type 1 interferons (IFNs) (21). IL-15 in particular has recently been shown to enhance NK cell-mediated antiviral activity in humanized mice (22) and in vitro following latency reversal in CD4+ T cells from patients on ART (23). In this study, we focused on alpha interferon (IFN-α) since it has been used clinically for the treatment of hepatitis C (24). As a member of the type 1 IFN family, IFN-α helps create antiviral immune states that in turn help control the spread of viral infections (25). The efficiency of this IFN-mediated control, however, is principally dependent on the timing and concentration levels of IFN-α (25, 26). Specifically, in the SIV model of HIV-1 infection, type 1 IFNs ramp up the immune system and tip the scales in favor of an antiviral state that helps control infection spread in primary infection (27). However, several studies have suggested that IFN-α also plays a role in the immune dysfunction seen in the chronic stages of HIV-1 infection (28, 29). Therefore, to maximize the benefits of type 1 IFN (IFN-α) for NK cells, a fine line must be drawn in choosing a concentration and regimen that facilitates an antiviral response without causing excessive immune activation that triggers a transition away from that antiviral state.
While several studies have shown that IFN-α enhances NK cell cytokine secretion and cytotoxic responses in healthy donors (30–33) and, in a subset of HIV controllers (34), similar studies have not been performed with chronic progressor (CP) NK cells. We therefore sought to determine via in vitro assays, whether treatment of CP NK cells over short periods of time (termed a “pulse”) with IFN-α would enhance their cytokine-producing capacities, as well as their cytotoxic responses, without inhibiting general CD8+ T cell function. We also sought to determine whether similar treatments would enhance NK cell-mediated HIV-1 replication suppression in elite suppressor (ES) NK cells, again without inhibiting the HIV-specific suppressive capacities of CD8+ T cells. Our results suggest that IFN-α pulse therapy could be employed for enhancing NK cell effector function in HIV-1 cure strategies.
RESULTS
IFN-α enhances degranulation and cytokine production in CP NK cells.
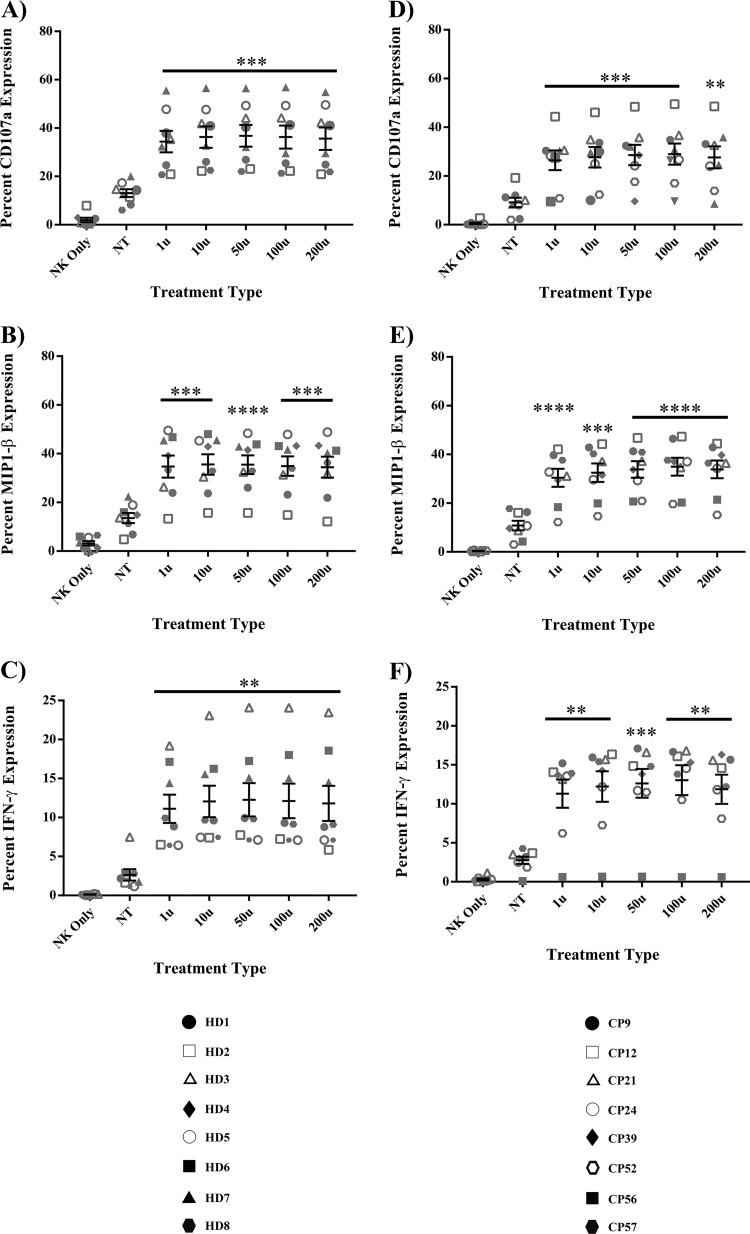
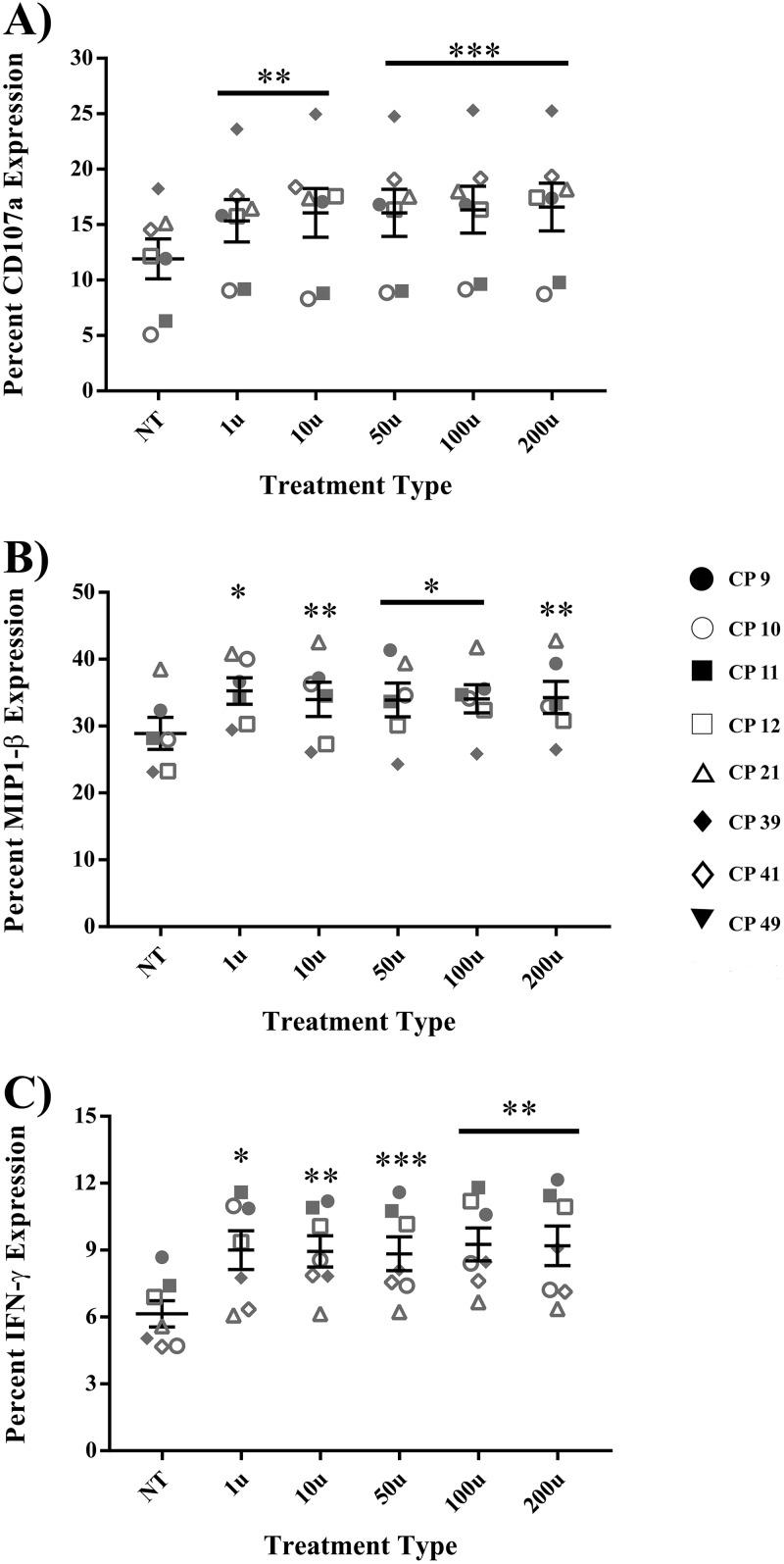
The goal of this study was to determine whether short treatments of IFN-α could be used to enhance CP NK cell effector function without incapacitating the effector functions of CD8+ T cells. To answer this question, assays measuring NK cell degranulation and cytokine production were used. The presence of residual intracellular drugs prevents effective superinfection of CP CD4+ T cells. We therefore assessed the effects of IFN-α on CP NK cell function by measuring the response of these cells to K562 cells. Briefly, isolated CP NK cells were pulsed with media alone (baseline control) or various concentrations of IFN-α and subsequently cocultured with K562 targets at a 1:1 effector/target ratio in the presence of a CD107a antibody and protein transport inhibitors. NK cells from healthy donors (HD) were treated in a similar fashion as a means of comparison to results from CP-treated NK cells. The percent expression of various cytokines was then assessed via flow cytometry. Relative to untreated NK cells, there was little to no nonspecific production of cytokines from NK cells treated with IFN-α and cultured in the absence of target cells (data not shown). However, in the presence of targets, IFN-α treatment significantly increased the cytokine-producing capacity of HD NK cells. As shown in Fig. 1A, IFN-α-treated HD NK cells, relative to baseline, showed a marked increase in CD107a degranulation (P < 0.0008). IFN-α treatment also significantly increased the production of MIP-1β (Fig. 1B, P < 0.0006) and IFN-γ (Fig. 1C, P < 0.004). CP NK cells also yielded significant amounts of CD107a (Fig. 1D, P < 0.001), MIP-1β (Fig. 1E, P < 0.0001), and IFN-γ (Fig. 1F, P < 0.002), with stimulation of K562 cells following IFN-α pulses.
FIG 1.
IFN-α enhances degranulation and cytokine production in HD and CP NK cells. NK cells from 8 HD and 8 CP were treated with either medium alone (NT baseline) or various concentrations of IFN-α for 6 h, washed, and then cocultured for 4 h with target K562 cells or medium alone (NK only) in the presence of a CD107a antibody and protein transport inhibitors. Enhancement of degranulation and cytokine production was subsequently studied by flow cytometry following surface and intracellular staining. (A) CD107a degranulation in HD; (B) MIP-1β in HD; (C) IFN-γ in HD; (D) CD107a degranulation in CP; (E) MIP-1β in CP; (F) IFN-γ in CP. Triplicates were performed for each patient with error bars representing standard deviation from mean within treatment groups. One-way repeated-measures ANOVA was used to determine difference between baseline and specific treatments. Asterisks indicate differences from a no-treatment baseline control (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
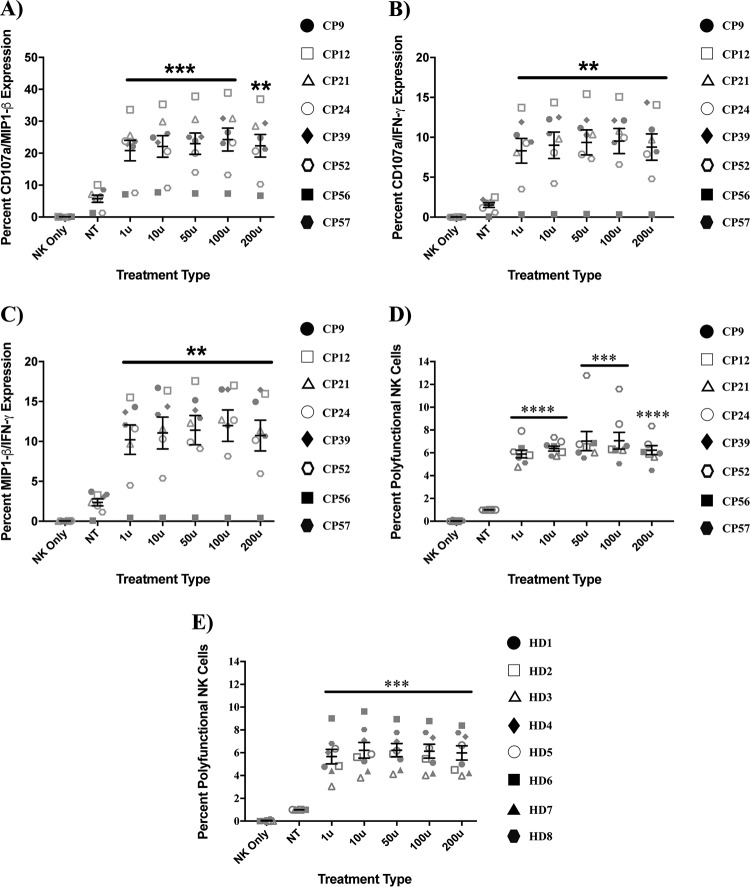
More importantly, not only were individual cytokine secretion and degranulation profiles of IFN-α-treated NK cells enhanced, but their polyfunctional profiles were enhanced as well. As shown in Fig. 2, pretreatment of CP NK cells with IFN-α significantly increased the coexpression of CD107a and MIP-1β (Fig. 2A, P < 0.002), CD107a and IFN-γ (Fig. 2B, P < 0.005), and MIP-1β and IFN-γ (Fig. 2C, P < 0.004). In addition, there was also enhanced coexpression of all three proteins in CP (Fig. 2D, P < 0.0007) and HD (Fig. 2E, P < 0.0008). There was no significant difference in the percentage of polyfunctional NK cell responses between HD and CP. We also assessed whether longer treatments with IFN-α would further increase CP NK cell polyfunctionality and found that while 6 h of treatment resulted in a more potent response than one hour, no added benefit was seen with 18 or 24 h of preincubation with the cytokine (see Fig. S1 in the supplemental material).
FIG 2.
IFN-α enhances HD and CP NK cell polyfunctionality. NK cells from 8 HD and 5 to 8 CP were pulsed with either medium (NT baseline) or various concentrations of IFN-α for 6 h, washed, and then cultured alone (NK only) or for 4 h with target K562 cells in the presence of antibodies to CD107a and protein transport inhibitors. The assessment of enhancement of degranulation, bi- and trifunctional protein production was subsequently studied via flow cytometry following surface and intracellular staining. In our analysis, bulk CP NK cells were probed for the simultaneous production of either CD107a and MIP-1β (A), CD107a and IFN-γ (B), and MIP-1β and IFN-γ (C). Bulk CP (D) and HD (E) NK cells were then also probed for the simultaneous production of CD107a, MIP-1β, and IFN-γ. In all experiments, triplicates were performed for each subject, with error bars representing standard deviations from mean within treatment groups. One-way repeated-measures ANOVA was used to determine the differences between baseline and specific treatments. Asterisks indicate differences from a no-treatment baseline control (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
IFN-α significantly enhances CP NK cytotoxicity.
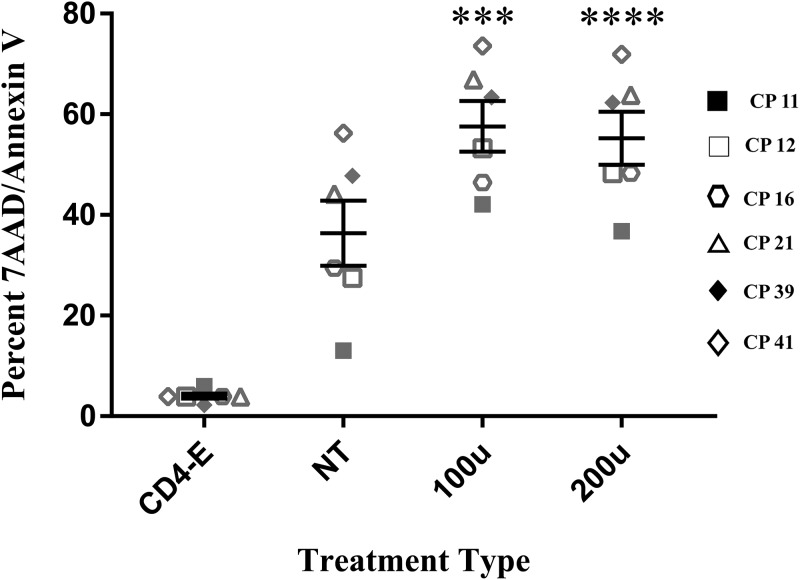
In assessing whether IFN-α treatment actually enhanced CP NK cell cytotoxic responses against target cells, we labeled K562 cells with CFSE and used that as a means of detecting cell death in the target cell population within an effector-target coculture. As described in Materials and Methods, death was assessed via flow cytometry as CFSE+ cells that were double positive for 7-aminoactinomycin D (7AAD) and annexin V (Fig. S2). Of the six CP studied at the 5:1 effector/target ratio, brief IFN-α treatments significantly enhanced CP NK cell cytotoxicity with an approximate net difference in death of 20% in IFN-α-treated NK cells relative to untreated baseline NK cells (Fig. 3, P = 0.0004 for 100 U and 0.0001 for 200 U). To ensure that cytotoxic responses in these experiments were NK cell specific, coculture of K562 cells with CD4+ T cells (as effectors) was performed simultaneously with cocultures of K562 cells with NK cells. Death observed in these wells was subtracted from that observed in the NK/K562 cocultures.
FIG 3.
IFN-α enhances CP NK cell cytotoxicity. NK cells and CD4+ T cells were isolated from 6 CPs and treated with either medium alone (NT baseline) or various concentrations of IFN-α for 6 h, washed, and then cocultured for 4 h with CFSE-labeled target K562 cells. Cell death was assessed based on the coexpression of 7AAD and annexin V on K562 cells. Experiments were conducted at a 5:1 effector/target ratio. In each experiment, CD4+ T cell effectors (CD4-E) were used at the same effector/target ratio as a control to affirm NK-specific cytotoxicity. Asterisks indicate differences from a no-treatment baseline control (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). Duplicates were performed for each patient with error bars representing standard deviations from the mean within treatment groups. One-way repeated-measures ANOVA was used to determine the difference between baseline and specific treatments.
IFN-α enhances the suppressive capacity of HD and ES NK cells.
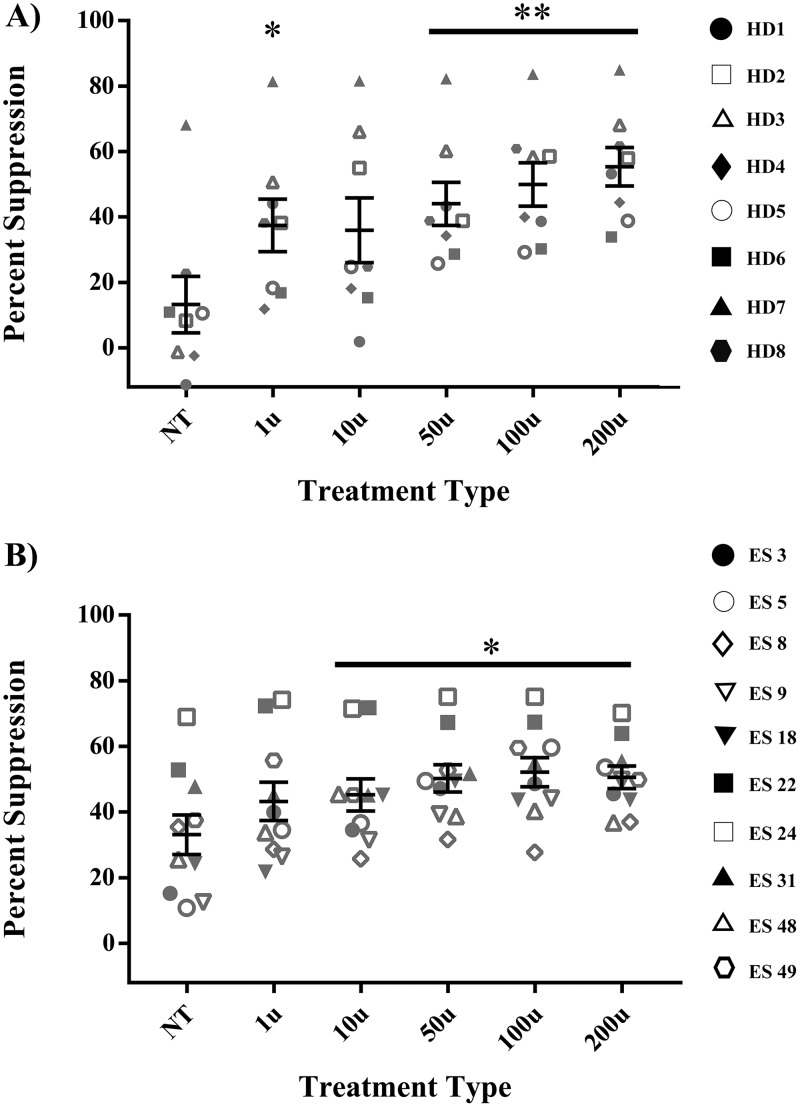
To determine whether IFN-α treatment of NK cells could impact their control of HIV-1 replication in vitro, we assessed the suppressive capacities of HD and ES NK cells with or without IFN-α treatment. CP were not used for these experiments, since the presence of residual antiretroviral drugs in CD4+ T cells from these patients prevents efficient superinfection of their cells in vitro. NK cells from 8 HD and 10 ES were treated for 6 h with either medium alone (baseline control) or various concentrations of IFN-α. The cytokine was then washed off, and the NK cells were cocultured with autologous CD4+ T cells infected with a lab strain of HIV-1 as described in Materials and Methods. Suppression, defined as a reduction in the percentage of target cells expressing GFP, was calculated following flow cytometry, with infected targets cultured without effectors being used to normalize the percent infection. As expected, not all HD NK cells were able to suppress viremia at baseline. However, some concentrations of IFN-α, particularly 50 U and higher, significantly improved NK cell suppression capacity in these subjects (Fig. 4A, P < 0.04). A higher baseline level of suppression was seen with ES NK cells, and this was further enhanced after treatment with 10 U or higher of IFN-α (Fig. 4B, P < 0.04).
FIG 4.
IFN-α enhances the suppressive capacity of HD and ES NK cells. NK cells from 8 HD (A) and 10 ES (B) were treated with either medium alone (NT) or various concentrations of IFN-α for 6 h, washed, and then cocultured over 3 days at a 1:1 ratio with autologous CD4+ T cells infected with a GFP-tagged pseudotyped lab strain of HIV-1. Viral replication suppression was measured as a percentage of GFP expression via flow cytometry. Triplicates were performed for each patient, with error bars representing the standard deviation, from means within treatment groups. One-way repeated-measures ANOVA was used to determine the difference between baseline and specific treatment groups. Asterisks indicate the differences from a no-treatment baseline control (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
IFN-α treatment enhances degranulation and the cytokine-producing capacity of CP CD8+ T cells.
Having shown that IFN-α significantly enhanced NK cell polyfunctionality and HIV-1 suppressive capacity, we next sought to determine whether this cytokine had inhibitory effects on CD8+ T cell responses, as has been previously shown in untreated HIV-1 infection. To answer this question, assays measuring CD8+ T cell cytokine production and degranulation after treatment with anti-CD3 monoclonal antibody (MAb) were employed. Figure 5 summarizes results for CD8+ T cells. Specifically, IFN-α treatment increased degranulation in CD8+ T cells following stimulation, as measured by CD107a (Fig. 5A, P < 0.0008). MIP-1β production was also similarly increased after IFN-α treatment relative to untreated, stimulated, CD8+ T cells (Fig. 5B, P < 0.04). In addition, CD8+ T cells from CP treated with IFN-α also showed significant increases in IFN-γ production (Fig. 5C, P < 0.02). Stimulation of CD8+ T cells with IFN-α alone resulted in minimal MIP-1β and IFN-γ production (data not shown).
FIG 5.
IFN-α treatment enhances CP CD8+ T cell degranulation and cytokine production. CD8+ T cells from 6 to 8 CP were pulsed with either medium alone (baseline) or various concentrations of IFN-α for 6 h, washed, and then cultured for 4 h in the presence of a CD107a antibody and protein transport inhibitors. Enhancement of degranulation and cytokine production was subsequently studied via flow cytometry following surface and intracellular staining. Specifically, the cells were either not treated but stimulated (NT) or treated with various units of IFN-α and stimulated with anti-CD3 MAb. (A) CD107a degranulation; (B) MIP-1β; (C) IFN-γ. Triplicates were performed for each patient, with error bars representing the standard deviations from the mean within treatment groups. One-way repeated-measures ANOVA was used to determine the differences between baseline and specific treatments. Asterisks indicate the differences from a no-treatment baseline control (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
IFN-α treatment does not inhibit CP HIV-specific CD8+ T cell IFN-γ responses.
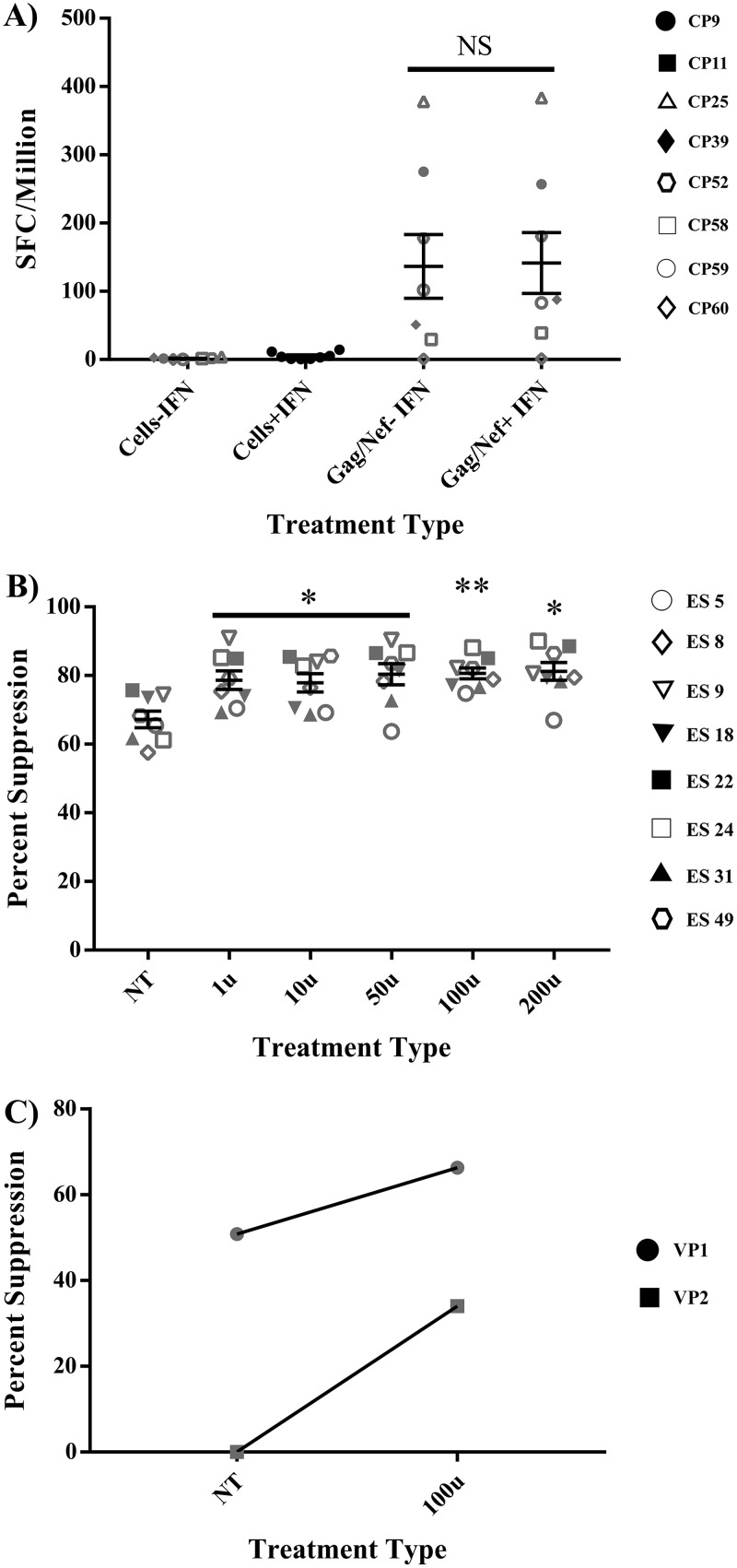
Having demonstrated that IFN-α treatment enhanced global CP CD8+ T cell responses, we next analyzed its effect on CP HIV-specific CD8+ T cells responses. To do this, we performed enzyme-linked immunospot (ELISpot) studies using peripheral blood mononuclear cells (PBMCs) from CP pulsed with a 100-U dose of IFN-α for 6 h prior to stimulation with overlapping Gag and Nef peptides. As shown in Fig. 6A, there was minimal nonspecific production of IFN-γ with IFN-α treatment. Furthermore, pretreatment of PBMCs with IFN-α had no effect on the number of IFN-γ-producing cells following stimulation with Gag and Nef peptides.
FIG 6.
IFN-α enhances the suppressive capacity of VP and ES CD8+ T cells without inhibiting HIV-specific IFN-γ responses of CP CD8+ T cells. (A) PBMCs from 8 CP were pulsed with either medium alone (baseline) or 100 U/ml of IFN-α for 6 h, washed, and then cultured for 18 h with or without CMV, Gag, or Nef peptides. An IFN-γ ELISpot assay was then used to assess the effect of IFN-α treatment on the production of IFN-γ by CP CD8+ T cells. Duplicates were performed for each patient, with error bars representing the standard deviations from the mean within treatment groups; saturated responses (CMV) were not included in data. One-way repeated-measures ANOVA was used to determine the difference between baseline and 100-U treatments. (B) CD8+ T cells from 8 ES were pulsed with either medium alone (NT) or various concentrations of IFN-α for 6 h, washed, and then cocultured over 3 days at a 1:1 ratio with autologous CD4+ T cells infected with a GFP-tagged pseudotyped lab strain of HIV-1. Viral replication suppression was measured as a percentage of GFP expression via flow cytometry. Triplicates were performed for each patient, with error bars representing the standard deviations from the mean within treatment groups. One-way repeated-measures ANOVA was used to determine the difference between baseline and specific treatments. Asterisks indicate the differences from a no-treatment baseline control *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (C) CD8+ T cells from 2 VP were pulsed with either medium alone (NT) or 100 U/ml of IFN-α for 6 h, washed, and then cocultured over 3 days at a 1:1 ratio with autologous CD4+ T cells infected with a GFP-tagged pseudotyped lab strain of HIV-1. Viral replication suppression was measured as a percentage of GFP expression via flow cytometry. Triplicates were performed for each patient.
IFN-α enhances the suppressive capacity of ES CD8+ T cells.
We next analyzed the effect of IFN-α on the suppressive capacity of ES CD8+ T cells. Similar to the suppression experiments performed with ES NK cells, ES CD8+ T cells were isolated from eight ES and pulsed with either medium alone (baseline control) or various concentrations of IFN-α for 6 h. The cells were then washed and cocultured at a 1:1 effector/target ratio with autologous CD4+ T cells infected with a green fluorescent protein (GFP)-tagged pseudotyped lab strain of HIV-1. The culture was maintained over 3 days, and suppression, defined as a reduction in the percentage of target cells expressing GFP, was measured by flow cytometry. Similar to the suppression observed with NK cells, each IFN-α treatment significantly improved the suppressive capacities of CD8+ T cells (Fig. 6B, P < 0.03). In ES22 we demonstrated that suppression was contact dependent since separating the CD8+ T cells from the target cells with a transwell abrogated the inhibitory response (Fig. S3). In summary, IFN-α did not inhibit, but rather enhanced suppressive capacities of ES CD8+ T cells.
IFN-α enhances the suppressive capacity of VP CD8+ T cells.
Because we were unable to determine the effect of IFN-α on the suppressive capacity of CP CD8+ T cells due to the presence of residual ART, we looked at the effect on two viremic subjects. In both cases, we saw enhanced suppressive capacity when CD8+ T cells were preincubated for 6 h with IFN-α (Fig. 6C).
DISCUSSION
Several clinical trials with LRAs have not resulted in reductions in the size of the latent reservoir (6–12). One possible explanation for this might be that effector immune cells critical to the “killing” aspect of the strategy are inefficient. Several studies have also shown that some LRAs inhibit NK cell (35, 36) and CD8+ T cell (37–39) function. To address these concerns, efforts are now being directed at enhancing immune effector function for the control of HIV (13, 14). While many of these are directed toward CD8+ T cells, NK cells would be important effector cells to consider for the purpose of complementing CD8+ T cell function in HIV control (40, 41). In the present study, we confirm prior studies that showed that pretreatment of NK cells with IFN-α enhances cytokine secretion, viral suppression, and the cytotoxic potential of HD (30–33) and ES NK (34) cells against target K562 cells. While Portales et al. showed an enhancement in NK cell perforin and granzyme A expression in patients who were treated with pegylated IFN-α2b (42), this is to our knowledge the first demonstration of IFN-α directly enhancing cytokine secretion and the killing capacity of CP NK cells. IFN-α pulses also enhanced the ability of HD and ES NK cells to suppress HIV-1 replication in autologous CD4+ T cells. While we were not able to study this particular function in CP NK cells, the fact that CP and HD NK cells had similar levels of degranulation and cytokine production in response to IFN-α following stimulation with K562 cells strongly suggests that CP NK cells would also have enhanced antiviral activity. Furthermore, in addition to studies showing improvement in direct NK cell cytotoxic activity, Tomescu et al. showed that IFN-α enhances NK cell-mediated antibody-dependent cell-mediated cytotoxicity (43). Thus, it appears that this cytokine enhances multiple facets of NK cell function.
Betts et al. demonstrated that the polyfunctionality of CD8+ T cells was a major correlate of HIV-specific immunity (44). These results have since been corroborated not only in CD8+ T cell studies (45) but also in NK cell studies. Specifically, in two separate reports, Boulet et al. (46) and Kamya et al. (47) demonstrate that HIV+ patients with the HLA-Bw4 ligand and its cognate NK receptor, KIR3DL1, had more polyfunctional NK cells, which accordingly contributed to slower disease progression. We show here for the first time that IFN-α enhances polyfunctionality in CP NK cells to a level that is similar to that seen in HD NK cells. This enhanced NK cell polyfunctionality may lead to better control of HIV-1 replication in shock-and-kill strategies.
We minimized our IFN-α preincubation exposure for two reasons. The first was to determine the feasibility of efficiently enhancing NK cell effector function within the narrow time frame that effector cells have for the elimination of infected CD4+ T cells prior to the release of virions after latency reversal (48). We show here that 6 h of preincubation with IFN-α was as effective as 24 h. Our finding that this short window of exposure was sufficient to efficiently and significantly enhance the suppressive, cytotoxic, and polyfunctional responses of NK cells in CP is exciting and suggests that it may be possible to optimize NK cell activity while avoiding IFN-α-mediated immune exhaustion in both NK cells and CD8+ T cells (25, 26).
Several studies have suggested that type I IFNs can lead to apoptosis of memory T cells in mice, thereby compromising the adaptive immune response (49–51). Other studies have shown that IFN-α induces immune activation of CD8+ T cells from HIV-positive subjects in vitro (52, 53) and in vivo (54). Furthermore, studies in humanized mice have shown that blocking the IFN-α/β receptor enhances HIV-specific immune responses (55, 56). In contrast to these studies, we show here for the first time that pretreatment with IFN-α for a limited period of time did not inhibit HIV-specific CD8+ T cell IFN-γ responses and resulted in a moderate enhancement of global CD8+ T cell responses in CP. Furthermore, there was also a modest enhancement in HIV-suppressive CD8+ T cell responses in ES and in two VP. These responses are not due to nonspecific activation of CD8+ T cells by IFN-α; instead, we show here that the suppression was contact dependent, which is consistent with our prior studies (57–59). However, further studies will be needed to definitively determine whether this is due to direct cytotoxic activity, as has been previously demonstrated for IFN-α-treated NK cells (33). If this enhanced HIV-specific CD8+ T cell response is confirmed with in vivo studies, it would suggest that pulse therapy with IFN-α could enhance both innate and adaptive antiviral immune responses in CP. A clinical trial has already shown that treatment with IFN-α prolongs the time to viral rebound following the cessation of ART (60). In another study, NK cell activation was associated with a decline in cell-associated HIV-1 DNA in HIV-1/HCV-coinfected subjects treated with pegylated IFN-α and ribavirin (61). While a recent study in SIV-infected monkeys on ART suggested that pegylated IFN-α2a treatment did not significantly affect the size of the viral reservoir (62), this could potentially be because there was no LRA given in conjunction with this immunomodulatory agent. An exciting new direction, given these data, would be the coupling of short course IFN-α therapy with LRAs in shock-and-kill trials with the goal of eliminating reactivated reservoirs without exacerbating preexisting immune exhaustion profiles of CP effector cells.
MATERIALS AND METHODS
Subjects.
Blood samples from HIV-negative and HIV-positive donors were obtained with written informed consent and subsequently handled in accordance with protocols approved by the Johns Hopkins University IRB. An elite suppressor (ES) refers to a patient who has maintained undetectable viral loads in the absence of ART (63), while a chronic progressor (CP) refers to a patient who has maintained undetectable viral loads for more than a year on ART. Viremic progressors (VP) were patients who were not on ART and were viremic. VP1 had a CD4 count of 192 cells/μl and a viral load of 7,250 copies/ml and VP2 had a CD4 count of 296 cells/μl and a viral load of 17,100 copies/ml. Healthy donors (HD) refer to HIV-negative subjects.
NK cell cytokine secretion and degranulation assay.
PBMCs were collected from donor whole blood after Ficoll-Paque Plus gradient centrifugation (GE Healthcare Life Sciences, Baltimore, MD). Next, NK cells were isolated from HD and CP PBMCs with NK cell-specific Miltenyi beads (Miltenyi Biotec, Gaithersburg, MD). The NK cell purity was generally in the range of 90%. Sample purity levels can be found in Fig. S4. Purified NK cells were treated for 6 h (termed a pulse) with either medium (RPMI supplemented with 10% fetal bovine serum [FBS], 1% Pen-Strep, and 10 U/ml IL-2) or various concentrations of IFN-α (2a) diluted in medium (1 U/ml to 200 U/ml; PBL Assay Science, Piscataway, NJ). At the end of IFN-α pulses, cells were washed three times and cocultured for 4 h at a 1:1 effector/target ratio with K562 cells to assess the relative effect of IFN-α on NK degranulation and cytokine production. NK cells treated with IFN-α were also washed and cultured alone to determine any nonspecific effects of IFN-α on NK cell function in the absence of target cell stimulation. Untreated NK cells were also cultured alone as a negative control. All cultures were performed in medium supplemented with protein transport inhibitors (Golgi Plug, 1 μg/ml; Golgi Stop, 0.7 μg/ml; CD49d, 1 μg/ml [BD Bioscience, San Diego, CA]), as well as an antibody to CD107a (FITC; BD Bioscience, clone H4A3). K562 cells were used because their lack of MHC-I surface expression makes them targets for NK cells. At the end of the 4 h, the cells were washed three times and stained for surface markers: CD3 (PB; BD Bioscience, clone UCHT1), CD16 (PerCPcy5.5; BD Bioscience, clone 3G8), and CD56 (APCH7 [BioLegend, San Diego, CA], clone HCD56). Cells were also probed for the production of cytokines via intracellular staining with a focus on IFN-γ (PECy7; BioLegend, clone B27) and MIP-1β (PE; BD Bioscience, clone D21-1351).
NK cell cytotoxicity assay.
The robust production of cytokines by an effector cell may not always correlate with cytotoxicity. Therefore, to assess NK cytotoxicity, we used the following assay adapted from Derby et al. (65). Briefly, effector NK cells from CP PBMCs were isolated as previously described. CD4+ T cells were also isolated from a portion of patients PBMCs. Next, NK cells were pulsed as described above with media for a baseline control or media with 100 or 200 U of IFN-α. CD4+ T cells, however, were pulsed with medium only. To distinguish between effector and target cells, K562 cells were labeled with 2.5 μM of CFSE (carboxyfluorescein succinimidyl ester) at 106 cells/ml of CFSE with gentle vortexing. CFSE was then quenched with lukewarm FBS. The K562 cells were then washed three times and cocultured for 4 h with pretreated, washed, NK cells at a 5:1 effector/target ratio (targets at 100,000 cells per well in 96-well flat bottom plates). Killing was determined by flow cytometry as CFSE-positive cells that coexpressed 7AAD and annexin V. To affirm that any cytotoxicity observed was NK cell specific, CFSE-labeled K562s were also cultured with CD4+ T cells at the same effector/target ratios. Any background death observed in CD4/K562 wells was subtracted from NK:K562 cultures at each effector/target ratio.
NK cell and CD8+ T cell suppression assay.
PBMCs were isolated from HD and ES blood as described above. In ES these PBMCs were divided into two sets: from one set CD8+ T cells were isolated (by positive selection) and from the other NK cells were isolated by negative selection. CD4+ T cells were then isolated, by negative selection, from the flowthrough of the CD8+ T cell isolation. In HD, PBMCs were split into two sets with one set used for NK cell isolation and the other for CD4+ T cell isolation. All cells were isolated with cell type-specific Miltenyi beads. As with NK cells, CD8+ T cell purity levels were generally in the 90% range (Fig. S4). Effector cells (CD8+ T cells or NK cells) were then pulsed with either medium or various concentrations of IFN-α as described above. Next, target cells (bulk CD4+ T cells) were infected by spinoculation at 30 ng of HIV-1 p24/100,000 cells with a pseudotyped virus (HIV-1-NL4-3ΔEnv–GFP) for 2 h at 1,200 × g and 37°C. HIV-1-NL4-3ΔEnv–GFP is a lab strain of HIV-1 that has env replaced with gfp. At the end of the cytokine pulse, effector cells were washed three times and cocultured over 72 h with infected CD4+ T cells at an effector/target ratio of 1:1. The percent GFP expression, as measured by flow cytometry, was subsequently used to assess the percent viral suppression according to the following formula: [1 – (%GFP + CD4+ T cells cultured with effectors)/(%GFP + CD4+ T cells without effectors)] × 100.
The median percentage of GFP infection in CD4+ T cells that were cultured in medium alone was 6% for HD and 13% for ES. In VP, an average of 6% GFP in infected CD4+ T cells was seen in the two subjects studied. For transwell experiments, 1 million infected CD4+ T cells were cultured at the bottom of 12-well transwell plates, while 1 million CD8+ T cell effectors were cultured in the transwell inserts. Infected CD4+ T cells were cultured alone to determine the maximum percent infection, and infected cells were cocultured in the same well with CD8+ T cells to determine the effect of direct contact of the effector and target cells. Cultures were maintained for 3 days, and viral replication suppression was measured as a percentage of GFP expression via flow cytometry.
CD8+ T cell cytokine secretion and degranulation assay.
To ensure that the concentrations of IFN-α used in this assay to enhance NK cell function did not impair the cytokine-producing abilities of patient CD8+ T cells, general CD8+ T cell cytokine production was also assessed in CP. Specifically, CD8+ T cells were isolated from CP PBMCs (Miltenyi Biotec) and pulsed with either medium or various concentrations of IFN-α diluted in medium as before. At the end of this pulse, cells were washed three times and stimulated for 4 h with either medium alone (baseline control) or anti-CD3 MAb at 1 μg/ml (BD Bioscience), all in the presence of protein transport inhibitors, as well as an antibody to CD107a. The cells were then washed and stained for surface markers CD3 (PB; BD Bioscience, clone UCHT1) and CD8 (APCH7; BD Biosciences, clone SK1), as well as cytokine production via intracellular staining for IFN-γ (PECy7; BioLegend, clone B27) and MIP-1β (PE; BD Bioscience, clone D21-1351).
CD8+ T cells IFN-γ ELISpot assays.
Beyond general CD8+ T cell function, we also sought to determine whether IFN-α treatment of CP CD8+ T cells inhibited their ability to release the type II interferon (IFN-γ) in an HIV-specific manner. We performed IFN-γ-specific ELISpot assays as previously described (64), following pretreatment of patient cells with the candidate type I IFN (IFN-α2a). Specifically, PBMCs were isolated from CP whole blood, seeded at 250,000 cells per well, and treated for 6 h with either medium as a baseline control or 100 U of IFN-α. Cells were then washed three times, transferred into 96-well ELISpot plates precoated with IFN-γ-specific antibodies (Mabtech, Human IFN-γ ELISpot Plus), and stimulated for 18 h with either medium alone or 10 μg/ml of overlapping consensus B Gag and Nef peptides (NIH AIDS Reagent Program). The plate was then processed according to the manufacturer’s protocol and read, blinded, by an independent investigator using an automated reading system.
Statistics.
Statistics were generated by GraphPad Prism 7. For each set of experiments, the one-way repeated-measures analysis of variance (ANOVA) was used to assess statistical significance, and levels of significance were parsed out with the ensuing P value demarcations as follows: ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001. To account for any violations in sphericity, the Geisser-Greenhouse correction was used during data analysis. Finally, in all cases, a Dunnett’s multiple-comparison test was used to assess variations of treatment groups from no-treatment baseline controls. Standard deviations were used to determine the variations from the mean within treatment groups.
Supplementary Material
ACKNOWLEDGMENTS
We thank Eileen Scully, Rebecca Veenhuis, Chris Thoburn, and Caroline Garliss for helpful discussions.
This study was funded by the Johns Hopkins University Center for AIDS Research (P30AI094189), The BEAT-HIV Delaney Collaboratory (UM1AI126620), and NIAID grants 2R56AI080328-05A1 and 1R01AI120024-01 (J.N.B.). A.K.R.K. was funded by T32GM008752. C.A.G.T. was funded by a Careers in Science and Medicine Summer Internship Program and the Health Careers Opportunity Program (DHHS D18HP29037-01-00).
The authors have no competing interests to disclose.
A.K.R.K. and J.N.B. conceptualized the study. A.K.R.K. and C.A.G.T. performed investigations. A.K.R.K. and J.N.B. were responsible for data curation, formal analysis, and interpretation.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JVI.01541-18.
REFERENCES
- 1.Dahabieh M, Battivelli E, Verdin E. 2015. Understanding HIV latency: the road to an HIV cure. Annu Rev Med 66:407–421. doi: 10.1146/annurev-med-092112-152941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siliciano RF, Greene WC. 2011. HIV latency. Cold Spring Harbor Perspect Med 1:a007096. doi: 10.1101/cshperspect.a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cary DC, Peterlin BM. 2016. Targeting the latent reservoir to achieve functional HIV cure. F1000Res 5:1009. doi: 10.12688/f1000research.8109.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsden MD, Zack JA. 2015. Experimental approaches for eliminating latent HIV. Forum Immun Dis Ther 6:91–99. doi: 10.1615/ForumImmunDisTher.2016015242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin AR, Siliciano RF. 2016. Progress toward HIV eradication: case reports, current efforts, and the challenges associated with cure. Annu Rev Med 67:215–228. doi: 10.1146/annurev-med-011514-023043. [DOI] [PubMed] [Google Scholar]
- 6.Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Østergaard L, Tolstrup M. 2015. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. 2012. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487:482. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang K-H, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, Richman DD, Robertson KR, Kashuba AD, Bosch RJ, Hazuda DJ, Kuruc JD, Eron JJ, Margolis DM. 2014. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis 210:728–735. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN, Emad F, Buckheit R, McCance-Katz EF, Lai J, Kennedy M, Chander G, Siliciano RF, Siliciano JD, Deeks SG. 2014. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis 58:883–890. doi: 10.1093/cid/cit813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 11.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G, Roney J, Watson J, Prince MH, Hoy JF, Chomont N, Fromentin R, Procopio FA, Zeidan J, Palmer S, Odevall L, Johnstone RW, Martin BP, Sinclair E, Deeks SG, Hazuda DJ, Cameron PU, Sékaly R-P, Lewin SR. 2014. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N, Savic R, Roney J, Hoh R, Solomon A, Piatak M, Gorelick RJ, Lifson J, Bacchetti P, Deeks SG, Lewin SR. 2015. Short-term disulfiram to reverse latent HIV infection: a phase 2 dose escalation study. Lancet HIV 2:e520–e529. doi: 10.1016/S2352-3018(15)00226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perreau M, Banga R, Pantaleo G. 2017. Targeted immune interventions for an HIV-1 cure. Trends Mol Med 23:945–961. doi: 10.1016/j.molmed.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Riley JL, Montaner LJ. 2017. Cell-mediated immunity to target the persistent human immunodeficiency virus reservoir. J Infect Dis 215:S160–S171. doi: 10.1093/infdis/jix002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 16.Folkvord JM, Armon C, Connick E. 2005. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Human Retrovir 21:363–370. doi: 10.1089/aid.2005.21.363. [DOI] [PubMed] [Google Scholar]
- 17.Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, McCarter MD, MaWhinney S, Hage A, White C, Skinner PJ. 2007. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol 178:6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- 18.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Picker LJ. 2015. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 21:132. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huot N, Jacquelin B, Garcia-Tellez T, Rascle P, Ploquin MJ, Madec Y, Reeves RK, Derreudre-Bosquet N, Müller-Trutwin M. 2017. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med 23:1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671. doi: 10.1016/S1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 21.Zwirner NW, Domaica CI. 2010. Cytokine regulation of natural killer cell effector functions. BioFactors 36:274–288. doi: 10.1002/biof.107. [DOI] [PubMed] [Google Scholar]
- 22.Seay K, Church C, Zheng JH, Deneroff K, Ochsenbauer C, Kappes JC, Liu B, Jeng EK, Wong HC, Goldstein H. 2015. In vivo activation of human NK cells by treatment with an interleukin-15 superagonist potently inhibits acute in vivo HIV-1 infection in humanized mice. J Virol 89:6264–6274. doi: 10.1128/JVI.00563-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carolina G, Maria A-F, Marina T, Justin JP, Guido F, Soriano-Sarabia N, Margolis D. 2018. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J Virol 92:e00235-18. doi: 10.1128/JVI.00235-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maughan A, Ogbuagu O. 2018. Pegylated interferon alpha 2a for the treatment of hepatitis C virus infection. Expert Opin Drug Metab Toxicol 14:219–227. doi: 10.1080/17425255.2018.1421173. [DOI] [PubMed] [Google Scholar]
- 25.Odorizzi PM, Wherry EJ. 2013. An interferon paradox. Science 340:155–156. doi: 10.1126/science.1237568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telenti A. 2014. The mixed blessing of interferon. Nature 511:537. doi: 10.1038/nature13517. [DOI] [PubMed] [Google Scholar]
- 27.Sandler NG, Bosinger SE, Estes JD, Zhu RTR, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, Hill BJ, Timmer JK, Reiss E, Yarden G, Darko S, Contijoch E, Todd JP, Silvestri G, Nason M, Norgren RB Jr, Keele BF, Rao S, Langer JA, Lifson JD, Schreiber G, Douek DC. 2014. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy GAD, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W, Mudd J, Schacker T, Funderburg NT, Pilch-Cooper HA, Debernardo R, Rabin RL, Lederman MM, Harding CV. 2013. Interferon-α is the primary plasma type I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS One 8:e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedaghat AR, German J, Teslovich TM, Cofrancesco J, Jie CC, Talbot CC, Siliciano RF. 2008. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol 82:1870–1883. doi: 10.1128/JVI.02228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis T, McKenzie R, Simms P, Helfrich B, Fisher RH, Fisher RI. 1989. Induction of human lymphokine-activated killer cells by IFN-α and IFN-γ. J Immunol 143:4282–4286. [PubMed] [Google Scholar]
- 31.Jewett A, Bonavida B. 1995. Interferon-alpha activates cytotoxic function but inhibits interleukin-2-mediated proliferation and tumor necrosis factor-alpha secretion by immature human natural killer cells. J Clin Immunol 15:35–44. doi: 10.1007/BF01489488. [DOI] [PubMed] [Google Scholar]
- 32.Tomescu C, Chehimi J, Maino VC, Montaner LJ. 2007. NK cell lysis of HIV-1-infected autologous CD4 primary T cells: requirement for IFN-mediated NK activation by plasmacytoid dendritic cells. J Immunol 179:2097–2104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- 33.Tomescu C, Mavilio D, Montaner LJ. 2015. Lysis of HIV-1 infected autologous CD4+ primary T cells by interferon-alpha activated NK cells requires NKp46 and NKG2D. AIDS 29:1767–1773. doi: 10.1097/QAD.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomescu C, Duh F-M, Hoh R, Viviani A, Harvill K, Martin MP. 2012. Impact of protective KIR/HLA genotypes on NK cell and T cell function in HIV-1-infected controllers. AIDS 26:1869–1878. doi: 10.1097/QAD.0b013e32835861b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garrido C, Spivak AM, Soriano-Sarabia N, Checkley MA, Barker E, Karn J, Planelles V, Margolis DM. 2016. HIV latency-reversing agents have diverse effects on natural killer cell function. Front Immunol 7:356. doi: 10.3389/fimmu.2016.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pace M, Williams J, Kurioka A, Gerry AB, Jakobsen B, Klenerman P, Nwokolo N, Fox J, Fidler S, Frater J, Investigators C. 2016. Histone deacetylase inhibitors enhance CD4 T cell susceptibility to NK cell killing but reduce NK cell function. PLoS Pathog 12:e1005782. doi: 10.1371/journal.ppat.1005782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones RB, O’Connor R, Mueller S, Foley M, Szeto GL, Karel D, Lichterfeld M, Kovacs C, Ostrowski MA, Trocha A, Irvine DJ, Walker BD. 2014. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog 10:e1004287. doi: 10.1371/journal.ppat.1004287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker-Sperling VE, Pohlmeyer CW, Tarwater PM, Blankson JN. 2016. The effect of latency reversal agents on primary CD8+ T cells: implications for shock and kill strategies for human immunodeficiency virus eradication. EBioMedicine 8:217–229. doi: 10.1016/j.ebiom.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwaa AK, Goldsborough K, Walker-Sperling VE, Pianowski LF, Gama L, Blankson JN. 2017. The effect of Ingenol-B on the suppressive capacity of elite suppressor HIV-specific CD8+ T cells. PLoS One 12:e0174516. doi: 10.1371/journal.pone.0174516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scully E, Alter G. 2016. NK cells in HIV disease. Curr HIV/AIDS Rep 13:85–94. doi: 10.1007/s11904-016-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrington M, Alter G. 2012. Innate immune control of HIV. Cold Spring Harbor Perspect Med 2:a007070. doi: 10.1101/cshperspect.a007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portales P, Reynes J, Pinet V, Rouzier-Panis R, Baillat V, Clot J, Corbeau P. 2003. Interferon-alpha restores HIV-induced alteration of natural killer cell perforin expression in vivo. AIDS 17:495–504. doi: 10.1097/01.aids.0000050816.06065.b1. [DOI] [PubMed] [Google Scholar]
- 43.Tomescu C, Tebas P, Montaner LJ. 2017. IFN-α augments natural killer-mediated antibody-dependent cellular cytotoxicity of HIV-1-infected autologous CD4+ T cells regardless of major histocompatibility complex class 1 downregulation. AIDS 31:613–622. doi: 10.1097/QAD.0000000000001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betts MR, Nason MC, West SM, De R SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Migueles SA, Connors M. 2015. Success and failure of the cellular immune response against HIV-1. Nat Immunol 16:563. doi: 10.1038/ni.3161. [DOI] [PubMed] [Google Scholar]
- 46.Boulet S, Song R, Kamya P, Bruneau J, Shoukry NH, Tsoukas CM, Bernard NF. 2010. HIV protective KIR3DL1 and HLA-B genotypes influence NK cell function following stimulation with HLA-devoid cells. J Immunol 184:2057–2064. doi: 10.4049/jimmunol.0902621. [DOI] [PubMed] [Google Scholar]
- 47.Kamya P, Boulet S, Tsoukas CM, Routy J-P, Thomas R, Cote P, Boulassel M-R, Baril J-G, Kovacs C, Migueles SA, Connors M, Suscovich TJ, Brander C, Tremblay CL, Bernard N. 2011. Receptor-ligand requirements for increased NK cell polyfunctional potential in slow progressors infected with HIV-1 coexpressing KIR3DL1*h/*y and HLA-B*57. J Virol 85:5949–5960. doi: 10.1128/JVI.02652-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker-Sperling VEK, Cohen VJ, Tarwater PM, Blankson JN. 2015. Reactivation kinetics of HIV-1 and susceptibility of reactivated latently infected CD4+ T cells to HIV-1-specific CD8+ T cells. J Virol 89:9631–9638. doi: 10.1128/JVI.01454-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welsh RM, Bahl K, Marshall HD, Urban SL. 2012. Type 1 interferons and antiviral CD8+ T-cell responses. PLoS Pathog 8:e1002352. doi: 10.1371/journal.ppat.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNally JM, Zarozinski CC, Lin M-Y, Brehm MA, Chen HD, Welsh RM. 2001. Attrition of bystander CD8+ T cells during virus-induced T-cell and interferon responses. J Virol 75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RMJ. 2006. IFN-induced attrition of CD8+ T cells in the presence of absence of cognate antigen during the early stages of viral infections. J Immunol 176:4284–4295. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez B, Lederman M, Jiang W, Bazdar D, Gàrate K, Harding C, Sieg S. 2006. Interferon-α differentially rescues CD4 and CD8 T cells from apoptosis in HIV infection. AIDS 20:1379–1389. doi: 10.1097/01.aids.0000233571.51899.ab. [DOI] [PubMed] [Google Scholar]
- 53.Hua S, Lécuroux C, Sáez-Cirión A, Pancino G, Girault I, Versmisse P, Boufassa F, Taulera O, Sinet M, Lambotte O, Venet A. 2014. Potential role for HIV-specific CD38-/HLA-DR+ CD8+ T cells in viral suppression and cytotoxicity in HIV controllers. PLoS One 9:e101920. doi: 10.1371/journal.pone.0101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manion M, Rodriguez B, Medvik K, Hardy G, Harding CV, Schooley RT, Pollard R, Asmuth D, Murphy R, Barker E, Brady KE, Landay A, Funderburg N, Sieg SF, Lederman MM. 2012. Interferon-alpha administration enhances CD8+ T cell activation in HIV infection. PLoS One 7:e30306. doi: 10.1371/journal.pone.0030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng L, Yu H, Li G, Li F, Ma J, Li J, Chi L, Zhang L, Su L. 2017. Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction during chronic HIV-1 infection. JCI Insight 2:e94366. doi: 10.1172/jci.insight.94366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhen A, Rezek V, Youn C, Lam B, Chang N, Rick J, Carrillo M, Martin H, Kasparian S, Syed P, Rice N, Brooks DG, Kitchen SG. 2016. Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J Clin Invest 127:260–268. doi: 10.1172/JCI89488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. 2012. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker-Sperling VE, Buckheit RW, Blankson JN. 2014. Comparative analysis of the capacity of elite suppressor CD4+ and CD8+ T cells to inhibit HIV-1 replication in monocyte-derived macrophages. J Virol 88:9789–9798. doi: 10.1128/JVI.00860-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veenhuis RT, Kwaa AK, Garliss CC, Latanich R, Salgado M, Pohlmeyer CW, Nobles CL, Gregg J, Scully EP, Bailey JR, Bushman FD, Blankson JN. 2018. Long-term remission despite clonal expansion of replication-competent HIV-1 isolates. JCI Insight 3(18):122795. doi: 10.1172/jci.insight.122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, Tebas P, Jacobson JM, Frank I, Busch MP, Deeks SG, Carrington M, O’Doherty U, Kostman J, Montaner LJ. 2013. Pegylated interferon Alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis 207:213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hua S, Vigano S, Tse S, Zhengyu O, Harrington S, Negron J, Garcia-Broncano P, Marchetti G, Genebat M, Leal M, Resino S, Ruiz-Mateos E, Lichterfeld M, Yu XG. 2017. Pegylated IFN-, α-, induced NK cell activation is associated with HIV-1 DNA decline in ART-treated HIV-1/HCV coinfected patients. Clin Infect Dis 66:1910–1917. doi: 10.1093/cid/cix1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palesch D, Bosinger S, Mavigner M, Billingsley J, Mattingly C, Carnathan D, Paiardini M, Chahroudi A, Vanderford T, Silvestri G. 2018. Short-term pegylated interferon α2a treatment does not significantly reduce the viral reservoir of simian immunodeficiency virus-infected, antiretroviral therapy-treated rhesus macaques. J Virol 92:e00279-18. doi: 10.1128/JVI.00279-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blankson JN. 2010. Control of HIV-1 replication in elite suppressors. Discov Med 9:261–266. [PubMed] [Google Scholar]
- 64.Pohlmeyer CW, Laskey SB, Beck SE, Xu DC, Capoferri AA, Garliss CC, May ME, Livingston A, Lichmira W, Moore RD, Leffell MS, Butler NJ, Thorne JE, Flynn JA, Siliciano RF, Blankson JN. 2018. Cross-reactive microbial peptides can modulate HIV-specific CD8+ T cell responses. PLoS One 13(2):e0192098. doi: 10.1371/journal.pone.0192098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Derby E, Reddy V, Koop W, Nelson E, Baseler M, Sayers T, Malyguine A. 2001. Three-color flow cytometric assay for the study of the mechanisms of cell-mediated cytotoxicity. Immunol Lett 78:35–39. doi: 10.1016/S0165-2478(01)00226-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.