Abstract
Objectives:
Previous studies have demonstrated a diminution in the baseline and mycobacterial antigen – specific cytokines in low body mass index (LBMI) individuals with latent tuberculosis infection (LTBI). We hypothesized that LBMI might be also associated with alteration in the baseline and antigen – stimulated levels of chemokines in LTBI.
Methods:
To test this hypothesis, we examined baseline, TB-antigen and mitogen stimulated levels of chemokines in these individuals and compared them with those with LTBI and normal BMI (NBMI).
Results:
LBMI with LTBI is characterized by diminished baseline levels of CCL1, CCL4, CCL11, CXCL1, CXCL9, CXCL10 and CXCL11 in comparison to NBMI with LTBI. Similarly, LTBI with LBMI is also characterized by diminished TB-antigen stimulated levels of CCL1, CCL2, CCL3, CCL4, CCL11, CXCL1, CXCL2, CXCL9, CXCL10 and CXCL11. In contrast, there were no significant differences in the mitogen stimulated chemokine levels between the groups. Finally, there was a significant positive correlation between BMI and CCL1, CCL4, CCL11, CXCL11, CXCL2, CXCL9 and CXCL11 levels in LTBI individuals.
Conclusions
Therefore, our data reveal that LTBI subjects with low BMI are characterized by diminished levels of a variety of important chemokines, providing a novel biological mechanism for the increased risk of developing active TB.
Keywords: Chemokines, Malnutrition, BMI, Latent tuberculosis infection
Introduction
Malnutrition is a serious public health problem worldwide and it is estimated to affect more than 925 million people in the world.1,2 The World Health Organization defines malnutrition according to body mass index (BMI): mild malnutrition (17.00 ≤ BMI ≤ 18.49 kg/m2), moderate malnutrition (16.00 ≤ BMI ≤ 16.99 kg/m2), and severe malnutrition (BMI < 16.00 kg/m2). Malnu-trition is a clinical syndrome that encompasses a spectrum of an-thropometric defects from wasting (low weight for height) and stunting (low height for age) in undernutrition to other disorders of nutrition including high BMI such as overweight and obesity.3 The association between malnutrition and tuberculosis (TB) has been recognized for centuries.4 Malnutrition is an important risk factor for TB disease and is also associated with elevated risk of mortality and relapse.4–6 In addition, malnutrition is known to affect the response to anti-TB treatment with reports of increased mortality and acute respiratory failure.4–6 Prospective studies have clearly shown that low BMI is associated with higher incidence of active TB with a log-linear inverse relationship between TB incidence and BMI within the range of 18.5–30 kg/m2.7
Multiple functions of chemokines including mediating inflammation, regulation of immune responses and promotion of cell growth, angiogenesis and apoptosis have been described in both homeostatic and pathological conditions. 8 In addition, recent data has revealed an important role for chemokines in the priming of naïve T cells, effector and memory T cell differentiation and regulatory T cell function. 8 The role of chemokines in the formation and maintenance of quiescent granulomas in latent TB infection is well established. 9 Chemokines are known to govern the establishment of the chemical gradient that drives the recruitment of cells from the periphery to the site of infection and for positioning within the granuloma. 10 Moreover, chemokines levels in the circulation are known to influence the ability of the host immune response to maintain protective immunity in LTBI. 11 However, the role of these chemokines in human immune responses to TB in malnourished individuals has never been examined.
We hypothesized that malnutrition would predominantly diminish the levels of protective chemokine responses in LTBI and thereby predispose to an increased risk of developing active TB. To study the influence of malnutrition on LTBI, we examined baseline, TB antigen and mitogen stimulated levels of a large panel of CC and CXC family of chemokines in individuals with LTBI and coexistent low BMI (LBMI) and compared them to those with LTBI but with normal BMI (NBMI).
Materials and methods
Study population
We studied a group of 70 individuals with latent TB infection – 35 with low BMI (hereafter LBMI) and 35 with normal BMI (here-after NMBI) (Table 1). This study was conducted in South India and the individuals were screened as part of a community screening protocol in a rural village in the outskirts of Chennai. All enrolled individuals were adults between 18 and 65 years old. The subjects enrolled had low or normal BMI, and they were negative for diabetes, HIV and parasitic infection. LTBI was diagnosed based on positive results of Tuberculin skin test (TST) and Quantiferon (QFT) TB Gold in tube, with no symptoms or signs of active TB, no history of previous TB and normal chest radiographs. TST was performed using 2 tuberculin units of Tuberculin PPD RT 23 SSI (Serum Statens Institute). A positive skin test was defined as an induration of at least 12 mm diameter, based on the previously determined cut off norms for South India.12 QFT was performed according to the manufacturer’s instructions (Qiagen). Anthropo-metric measurements, including height and weight, and biochemical parameters, including plasma glucose, serum albumin, urea, creatinine, alanine aminotransferase (ALT), aspartate aminotrans-ferase (AST) and HbA1c were obtained using standardized techniques. Low and normal BMI were defined on the basis of the 2013 American Heart Association/ American College of Cardiology guidelines (LBMI < 18.5 and NBMI between 18.5 and 24.9 kg/m2). In addition, malnutrition was confirmed by the presence of low serum albumin (<3.4 g/dl) in all the low BMI individuals. Hematology was performed on all individuals using the Act-5 Diff hematology analyzer (Beckman Coulter). Stool microscopy was performed to rule out the presence of intestinal parasites. Also, filarial infections were excluded by TropBio ELISA. All individuals were examined as part of a clinical protocol approved by the Institutional Re-view Board of the National Institute of Research in Tuberculosis (NCT00375583 and NCT00001230), and informed written consent was obtained from all individuals.
Table 1.
Demographics and biochemical parameters of the study groups.
| Study characteristics | LBMI | NBMI |
|---|---|---|
| Males | 18 | 18 |
| Median age (range) | 36 (20–60) | 36 (20–60) |
| Albumin (g/dl) | 2.9 (2.43.1) | 4.8 (4.1–5.5) |
| RBG (mg/dl) | 95 (84–113) | 107 (60–129) |
| HbA1c (%) | 5.8 (4.8–6.3) | 5.8 (4.9–6.1) |
| Urea (mg/dl) | 21 (12–34) | 23 (11–42) |
| Creatinine (mg/dl) | 0.8 (0.3–1) | 0.8 (0.4–1.3) |
| ALT (U/L) | 20.1 (7–60) | 24.2 (7–92) |
| AST (U/L) | 27.6 (16–110) | 27.3 (11–68) |
| Body mass index | 16.4 (14.32–17.9) | 22.2 (19.30–24.5) |
| Median height, cm | 158 (151–169) | 168 (145–180) |
| Median weight, kg | 41.3 (28–51) | 69 (41–76) |
The values represent median and range.
QFT ELISA
Whole blood obtained from LBMI or NMBI individuals was incubated in vitro with either no antigen (NIL) or a cocktail of TB antigens (ESAT-6, CFP-10, TB 7.7) (TB Ag) or mitogen for 18 h, ac-cording to the manufacturers instructions using the QFT kit (Qia-gen). The baseline (unstimulated) or TB antigen or mitogen stimulated whole blood supernatants were then used to analyze the levels of chemokine panel using a Human magnetic Luminex Assay Kit from R&D Systems. The parameters analyzed were CCL1, CCL2, CCL3, CCL4, CCL11, CXCL1, CXCL2, CXCL9, CXCL10 and CXCL11.
Statistical analysis
Geometric means and Medians were used for measurements of central tendency. Statistically significant differences between two groups were analyzed using the nonparametric Mann–Whitney U test. Correlations were calculated using Spearman rank correlation. p values ≤ 0.05 were considered significant with the following ranges: high correlation (r = 0.5–1), moderate correlation (r = 0.3– 0.5) and weak correlation (r < 0.3). Principle component Analysis (PCA) analysis was performed as a tool in explorative analysis and for making predictions about the multivariate dataset. Analyses were performed using GraphPad PRISM Version 5.01. JMP 13 (SAS) software was used to perform Spearman rank correlation matrix and the PCA analysis.
Results
Study population characteristics
As shown in Table 1, BMI was used to classify LTBI individuals into two groups – LBMI (BMI < 18.5 kg/m2) and NBMI (18.5 ≤ BMI ≤ 24.9 kg/m2). In addition, this classification was con-firmed by the measurement of serum albumin, which provided an additional verification of malnutrition. All the LBMI individuals had serum albumin < 3.4 g/dl, while the NBMI individuals has serum albumin levels (4.1–5.5 g/dl) in the normal range. The two groups did not significantly differ in the levels of random blood glucose, HbA1c, urea, creatinine, ALT and AST. The hematological parameters are depicted in Table 2. As shown, the two groups did not significantly differ in their baseline hematological parameters. There-fore, LBMI individuals with LTBI did not differ significantly from their NBMI counterparts in age, gender, biochemical and hemato-logical parameters, excluding a role for these confounding variables in the interpretation of the study findings.
Table 2.
Hematological parameters of the study groups.
| Study groups | LBMI (n = 35) | NBMI (n = 35) | p value |
|---|---|---|---|
| Hb gm/dL Median (range) | 11.7 (8.4–13.3) | 12.4 (9.1–14.6) | NS |
| RBC 106 /ml Median (range) | 4.8 (3.8–6.09) | 4.9 (3.8–6.04) | NS |
| WBC 103 /mlMedian (range) | 9 (5.7–11.2) | 9.6 (5.11–12.5) | NS |
| HCT % Median (range) | 45.62 (32.2–71.1) | 42.52 (27.9–58.6) | NS |
| PLT 103 /ml Median (range) | 238 (72–352) | 247 (172–397) | NS |
| Neutrophil 103 /ml Median (range) | 53.2 (33.1–72.2) | 58.2 (42.0–68.7) | NS |
| Lymphocyte 103 /ml Median (range) | 2.5 (1.52–3.49) | 2.3 (1.17–3.0) | NS |
| Monocyte 103 /ml Median (range) | 7.0 (4.1–10.4) | 7.6 (4.3–10.9) | NS |
| Eosinophil 103 /ml median (Range) | 0.36 (0.05–0.68) | 0.29 (0.10–0.59) | NS |
| Basophil 103 /ml Median (range) | 0.05 (0.04–0.09) | 0.06 (0.02–0.08) | NS |
The values represent median and range. p values were calculated using the Mann–Whitney test.
LBMI is associated with decreased baseline (unstimulated) levels of CC and CXC chemokines
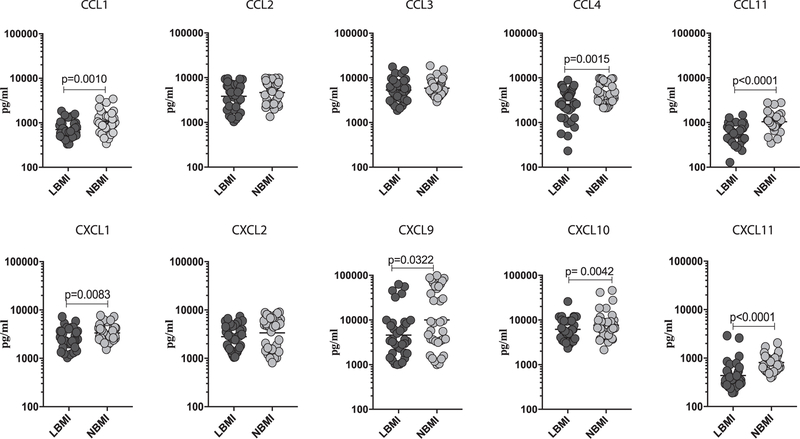
To determine the influence of BMI on homeostatic levels of and CXC chemokines in LTBI, we measured the baseline (un-stimulated) levels of CCL1, CCL2, CCL3, CCL4, CCL11, CXCL1, CXCL2, CXCL9, CXCL10 and CXCL11 in LBMI and NBMI individuals with concomitant LTBI (Fig. 1). As shown in Fig. 1, the baseline (un-stimulated) levels of CCL1 (Geometric Mean of 705.9 pg/ml in LBMI vs. 1075 pg/ml in NBMI), CCL4 (GM of 25 54 pg/ml vs. 4780 pg/ml), CCL11 (GM of 449.5 pg/ml vs. 1041 pg/ml), CXCL1 (GM of 2535 pg/ml vs. 3348 pg/ml), CXCL9 (GM of 4594 pg/ml vs. 10,019 pg/ml), CXCL10 (GM of 6178 pg/ml vs. 7636 pg/ml) and CXCL11 (GM of 436.7 pg/ml vs. 808.4 pg/ml) were significantly lower in LBMI compared to NBMI individuals. Thus, LBMI is associated with diminished unstimulated levels of certain CC and CXC chemokines in LTBI individuals.
Fig. 1.
Diminished baseline (unstimulated) levels of chemokines in LBMI individuals. The baseline (unstimulated) levels of CC (CCL1, CCL2, CCL3, CCL4, CCL11) and CXC (CXCL1, CXCL2, CXCL9, CXCL10, CXCL11) chemokines were measured by multiplex ELISA in LBMI (n = 35) and NBMI (n = 35) individuals with LTBI. The data are represented as scatter plots with each circle representing a single individual (dark grey – LBMI and light grey – NBMI). p values were calculated using the Mann–Whitney test.
LBMI is associated with decreased TB-antigen stimulated levels of CC and CXC chemokines
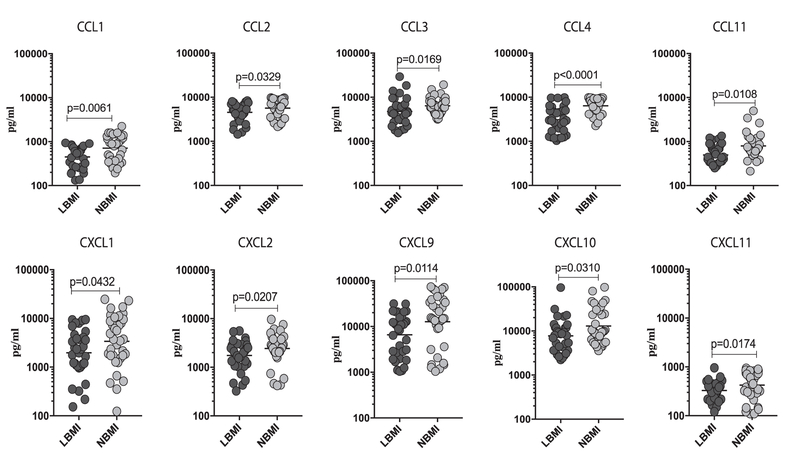
To determine the influence of BMI on antigen-stimulated levels of CC and CXC chemokines in LTBI, we measured the TB-antigen stimulated levels of CCL1, CCL2, CCL3, CCL4, CCL11, CXCL1, CXCL2, CXCL9, CXCL10 and CXCL11 in LBMI and NBMI individuals with concomitant LTBI (Fig. 2). As shown in Fig. 2, the TB-antigen stim-ulated levels of CCL1 (Geometric Mean of 449.6 pg/ml in LBMI vs. 710.5 pg/ml in NBMI), CCL2 (GM of 4543 pg/ml in LBMI vs. 5677 pg/ml in NBMI), CCL3 (GM of 4770 pg/ml in LBMI vs. 6405 pg/ml in NBMI), CCL4 (GM of 3087 g/ml vs. 6408 pg/ml), CCL11 (GM of 328.4 pg/ml vs. 424.3 pg/ml), CXCL1 (GM of 1971 pg/ml vs. 3391 pg/ml), CXCL2 (GM of 1746 pg/ml vs. 2434 pg/ml), CXCL9 (GM of 6600 pg/ml vs. 12,712 pg/ml), CXCL10 (GM of 7749 pg/ml vs. 12,986 pg/ml) and CXCL11 (GM of 328.4 pg/ml vs. 424.3 pg/ml) were significantly lower in LBMI compared to NBMI individuals. Thus, LBMI is associated with diminished TB-antigen stimulated levels of certain CC and CXC chemokines in LTBI individuals.
Fig. 2.
Diminished TB-antigen stimulated levels of chemokines in LBMI individuals. The TB-antigen stimulated levels of CC (CCL1, CCL2, CCL3, CCL4, CCL11) and CXC (CXCL1, CXCL2, CXCL9, CXCL10, CXCL11) chemokines were measured by multiplex ELISA in LBMI (n = 35) and NBMI (n = 35) individuals with LTBI. The data are represented as scatter plots with each circle representing a single individual (dark grey – LBMI and light grey – NBMI). p values were calculated using the Mann-Whitney test.
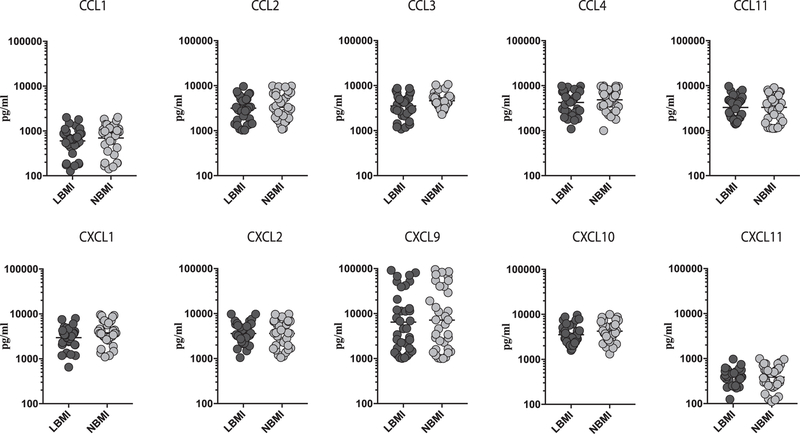
LBMI is not associated with changes in the mitogen-stimulated levels of CC and CXC chemokines
To determine the influence of BMI on mitogen-stimulated lev-els of CC and CXC chemokines in LTBI, we measured the mitogen stimulated levels of CCL1, CCL2, CCL3, CCL4, CCL11, CXCL1, CXCL2, CXCL9, CXCL10 and CXCL11 in LBMI and NBMI individuals with concomitant LTBI (Fig. 3). As shown in Fig. 3, The mitogen stim-ulated levels of the CC (CCL1, CCL2, CCL3, CCL4, CCL11) and CXC (CXCL1, CXCL2, CXCL9, CXCL10, CXCL11) chemokines were not sig-nificantly different between LBMI and NBMI individuals with concomitant LTBI. Thus, LBMI is not associated with altered mitogen stimulated levels of certain CC and CXC chemokines in LTBI individuals.
Fig. 3.
No differences in mitogen stimulated levels of chemokines in LBMI individuals. The mitogen stimulated levels of CC (CCL1, CCL2, CCL3, CCL4, CCL11) and CXC (CXCL1, CXCL2, CXCL9, CXCL10, CXCL11) chemokines were measured by multiplex ELISA in LBMI (n = 35) and NBMI (n = 35) individuals with LTBI. The data are represented as scatter plots with each circle representing a single individual (dark grey – LBMI and light grey – NBMI). p values were calculated using the Mann–Whitney test.
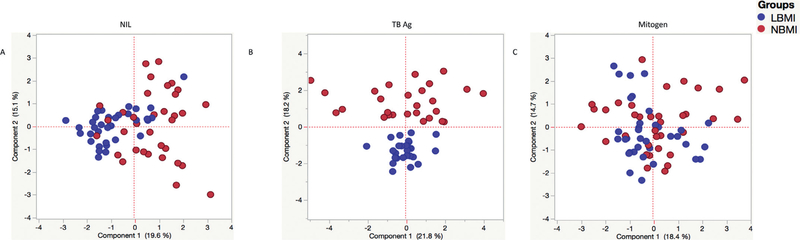
PCA analysis of chemokines as markers to distinguish LBMI from NBMI
Having demonstrated the differences in the expression patterns of chemokines at baseline, TB antigen or mitogen stimulation, we next used the data to create PCA models to better define the role of the chemokines in discrimination of the immune response between LBMI and NBMI individuals. As seen in Fig. 4, we de-tect distinct clustering of markers in PCA analysis only following TB antigen stimulation with a great degree of overlap at base-line and mitogen stimulation. This approach confirms the utility of chemokines responses to discriminate between TB antigen driven responses in LBMI and NBMI individuals with LTBI.
Fig. 4.
PCA plots of chemokine values in LBMI and NBMI individuals. PCA (Principle component analysis) plot computing normalized ELISA data from baseline (unstimulated) or TB-antigen stimulated or mitogen stimulated conditions in combination of two experimental groups LBMI (blue circles) and LTBI NBMI (red circles). The PCA shows the two principal components of variation, under the different conditions. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
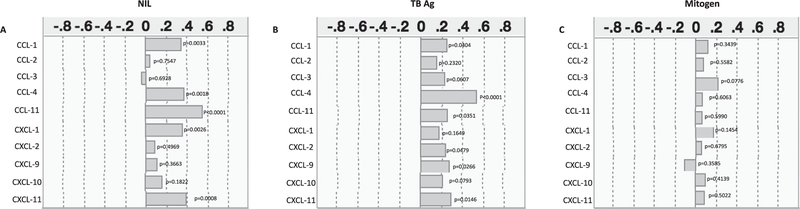
Relationship between unstimulated or TB antigen stimulated or mitogen stimulated levels of chemokines and BMI
Low BMI is an accurate indicator of the level of malnutrition or undernutrition in the individual.13 Thus, to examine the relationship between the baseline (unstimulated) or TB antigen or mitogen stimulated levels of chemokines, we assessed the association of all of the above mentioned chemokines with BMI (in kg/m2) in all the individuals in the study. As shown in Fig. 5(A), the baseline (unstimulated) levels of CCL1 (r = 0.3465, p = 0.0033), CCL4 (r = 0.3673, p = 0.0018), CCL11 (r = 0.5543, p < 0.0001), CXCL1 (r = 0.3551, p = 0.0026) and CXCL11 (r = 0.3924, p = 0.0008) exhibited a positive association with BMI in all LTBI individuals. As shown in Fig. 5(B), the TB-antigen stimulated levels of CCL1 (r = 0.2511, p = 0.0404), CCL4 (r = 0.5299, p < 0.0001), CCL11 (r = 0.2579, p = 0.0351), CXCL2 (r = 0.2426, p = 0.0479), CXCL9 (r = 0.2709, p = 0.0266) and CXCL11 (r = 0.2971, p = 0.0146) exhibited a positive association with BMI in all LTBI individuals. In contrast, as shown in Fig. 5(C), the mitogen stimulated levels of chemokines did not exhibit any significant relationship with BMI.
Fig. 5.
Positive and negative relationship between chemokine levels and BMI indices in LTBI individuals. The relationship between the (A) baseline (unstimulated) or (B) TB – antigen stimulated or (C) mitogen stimulated levels of chemokines and BMI indices was examined in all LTBI (n = 70) individuals. The data are represented correlation rank matrices with r values being denoted by horizontal bars. p and r values were calculated using the Spearman rank correlation test at 95% confidence intervals using JMP software.
Discussion
Chemokines, by their ability to mobilize, recruit and retain cells at the site of infection as well as due to their ability to influence the subsequent cytokine response, play a vital role in protective immunity to TB.9 Chemokines are typically divided into four fam-ilies based on the presence of cysteines and the presence or ab-sence of amino acids between those cysteines.8 Chemokines of the and CXC family have been extensively investigated for their role in immune response to TB, mainly in animal models.10 Thus, CCL1, 2, 3 and 4 are known to be upregulated during Mycobacterium tuberculosis (Mtb) infection and mediate early dendritic cell recruitment in the lymph nodes.14,15 CXCL1 and 2 are mainly involved in neutrophil recruitment, which is important in the early phase of the immune response.8 CXCL9, 10 and 11 are known to be key requirements for granuloma formation and maintenance.16,17 The role of CCL11 in Mtb infection is not well studied. Our examination of the above-mentioned chemokines clearly reveals a deficiency in the ability of LBMI individuals to upregulate the production of these cytokines either at baseline (unstimulated) or following TB – antigen stimulated conditions.
First, our data demonstrated diminution in the baseline level of certain chemokines, specifically, CCL1, CCL4, CCL11, CXCL1, CXCL9, CXCL10 and CXCL11. Second, our data revealed that CCL2, CCL3 and CXCL2 are additional chemokines with altered levels upon TB anti-gen stimulation. CCL2 is a known ligand of CCR2 and increased levels of CCL2 have been described in the circulation of active TB individuals and are associated with severity of TB disease.18,19 Our data on changes in CCL2 levels would therefore suggest an effect on the recruitment of monocytes. CCL3 and CCL4 are known lig-ands for CCR1 and CCR5 and are known to be induced in the lung upon Mtb infection.14 Moreover, CD4+ T cells expressing CCR1 are elevated in the pleural fluid and in the periphery of individuals with active TB.20,21 Therefore, altered levels of CCL3 and 4 could potentially modify the recruitment of T cells in latent TB.
CXCL1 and 2 are known ligands of CXCR1 and 2 and individuals with TB have increased CXCR1 expression, while individuals with latent TB have increased CXCR2 expression.22 The role of CXCL1 and 2 are not well understood in TB infection or disease, although they are mainly important in neutrophil recruitment in infections. Our data also suggest that altered levels of these chemokines could contribute to perturbations in the recruitment of neutrophils in la-tent TB in the context of LBMI. CXCL9, 10 and 11 are ligands of CXCR3 and elevated expression of these chemokines within granulomas has been described in animal models of Mtb infection.16,17 Studies from human disease reveal that disease severity is associated with the levels of CXCL9, CXCL10 and CXCL11.19,23 CXCL10 is an important mediator of TB specific responses and TB mediated inflammation.24–26 Thus, our data, which show diminished levels of CXCR3 ligands suggest that BMI influences the important process of granuloma maintenance in LTBI. Finally, our data on mitogen stimulated chemokines provides evidence that LBMI or NBMI individuals do not differ in the ability to produce chemokines per se but rather express certain chemokines at differential levels upon latent infection or in vitro re-stimulation.
Our data provides additional evidence on BMI being a major influence on chemokine levels, when analyzed by PCA analysis and using a correlation matrix. Thus, most of the altered chemokines exhibit a direct positive relationship with BMI, suggesting that these changes are directly associated with changes in the BMI in LTBI individuals. In addition, our composite analysis of the data using principal components also reinforces the differences between LBMI and NBMI, especially in the TB-antigen stimulated scenario, wherein chemokines can be used clearly distinguish the two groups. Chemokines control both homeostatic and infection induced immune cell movement between the bone marrow, blood and peripheral tissues or infection sites. Our data on diminished levels of chemokines thus suggests that BMI causes perturbations in both the homeostatic and infection induced tracking of different cell types in infected individuals and therefore, could con-tribute to altered capacity of the host to form and maintain protective granulomas. Whether the diminished levels of chemokines in LBMI individuals actually translates to diminished cellular retention in the lung granulomas and contributes to breakdown of protection remains to be determined.
Our study suffers from the limitation of being a cross-sectional study examining only associations and not cause and effect. In addition, while we observe an association between malnutrition and chemokine diminution, we cannot infer any causality. While we have excluded most of the known co-morbidities that are known to influence chemokine responses in TB, including HIV, diabetes and intestinal parasites,27 it is theoretically possible that other factors not examined in this study could have contributed to the differential responses. Nevertheless, to our knowledge, this study is the first ever study in humans to examine the relationship be-tween chemokine responses and BMI in TB infection. Chemokines can mediate protective anti-TB immune responses by inducing inflammation; however, the overwhelming lung inflammation induced by chemokines can also drive lung pathology, including cavitation and tissue destruction.11 Low chemokine levels could promote Mtb proliferation or alter anti-Mtb immune responses and therefore our data provides an important biological mechanism by which nutrition and BMI influence the host immune response. Our study provides important insights into the influence of nutrition on the pathogenesis of TB. Our study also provides an impetus to perform longitudinal studies examining the role of immunological biomarkers in the development of TB in malnourished patients.
Acknowledgements
We thank the staff of Department of Epidemiology, NIRT, for valuable assistance in recruiting the patients for this study, and M. Satiswaran, J. Jeevan, Prabbu Balakrishnan and Jovvian George of the NIH-NIRT-ICER for technical assistance.
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Al-lergy and Infectious Diseases, National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the De-partment of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Conflict of interest
None reported.
Author disclosure
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 2.Rahman SA, Adjeroh D. Surface-based body shape index and its relationship with all-cause mortality. PLoS One 2015;10(12):e0144639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and con-sequence of malnutrition. Trends Immunol 2016;37(6):386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004;8(3):286–98. [PubMed] [Google Scholar]
- 5.Koethe JR, von Reyn CF. Protein-calorie malnutrition, macronutrient supplements, and tuberculosis. Int J Tuberc Lung Dis 2016;20(7):857–63. [DOI] [PubMed] [Google Scholar]
- 6.Padmapriyadarsini C, Shobana M, Lakshmi M, Beena T, Swaminathan S. Under-nutrition & tuberculosis in India: situation analysis & the way forward. Indian J Med Res 2016;144(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relation-ship between tuberculosis incidence and body mass index. Int J Epidemiol 2010;39(1):149–55. [DOI] [PubMed] [Google Scholar]
- 8.Griffth JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014;32:659–702. [DOI] [PubMed] [Google Scholar]
- 9.Domingo-Gonzalez R, Prince O, Cooper A, Khader SA. Cytokines and chemokines in mycobacterium tuberculosis infection. Microbiol Spectr 2016;4(5). doi: 10.1128/microbiolspec.TBTB2-0018-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slight SR, Khader SA. Chemokines shape the immune responses to tuberculosis. Cytokine Growth Factor Rev 2013;24(2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monin L, Khader SA. Chemokines in tuberculosis: the good, the bad and the ugly. Semin Immunol 2014;26(6):552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radhakrishna S, Frieden TR, Subramani R. Association of initial tuberculin sensitivity, age and sex with the incidence of tuberculosis in south India: a 15-year follow-up. Int J Tuberc Lung Dis 2003;7(11):1083–91. [PubMed] [Google Scholar]
- 13.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res 1995;3(1):73–95. [DOI] [PubMed] [Google Scholar]
- 14.Saukkonen JJ, Bazydlo B, Thomas M, Strieter RM, Keane J, Kornfeld H. Beta-chemokines are induced by Mycobacterium tuberculosis and inhibit its growth. Infect Immun 2002;70(4):1684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vesosky B, Rottinghaus EK, Stromberg P, Turner J, Beamer G. CCL5 partic-ipates in early protection against Mycobacterium tuberculosis. J Leukoc Biol 2010;87(6):1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiler P, Aichele P, Bandermann S, Hauser AE, Lu B, Gerard NP, et al. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur J Immunol 2003;33(10):2676–86. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarty SD, Xu J, Lu B, Gerard C, Flynn J, Chan J. The chemokine receptor CXCR3 attenuates the control of chronic Mycobacterium tuberculosis infection in BALB/c mice. J Immunol 2007;178(3):1723–35. [DOI] [PubMed] [Google Scholar]
- 18.Hasan Z, Cliff JM, Dockrell HM, Jamil B, Irfan M, Ashraf M, et al. CCL2 responses to Mycobacterium tuberculosis are associated with disease severity in tuberculosis. PLoS One 2009;4(12):e8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasan Z, Jamil B, Khan J, Ali R, Khan MA, Nasir N, et al. Relationship between circulating levels of IFN-gamma, IL-10, CXCL9 and CCL2 in pulmonary and extrapulmonary tuberculosis is dependent on disease severity. Scand J Immunol 2009;69(3):259–67. [DOI] [PubMed] [Google Scholar]
- 20.Pokkali S, Das SD. Augmented chemokine levels and chemokine receptor expression on immune cells during pulmonary tuberculosis. Hum Immunol 2009;70(2):110–15. [DOI] [PubMed] [Google Scholar]
- 21.Pokkali S, Das SD, Logamurthy R. Expression of CXC and CC type of chemokines and its receptors in tuberculous and non-tuberculous effusions. Cytokine 2008;41(3):307–14. [DOI] [PubMed] [Google Scholar]
- 22.Alaridah N, Winqvist N, Hakansson G, Tenland E, Ronnholm A, Sturegard E, et al. Impaired CXCR1-dependent oxidative defence in active tuberculosis patients. Tuberculosis (Edinb) 2015;95(6):744–50. [DOI] [PubMed] [Google Scholar]
- 23.Hasan Z, Jamil B, Ashraf M, Islam M, Dojki M, Irfan M, et al. Differential live Mycobacterium tuberculosis-, M. bovis BCG-, recombinant ESAT6-, and culture filtrate protein 10-induced immunity in tuberculosis. Clin Vaccine Immunol 2009;16(7):991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goletti D, Raja A, Ahamed Kabeer BS, Rodrigues C, Sodha A, Butera O, et al. IFN-gamma, but not IP-10, MCP-2 or IL-2 response to RD1 selected pep-tides associates to active tuberculosis. J Infect 2010;61(2):133–43. [DOI] [PubMed] [Google Scholar]
- 25.Petrone L, Cannas A, Vanini V, Cuzzi G, Aloi F, Nsubuga M, et al. Blood and urine inducible protein 10 as potential markers of disease activity. Int J Tuberc Lung Dis 2016;20(11):1554–61. [DOI] [PubMed] [Google Scholar]
- 26.Petrone L, Cannas A, Aloi F, Nsubuga M, Sserumkuma J, Nazziwa RA, et al. Blood or urine IP-10 cannot discriminate between active tuberculosis and respiratory diseases different from tuberculosis in children. Biomed Res Int 2015;2015:589471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates M, Marais BJ, Zumla A. Tuberculosis comorbidity with communicable and noncommunicable diseases. Cold Spring Harb Perspect Med 2015. [DOI] [PMC free article] [PubMed]