Abstract
BACKGROUND:
Circulating angiogenic factors of the vascular endothelial growth factor family are important biomarkers of disease severity in pulmonary tuberculosis (PTB). However, the role of angiopoietins, which are also involved in angiogenesis, in PTB is not known.
OBJECTIVE AND DESIGN:
To examine the association of circulating angiopoietins with TB disease or latent tuberculous infection (LTBI), we examined the systemic levels of angiopoietin (Ang) 1, Ang 2 and Tie-2 receptor in individuals with PTB (n = 44), LTBI (n = 44) or no tuberculous infection (NTBI) (n = 44).
RESULTS:
Circulating levels of Ang-1, Ang-2 and Tie-2 were significantly higher in PTB than in individuals with LTBI or NTBI. Moreover, Ang-1, Ang-2 and Tie-2 levels were significantly higher in PTB with bilateral disease. The levels of these factors also exhibited a significant positive relationship with bacterial burdens in PTB. Receiver operating characteristics curve analysis revealed Ang-2 as a marker distinguishing PTB from LTBI or NTBI. Finally, the circulating levels of Ang-1, Ang-2 and Tie-2 were significantly reduced following anti-tuberculosis chemotherapy.
CONCLUSIONS:
Our data demonstrate that PTB is associated with elevated levels of circulating angiopoietins, possibly reflecting endothelial dysfunction. In addition, Ang-2 could prove useful as a biomarker to monitor disease severity, bacterial burden and therapeutic responses.
Keywords: tuberculosis, angiopoietins angiogenesis, lymphangiogenesis
RÉSUMÉ
CONTEXTE:
Les facteurs circulants angiogéniques de la famille des facteurs de croissance de l’endothélium vasculaire sont d’importants biomarqueurs de gravité de la maladie en cas de tuberculose pulmonaire (TBP). Cependant, le rôle des angiopoiétines (Ang), qui sont également impliquées dans l’angiogenése, dans la TBP n’est pas connu.
OBJECTIF ET SCHÉMA:
Pour étudier l’association des Ang circulantes avec la maladie ou l’infection tuberculeuse latente (LTBI), nous avons examiné les niveaux systémiques des récepteurs de l’Ang-1, de l’Ang-2 et de Tie-2 chez des individus atteints de TBP (n = 44), de LTBI (n = 44) ou sans infection tuberculeuse (NTBI) (n = 44).
RÉSULTATS:
Les taux circulants d’Ang-1, d’Ang-2 et de Tie-2 ont été significativement plus élevés chez les patients atteints de TBP compares aux personnes avec LTBI ou NTBI. Plus encore, les niveaux d’Ang-1, d’Ang-2 et de Tie-2 ont été significativement plus élevés en cas de TBP avec atteinte bilatérale. Les niveaux de ces facteurs ont également démontré une relation positive significative avec la charge bactérienne dans la TBP. L’analyse de la courbe de la fonction d’efficacité du récepteur a révélé que l’Ang-2 était un marqueur distinguant la TBP de la LTBI ou de la NTBI. Enfin, les niveaux circulants d’Ang-1, d’Ang-2 et de Tie-2 ont été significativement réduits aprés chimiothérapie antituberculeuse.
CONCLUSION:
Nos données démontrent donc que la TBP est associée à des niveaux circulants élevés d’Ang, témoignant peut-être d’une dysfonction de l’endothélium. De plus, l’Ang-2 pourrait s’avérer un biomarqueur utile pour suivre la gravité de la maladie, la charge bactérienne et les réponses thérapeutiques.
RESUMEN
MARCO DE REFERENCIA:
Los factores angiógenos circulantes, de la familia del factor de crecimiento del endotelio vascular, son importantes biomarcadores de la gravedad de la tuberculosis pulmonar (TBP). Sin embargo, se desconoce el papel que pueden tener en esta enfermedad las angiopoyetinas (Ang) que también participan en la angiogénesis.
OBJETIVO Y MÉTODOS:
Con el objeto de examinar la asociación entre las Ang circulantes y la infección tuberculosa o la enfermedad activa, se evaluaron las concentraciones sistémicas de Ang-1, Ang-2 y del receptor Tie-2 en personas con TBP (n = 44), infección tuberculosa latente (LTBI) (n = 44) o sin infección tuberculosa (NTBI) (n = 44).
RESULTADOS:
Las concentraciones de Ang-1, Ang-2 y Tie 2 circulantes fueron significativamente más altas en las personas con TBP que en las personas con LTBI o NTBI. Además, las concentraciones de Ang-1, Ang-2 y Tie-2 fueron considerablemente mayores en los casos con afectación bilateral. Las concentraciones de estos factores ofrecieron también una correlación positiva con la carga de micobacterias en los casos de TB. Las curvas de eficacia diagnóstica revelaron que la Ang-2 es un marcador que permite distinguir entre la TBP, la LTBI y la NTBI. Por último, las concentraciones de Ang-1, Ang2 y Tie-2 circulantes disminuyeron de manera significativa con la administración del tratamiento antituberculoso.
CONCLUSIÓN:
Los resultados del presente estudio demuestran que la TBP se asocia con altas concentraciones de Ang circulantes, lo cual puede corresponder a una disfunción endotelial. Además, la Ang-2 aparece como un biomarcador útil en la vigilancia de la gravedad de la enfermedad, la carga de micobacterias y la respuesta al tratamiento.
ANGIOPOIETIN-1 and −2 (Ang-1 and Ang-2) are two of the most broadly studied biomarkers of endothelial activation/dysfunction in infectious diseases.1 Ang-1 and Ang-2 and their endothelial tyrosine kinase receptor Tie-2 form a major signaling system in endothelial permeability.2 Pathogenic mycobacteria prompt the development of complex cellular aggregates called granulomas that are the hallmark of tuberculosis (TB).3,4 The human tuberculous granuloma, a strongly solid cellular structure that houses infecting mycobacteria, develops hypoxic areas around its necrotic core,5 which in turn acts as stimulus for vascularization.6 Vascularization in animal models of TB has been shown to be mediated by angiogenesis and lymphangiogenesis;7 in addition, angiogenesis has been shown to promote mycobacterial growth and increase the spread of infection.8 Finally, lymphangiogenesis stimulated by mycobacterial infection has been shown to promote systemic T-cell responses against tuberculous infection.9 We and others have previously shown that VEGF-A, VEGF-C and VEGFR2 are prominent biomarkers of active TB disease severity and bacterial burden.10–13 Angiogenesis and lymphangiogenesis thus appear to be vital players in the pathogenesis of TB.
The angiopoietin-Tie system is the second endothelial-specific receptor tyrosine kinase pathway involved in angiogenesis and lymphangiogenesis.14 The angiopoietin growth factors (Ang-1, Ang-2 and Ang-4) function as ligands for the Tie-2 receptor to stimulate angiogenesis, although in some situations Ang-2 can function as an antagonist to Ang-1.14 However, the association of angiopoietins with tuberculous infection or TB disease has been poorly studied. We hypothesized that pulmonary TB (PTB) is associated with endothelial dysfunction, leading to the enrichment of angiopoiteins.
We aimed to examine the circulating levels of angiogenic factors in individuals with PTB, latent tuberculous infection (LTBI) and no tuberculous infection (NTBI). Our data reveal a significant association of systemic levels of Ang-1, Ang-2 and Tie-2 receptor with disease severity and bacterial burden and a significant ability of Ang-2 to distinguish PTB from LTBI or NTBI and also a potential host-directed therapeutic target for TB disease. Our data also suggest that the factors mentioned above could serve as accurate biomarkers for monitoring therapeutic responses.
MATERIALS AND METHODS
Study design
In this prospective case-control study, patients attending the out-patient clinics of the National Institute for Research in Tuberculosis (NIRT), Chennai, India, and community controls were consecutively enrolled in the study. Plasma samples from 44 individuals with active PTB, 44 with LTBI and 44 with NTBI were collected in sodium heparin tubes and stored at –80°C after centrifugation. The diagnosis of PTB was based on acid-fast bacilli (AFB) smear and Lo¨wenstein-Jensen (LJ) medium solid culture positivity. Smear grade was used to determine bacterial burden, and was classified as 1+, 2+ and 3+. Chest X-ray (CXR) was used to define cavitary disease as well as unilateral vs. bilateral involvement. Severity of disease was estimated based on bilateral involvement and cavitary disease.
At the time of enrollment, none of the active TB cases had a record of previous TB disease or anti-tuberculosis treatment. LTBI diagnosis was based on the tuberculin skin test (TST) and QuantiFERON® TB-Gold In-Tube (Qiagen/Cellestis, Carnegie, VIC, Australia) enzyme-linked immunosorbent assay (ELISA) positivity, absence of CXR abnormalities or pulmonary symptoms and negative sputum smears. A positive TST result was defined as an induration at the site of TST inoculation of at least 12 mm in diameter, to minimize false positivity due to exposure to environmental mycobacteria. NTBI individuals were asymptomatic, with normal CXR, negative TST (indurations <5 mm in diameter) and QuantiFERON ELISA results. All participants were bacille Calmette-Gue´rin vaccinated, human immunodeficiency virus negative and non-diabetic, with normal body mass index. None of the participants exhibited signs or symptoms of any associated lung or systemic disease. The study groups were similar with regard to age and sex; baseline characteristics of study participants are shown in the Table.
Table.
Demographics of the study groups
| Study demographics | Pulmonary TB | LTBI | NTBI |
|---|---|---|---|
| Subjects recruited, n | 44 | 44 | 44 |
| Sex: male/female | 35/9 | 34/10 | 36/8 |
| Age, years, median (range) | 35 (18–64) | 39 (21–60) | 42 (19–60) |
| Smear grade (1+ /2+ /3+) | 13/11/18 | Not applicable | Not applicable |
| QuantiFERON® TB Gold In-Tube | Not done | Positive | Negative |
| Tuberculin skin test, mm | Not done | >12 mm | <12 mm |
TB = tuberculosis; LTBI = latent tuberculous infection; NTBI = no tuberculous infection.
Standard anti-tuberculosis treatment was administered to PTB individuals using the DOTS strategy. Fresh plasma samples were obtained both at baseline and 6 months following the initiation of anti-tuberculosis treatment. All PTB individuals were culture-negative at the end of anti-tuberculosis treatment.
All individuals were examined as part of a study protocol approved by the Internal Ethics Committee of NIRT, Chennai, India. Written informed consent was obtained from all participants.
ELISA
Circulating Ang-1 and Ang-2 levels were measured using the Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA); the Mybiosource ELISA kit (San Diego, CA, USA) was used to measure angiopoietin receptor Tie-2 in plasma samples. The lowest detection limits were as follows: Ang-1, 156.25 pg/ml; Ang-2, 48.87 pg/ml; and Tie-2, 0.313 ng/ml.
Statistical analysis
Geometric means (GMs) were used for measurements of central tendency. Statistically significant differences between the three groups were analyzed using the Kruskal-Wallis test with Dunn’s multiple comparisons. The Mann-Whitney test was used to compare angiogenic factor concentrations between individuals with PTB with unilateral or bilateral lung lesions or cavitary or non-cavitary disease. Linear trend post-test was used to compare angiogenic factor concentrations with smear grades reflecting bacterial burden. Receiver operator characteristics (ROC) curves were designed to test the power of each candidate angiogenic factor to distinguish LTBI or NTBI from PTB. Wilcoxon signed rank test was used to compare angiogenic factor concentrations before and after anti-tuberculosis treatment. Analyses were performed using Graph Pad PRISM Version 5.01 (Graph Pad, La Jolla, CA, USA).
RESULTS
Circulating angiopoietin levels are elevated in pulmonary tuberculosis
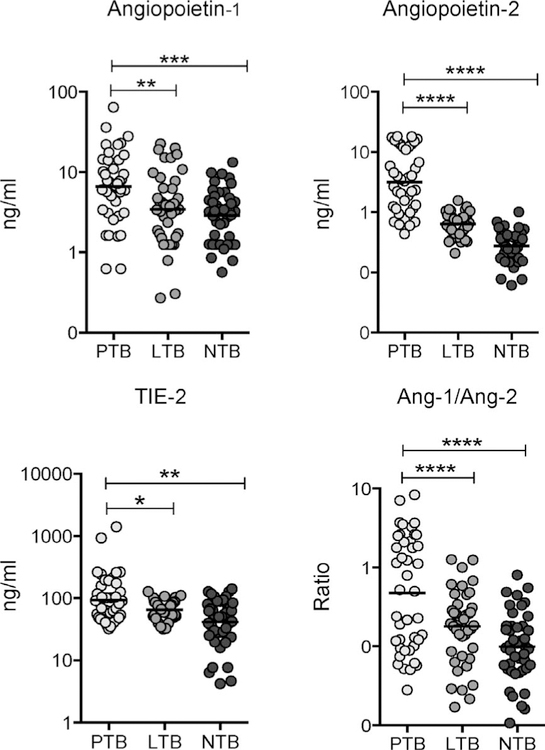
To determine the systemic levels of angiogenic factors in LTBI and TB disease, we measured the circulating levels of Ang-1, Ang-2 and angiopoietin receptor Tie-2 in PTB, LTBI and NTBI individuals (Figure 1). As shown in Figure 1, the systemic levels of Ang-1 (GM 6.61 ng/ml in PTB vs. 3.44 ng/ml in LTBI and 2.88 ng/ ml in NTBI), Ang-2 (GM 3.15 ng/ml in PTB vs. 0.63 ng/ml in LTBI and 0.27 ng/ml in NTBI), Tie-2 (GM 92.27 ng/ml in PTB vs. 65.03 ng/ml in LTBI and 41.35 ng/ml in NTBI) and Ang-1/Ang-2 ratio (GM 0.4765 ng/ml in PTB vs. 0.1784 ng/ml in LTBI and 0.0989 ng/ml in NTBI) were significantly higher in PTB than in LTBI and NTBI individuals. PTB is thus associated with elevated systemic levels of circulating angiogenic factors.
Figure 1.
Elevated systemic levels of circulating angiopoietins in individuals with PTB. Plasma angiopoietins (Ang-1 and 2) and Tie-2 levels, as well as the Ang-1/Ang-2 ratio, were measured in individuals with PTB (n = 44), LTBI (n = 44) and NTBI (n = 44). Data are represented as scatter plots: each circle represents a single individual, horizontal bars indicate the geometric mean P values calculated using the Kruskal-Wallis test with Dunn’s post hoc comparison. PTB = pulmonary tuberculosis; LTB = latent tuberculous infection; NTB = no tuberculous infection.
Circulating angiopoietins are markers of disease severity in PTB
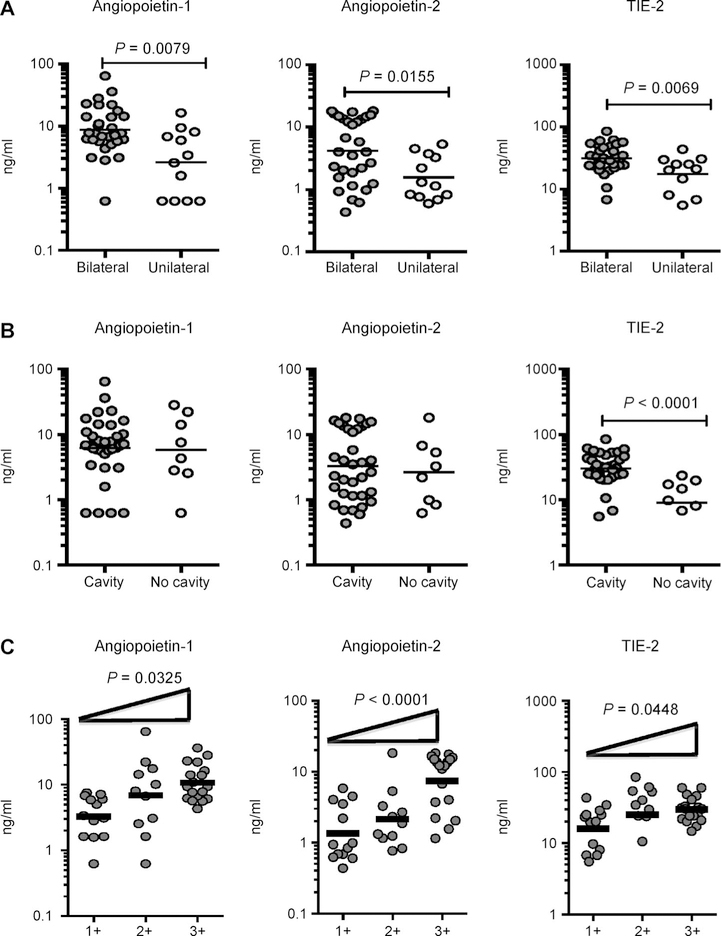
To determine the association between the systemic levels of angiogenic factors and disease severity in PTB, we measured circulating Ang-1, Ang-2 and Tie-2 levels in individuals with PTB with unilateral vs. bilateral disease and with cavitary vs. non-cavitary disease (Figure 2). As shown in Figure 2A, the systemic levels of Ang-1 (GM 8.71 ng/ml in bilateral vs. 2.62 ng/ml in unilateral disease), Ang-2 (GM 4.17 ng/ml in bilateral vs. 1.56 ng/ml in unilateral disease) and Tie-2 (GM 31.33 ng/ml in bilateral vs. 13.35 ng/ ml in unilateral disease) were significantly higher in PTB individuals with bilateral disease than in those with unilateral disease. Similarly, circulating Tie-2 levels (GM 30.04 ng/ml in cavitary vs. 9 ng/ml in noncavitary disease) were significantly higher in PTB individuals with cavitary disease than in those without cavitary disease (Figure 2B). In contrast, no significant differences were seen in Ang-1 and Ang-2 levels in those with cavitary disease and in those without. Disease severity in PTB is thus associated with elevated systemic levels of circulating angiopoietins.
Figure 2.
Elevated systemic levels of angiopoietins in bilateral disease and positive relationship between systemic levels of angiopoietins and smear grades in individuals with PTB. A) Plasma angiopoietins (Ang-1 and 2) and Tie-2 levels were measured in PTB individuals with bilateral vs. unilateral disease. B) Plasma angiopoietins (Ang-1 and 2) levels and Tie-2 were measured in PTB individuals with cavitary vs. non-cavitary disease. Data are represented as scatter plots: each circle represents a single individual. P values were calculated using the Mann-Whitney test. C) The relationship between angiopoietin (Ang-1 and 2) plasma levels and Tie-2 and smear grades as estimated using sputum smears was examined in all individuals with PTB. Data are represented as scatter plots: each circle represents a single individual, horizontal bars indicate the geometric mean P values calculated using linear trend post-test. PTB = pulmonary tuberculosis.
Circulating angiopoietins are markers of bacterial burden in PTB
To determine the association between systemic levels of angiogenic factors and bacterial burden in PTB, we correlated circulating Ang-1, Ang-2 and Tie-2 levels in PTB individuals with smear grade classified as 1+, 2+and 3+ (Figure 2C). As shown, the systemic Ang-1, Ang-2 and Tie-2 levels exhibited a significant positive correlation with smear grade in PTB individuals, indicating a positive association of these factors with bacterial burden.
Circulating angiopoietin-2 can distinguish PTB from LTBI and NTBI
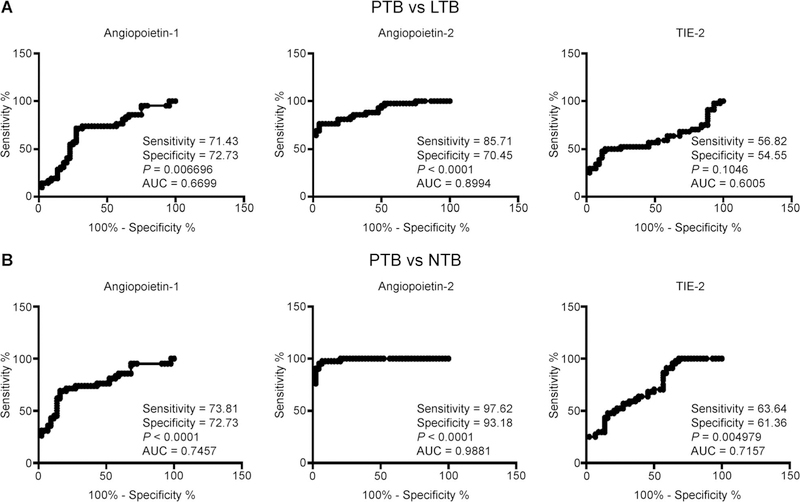
To determine the discriminatory power of circulating angiogenic factors in distinguishing PTB from LTBI and NTBI, we performed on ROC analysis of Ang-1, Ang-2 and Tie-2 in PTB vs. LTBI and PTB vs. NTBI individuals (Figure 3). As shown in Figure 3A, Ang-2 exhibited significant discriminatory power with high area under the curve (AUC) values and sensitivity and specificity, in discriminating PTB from LTBI individuals (AUC 0.8994, sensitivity 85.71, specificity 70.45). Similarly, as shown in Figure 3B, Ang-2 exhibited the highest AUC and sensitivity and specificity in discriminating PTB from NTBI individuals (AUC 0.9881, sensitivity 97.62, specificity 93.18). Ang-2 thus exhibits the potential to serve as a biomarker to distinguish active TB from LTBI or NTBI.
Figure 3.
ROC analysis to estimate the discriminatory power of systemic angiopoietins in individuals with PTB. A) ROC analysis to estimate the sensitivity, specificity and AUC was performed using Ang-1, Ang-2 and Tie-2 to estimate the capacity of these factors to distinguish individuals with PTB vs. LTBI. B) ROC analysis to estimate the sensitivity, specificity and AUC was performed using Ang-1, Ang-2 and Tie-2 to estimate the capacity of these factors to distinguish individuals with PTB vs. NTBI. PTB = pulmonary tuberculosis; LTBI = latent tuberculous infection; AUC = area under the ROC curve; NTBI = no tuberculous infection; ROC = receiver operating characteristic.
Circulating angiopoietin levels are significantly diminished following anti-tuberculous treatment
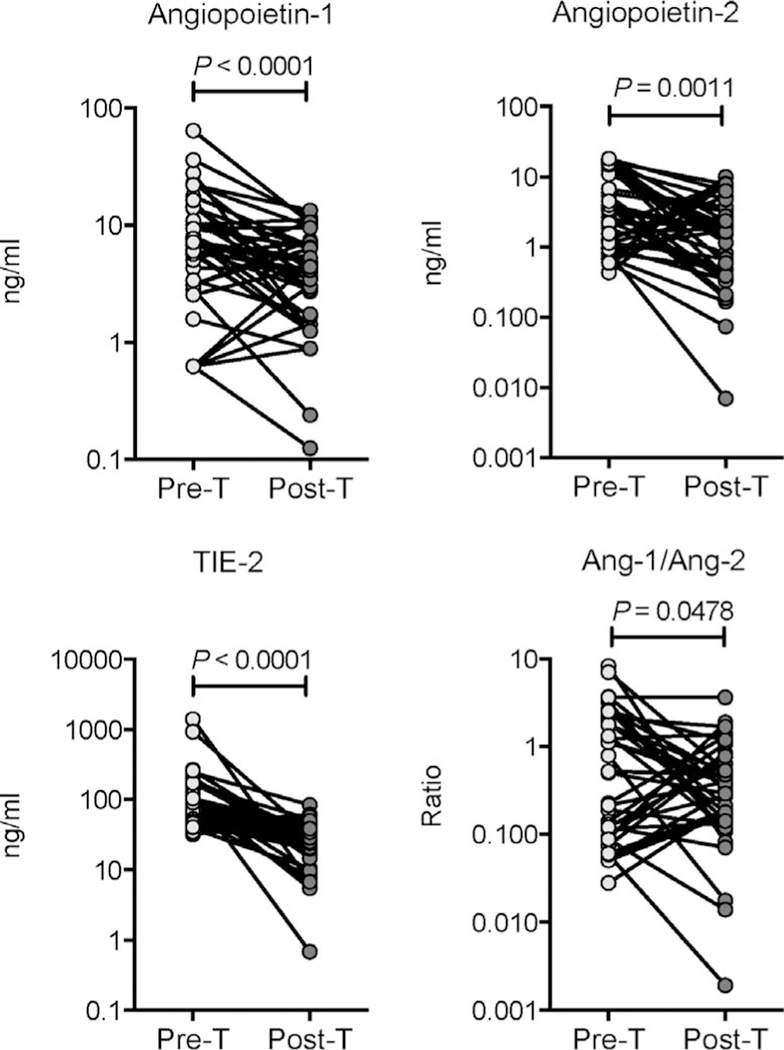
To determine whether elevated levels of circulating angiogenic factors are directly associated with TB disease, we determined the levels of these factors in PTB individuals before and after a standard course of anti-tuberculosis treatment (pre-T vs. post-T). As shown in Figure 4, at the end of anti-tuberculous treatment, circulating Ang-1 (GM 6.61 ng/ml pre-T vs. 3.28 ng/ml post-T), Ang-2 (GM 3.15 ng/ml pre-T vs. 1.18 ng/ml post-T) and Tie-2 levels (GM 92.27 ng/ ml pre-T vs. 24.55 ng/ml post-T) and Ang-1/Ang-2 ratio (GM 0.4765 ng/ml in pre-T vs. 0.2759 ng/ml post-T) were all significantly lower post-treatment than pre-treatment in PTB individuals. Successful treatment of active TB thus results in significantly diminished levels of circulating angiogenic factors in PTB.
Figure 4.
Diminished systemic levels of angiopoietins following anti-tuberculosis treatment in individuals with PTB. Plasma angiopoietins (Ang-1 and 2) and Tie-2 levels, as well as the Ang-1/Ang-2 ratio, were measured in PTB individuals before (pre-T) and after (post-T) standard anti-tuberculosis chemotherapy. Data are represented as line graphs, with each line representing a single individual. P values were calculated using the Wilcoxon signed rank test. PTB = pulmonary tuberculosis.
DISCUSSION
Ang-1 and Ang-2 are two of the most widely studied biomarkers of endothelial activation/dysfunction in infectious diseases.1 Our results reveal a substantial association of circulating Ang-1, Ang-2 and Tie-2 receptor levels with disease severity and bacterial burden and a significant ability of Ang-2 to distinguish PTB from LTBI or NTBI.
While Ang-1 is produced constitutively in pericytes and smooth muscle cells, Ang-2 is produced by endothelial cells and pre-stored in granules for rapid release.2 Under biological conditions, Ang-1 levels exceed those of Ang-2 and inhibit the pro-inflammatory pathways, resulting in endothelial cell quiescence. In contrast, following inflammation, Ang-2 is quickly released and supports pro-inflammatory and prothrombotic pathways, resulting in endothelial dysfunction.1,15 Ang-2 expression is firmly regulated, and its expression is mostly restricted to the vascular endothelium.16,17 Ang-2 levels are known to be elevated in sepsis,18 severe malaria19 and dengue hemorrhagic fever.20 Tie-2 protein kinase receptors are expressed primarily on endothelial cells, and this signaling pathway reduces endothelial activation.21 Endothelial dysfunction contributes to the pathogenesis of a mixture of potentially serious infectious diseases and syndromes, including sepsis and septic shock, hemolytic-uremic syndrome, severe malaria, and dengue hemorrhagic fever.1,15 Angiogenesis, lymphangiogenesis and vascular dysfunction are progressively being accepted as major hallmarks of active TB, and the use of host-directed therapies targeted at blocking these functions has been proposed as an adjunct measure for anti-tuberculosis treatment.8,22 However, the role of circulating angiopoietins has been poorly studied in human TB disease.
Ang-2 is significantly enhanced in serum and is associated with both disease severity and prognosis in sepsis, while reduced Ang-1 in the peripheral blood is associated with disease severity and results in Plasmodium falciparum malaria.1 Ang/Tie levels have been shown to contribute to acute illness in patients with pulmonary conditions, and it has also been postulated that Ang-1 and Ang-2 concentrations in sputum from asthma patients correlate with airway microvascular permeability.23 Studies have also shown that the Ang-2 level was improved in patients with exudative pleural effusion, whereas Ang-1 levels remained unchanged in comparison to transudative pleural effusion.24 Other studies have shown that serum Ang-2 levels were significantly higher in tuberculous than in non-tuberculous pneumonic pleural effusion patients; these higher levels of Ang-2 in tuberculous pleural effusion may suggest that Ang-2 plays a key role in the pathogenesis of pleural exudates.25 Moreover, recent studies have shown that serum Ang-2 levels were significantly higher in patients with acute chronic obstructive pulmonary disease (COPD) than in those with stable COPD or control subjects, and Ang-2 appears to be a consistent biomarker of active disease.26
Our data clearly expand on these reports, confirming that Ang-1, Ang-2 and Tie-2 levels are elevated in PTB patients and that Ang-2 can serve as an accurate biomarker to distinguish PTB from LTBI and NTBI with a high degree of sensitivity and specificity. Ang-2 is also useful for assessing the degree of inflammation and monitoring the inflammatory process. In addition, elevated Ang-1 Ang-2 and Tie-2 levels are also significantly diminished following treatment, indicating that the elevated levels of these markers are intimately related to the disease process. It is to be noted that Ang-1, Ang-2 and Tie-2 levels are also correlated with disease severity or bacterial burden in PTB. Ang-1/Ang-2 ratio was also used to analyze the role of angiopoietins and potentially used as predictors of vascular dysfunction. However, unlike other inflammatory conditions where Ang-2 is predominantly elevated, we find that in PTB, significant elevations of Ang-1 levels accompany elevated levels of Ang-2 as well as an increase in the Ang-1/Ang-2 ratio. Whether this represents a compensatory increase in Ang-1 or whether the increases in both angiopoietins are important contributors to the pathogenesis of TB disease needs to be determined in the future.
Our study is the first to systemically study the relationship of circulating angiopoietins in human PTB patients and controls. As this was an exploratory study with a small sample size, we are unable to draw any inferences on cause and effect. However, our study provides important evidence of a relationship and, therefore, the need to further investigate the role of these factors in pathogenesis. In addition, our data do not support any claim to demonstrate angiopoietins as diagnostic biomarkers in TB, as elevation of angiopoietins is known to occur in a variety of inflammatory and infectious diseases. We do, however, report the utility of a certain biomarker (Ang-2) that serves to increase our understanding of host immune factors that might contribute to the pathogenesis of TB and accelerate or impede its extra-pulmonary spread in TB-infected patients. In addition, as angiogenesis has also been implicated in the dissemination of mycobacterial infection, the investigation of these factors in human extra-pulmonary TB should provide further input on the pathogenesis of disseminated TB. Finally, our data also add to the growing portfolio of host factors and provide valuable insights on factors that might need further exploration as biomarkers in PTB.
Acknowledgements
The authors thank the staff of the Department of Clinical Research and Department of Social Work, National Institute for Research in Tuberculosis, Chennai, India, for valuable assistance in recruiting patients for this study, the staff of the Department of Bacteriology for Cultures and P Balakrishnan, Y Bhootra and J George of the National Institutes of Health-International Center for Excellence in Research for technical assistance.
The Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA, supported this work.
Footnotes
Conflicts of interest: none declared.
References
- 1.Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence 2013; 4: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Meurs M, Kumpers P, Ligtenberg JJ, Meertens JH, Molema G, Zijlstra JG. Bench-to-bedside review: angiopoietin signalling in critical illness—a future target? Crit Care 2009; 13: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan L Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 2012; 12: 352–366. [DOI] [PubMed] [Google Scholar]
- 4.Ernst JD The immunological life cycle of tuberculosis. Nat Rev Immunol 2012; 12: 581–591. [DOI] [PubMed] [Google Scholar]
- 5.Rittershaus ES, Baek SH, Sassetti CM. The normalcy of dormancy: common themes in microbial quiescence. Cell Host Microbe 2013; 13: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matty MA, Roca FJ, Cronan MR, Tobin DM. Adventures within the speckled band: heterogeneity, angiogenesis, and balanced inflammation in the tuberculous granuloma. Immunol Rev 2015; 264: 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagan AJ, Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med 2014; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oehlers SH, Cronan MR, Scott NR, et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature 2015; 517: 612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding J, Ritter A, Rayasam A, Fabry Z, Sandor M. Lymphangiogenesis is induced by mycobacterial granulomas via vascular endothelial growth factor receptor-3 and supports systemic T-cell responses against mycobacterial antigen. Am J Pathol 2015; 185: 432–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar NP, Banurekha VV, Nair D, Babu S. Circulating angiogenic factors as biomarkers of disease severity and bacterial burden in pulmonary tuberculosis. PLOS ONE 2016; 11: e0146318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe Y, Nakamura M, Oshika Y, et al. Serum levels of vascular endothelial growth factor and cavity formation in active pulmonary tuberculosis. Respiration 2001; 68: 496–500. [DOI] [PubMed] [Google Scholar]
- 12.Alatas F, Alatas O, Metintas M, Ozarslan A, Erginel S, Yildirim H. Vascular endothelial growth factor levels in active pulmonary tuberculosis. Chest 2004; 125: 2156–2159. [DOI] [PubMed] [Google Scholar]
- 13.Matsuyama W, Hashiguchi T, Matsumuro K, et al. Increased serum level of vascular endothelial growth factor in pulmonary tuberculosis. Am J Respir Crit Care Med 2000; 162: 1120–1122. [DOI] [PubMed] [Google Scholar]
- 14.Jeltsch M, Leppanen VM, Saharinen P, Alitalo K. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb Perspect Biol 2013; 1; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee WL, Liles WC. Endothelial activation, dysfunction and permeability during severe infections. Curr Opin Hematol 2011; 18: 191–196. [DOI] [PubMed] [Google Scholar]
- 16.16-Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell 2002; 3: 411–423. [DOI] [PubMed] [Google Scholar]
- 17.Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol 1998; 153: 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLOS MED 2006; 3: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo TW, Lampah DA, Gitawati R, et al. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci USA 2008; 105: 17097–17102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michels M, van der Ven AJ, Djamiatun K, et al. Imbalance of angiopoietin-1 and angiopoetin-2 in severe dengue and relationship with thrombocytopenia, endothelial activation, and vascular stability. Am J Trop Med Hyg 2012; 87: 943–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 2006; 27: 552–558. [DOI] [PubMed] [Google Scholar]
- 22.Datta M, Via LE, Kamoun WS, et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci USA 2015; 112: 1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanazawa H, Nomura S, Asai K. Roles of angiopoietin-1 and angiopoietin-2 on airway microvascular permeability in asthmatic patients. Chest 2007; 131: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 24.Moschos C, Psallidas I, Kollintza A, et al. The angiopoietin/Tie 2 axis mediates malignant pleural effusion formation. Neoplasia 2009; 11: 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanad M, Shouman W, Gharib AF. Evaluation of serum and pleural levels of angiopoietin-1 and angiopoietin-2 in children with transudative and exudative pleural effusions. Iran J Pediatr 2011; 21: 278–286. [PMC free article] [PubMed] [Google Scholar]
- 26.Lazovic B Correlation of CRP and serum level of fibrinogen with severity of disease in chronic obstructive pulmonary disease patients. Med Arch 2012; 66: 159–160. [DOI] [PubMed] [Google Scholar]