Abstract
Aims
To determine the impact of smoking and alcohol exposure during adolescence on arterial stiffness at 17 years.
Methods and results
Smoking and alcohol use were assessed by questionnaires at 13, 15, and 17 years in 1266 participants (425 males and 841 females) from the ALSPAC study. Smoking status (smokers and non-smoker) and intensity (‘high’ ≥100, ‘moderate’ 20–99, and ‘low or never’ <20 cigarettes in lifetime) were ascertained. Participants were classified by frequency (low or high) and intensity of drinking [light (LI <2), medium (MI 3–9), and heavy (HI >10 drinks on a typical drinking day)]. Carotid to femoral pulse wave velocity (PWV) was assessed at 17 years [mean ± standard deviation and/or mean difference (95% confidence intervals)]. Current smokers had higher PWV compared with non-smokers (P = 0.003). Higher smoking exposure was associated with higher PWV compared with non-smokers [5.81 ± 0.725 vs. 5.71 ± 0.677 m/s, mean adjusted difference 0.211 (0.087–0.334) m/s, P = 0.001]. Participants who stopped smoking had similar PWV to never smokers (P = 0.160). High-intensity drinkers had increased PWV [HI 5.85 ± 0.8 vs. LI 5.67 ± 0.604 m/s, mean adjusted difference 0.266 (0.055–0.476) m/s, P = 0.013]. There was an additive effect of smoking intensity and alcohol intensity, so that ‘high’ smokers who were also HI drinkers had higher PWV compared with never-smokers and LI drinkers [mean adjusted increase 0.603 (0.229–0.978) m/s, P = 0.002].
Conclusion
Smoking exposure even at low levels and intensity of alcohol use were associated individually and together with increased arterial stiffness. Public health strategies need to prevent adoption of these habits in adolescence to preserve or restore arterial health.
Keywords: Smoking , Alcohol , Arterial stiffness , Adolescence
Introduction
Cigarette smoking and consumption of alcohol are associated with increased cardiovascular (CV) risk in adult life.1,2 Smokers are twice as likely to suffer a myocardial infarction compared with people who have never smoked. For alcohol, a J-shaped association has been reported, with CV benefit for those consuming small or moderate amount of alcohol daily. This has however been questioned in recent studies,3 which showed that higher intake or binge drinking are associated with increased CV morbidity and mortality4.
Most adult users of alcohol or tobacco first start in their early teens.5,6 In the United States, each day approximately 6000 adolescents, aged 12–18 years, smoke a cigarette for the first time, and 3000 adolescents become daily smokers.7 A large body of research suggests that adolescents who participate in one health-risk behaviour are more likely to engage in additional risk behaviours.7 According to the Center of Disease Classification (CDC), 75% of high school students report drinking alcoholic beverages at least once.7 The early impact of unhealthy behaviours on contemporary arterial disease is not well documented.
The Avon Longitudinal Study of Parents and Children was established in 1991 to provide environmental information on participants from childhood to adolescence and young adulthood and to study the impact on their health (http://www.alspac.bris.ac.uk).8 The cohort has been followed with questionnaires through childhood and at regular annual clinics since age of 7 years. Detailed information about alcohol and smoking is available for participants through adolescence from questionnaires, and vascular phenotyping was performed at age 17 years. This provided a unique opportunity to assess the impact of exposure to these substances on arterial phenotype and CV risk factor profile in young adulthood.
Methods
Vascular study population
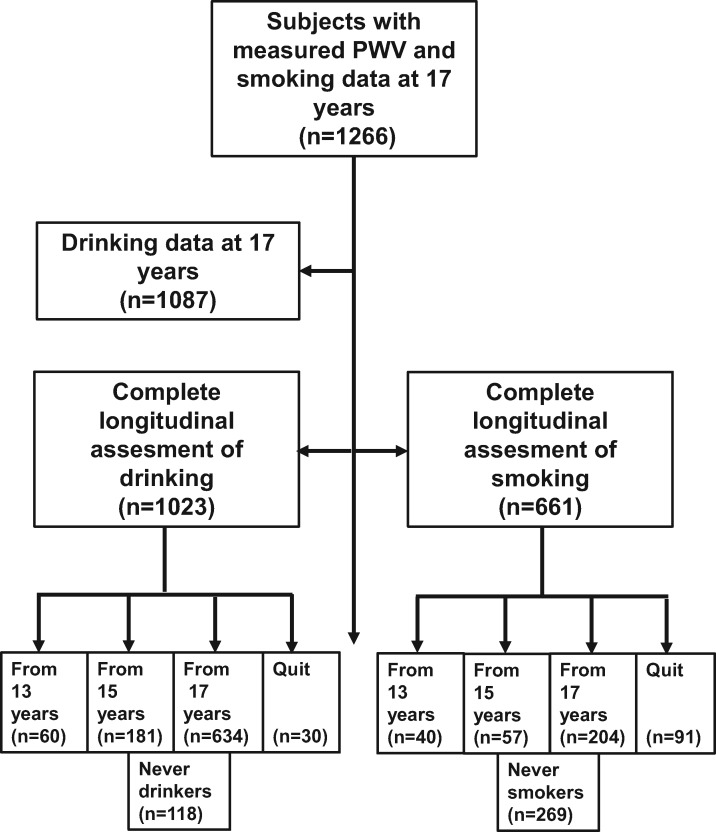
We studied adolescents who attended the ALSPAC clinics in Bristol at 17 years (Figure 1). The study was approved by the ALSPAC Ethics and Law Committee and written informed consent was obtained from all participants.
Figure 1.
Study population. Flow chart shows the number of participants who responded to questionnaires exploring smoking and alcohol use from 13 to 17 years.
Tobacco use in adolescence
Information about tobacco use was obtained from questionnaires at ages 13, 15, and 17 years. Participants were classified as smokers or no-smokers at each period. Smoking exposure over the 5-year period was assessed. Participants who stopped smoking were also identified.
Numbers of cigarettes smoked over lifetime were assessed by questionnaire at 17 years and participants were classified in three groups to reflect smoking intensity: ‘high’ (≥100 cigarettes), moderate (20–99 cigarettes), and low/never smokers (<20 cigarettes). Cotinine levels were not measured. Exposure to parental smoking (passive smoking) was also assessed by questionnaires.
Alcohol consumption in adolescence
Information about alcohol consumption was obtained from questionnaires at the ages of 13, 15, and 17 years, and participants were classified as drinkers or non-drinkers at each period. At 17 years, more detailed information about patterns of alcohol use was obtained. The participants were asked to report the age when they started drinking alcohol, frequency of alcohol consumption/month, and intensity of alcohol consumption (number of drinks on a typical drinking day, with one drink equating to 8 g of alcohol). Heavy (HI), medium (MI), and light (LI) intensity drinkers were defined as subjects consuming >10 drinks, 3–9 drinks, and < 2 drinks, respectively on a typical day that they were drinking alcohol. Preferences for different beverages (i.e. beer, wine, and spirits) were evaluated at 17 years by questionnaires, which assessed the number of each of the aforementioned drinks consumed. Participants were classified as never, light, moderate and heavy beer, wine or spirit-drinking if they reported no consumption or consumption of 1–5, 6–20, and over 20 drinks in the last 30 days.
Aortic stiffness
Pressure-pulse waveforms were obtained using the Vicorder device by placing a 100 mm wide blood pressure cuff around the upper thigh to measure the femoral pulse pressure, and a 30 mm partial cuff around the neck at the level of the carotid artery. To measure carotid to femoral pulse wave velocity (PWV), high quality waveforms were recorded simultaneously for 3 s with the subject in the supine position, and the foot-to-foot transit time was determined using an in-built cross-correlation algorithm centred around the peak of the second derivative of pressure. All measurements were performed independently by one of two trained vascular technicians [inter-observer mean difference (standard deviation of difference) 0.2 (0.1) m/s].
Cardiovascular risk factors (confounders and mediators)
Three seated right arm blood pressure measurements were made (Omron 705 IT oscillometric BP monitor) after a 10 min rest and the mean of the last two were analysed. Simultaneous heart rate was measured. Waist circumference, weight, and height were measured, and body mass index (BMI) was calculated. At 17 years, fasting blood samples were taken; all samples were immediately centrifuged and stored at −80°C for lipid profile [total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides], inflammatory markers [C-reactive protein (CRP)], and liver function tests.9 Socioeconomic status (SES) was designated from 1 to 5 according to National Readership Survey (NRS) grading criteria based on father’s occupation, with Class 5 denoting unskilled workers (http://www.nrs.co.uk/nrs-print/lifestyle-and-classification-data/social-grade). Physical activity (PA) was assessed by actigraph accelerometer at 15 years. Time spent in moderate to vigorous PA (MVPA) such as cycling, swimming, and running was calculated, the cut-point being four times resting metabolic rate (equivalent to brisk walking). Minutes of MVPA was calculated as the average minutes of such activity/valid day of measurement.
Statistical analysis
Standard analytical approaches to normally and non-normally distributed measures were used and values are expressed as mean ± standard deviation and median (interquartile range), respectively. Categorical variables are expressed as N, percentage. Unadjusted comparisons for continuous variables between groups of interest were performed using analysis of variance (ANOVA), with a test for linear trend carried out across pre-specified categories. Categorical variables were compared using the χ2 test. Multivariable linear regression analysis was performed to assess the association between smoking, alcohol and CV risk factors, and PWV. Numeric estimates for comparisons of interest are provided as mean difference in PWV along with 95% confidence intervals around the mean difference. Multivariable models were built through an unbiased method of a priori variable selection, based on previous medical literature and conventional knowledge with adjustment for established CV risk factors and exposure to variables previously associated with increased PWV (Supplementary material online). An interaction effect between gender and smoking/alcohol consumption behaviour was tested as well as a potential additive or multiplicative adverse effect of combination of bad behaviours on PWV at 17 years (Supplementary material online).
To assess smoking and alcohol exposure across the range of 13–17 years, adolescents with complete questionnaires for smoking behaviour and alcohol consumption were included in longitudinal analyses (Supplementary material online, Tables S1 and S2).
We replaced missing data for all variables of interest by multiple imputation using the MCMC method.10 Variables with <5% missing values were not imputed. Statistical analysis was performed using STATA package, version 11.1 (StataCorp, College Station, TX, USA). We deemed statistical significance at alpha = 0.05.
Results
Participant characteristics
There was no difference in either smoking or alcohol use between males and females (Table 1). At 17 years, 23.8% of participants were smokers. One hundred and fifty-four (14.2%) participants drank 0–2 drinks/typical day, 822 (75.6%) 3–9/typical day, and 111 (10.2%) >10 drinks/typical day. Seven hundred and twenty-four (66.6%) participants were classified as low-frequency drinkers [10 never drinkers, 203 monthly or less, 511 (2–4 times/month), and 363 (33.4%)] and were high-frequency drinkers (316 drank 2–3 times/week and 47 drank ≥4 times/week).
Table 1.
Demographic characteristics according to lifetime smoking exposure and drinking intensity groups
| ‘Low/never’ smoking exposure | ‘Moderate’ smoking exposure | ‘High’ smoking exposure | P-value | Low- intensity drinkers | Medium- intensity drinkers | High-intensity drinkers | P-value | |
|---|---|---|---|---|---|---|---|---|
| N (% males) | 710 (36.2) | 257 (29.2) | 281 (31.7) | 0.089 | 154 (31.8) | 822 (33.2) | 111 (36.04) | 0.768 |
| Socioeconomic status (I/V)% | 14.3/6.58 | 15.7/9.17 | 11.1/9.36 | 0.012 | 15.4/6.62 | 15.2/6.95 | 9.68/14 | 0.130 |
| Anthropometric | ||||||||
| Weight (kg) | 65.2 (11.9) | 66.3 (12.4) | 65.4 (12) | 0.450 | 66.2 (12.2) | 65.0 (11.9) | 68.3 (13.2) | 0.018 |
| Height (cm) | 171 (8.83) | 170 (8.25) | 170 (8.92) | 0.471 | 171.2 (9.4) | 171.3 (9.1) | 173.1 (9.0) | 0.010 |
| BMI (kg/m2) | 22.3 (3.44) | 23 (4.02) | 22.5 (3.71) | 0.048 | 22.9 (4.01) | 22.4 (3.53) | 23 (4.14) | 0.107 |
| Heart rate (b.p.m.)* | 64 (57.5–70.9) | 65.4 (59.6–71.6) | 64 (57.6–71.8) | 0.335 | 66 (58.3–71) | 63.8 (57.9–71) | 65.5 (57.8–71.3) | 0.746 |
| Risk factors | ||||||||
| Passive smoking, n (%) | 187 (26.3) | 61 (23.7) | 81 (28.8) | 0.046 | 44 (28.6) | 206 (25.1) | 34 (30.6) | 0.588 |
| Family history of CAD, n (%) | 47 (6.6) | 15 (5.8) | 18 (6.4) | 0.88 | 10 (6.5) | 55 (6.7) | 3 (2.7) | 0.351 |
| Physical activity (min)* | 446 (364–555) | 419 (365–535) | 489 (411–616) | 0.059 | 422 (352–553) | 455 (377–565) | 418 (359–497) | 0.897 |
| SBP (mmHg) | 116 (9.38) | 115 (8.99) | 115 (9.21) | 0.772 | 115 (9.79) | 115 (9.03) | 116 (9.84) | 0.508 |
| DBP (mmHg) | 63.4 (5.71) | 64.3 (5.74) | 63.9 (5.86) | 0.081 | 64.2 (6.41) | 63.5 (5.61) | 64.3 (6.33) | 0.191 |
| Lipids | ||||||||
| Tchol (mmol/L) | 3.8 (0.675) | 3.83 (0.668) | 3.75 (0.693) | 0.541 | 3.88 (0.803) | 3.81 (0.666) | 3.62 (0.617) | 0.044 |
| LDL (mmol/L) | 2.13 (0.615) | 2.13 (0.585) | 2.08 (0.654) | 0.651 | 2.2 (0.722) | 2.13 (0.609) | 2 (0.589) | 0.119 |
| HDL (mmol/L) | 1.28 (0.302) | 1.32 (0.336) | 1.3 (0.295) | 0.246 | 1.29 (0.32) | 1.29 (0.302) | 1.26 (0.325) | 0.801 |
| Triglycerides (mmol/L)* | 0.79 (0.620–1) | 0.8 (0.600–1) | 0.77 (0.625–1) | 0.793 | 0.76 (0.630–1.02) | 0.82 (0.620–1) | 0.75 (0.600–0.940) | 0.464 |
| Inflammatory markers | ||||||||
| CRP (mg/L)* | 0.6 (0.3–1.43) | 0.660 (0.360–1.28) | 0.695 (0.405–1.565) | 0.371 | 0.635 (0.390–1.55) | 0.62 (0.320–1.43) | 0.69 (0.330–1.4) | 0.896 |
| Liver function | ||||||||
| AST (U/L)* | 19.3 (16.6–22.6) | 18.8 (16.8–22.8) | 19.3 (16.5–22) | 0.615 | 18.9 (16.6–22.9) | 19.2 (16.5–22.4) | 19.7 (17–24.1) | 0.422 |
| ALT (U/L)* | 15.1 (11.7–19.2) | 14.4 (11.5–18.6) | 15 (11.8–19) | 0.809 | 14.5 (11.6–18.6) | 14.9 (11.6–18.7) | 17.2 (12.9–21.8) | 0.024 |
| GGT (U/L)* | 17 (13–21) | 16 (13–21) | 16 (13–21) | 0.930 | 17 (13–22) | 17 (13–21) | 18 (14–24) | 0.178 |
Comparisons between groups were performed using one-way analysis of variance (ANOVA) for continuous variables and χ2 test for categorical variables. Non-parametric variables (*) were log transformed for analysis to approximate normality. Continuous variables are presented as mean (SD). Values for non-parametric data are presented as median (IQR).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAD, coronary artery disease; CRP, C-reactive protein; DBP, diastolic blood pressure; GGT, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; SBP, systolic blood pressure; SD, standard deviation; Tchol, total cholesterol.
Smoking rates (P = 0.012) but not alcohol consumption (P = 0.130) in adolescence increased progressively from social Class I (high status) to V (manual unskilled). Parental smoking was associated with smoking in adolescence (P = 0.046). Physical activity did not differ across smoking and drinking intensity groups. Participants with higher alcohol intensity had increased weight but normal BMI, increased TC and mildly deranged liver function (Table 1). Following adjustment, drinking intensity was still related to increased ALT (P = 0.014).
Patterns of smoking behaviour during adolescence and arterial stiffness
Current smokers had increased arterial stiffness compared with non-smokers [mean difference 0.176 (0.058–0.293) m/s, P = 0.003]. Number of cigarettes smoked over lifetime (smoking intensity) was positively associated with PWV, with ‘high’ intensity smokers having higher PWV compared with low/never-smokers [5.81 ± 0.725 vs. 5.71 ± 0.677 m/s, mean difference 0.104 (0.01–0.199) m/s, P = 0.032]. This association remained after adjustment for other CV risk factors [mean increase in PWV for high smokers 3.7% or 0.211 (0.087–0.334) m/s, P = 0.001 compared with low/never smokers] (Table 2) and passive smoking (P = 0.004). Following imputation for missing values in covariates of interest [i.e. low-density lipoprotein (LDL), CRP, family history of coronary artery disease (CAD), physical activity, and passive smoking], the association of smoking intensity, and increased PWV remained significant [mean increase 0.122 (0.032–0.212), P = 0.008]. There was no interaction (Pforinteraction = 0.308) between gender and smoking exposure on vascular profile.
Table 2.
Multi-adjusted associations of smoking and drinking behaviour on arterial stiffness at 17 years
| N (% males) | Adjusted coefficient (95% CIs) | P-value* | P-value** | |
|---|---|---|---|---|
| Cross-sectional analysis | ||||
| Smoking intensity | 1248 (33.7) | 0.211 m/s (0.087/0.334) | 0.001 | 0.008 |
| Drinking intensity | 1087 (33.3) | 0.266 m/s (0.055/0.476) | 0.013 | 0.059 |
| Combined smoking and drinking | 1072 (33.5) | 0.603 m/s (0.229/0.978) | 0.002 | 0.006 |
| Longitudinal analysis | ||||
| Smokinga | 661 (28.8) | 0.313 m/s (0.01/0.618)c | 0.044 | 0.042 |
| Drinkingb | 1023 (34.02) | 0.029 m/s (−0.210/0.269)c | 0.812 | 0.401 |
All the associations were adjusted for gender, SES, CRP, BMI, SBP, and LDL in the cross-sectional analysis, plus height in the longitudinal analysis (*P-value). Additional adjustment for parental smoking, family history of CAD, and physical activity in the imputed sample is also provided (**P-value).
BMI, body mass index; CRP, C-reactive protein; LDL, low-density lipoprotein; SBP, systolic blood pressure; SES, parental socio-economic status.
A five-group variable was used to encode smoking behaviour during the adolescence as follows: smokers since 13 years, smokers since 15 years, smokers at 17 years, never smokers, and previous smokers that quit.
A five-group variable was used to encode drinking behaviour during the adolescence as follows: drinkers since 13 years, drinkers since 15 years, drinkers at 17 years, never drinkers, and previous drinkers that quit.
Coefficient corresponds to the comparison of never smokers/drinkers to smokers/drinkers since 13 years.
Of 661 participants with measurements of smoking status at 13, 15, and 17 years, longitudinal analysis indicated that never smokers (n = 269) had lower PWV at 17 years compared with those who had been smoking since 13 years [−0.313 (−0.01 to −0.618) m/s, P = 0.044] and to current smokers (i.e. at 17 years) [−0.196 (−0.034 to −0.357) m/s, P = 0.018]. The longitudinal effect of smoking across the period of 13–17 years on PWV was consistent across categories of increased duration [mean increase in PWV 0.143 (0.047–0.239) m/s per category, Pforlinear trend = 0.004] (Figure 2) and when adjusted for passive smoking [mean increase in PWV 0.211 (0.083–0.340) m/s per category, Pforlinear trend = 0.002] compared with never smokers (Table 2). Subjects (n = 91) who smoked between 13 and 17 years but who subsequently stopped, had comparable PWV with never smokers [mean difference −0.152 (−0.364 to 0.06), P = 0.160]. Finally, when missing values for longitudinal smoking status as well as for confounders were imputed, the association of increased duration of smoking with PWV at 17 years remained significant [mean expected increase 0.047 (0.01–0.089) m/s per category, P = 0.027] after adjustment. Adolescents who smoked since 13 or 15 years, presented an adjusted increase of 0.157 (0.01–0.308) m/s in PWV compared with participants who had never smoked (P = 0.042) (n = 1225) (Table 2).
Figure 2.
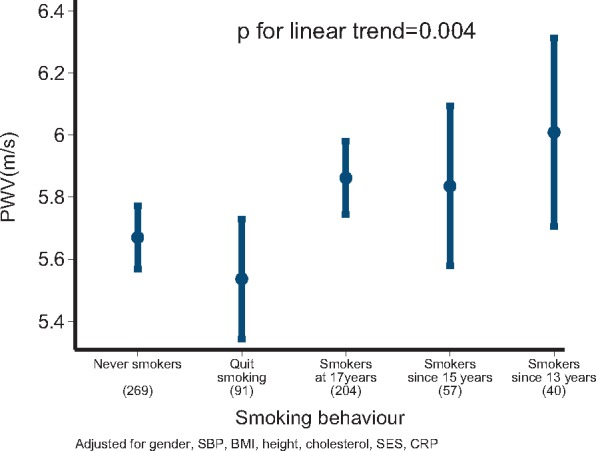
The longitudinal association between smoking exposure and arterial stiffness. Increased smoking exposure was associated with higher aortic pulse wave velocity compared with those who had never smoked. Participants who quit smoking had similar pulse wave velocity compared with those who never smoked. *P < 0.05. PWV, pulse wave velocity.
Patterns of alcohol consumption during adolescence and arterial stiffness
There was no association between age of starting drinking alcohol or frequency of drinking and PWV, whereas HI drinking was associated with increased PWV [HI 5.85 ± 0.8 vs. LI 5.67 ± 0.604 m/s, mean difference 0.182 (0.019–0.346) m/s, P = 0.029]. This association remained after adjustment for CV risk factors [relative mean increase 4.7% or 0.266 (0.055–0.476) m/s, P = 0.013] (Table 2). Complete analysis for imputed observations of drinking intensity at 17 years and additional exposure variables, including physical activity, revealed a similar association with increased PWV (P = 0.048) but additional adjustment for family history of CAD and parental smoking attenuated this effect (P = 0.059). There was no interaction between gender and alcohol consumption (P = 0.230). Increased drinking intensity as a proxy to binge drinking (≥10 drinks/typical drinking day) correlated with increased arterial stiffness [5.85 ± 0.8 vs. 5.7 ± 0.625 m/s, mean increase 0.147 (0.016–0.279), P = 0.028], even after controlling for gender, SBP, SES, LDL, BMI, CRP [mean increase 0.231 (0.057–0.405) m/s, P = 0.01]. In longitudinal analysis, drinking intensity was not associated with PWV (P > 0.05 for all categories) (Table 2).
Over a month period, adolescents consumed more beer than wine (P < 0.001) with no difference between consumption of beer- and spirit-based drinks (P = 0.426). Three hundred twenty-seven (26.7%) adolescents did not drink beer while 414 (33.8%), 371 (30.3%), and 112 (9.15%) were light, moderate, and heavy beer-drinkers. Almost half of participants, 558 (46.5%) did not consume wine, 458 (38.2%), 158 (13.16%), and 26 (2.17%) were light, moderate, and heavy wine-drinkers, respectively. Finally, 264 (21.48%) participants abstained from spirits in the last month, while 518 (42.14%), 372 (30.27%), and 75 (6.1%) adolescents were light, moderate, and heavy spirit-drinkers. Light and moderate wine drinking were associated, in univariate analysis, with decreased arterial stiffness (5.67 ± 0.677 m/s for light and 5.61 ± 0.649 m/s for moderate vs. 5.77 ± 0.684 m/s for no consumption, P = 0.02 and P = 0.008, respectively). Moderate and heavy consumption of beer were related to increased PWV (5.66 ± 0.672 m/s for moderate and 5.79 ± 0.690 m/s for heavy vs. 5.62 ± 0.597 m/s for non-consumers, P = 0.001 and P < 0.001, respectively) when compared with non-consumption. However, these associations lost their significance following adjustment for confounding exposure variables (P > 0.05 for all categories). Spirit drinking pattern was not associated with arterial stiffness at 17 years.
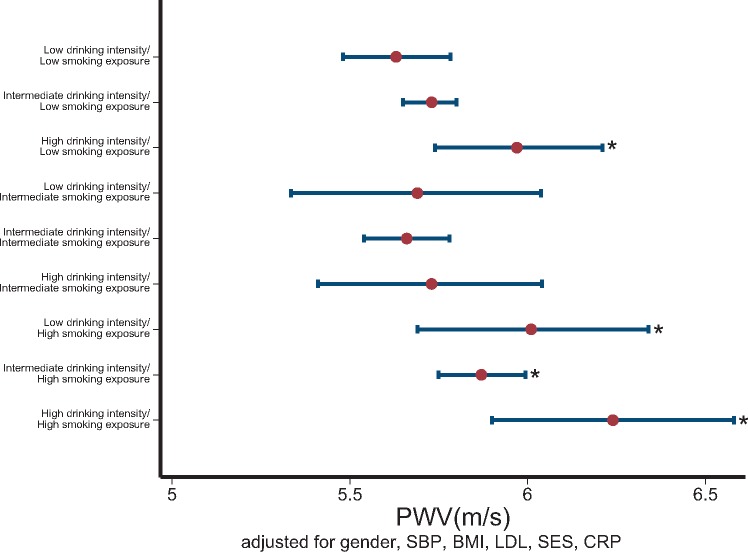
Higher alcohol intensity and smoking intensity had additive effects on arterial stiffness [high alcohol and smoking vs. never smokers and low alcohol; 5.89 ± 0.857 vs. 5.61 ± 0.589, mean increase 0.277 (0.028–0.525) m/s, P = 0.029] compared with never smoking and low alcohol consumption. After adjustment, the combined index of alcohol and smoking intensity remained an independent predictor of PWV [mean increase 10.8% or 0.603 (0.229–0.978) m/s in PWV, for adolescents with both high alcohol and smoking vs. never smokers and low alcohol, P = 0.002] (Table 2) (Figure 3). The association of the combined index of alcohol and smoking intensity with increased PWV remained significant after additional adjustment for parental smoking (P < 0.001) or imputation for missing values in confounders at 17 years [mean increase 0.331 (0.094–0.568) m/s, P = 0.006] (Table 2). There was a non-multiplicative combined effect of these unfavourable behaviours for the vascular profile of adolescents (P for interaction > 0.1 in both unadjusted and adjusted models, likelihood ratio test P > 0.1 for the addition of the interaction terms smoking*drinking intensity over the core model).
Figure 3.
The combined effect of smoking over lifetime and intensity of drinking on arterial stiffness. The combination of high-intensity drinking with lifetime smoking exposure is shown. Pulse wave velocity measurements are expressed as mean values and 95% confidence intervals around the mean on the x-axis. The participants who had ‘high’ drinking intensity and ‘high’ smoking exposure had the highest pulse wave velocity compared with the ‘low lifetime smoking exposure’ and ‘low drinking intensity’. *P < 0.05.
Take home figure.
Unhealthy behaviours in adolescents (drinking and cigarette smoking) are associated with increased carotid to femoral pulse wave velocity (stiffer arteries) and accelerated atherosclerosis. Stopping smoking in adolescence and reducing binge drinking has potential for reversibility of arterial stiffening.
Discussion
This study demonstrates that smoking and alcohol use up to age 17 years have both independent and additive associations with arterial stiffness, a marker of vascular damage that predicts later CV disease and events. Smoking in youth, even at low levels, was associated with increased arterial stiffness, but stopping during adolescence could restore arterial health. In addition, patterns of alcohol use had a significant impact on arterial stiffness with higher intensity, rather than frequency of consumption, showing the greatest adverse effect on PWV. These data demonstrate the importance of public health measures, which focus on preventing the establishment of unhealthy behaviours in children and adolescents.
Impact of smoking and excessive alcohol use on adverse CV outcomes in adults is well established.11,12 While most adult users of alcohol or tobacco first tried these ‘drugs’ during their early teens, the impact of smoking and alcohol consumption on the atherosclerotic process at this early stage of the life course is less clear. To evaluate the development of early atherosclerosis, we measured carotid to femoral PWV in a well-characterized cohort of 17 year olds. Previous studies have demonstrated that PWV is reproducible and, in adults, predicts CV outcomes independently from conventional risk factors.13
Epidemiological studies have consistently demonstrated that acute and chronic cigarette use adversely affects vascular health in adulthood and promotes atherosclerosis progression.14 We quantified exposure to cigarettes during adolescence and also assessed smoking frequency. Both current cigarette use and chronic smoking exposure had an adverse effect on arterial stiffness. This finding is consistent with reports in adults which show that the longer and earlier a person starts smoking, the higher the incidence of coronary artery disease and hypertension and the worse the impact on life expectancy.15 Passive smoking has consistently been shown to adversely affect arterial health in the young, however, no association with aortic stiffness in adolescence was demonstrated in our cohort.16 Exposure to parental smoking at home was assessed as binary variable, which precluded evaluation of potential differences in the degree in passive smoking.
The pathophysiological mechanisms linking smoking to vascular disease are still not fully elucidated.17,18 Tobacco contains many toxic and vasoactive compounds, which can exert damage on vascular endothelium and can activate inflammatory and thrombotic pathways relevant to CV events.17 Although cigarette smoking is discouraged in many countries with legislation and other initiatives, our findings confirm that smoking continues to be prevalent in adolescence in both genders in the UK. It was interesting that only a small percentage of our cohort was in the highest smoking category of >100 cigarettes in total. This is considerably less exposure than that of typical adult smokers, in whom life time exposure is measured in pack-years (≡20 cigarettes/day for 1 year). These data are consistent with a recent NHS survey, which demonstrated that the number of children trying cigarettes has fallen by three quarters since 2003. However, even at these low levels of smoking exposure, we could detect an adverse effect on arterial stiffness even when adjusted for other CV risk factors. Quitting smoking in adolescence could restore aortic stiffness, emphasizing the benefit and opportunity from implementing interventional and educational strategies in the young to preserve arterial health consistent with the recent European CV prevention guidelines.12
There are conflicting findings on the impact of alcohol consumption on atherosclerosis. Alcohol intake has been shown to prevent atherosclerosis in some animal studies, but not in all,19,20 and results from clinical studies are also inconsistent.21,22 High alcohol intake (>34 g/day) is associated with higher blood pressure, but observational evidence does not support an association of alcohol intake with blood pressure below this level.23 Mild alcohol consumption (≤2 alcoholic beverages/day), can independently improve endothelial function both in young healthy subjects and in those with Type 2 diabetes.24 In contrast, a Finnish study reported a direct linear adverse effect of alcohol consumption on carotid intima media thickness in the young, from as little as two alcoholic drinks per day.25 In support of this, a recent Mendelian randomization meta-analysis implicated alcohol consumption as a causal factor for CV disease.11
We used a combination of frequency and intensity questionnaires to describe better patterns of alcohol use. The age at which participants started consuming alcohol was not associated with vascular stiffness, suggesting that duration of exposure might not be that important at this young age, and this was confirmed in our longitudinal analysis. In contrast, higher intensity rather than frequency of alcohol consumption had the greatest adverse relationship with arterial stiffness. This finding is of particular concern since excessive drinking, with the aim of getting drunk, is increasingly the norm for teenagers.26
The mechanistic link between alcohol and arterial stiffness is not well explored. Light to moderate alcohol consumption is associated with an increase in HDL-C, a decrease in inflammatory mediators and improvement in metabolic pathways.27,28 In contrast, excess alcohol consumption has been associated with elevation in blood pressure, autonomic dysregulation, and derangements in coagulation and fibrinolytic pathways.27 We did not find any beneficial effect of alcohol consumption on arterial stiffness even at lower consumption levels. When we separated different alcoholic beverages, light and moderate wine drinking groups and moderate to heavy beer drinkers were associated with lower and higher PWV, respectively when compared with non-consumers. However, these associations were not significant in multivariable analysis, implying that drinking intensity rather than type of alcoholic beverage may be more important. The association between alcohol use and CV risk factors was modest and only mild derangements in liver function tests were noted in participants with high alcohol intensity.29 Interestingly, lower heart rate was noticed in adolescents with high drinking intensity and this might represent autonomic dysregulation.30
Our study has limitations as it is observational, so that causal associations between smoking, alcohol exposure, and arterial stiffness cannot be established. We relied on self-reported confidential questionnaires, collected every 2 years through adolescence, without any biomarker-based assessment of smoking or alcohol exposure. Although self-report of smoking and alcohol behaviour has been shown to be a valid measure compared with biochemical measures, we cannot exclude the possibility that some of our participants may have been misclassified, particularly those from the heavier exposure categories. This however, would be likely to result in underestimation of the effect sizes observed. The presence of unmeasured or residual confounders on our results cannot be excluded. In addition, a number of missing data were imputed in order not to reduce the power of our multivariable longitudinal analysis. This is a well-established statistical approach. Nevertheless, despite these limitations, we were able to demonstrate important associations between patterns of smoking and drinking use in adolescence with arterial stiffness.
In summary, in this large contemporary British cohort of adolescents, we have demonstrated that drinking intensity and smoking in adolescence, even at lower levels compared with those reported in adult studies, is associated with arterial changes relevant to atherosclerosis progression. The effect of these unhealthy behaviours was independent of one another and additive. Smoking cessation in adolescence was associated with normalization of aortic stiffness. These findings have significant public health implications and provide further support for public health measures to discourage young adults adopting smoking and drinking habits and the benefit of discontinuing these unhealthy behaviours.
Supplementary Material
Acknowledgements
The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALPSAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. G.D.S. and D.L. work within a unit supported by the MRC(MC_UU_00011/1,MC_UU_00011/6).
Funding
The UK Medical Research Council (MRC), British Heart Foundation (BHF), the Wellcome Trust, and the University of Bristol provide core support for the ALSPAC.
Conflict of interest: none declared.
Footnotes
See page 354 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy430)
References
- 1. Willett WC, Green A, Stampfer MJ, Speizer FE, Colditz GA, Rosner B, Monson RR, Stason W, Hennekens CH.. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med 1987;317:1303–1309. [DOI] [PubMed] [Google Scholar]
- 2. Roerecke M, Rehm J.. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med 2014;12:182.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stockwell T, Zhao J, Panwar S, Roemer A, Naimi T, Chikritzhs T.. Do “moderate” drinkers have reduced mortality risk? A systematic review and meta-analysis of alcohol consumption and all-cause mortality. J Stud Alcohol Drugs 2016;77:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K.. Alcohol and coronary heart disease: a meta‐analysis. Addiction 2000;95:1505–1523. [DOI] [PubMed] [Google Scholar]
- 5. Azagba S, Baskerville NB, Minaker L.. A comparison of adolescent smoking initiation measures on predicting future smoking behavior. Prev Med Rep 2015;2:174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulenberg JE, Maggs JL.. A developmental perspective on alcohol use and heavy drinking during adolescence and the transition to young adulthood. J Stud Alcohol Suppl 2002;54–70. [DOI] [PubMed] [Google Scholar]
- 7. Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Lim C, Brener ND, Wechsler H.. Youth risk behavior surveillance—United States, 2007. MMWR Surveill Summ 2008;57:1–131. [PubMed] [Google Scholar]
- 8. Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, Ring S, Nelson SM, Lawlor DA.. Cohort profile: the Avon Longitudinal Study of Parents and Children: aLSPAC mothers cohort. Int J Epidemiol 2013;42:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falaschetti E, Hingorani AD, Jones A, Charakida M, Finer N, Whincup P, Lawlor DA, Smith GD, Sattar N, Deanfield JE.. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. Eur Heart J 2010;31:3063–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Buuren S, Boshuizen HC, Knook DL.. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681–694. [DOI] [PubMed] [Google Scholar]
- 11. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA.. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C.. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016;252:207–274. [DOI] [PubMed] [Google Scholar]
- 13. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang K-L, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB.. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kubozono T, Miyata M, Ueyama K, Hamasaki S, Kusano K, Kubozono O, Tei C.. Acute and chronic effects of smoking on arterial stiffness. Circ J 2011;75:698–702. [DOI] [PubMed] [Google Scholar]
- 15. Jonas MA, Oates J, Ockene J, Hennekens C.. Statement on smoking and cardiovascular disease for health care professionals. American Heart Association. Circulation 1992;86:1664–1669. [DOI] [PubMed] [Google Scholar]
- 16. Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE.. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 1996;334:150–155. [DOI] [PubMed] [Google Scholar]
- 17. Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis 2003;46:91–111. [DOI] [PubMed] [Google Scholar]
- 18. Doonan RJ, Hausvater A, Scallan C, Mikhailidis DP, Pilote L, Daskalopoulou SS.. The effect of smoking on arterial stiffness. Hypertens Res 2010;33:398–410. [DOI] [PubMed] [Google Scholar]
- 19. Emeson EE, Manaves V, Emeson BS, Chen L, Jovanovic I.. Alcohol inhibits the progression as well as the initiation of atherosclerotic lesions in C57Bl/6 hyperlipidemic mice. Alcohol Clin Exp Res 2000;24:1456–1466. [PubMed] [Google Scholar]
- 20. Bentzon JF, Skovenborg E, Hansen C, Møller J, de Gaulejac NS-C, Proch J, Falk E.. Red wine does not reduce mature atherosclerosis in apolipoprotein E-deficient mice. Circulation 2001;103:1681–1687. [DOI] [PubMed] [Google Scholar]
- 21. Van Trijp MJ, Beulens JW, Bos WJ, Uiterwaal CS, Grobbee DE, Hendriks HF, Bots ML.. Alcohol consumption and augmentation index in healthy young men: the ARYA study. Am J Hypertens 2005;18:792–796. [DOI] [PubMed] [Google Scholar]
- 22. Mukamal KJ, Kronmal RA, Mittleman MA, O’Leary DH, Polak JF, Cushman M, Siscovick DS.. Alcohol consumption and carotid atherosclerosis in older adults: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 2003;23:2252–2259. [DOI] [PubMed] [Google Scholar]
- 23. Marmot MG, Elliott P, Shipley MJ, Dyer AR, Ueshima H, Beevers DG, Stamler R, Kesteloot H, Rose G, Stamler J.. Alcohol and blood pressure: the INTERSALT study. BMJ 1994;308:1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Puddey I, Zilkens R, Croft K, Beilin L.. Alcohol and endothelial function: a brief review. Clin Exp Pharmacol Physiol 2001;28:1020–1024. [DOI] [PubMed] [Google Scholar]
- 25. Juonala M, Viikari JSA, Kähönen M, Laitinen T, Taittonen L, Loo B-M, Jula A, Marniemi J, Räsänen L, Rönnemaa T, Raitakari OT.. Alcohol consumption is directly associated with carotid intima-media thickness in Finnish young adults: the Cardiovascular Risk in Young Finns Study. Atherosclerosis 2009;204:e93–e98. [DOI] [PubMed] [Google Scholar]
- 26. Seaman P, Ikegwuonu T.. Drinking to Belong: Understanding Young Adults’ Alcohol Use Within Social Networks. York: Joseph Rowntree Foundation; 2010. [Google Scholar]
- 27. Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ.. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ 1999;319:1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sierksma A, Patel H, Ouchi N, Kihara S, Funahashi T, Heine RJ, Grobbee DE, Kluft C, Hendriks HF.. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-α, and insulin sensitivity. Diabetes Care 2004;27:184–189. [DOI] [PubMed] [Google Scholar]
- 29. Mathews MJ, Liebenberg L, Mathews EH.. The mechanism by which moderate alcohol consumption influences coronary heart disease. Nutr J 2015;14:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evans BE, Greaves-Lord K, Euser AS, Tulen JH, Franken IH, Huizink AC.. Alcohol and tobacco use and heart rate reactivity to a psychosocial stressor in an adolescent population. Drug Alcohol Depend 2012;126:296–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.