Abstract
Aims
The E3-ligase CBL-B (Casitas B-cell lymphoma-B) is an important negative regulator of T cell activation that is also expressed in macrophages. T cells and macrophages mediate atherosclerosis, but their regulation in this disease remains largely unknown; thus, we studied the function of CBL-B in atherogenesis.
Methods and results
The expression of CBL-B in human atherosclerotic plaques was lower in advanced lesions compared with initial lesions and correlated inversely with necrotic core area. Twenty weeks old Cblb−/−Apoe−/− mice showed a significant increase in plaque area in the aortic arch, where initial plaques were present. In the aortic root, a site containing advanced plaques, lesion area rose by 40%, accompanied by a dramatic change in plaque phenotype. Plaques contained fewer macrophages due to increased apoptosis, larger necrotic cores, and more CD8+ T cells. Cblb−/−Apoe−/− macrophages exhibited enhanced migration and increased cytokine production and lipid uptake. Casitas B-cell lymphoma-B deficiency increased CD8+ T cell numbers, which were protected against apoptosis and regulatory T cell-mediated suppression. IFNγ and granzyme B production was enhanced in Cblb−/−Apoe−/− CD8+ T cells, which provoked macrophage killing. Depletion of CD8+ T cells in Cblb−/−Apoe−/− bone marrow chimeras rescued the phenotype, indicating that CBL-B controls atherosclerosis mainly through its function in CD8+ T cells.
Conclusion
Casitas B-cell lymphoma-B expression in human plaques decreases during the progression of atherosclerosis. As an important regulator of immune responses in experimental atherosclerosis, CBL-B hampers macrophage recruitment and activation during initial atherosclerosis and limits CD8+ T cell activation and CD8+ T cell-mediated macrophage death in advanced atherosclerosis, thereby preventing the progression towards high-risk plaques.
Keywords: Atherosclerosis, Innate and adaptive immune system, Macrophages, T cells, CBL-B
Introduction
Atherosclerosis, a lipid-driven inflammatory disease of the large arteries, is the underlying cause of the majority of cardiovascular diseases (CVD).1 Although primary and secondary preventive strategies have significantly lowered the incidence of CVD, atherosclerosis remains a major cause of morbidity and mortality.2 Additional therapeutic strategies, which target the residual cardiovascular risk that persists after optimal pharmacological treatment, are therefore required.2 In addition to dyslipidaemia, immune cell activation and subsequent inflammation drive atherogenesis.1–3 Inhibition of atherosclerosis-associated inflammation is therefore a strategy with a great therapeutic potential, as highlighted by the CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) trial, in which antibody-mediated inhibition of interleukin (IL)-1β reduced the incidence of recurrent CVD in patients with a previous myocardial infarction and high residual inflammatory risk.4
T cells constitute a variable but substantial proportion of the immune cell population in the atherosclerotic plaque and are significant drivers of the inflammatory responses that underlie atherosclerosis.1,3 CD4+ T cells are the predominant T cell subset in atherosclerotic lesions of Apolipoprotein E-deficient (Apoe−/−) mice. However, subsets of CD4+ T cells contribute differently to atherosclerosis.1 While T helper (Th)1 cells are considered pro-atherosclerotic, Th2 cells are still controversially discussed.1 Regulatory T cells (Tregs) are considered protective in atherosclerosis through the release of transforming growth factor (TGF)β and IL10.1 The function of CD8+ cytotoxic T cells in atherosclerosis is incompletely understood; however, they appear proatherogenic and are abundantly present in advanced human atherosclerotic lesions.3 Transfer of CD8+ T cells accelerates atherosclerosis and leads to a vulnerable plaque phenotype in Apoe−/− mice, whereas antibody-mediated depletion of CD8+ T cells impedes the formation of atherosclerotic lesions.3,5,6 Despite the well-described functions of T cell subsets in atherosclerosis, the regulatory mechanisms by which they undergo activation and polarization during atherogenesis are less extensively studied.
The Casitas B-cell lymphoma (CBL) E3 ubiquitin ligases—comprising CBL-B, C-CBL, and CBL-C—form one of the protein families that modulate T cell activation and polarization.7Casitas B-cell lymphoma-B promotes T cell tolerance through ubiquitination and degradation of downstream effectors, such as phosphoinositide phospholipase Cγ and phosphoinositide 3-kinase, and thus is a negative regulator of T cell activation.7,8Casitas B-cell lymphoma-B-deficient T cells have a hyper responsive phenotype that is accompanied by CD28-independent activation, due to their lower threshold for T cell receptor-mediated responses.9 Further, these T cells mount delayed responses to anergic signals, contributing to a state of hyper responsiveness.10
Notably, macrophages, an important cell type that abounds in atherosclerotic plaques, also expresses CBL-B, the function of which in this cell type remains incompletely described.7Casitas B-cell lymphoma-B deficiency is linked to enhanced toll-like receptor (TLR)4 signalling and increased macrophage activation and migration in diet-induced obesity11 and lung inflammation models,12 processes that are also relevant for the atherosclerosis.
Considering the significant regulatory activity of CBL-B in T cell and macrophage biology, we evaluated the expression pattern of CBL-B in human atherosclerotic lesions and investigated the function of CBL-B in experimental atherosclerosis.
Translational perspective
In this study, we demonstrate that the E3-ligase Casitas B-cell lymphoma-B (CBL-B) is expressed in human atherosclerotic plaques, and that its expression decreases with plaque progression. Using an atherosclerotic mouse model, we found that CBL-B exerts profound anti-atherogenic effects by regulating CD8+ T cell and macrophage activation. Activation of CBL-B, therefore, represents a promising anti-inflammatory therapeutic strategy in atherosclerosis.
Methods
Human studies
Coronary artery specimens were obtained from autopsy from the Department of Pathology of the Amsterdam UMC and immediately fixed in 10% formalin and processed for paraffin embedding. All use of tissue was in agreement with the ‘Code for Proper Secondary Use of Human Tissue in the Netherlands’. CBL-B expression was analysed by immunohistochemistry, as described in the Supplementary material online. Gene expression of CBL-B in human atherosclerosis was examined by microarray-based transcriptional profiling of carotid endarterectomy specimens (BiKE dataset13,14).
Animal studies
Male Cblb−/−Apoe−/− and Apoe−/− mice were bred and housed at the animal facility of the University of Amsterdam and kept on a normal chow diet. All mice were treated according to the study protocol (permit nos. 102601 and 102869) that were approved by the Committee for Animal Welfare of the University of Amsterdam, the Netherlands. Detailed methods are provided in the Supplementary material online.
Results
Casitas B-cell lymphoma-B co-localizes with macrophages and T cells in human atherosclerotic plaques
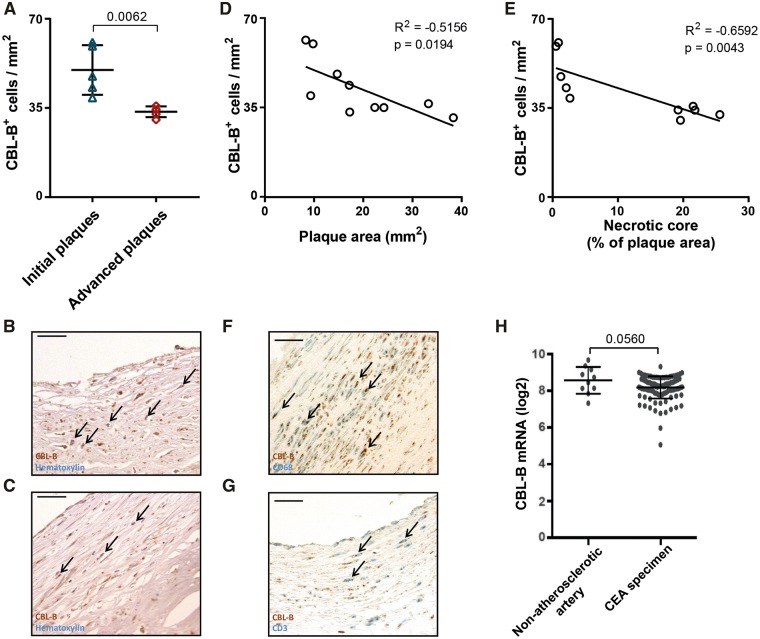
Human coronary atherosclerotic plaques, histologically classified as intimal xanthomas or pathological intimal thickenings (initial/intermediate atherosclerosis) expressed higher levels of CBL-B+ cells when compared with fibrous cap atheromata (advanced atherosclerosis) (Figure 1A–C). A negative correlation between plaque area and CBL-B expression (Figure 1D), and necrotic core area and CBL-B was observed (Figure 1E), indicating that CBL-B expression in the plaque decreased during the progression of atherosclerosis. The majority of CBL-B+ cells were CD68+ macrophages (Figure 1F) and CD3+ T cells (Figure 1G), whereas only few intraplaque vascular smooth muscle cells (VSMCs) and endothelial cells expressed CBL-B (data not shown).
Figure 1.
Casitas B-cell lymphoma-B is expressed in human atherosclerotic lesions and co-localizes with macrophages and T cells. (A) Immunohistochemical analysis of CBL-B expression in initial/intermediate and advanced human coronary atherosclerotic lesions. The percentage of CBL-B+ cells in the lesion decreased in the advanced atherosclerotic plaques (n = 5 per plaque phenotype). Representative pictures of (B) CBL-B expression in initial/intermediate and (C) advanced lesions are shown. Arrows indicate CBL-B+ cells. A negative correlation between (D) CBL-B expression and plaque area and (E) CBL-B and necrotic core area was observed. CBL-B expression co-localized with CD68+ cells (F) and CD3+ cells (G), arrows indicate CBL-B+CD68+ or CBL-B+CD3+ cells, respectively. Scale bar 25 μm for all pictures. (H) BiKE database: CBL-B mRNA expression in carotid endarterectomy specimens (n = 127) when compared with non-atherosclerotic arteries (n = 10). Data are presented as mean ± standard deviation.
To further evaluate the expression of CBL-B in human atherosclerosis, gene expression of CBL-B in carotid endarterectomy specimens was examined by microarray-based transcriptional profiling (BiKE dataset13,14) Carotid atherosclerotic lesions had a tendency to express less CBL-B mRNA when compared with non-atherosclerotic arteries (P = 0.056) (Figure 1H). Casitas B-cell lymphoma-B was not differentially expressed between atherosclerotic plaques from symptomatic and asymptomatic patients (data not shown), indicating that CBL-B predominantly affects plaque development and not plaque rupture.
Casitas B-cell lymphoma-B deficiency aggravates atherosclerosis in Apoe−/− mice
Casitas B-cell lymphoma-B is expressed in CD68+ macrophages and CD3+ T cells in murine atherosclerotic plaques (Supplementary material online, Figure S1). To study the function of CBL-B in atherosclerosis, Cblb−/−Apoe−/− and Apoe−/− mice were generated and fed a normal chow diet for 20 weeks. The extent and phenotype of atherosclerosis was determined in the aortic arch and the aortic root (Figure 2A). Body weight or basic haematologic parameters did not differ between genotypes (Supplementary material online, Table S1). Histological analysis of over 20 organs revealed no abnormalities, particularly no signs of autoimmunity, in Cblb−/−Apoe−/− or Apoe−/− mice.
Figure 2.
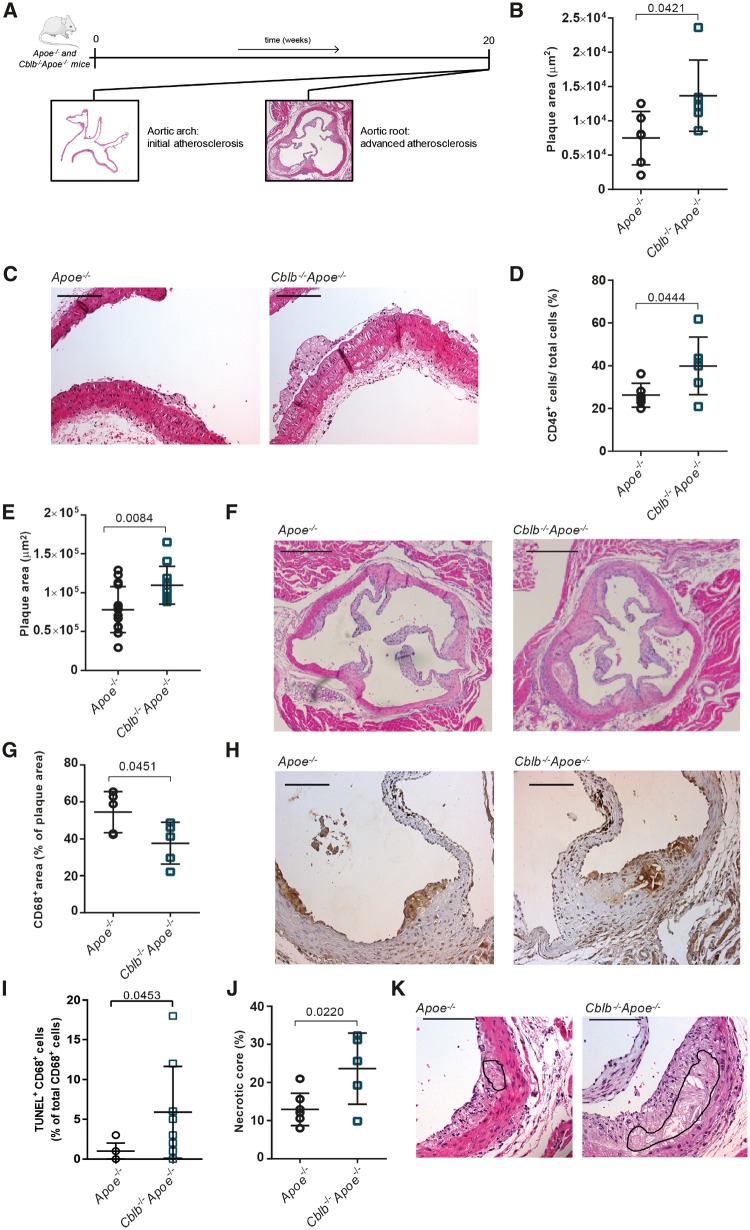
Casitas B-cell lymphoma-B deficiency aggravates atherosclerosis in Apoe−/− mice. (A) Atherosclerosis was analysed in the aortic arch, where initial plaques were present, and the aortic root, which contained advanced atherosclerotic plaques. (B) Atherosclerotic plaque area in the aortic arch of 20-week-old Apoe−/− (n = 6) and Cblb−/−Apoe−/− (n = 6) mice. (C) Representative longitudinal sections of aortic arches in Apoe−/− and Cblb−/−Apoe−/− mice (the brachiocephalic trunk is shown; haematoxylin and eosin staining). Scale bar: 50 μm. (D) Immunohistochemical quantification of the relative number of CD45+ cells per plaque (n = 6 per genotype). (E) Aortic roots of 20-week-old Apoe−/− (n = 15) and Cblb−/−Apoe−/− (n = 11) mice were used to analyse the amount of atherosclerosis. (F) Representative pictures of haematoxylin and eosin-stained aortic root cross-sections containing advanced atherosclerotic plaques in Apoe−/− and Cblb−/−Apoe−/− mice. Scale bar: 500 μm. (G, H) Plaque macrophage content and representative images of CD68 staining. Scale bar: 200 μm. (I) Percentage of apoptotic (TUNEL+CD68+) macrophages in the plaques. (J, K) Quantification of necrotic core area in plaques of aortic roots. Representative pictures are shown. The black line indicates the necrotic core. Scale bar: 100 μm. Data are presented as mean ± standard deviation.
Deficiency of CBL-B increased atherosclerotic plaque area in the aortic arch and its main branch points by 1.8-fold (Figure 2B and C). Most plaques in the aortic arch were early, macrophage rich lesions (Figure 2C). Immunohistochemistry demonstrated that the plaques of Cblb−/−Apoe−/− mice contained significantly more CD45+ cells (Figure 2D), reflecting a more inflammatory plaque phenotype.
Plaques in the aortic roots of Cblb−/−Apoe−/− and Apoe−/− mice were not only larger (Figure 2E), but also displayed hallmarks of advanced stages of atherosclerosis, especially necrotic core formation (Figure 2F). Deficiency of CBL-B resulted in a 1.4-fold increase in atherosclerotic plaque area. Plaques in the aortic root of Cblb−/−Apoe−/− mice contained fewer CD68+ macrophages when compared with Apoe−/− mice (Figure 2G and H), which resulted from increased macrophage apoptosis (Figure 2I) and a subsequent increase in necrotic core area (Figure 2J and K). In line with the more advanced plaque phenotype, collagen content increased in plaques of Cblb−/−Apoe−/− mice (30.4 ± 2.6% Apoe−/− vs. 45.0 ± 3.8% Cblb−/−Apoe−/−), whereas plaque VSMC content (2.1 ± 0.3 Apoe−/− vs. 2.0 ± 0.1% Cblb−/−Apoe−/−) did not differ. Thus, deficiency of CBL-B increased plaque inflammation and macrophage death, thereby accelerating the progression of atherosclerosis.
Casitas B-cell lymphoma-B deficiency induces an atherogenic phenotype in macrophages
Considering the profound increase in early, macrophage-rich lesions observed in the aortic arch and incremented necrotic core formation in the more advanced stages of atherosclerosis in Cblb−/−Apoe−/− mice, we analysed the effects of CBL-B on monocytes and macrophages.
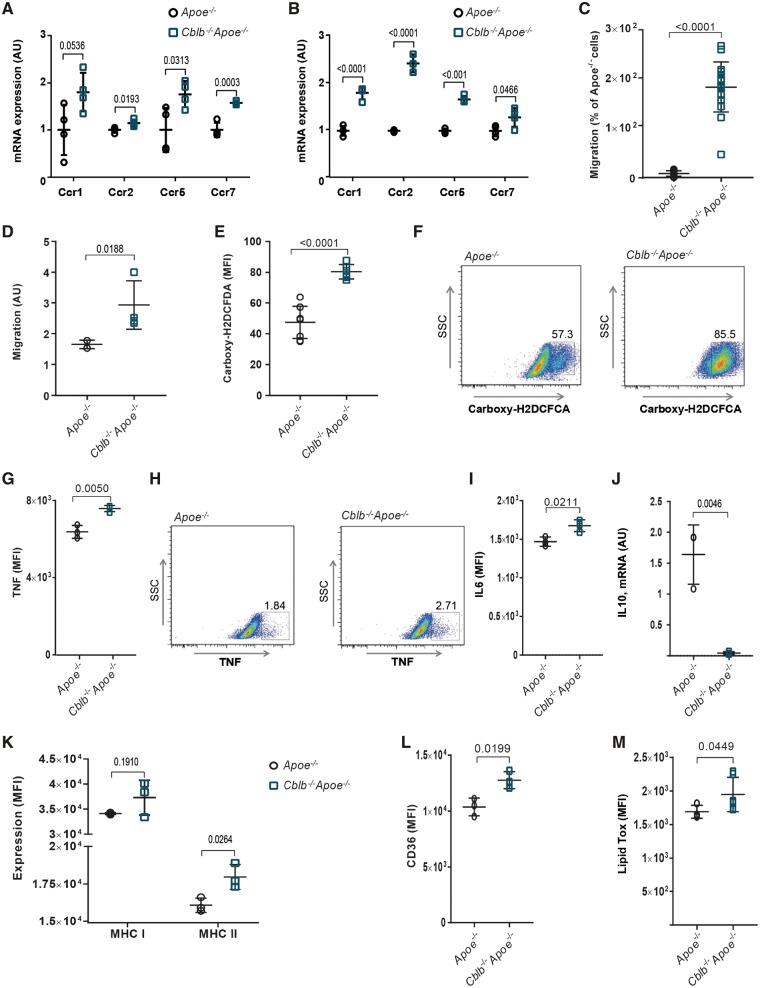
Deficiency of CBL-B increased the expression of the chemokine receptors CCR1, CCR2, and CCR5, all of which mediate leucocyte recruitment into the arterial wall, in primary monocytes and bone marrow-derived macrophages (BMDMs) (Figure 3A and B). Transcript levels of CCR7, a chemokine receptor that governs macrophage egress in atherosclerosis,15 also increased (Figure 3A and B). Consistent with these findings, Cblb−/−Apoe−/− monocytes and BMDMs exhibited an increased migratory capacity towards CCL2 (Figure 3C and D).
Figure 3.
Casitas B-cell lymphoma-B deficiency induces an atherogenic phenotype in macrophages. Quantification of mRNA expression of chemokine receptors CCR1, 2, 5, and 7 in monocytes (A) and bone marrow-derived macrophages (B) of Apoe−/− (n = 4) and Cblb−/−Apoe−/− (n = 4) mice. (C) CCL2-induced monocyte migration was increased in Cblb−/−Apoe−/− mice (n = 16 per genotype). (D) Migration of bone marrow-derived macrophages from Apoe−/− and Cblb−/−Apoe−/− mice towards 10 ng/mL MCP-1 by transwell assay (n = 3 experiments). (E, F) Flow cytometric analysis of reactive oxygen species production by Apoe−/− (n = 8) and Cblb−/−Apoe−/− (n = 6) bone marrow-derived macrophages after 48 h LPS stimulation. Representative dot plots; numbers indicate percentage of bone marrow-derived macrophages positive for carboxy-H2DCFCA. Representative dot plot and graph of TNF (G, H) and interleukin-6 (I) production after 24 h exposure to oxLDL (n = 3 experiments). (J) mRNA expression of interleukin-10 in bone marrow-derived macrophages from Apoe−/− and Cblb−/−Apoe−/− mice after 24 h exposure to oxLDL (n = 3 experiments). Flow cytometric analysis of MHC-I and MHC-II expression (K) and CD36 expression (L) of bone marrow-derived macrophages (n = 3 experiments). (M) Flow cytometric analysis of lipid uptake in bone marrow-derived macrophages (n = 6). Data are presented as mean ± standard deviation.
Lipopolysaccharide stimulated Cblb−/−Apoe−/− BMDMs produced significantly more reactive oxygen species (ROS) (Figure 3E and F), TNF (Figure 3G and H), and IL6 (Figure 3I), whereas IL10 expression was reduced (Figure 3J). Moreover, CBL-B-deficient BMDMs expressed significantly more MHC-II, pointing towards increased antigen presenting capacity of these cells (Figure 3K). Expression of the M1 macrophage marker iNOS was increased in aortic arch lysates of Cblb−/−Apoe−/− mice, the M2 markers arginase 1 and CD206 were not affected (Supplementary material online, Figure S2A).
Upon phagocytosis and cytoplasmic storage of lipoproteins, macrophages evolve into foam cells, the predominant constituent of atherosclerotic plaques. Notably, CBL-B transcript levels decreased during foam cell formation (Supplementary material online, Figure S2B). Casitas B-cell lymphoma-B-deficient BMDMs expressed higher protein levels of the scavenger receptor CD36 (Figure 3L) and ingested significantly more oxLDL (Figure 3M), whereas the cholesterol efflux genes ABCA1 and ABCG1 were not affected (Supplementary material online, Figure S2C). Thus, deficiency of CBL-B enhanced the migratory potential of macrophages, promoted the expression of inflammatory mediators and increased lipid uptake, resulting in an atherogenic macrophage phenotype.
Casitas B-cell lymphoma-B deficiency increases the abundance of CD8+ T cells by reducing apoptosis and regulatory T-cells-mediated suppression
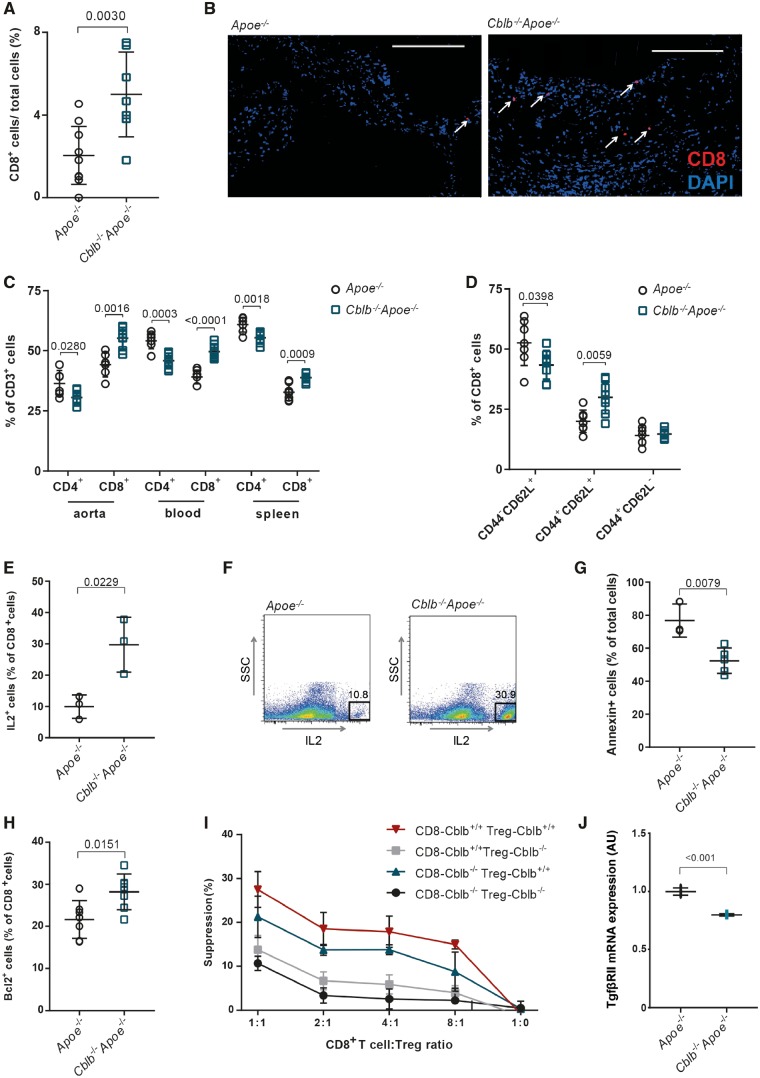
As CBL E3 ubiquitin ligases modulate T cell activation and polarization, we investigated T cell appearance in plaques of Cblb−/−Apoe−/− and Apoe−/− mice. Immunohistochemistry demonstrated a trend towards increased CD3+ T cell abundance in the advanced plaques of the aortic roots of Cblb−/−Apoe−/− mice (8.0 ± 3.2% Apoe−/− vs. 12.0 ± 3.2% Cblb−/−Apoe−/−; P = 0.08), specifically due to a significant increase in cytotoxic CD8+ T cells (2.05 ± 1.41% Apoe−/− vs. 5.00 ± 2.05% Cblb−/−Apoe−/−; P = 0.003) (Figure 4A and B). These findings were supported by flow cytometry, verifying skewing towards more CD8+ T cells in the aortic arch, blood and spleen (Figure 4C). In the absence of CBL-B, CD8+ T cells shifted from naïve (CD44−CD62L+) to central memory T cells (CD44+CD62L+) with no differences in the effector memory T cell compartment (CD62L−CD44+) (Figure 4D), suggesting enhancement of their activation status.
Figure 4.
Casitas B-cell lymphoma-B deficiency increases CD8+ T cell abundance in Cblb−/−Apoe−/− mice by reducing apoptosis and regulatory T cell-mediated suppression. (A) Percentages of CD8+ T cells in advanced atherosclerotic plaques of aortic roots of 20-week-old Apoe−/− (n = 10) and Cblb−/−Apoe−/− mice (n = 7). (B) Representative pictures of anti-CD8 (Alexa Fluor 594, red) staining (DAPI staining: blue). White arrows indicate CD8+ T cells. Scale bar: 100 μm. (C) Flow cytometric analysis of CD4+ and CD8+ T cells in aortic arch, blood, and spleen of Apoe−/− and Cblb−/−Apoe−/− mice (n = 7) (D) Quantification of naïve (CD44−CD62L+), central memory (CD44+CD62L+), and effector memory (CD44+CD62L−) CD8+ T cells in spleens of Apoe−/− and Cblb−/−Apoe−/− mice. (E, F) interleukin-2 production by CD8+ T cells isolated from in vitro-restimulated splenocytes (n = 3), Representative dot plots are shown. (G) Fraction of apoptotic (Annexin V+) cells of CD3/CD28-activated isolated splenic CD8+ T cells from Apoe−/− (n = 3) or Cblb−/−Apoe−/− (n = 5) mice that were incubated with TNF for 96 h. (H) Flow cytometric analysis of BCL2 expression in CD8+ T cells (n = 7). (I) Regulatory T cell suppression assay using splenic CD8+ T cells and CD4+CD25+ regulatory T cells from Apoe−/− and Cblb−/−Apoe−/− mice, co-cultured at various ratios (n = 3 experiments). (J) TGFβRII mRNA expression in CD8+ T-cells isolated from Apoe−/− and Cblb−/−Apoe−/− mice (n = 3). Data are presented as mean ± standard deviation.
Next, we studied the potential mechanism underlying the increased abundance of CD8+ T cells and found that CBL-B deficiency enhanced the production of IL2 (Figure 4E and F), a potent growth factor for T cells. Casitas B-cell lymphoma-B-deficient CD8+ T cells were also protected against TNF-induced apoptosis as demonstrated by lower expression of annexin V (Figure 4G). Corroborating this finding, more Cblb−/−Apoe−/− CD8+ T cells contained the anti-apoptotic B cell lymphoma 2 (Bcl2) protein (Figure 4H). Moreover, Cblb−/−Apoe−/− CD8+ T cells were more resistant to Treg-mediated suppression than Apoe−/− CD8+ T cells and underwent more vigorous proliferation at various CD8+ T cell:Treg ratios (Figure 4I). Additionally, TGFβ receptor II (TgfβR2) gene expression was decreased in Cblb−/−Apoe−/− CD8+ T cells, rendering them less sensitive to TGFβ-induced Treg-mediated suppression16 (Figure 4J).
Casitas B-cell lymphoma-B deficiency increases the cytotoxicity of CD8+ T cells and provokes macrophage death
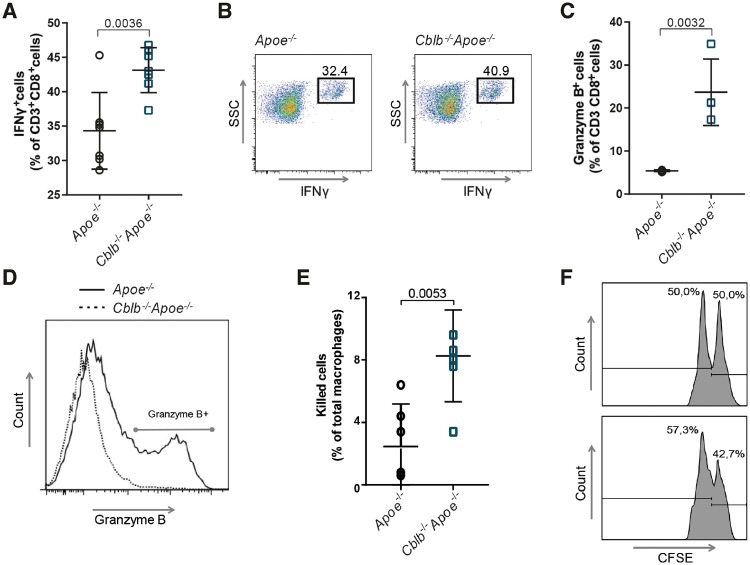
To further characterize, the effects of CBL-B deficiency on cytotoxic T cell function, splenic CD8+ T cells were isolated and the production of effector proteins was analysed. Cblb−/−Apoe−/− CD8+ T cells showed a significant increase in IFNγ protein levels compared with Apoe−/− CD8+ T cells (Figure 5A and B). Moreover, CBL-B-deficient cytotoxic T cells expressed higher levels of granzyme B (Figure 5C and D), a protein described to promote atherosclerosis by inducing apoptosis in plaque-associated cells.5 Perforin (Apoe−/− 1.0 ± 0.1 vs. Cbl-b−/−Apoe−/− 1.2 ± 0.3) and granzyme A (Apoe−/− 0.9 ± 0.1 vs. Cblb−/−Apoe−/− 1.2 ± 0.3) levels remained unchanged.
Figure 5.
Casitas B-cell lymphoma-B deficiency increases the inflammatory and cytotoxic propensity of CD8+ T cells. IFNγ (A, B) and granzyme B (C, D) producing CD3+CD8+ cells among in vitro-restimulated splenocytes isolated from Apoe−/− and Cblb−/−Apoe−/− mice (n = 7 for IFNγ; n = 4 for Granzyme B). Representative dot plots are shown (dotted line: Apoe−/−; solid line Cbl-b−/−Apoe−/−). (E, F) In vitro macrophage killing assay; Apoe−/− and Cblb−/−Apoe−/− splenocytes were cultured in the presence of ovalbumin peptide257–264 for 6 days, subsequently CD8+ T cells were isolated and co-cultured with ovalbumin peptide257–264-pulsed CFSEhigh labelled bone marrow-derived macrophages and unpulsed CFSElow labelled bone marrow-derived macrophages (n = 4). Representative histograms are shown. Data are presented as mean ± standard deviation.
To investigate whether the increase in effector protein production in CBL-B-deficient CD8+ T cells affected the cytotoxicity of these cells, a macrophage killing assay was performed. Ovalbumin peptide (OVA257–264) primed CD8+ T cells were co-cultured with OVA257–264-pulsed CFSEhigh-labelled BMDMs and unpulsed CFSElow-labelled BMDMs. In comparison with OVA257–264-primed Apoe−/− CD8+ T cells, incubation with OVA257–264-primed Cblb−/−Apoe−/− CD8+ T cells significantly reduced the survival of OVA257–264-pulsed BMDMs (Figure 5E and F). These data indicate that the enhanced cytotoxicity of CD8+ T cells, in conjunction with their increased abundance (Figure 4A–C), provoked macrophage killing and necrotic core formation in the plaques of Cblb−/−Apoe−/− mice.
CD8+ T cell are the main drivers of atherogenesis in Cblb−/−Apoe−/− mice
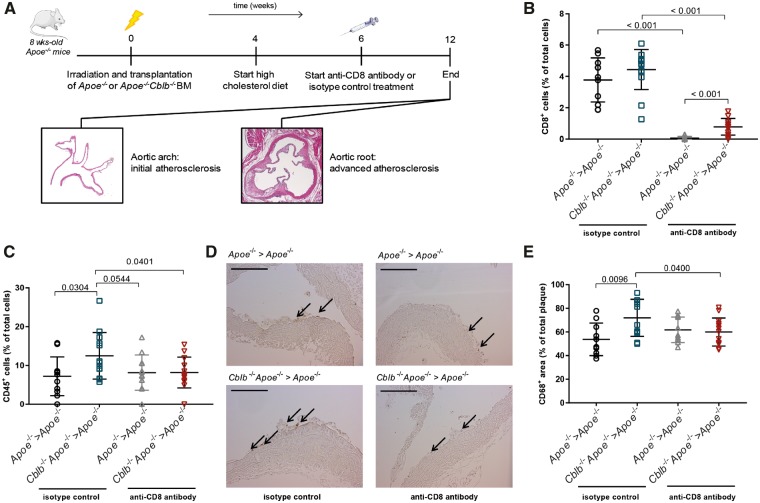
To further evaluate the contribution of Cblb−/−Apoe−/− CD8+ T cells to atherosclerosis, Cblb−/−Apoe−/− or Apoe−/− bone marrow was transplanted into lethally irradiated Apoe−/− recipient mice. Following 6 weeks of recovery, antibody-mediated depletion of CD8+ T cells was initiated and continued for 6 weeks until the assessment of atherosclerosis in the aortic arch and aortic root (Figure 6A). Anti-CD8 treatment successfully depleted circulating CD8+ T cells in both Cblb−/−Apoe−/− and Apoe−/− recipients (Figure 6B). CD8+ T cells were also successfully depleted in the lymphoid organs and only a minor increase in CD4+ T cells was observed in Cblb−/−Apoe−/− chimeras (Supplementary material online, Figure S3).
Figure 6.
Depletion of Cblb−/−Apoe−/− CD8+ T cells reduces inflammation in initial atherosclerotic plaques. (A) Apoe−/− mice were lethally irradiated and reconstituted with Apoe−/− or Cblb−/−Apoe−/− bone marrow and either treated with a CD8+ T cell depleting antibody or isotype control for 6 weeks. (B) CD8+ T cell numbers in the blood of isotype and anti-CD8-treated mice. (C, D) Immunohistochemical quantification of the relative number of CD45+ cells. And representative pictures of CD45-stained aortic arch sections containing initial atherosclerotic plaques. Scale bar: 100 μm. (E) Quantification of plaque macrophage content. Data are presented as mean ± standard deviation (n = 11–14).
Haematopoietic CBL-B deficiency did not affect plaque area in the aortic arch, which contained only very initial plaques (Supplementary material online, Figure S4), but markedly increased plaque inflammation as reflected by an increased abundance of CD45+ cells (Figure 6C and D) and MAC3+ macrophages (Figure 6E). A trend towards increased CD3+ T cell content in the plaques of haematopoietic CBL-B-deficient mice was observed (Supplementary material online, Figure S4). Depletion of CD8+ T cells prevented the increase in CD45+ and CD68+ cells in the plaques of haematopoietic CBL-B-deficient mice (Figure 6C–E), demonstrating that Cblb−/−Apoe−/− cytotoxic T cells drive plaque inflammation in the early stages of atherosclerosis.
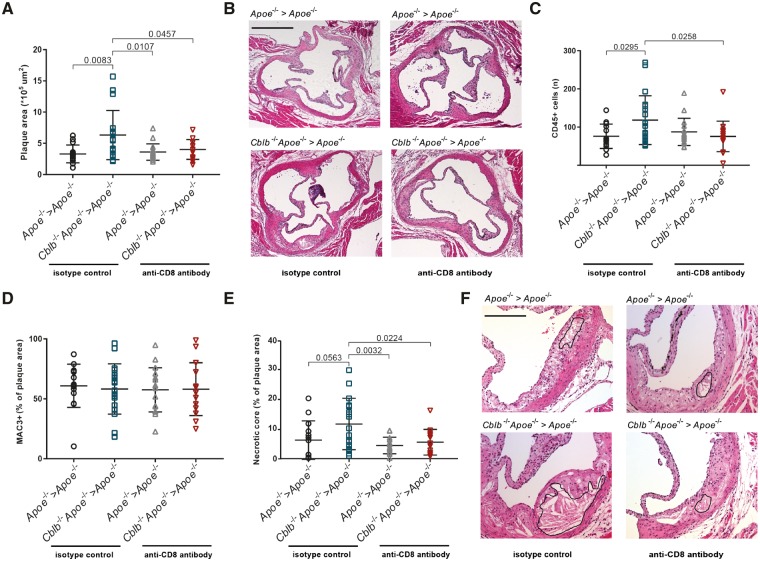
In the aortic root, where more advanced plaques were present, haematopoietic deficiency of CBL-B resulted in a 1.8-fold increase in lesion area (Figure 7A and B). Depletion of Cblb−/−Apoe−/− CD8+ T cells prevented this increase (Figure 7A and B) and ameliorated plaque inflammation, as reflected by the decrease in CD45+ cells (Figure 7C). Although MAC3+ content was not affected by haematopoietic CBL-B deficiency (Figure 7D), depletion of CD8+ T cells prevented the increase in necrotic core formation that was observed in Cblb−/−Apoe−/− bone marrow chimeras (Figure 7E and F). This experiment, which demonstrates that depletion of CD8+ T cells improves plaque inflammation and halts the progression of atherosclerosis in CBL-B-deficient bone marrow chimeras, indicates that the atheroprotective effect of CBL-B predominantly relies on its function in cytotoxic T cells.
Figure 7.
The progression of atherosclerosis is hampered in CD8+ T cell-depleted haematopoietic Cbl-b−/−Apoe−/− chimeras. (A) Aortic roots of 20-week-old haematopoietic Apoe−/− and Cblb−/−Apoe−/− chimeras analysed for the amount of atherosclerosis. (B) Representative pictures of haematoxylin and eosin-stained aortic root cross-sections containing advanced atherosclerotic plaques. Scale bar: 500 μm. (C–E) Quantification of plaque CD45+ cells, MAC3+ cells and necrotic core area in plaques of the aortic roots. Representative pictures are shown. The black line indicates the necrotic core. Scale bar: 200 μm. Data are presented as mean ± standard deviation (n = 14–18).
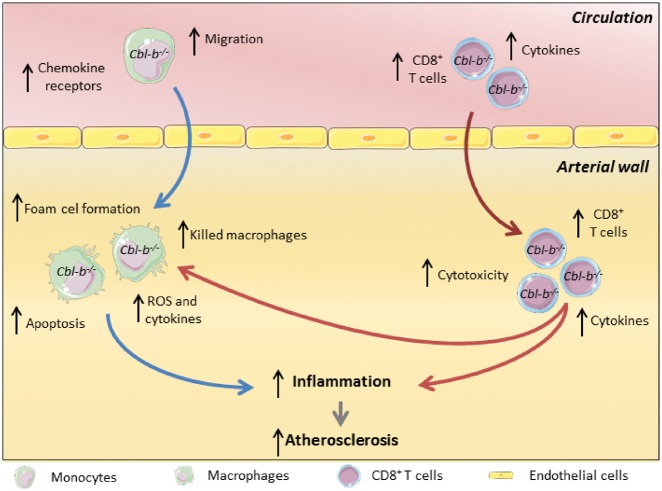
Take home figure.
Proposed model of the role of Casitas B-cell lymphoma-B in atherosclerosis. During the initial stages of atherosclerosis, Casitas B-cell lymphoma-B deficiency increases lesion formation by enhancing monocyte influx into the arterial wall; these monocytes subsequently develop into macrophages with an atherogenic phenotype (indicated by the blue arrows). When atherosclerosis progresses, Casitas B-cell lymphoma-B deficiency increases plaque CD8+ T-cell abundance, which aggravates plaque inflammation and provokes macrophage death (indicated by the red arrows), thereby enhancing the progression of plaques towards clinically unfavourable high-risk plaques with large necrotic cores. Agonizing, the function of Casitas B-cell lymphoma-B therefore represents a novel therapeutic strategy to target detrimental CD8+ T-cell-driven responses in atherosclerosis.
Discussion
Here, we report that CBL-B, the ‘natural inhibitor’ of T cell activation, has a critical function in atherosclerosis. The expression of CBL-B in human atherosclerotic plaques is lower in advanced lesions when compared with initial lesions and negatively correlated with necrotic core area, indicating that CBL-B expression decreases during the progression of atherosclerosis. Absence of CBL-B aggravates initial atherosclerosis in Apoe−/− mice by inducing an atherogenic phenotype in macrophages and accelerates the progression towards advanced atherosclerotic lesions with large necrotic cores. This phenotype results from an increase in CD8+ T cell numbers in CBL-B-deficient mice, in conjunction with an enhanced inflammatory and cytotoxic potential of Cblb−/−Apoe−/− CD8+ T cells, which provoked macrophage death, as illustrated in our schematic model (Take home figure).
Circulating levels of activated CD8+ T cells are increased in patients with coronary artery disease and CD8+ T cells are abundantly present in human atherosclerotic lesions, where they outnumber CD4+ T cells.3 Experimental studies have attributed a detrimental role to CD8+ T cells in atherosclerosis as antibody-mediated depletion of CD8+ T cells in Apoe−/− mice mitigated atherosclerosis by reducing the number of circulating proinflammatory monocytes and hampering macrophage accumulation and apoptosis in the plaque.6 Accordingly, adoptive transfer of CD8+ T-cells aggravated atherosclerosis and increased necrotic core formation in Apoe−/−Rag−/− mice, due to granzyme B- and perforin-induced macrophage death, resulting in clinically unfavourable high-risk plaques.5 In the current study, we confirmed that an excess of CD8+ T cells is detrimental in atherosclerosis, particularly due to the increase in CD8+ T cell-mediated macrophage apoptosis and necrotic core formation.
One cause of the increase in CD8+ T cells in Cblb−/−Apoe−/− mice is the lower susceptibility to Treg-mediated suppression. A similar phenotype has been found in Cblb−/− CD4+ T cells, which had developed resistance to TGFβ due to SMAD7-mediated down-regulation of TGFβR-II.16 In our study, CBL-B-deficient CD8+ T cells also expressed less TGFβR-II, rendering them less prone to TGFβ-mediated Treg suppression. Furthermore, Tregs suppress T cell proliferation by capturing IL2, thereby limiting IL2-dependent T-cell proliferation.17 We found that Cblb−/−Apoe−/− CD8+ T cells secrete more IL2 than Apoe−/− CD8+ T cells, lowering their sensitivity to Treg-mediated reductions in IL2. In addition, we demonstrate that CBL-B promotes the suppressive effects of Tregs, which contrasts previous findings that showed no effect of CBL-B ablation on Treg-mediated suppression in in vitro polyclonal CD8+ T-cell proliferation assays.18 This discrepancy might be due to the use of stimulating anti-CD28 and anti-CD3 beads in our study vs. irradiated splenocytes and CD3 stimulation in the earlier reports.18 In such an experimental setup, Tregs can modulate antigen-presenting cells, interfering with T cell activation, in addition to the suppressive effects on CD8+ T cells.
In addition to the significant effect on CD8+ T cells, CBL-B deficiency also resulted in an atherogenic phenotype in monocytes and macrophages, characterized by an increased migratory potential, increased cytokine production and lipid uptake. Little is known about the function of CBL-B in cells of myeloid origin, but it has been demonstrated that CBL-B mediates TLR4 ubiquitination and impedes the association of the adhesion proteins Lymphocyte Function-associated Antigen 1 (LFA-1) and Intercellular Adhesion Molecule 1 (ICAM-1), thereby inhibiting adhesion and diapedesis.19 In other disease models, such as diet-induced obesity and sepsis, CBL-B deficiency enhanced the infiltration of macrophages into adipose tissue, causing insulin resistance in obesity, and excessive macrophage infiltration into the lung during sepsis.11,12 Our study shows that CBL-B deficiency not only increased the migratory potential of monocytes and macrophages, but also increased the production of inflammatory mediators, which accelerates plaque initiation. Accordingly, we found that depletion of CD8+ T cells in haematopoietic Cblb−/−Apoe−/− mice did not affect lesion formation in the very early stage of atherosclerosis, which is primarily monocyte/macrophage-driven. In the later stages of atherosclerosis, depletion of CD8+ T cells reduced plaque area, plaque inflammation and necrotic core formation, indicating that the progression of atherosclerosis in CBL-B-deficient mice was predominantly driven by CD8+ T cells.
Conclusion
In summary, this study demonstrates that CBL-B puts a brake on CD8+ T cell activation during atherogenesis, thereby inhibiting plaque inflammation and progression towards a clinically unfavourable high-risk plaque phenotype. Although our experimental results should be extrapolated to patients with caution and the effects of targeting ubiquitination in specific immune cells must be scrutinized before being translated into a clinical application, our study attributes a critical role to CBL-B in the regulation of cytotoxic T cell-driven responses in atherosclerosis and provides the basis for novel CBL-B-targeting therapeutic strategies.
Supplementary Material
Acknowledgements
The authors thank Myrthe den Toom, Linda Beckers, Cindy van Roomen, Quinte Braster, Johannes Levels, and Saskia van der Velden for technical assistance.
Funding
This work was supported by The Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation, Dutch Federation of University Medical Centers, the Netherlands, Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences for the GENIUS-II project ‘Generating the best evidence-based pharmaceutical targets for atherosclerosis-II’ (CVON2018-19). This study was also supported by the Netherlands Organization for Scientific Research (NWO) (VICI grant 016.130.676 to E.L.), the EU (H2020-PHC-2015-667673, REPROGRAM to E.L.), the European Research Council (ERC consolidator grant CD40-INN 681492 to E.L.), the German Science Foundation (DFG, CRC1123, project A5), Amsterdam Cardiovascular Sciences (MD/PhD grant to T.S.), The Netherlands Heart Institute (Young@heart grant to T.S.), and the Dutch Heart Foundation (Dr Dekker Physician-in-specialty-training grant to T.S.).
Conflict of interest: none declared.
References
- 1. Ketelhuth DF, Hansson GK.. Adaptive response of T and B cells in atherosclerosis. Circ Res 2016;118:668–678. [DOI] [PubMed] [Google Scholar]
- 2. Patel KV, Pandey A, de Lemos JA.. Conceptual framework for addressing residual atherosclerotic cardiovascular disease risk in the era of precision medicine. Circulation 2018;137:2551–2553. [DOI] [PubMed] [Google Scholar]
- 3. Kyaw T, Peter K, Li Y, Tipping P, Toh BH, Bobik A.. Cytotoxic lymphocytes and atherosclerosis: significance, mechanisms and therapeutic challenges. Br J Pharmacol 2017;174:3956–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ.. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 5. Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, Li P, Tipping P, Bobik A, Toh BH.. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 2013;127:1028–1039. [DOI] [PubMed] [Google Scholar]
- 6. Cochain C, Koch M, Chaudhari SM, Busch M, Pelisek J, Boon L, Zernecke A.. CD8+ T cells regulate monopoiesis and circulating ly6c-high monocyte levels in atherosclerosis in mice. Circ Res 2015;117:244–253. [DOI] [PubMed] [Google Scholar]
- 7. Lutz-Nicoladoni C, Wolf D, Sopper S.. Modulation of immune cell functions by the E3 ligase Cbl-b. Front Oncol 2015;5:58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fang D, Wang HY, Fang N, Altman Y, Elly C, Liu YC.. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J Biol Chem 2001;276:4872–4878. [DOI] [PubMed] [Google Scholar]
- 9. Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H.. Cbl-b regulates the CD28 dependence of T-cell activation. Nature 2000;403:216–220. [DOI] [PubMed] [Google Scholar]
- 10. Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu YC, Penninger JM.. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 2004;21:167–177. [DOI] [PubMed] [Google Scholar]
- 11. Abe T, Hirasaka K, Kagawa S, Kohno S, Ochi A, Utsunomiya K, Sakai A, Ohno A, Teshima-Kondo S, Okumura Y, Oarada M, Maekawa Y, Terao J, Mills EM, Nikawa T.. Cbl-b is a critical regulator of macrophage activation associated with obesity-induced insulin resistance in mice. Diabetes 2013;62:1957–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bachmaier K, Toya S, Gao X, Triantafillou T, Garrean S, Park GY, Frey RS, Vogel S, Minshall R, Christman JW, Tiruppathi C, Malik AB.. E3 ubiquitin ligase Cblb regulates the acute inflammatory response underlying lung injury. Nat Med 2007;13:920–926. [DOI] [PubMed] [Google Scholar]
- 13. Perisic L, Hedin E, Razuvaev A, Lengquist M, Osterholm C, Folkersen L, Gillgren P, Paulsson-Berne G, Ponten F, Odeberg J, Hedin U.. Profiling of atherosclerotic lesions by gene and tissue microarrays reveals PCSK6 as a novel protease in unstable carotid atherosclerosis. Arterioscler Thromb Vasc Biol 2013;33:2432–2443. [DOI] [PubMed] [Google Scholar]
- 14. Perisic L, Aldi S, Sun Y, Folkersen L, Razuvaev A, Roy J, Lengquist M, Akesson S, Wheelock CE, Maegdefessel L, Gabrielsen A, Odeberg J, Hansson GK, Paulsson-Berne G, Hedin U.. Gene expression signatures, pathways and networks in carotid atherosclerosis. J Intern Med 2016;279:293–308. [DOI] [PubMed] [Google Scholar]
- 15. Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA.. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci USA 2006;103:3781–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gruber T, Hinterleitner R, Hermann-Kleiter N, Meisel M, Kleiter I, Wang CM, Viola A, Pfeifhofer-Obermair C, Baier G.. Cbl-b mediates TGFbeta sensitivity by downregulating inhibitory SMAD7 in primary T cells. J Mol Cell Biol 2013;5:358–368. [DOI] [PubMed] [Google Scholar]
- 17. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ.. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 2007;8:1353–1362. [DOI] [PubMed] [Google Scholar]
- 18. Loeser S, Loser K, Bijker MS, Rangachari M, van der Burg SH, Wada T, Beissert S, Melief CJ, Penninger JM.. Spontaneous tumor rejection by cbl-b-deficient CD8+ T cells. J Exp Med 2007;204:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi EY, Orlova VV, Fagerholm SC, Nurmi SM, Zhang L, Ballantyne CM, Gahmberg CG, Chavakis T.. Regulation of LFA-1-dependent inflammatory cell recruitment by Cbl-b and 14-3-3 proteins. Blood 2008;111:3607–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.