SUMMARY
KCC2 is the neuron-specific K+-Cl− cotransporter required for maintaining low intracellular Cl−, which is essential for fast inhibitory synaptic transmission in the mature CNS. Despite the requirement of KCC2 for inhibitory synaptic transmission, understanding of the cellular mechanisms that regulate KCC2 expression and function is rudimentary. We examined KCC2 in its native protein complex in vivo to identify key KCC2-interacting partners that regulate KCC2 function. Using blue native-polyacrylamide gel electrophoresis (BN-PAGE), we determined that native KCC2 exists in a macromolecular complex with kainate-type glutamate receptors (KARs). We found that KAR subunits are required for KCC2 oligomerization and surface expression. In accordance with this finding, acute and chronic genetic deletion of KARs decreased KCC2 function and weakened synaptic inhibition in hippocampal neurons. Our results reveal KARs as regulators of KCC2, significantly advancing our growing understanding of the tight interplay between excitation and inhibition.
INTRODUCTION
Hyperpolarizing GABAergic synaptic transmission in the mature CNS depends upon a low concentration of intracellular Cl− [Cl−]i KCC2 is the neuron-specific member of the K+-Cl− cotransporter gene family that primarily extrudes Cl− from neurons, making it essential for inhibitory synaptic transmission (Acton et al., 2012; Blaesse et al., 2009; Rivera et al., 1999). Physiological levels of neuronal activity can regulate KCC2 in a Ca2+-dependent manner to induce inhibitory synaptic plasticity, which plays a key role in the delicate balance between inhibition and excitation (Fiumelli and Woodin, 2007; Lamsa et al., 2010; Woodin et al., 2003). However, aberrant KCC2 regulation results in increased neuronal Cl− and contributes toward the pathophysiology of numerous neurological disorders including epilepsy, autism, and neuropathic pain (Coull et al., 2005; Kahle et al., 2008; Tyzio et al., 2014; Woo et al., 2002).
KCC2 membrane expression and function are regulated by multiple posttranslational mechanisms, including alterations in phosphorylation state, oligomerization, association with lipid rafts, and cleavage by proteases (Blaesse et al., 2006; Lee et al., 2011; Puskarjov et al., 2012; Rinehart et al., 2009; Watanabe et al., 2009). Recently, we made an important addition to this list of mechanisms that regulate KCC2 function by identifying a KCC2-interacting protein termed Neto2 (Ivakine et al., 2013). We found that Neto2 is required to maintain KCC2 abundance in neurons and for efficient KCC2-mediated Cl− transport. Thus, the KCC2-Neto2 interaction is vital for normal synaptic inhibition in mature neurons.
Neto2 is a CUB domain containing transmembrane protein that also acts as an auxiliary subunit of native kainate-type glutamate receptors (KARs). Neto2 regulates both the kinetics and synaptic localization of KAR subunits (Copits et al., 2011; Tang et al., 2012; Wyeth et al., 2014; Zhang et al., 2009). KARs are unique ionotropic glutamate receptors that perform multiple functions during synaptic transmission and plasticity (Lerma and Marques, 2013). They regulate GABAergic release from presynaptic terminals (Rodríguez-Moreno et al., 1997), mediate slow excitatory currents postsynaptically (Castillo et al., 1997), and are involved in mossy fiber-pyramidal neuron long-term potentiation in the CA3 area (Contractor et al., 2001).
Our identification of the Neto2-KCC2 interaction, coupled with the previous demonstrations that Neto2 is an auxiliary subunit of KARs, led us to ask whether KCC2 and KARs coexist in a macromolecular complex. In particular, we examined the role of GluK2 subunits that were previously shown to interact with Neto2 (Copits et al., 2011; Tang et al., 2011; Zhang et al., 2009). In this study, we have made a surprising discovery that native oligomeric KCC2 coexists in an ensemble with the GluK2 KAR subunit in the CNS. Moreover, we determined that KARs are required to maintain both KCC2 oligomerization and the expression of this transporter in the membrane. When we performed an electrophysiological characterization of KCC2 function following KAR subunit disruption, we found neurons had a depolarized reversal potential for GABA (EGABA). Hence, our findings represent a regulation of KCC2 function and fast synaptic inhibition by components of excitatory transmission.
RESULTS
KCC2 and GluK2 KARs Interact In Vivo and In Vitro
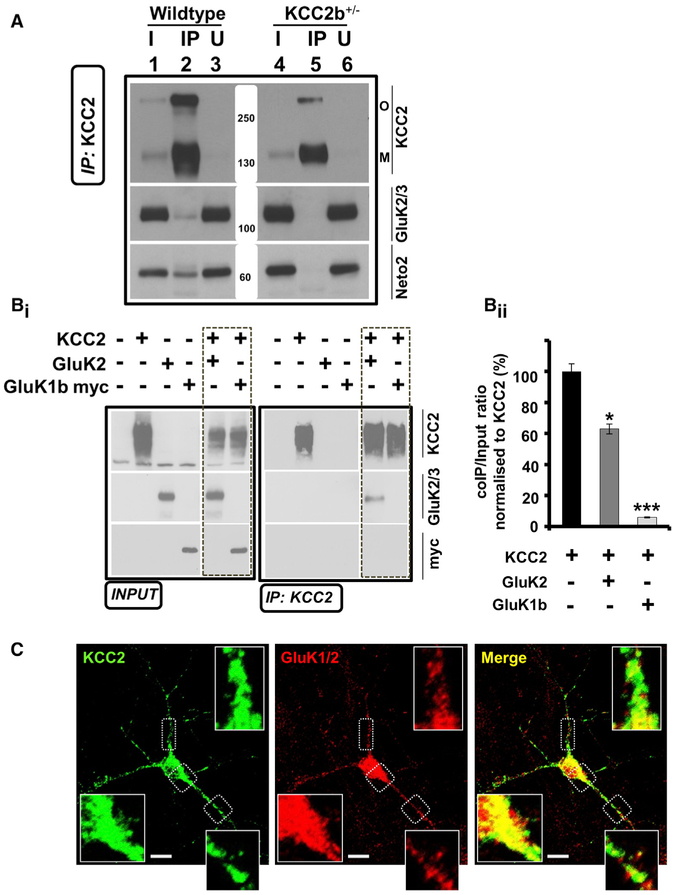
We have recently discovered that KCC2 binds to the single-pass CUB domain protein Neto2, and that this interaction is required for efficient Cl− extrusion in hippocampal neurons (Ivakine et al., 2013). Several groups have previously established that Neto2 is a critical auxiliary subunit of native KARs, including GluK2 (Copits et al., 2011; Tang et al., 2011; Wyeth et al., 2014; Zhang et al., 2009). This led us to hypothesize that KARs might be a putative candidate that could interact with KCC2. In order to determine whether KCC2 interacts with KAR subunits in vivo, we performed a coimmunoprecipitation assay from whole-brain native membrane preparations. We found that anti-KCC2 antibodies coimmunoprecipitated GluK2/3 primarily from wild-type mice in comparison to KCC2b+/− mice, indicating the existence of a KCC2-KAR complex in vivo (Figures 1A and S1A; n = 3). To determine whether KCC2 can interact with KARs independent of exogenous Neto2, we performed coimmunoprecipitation experiments in HEK293 cells transfected with KCC2 and KAR subunits alone. In this assay, we found that KCC2 could coimmunoprecipitate GluK2, but not GluK1 (Figures 1B and S1B; n = 4). We also performed the experiment in the reverse direction and found that GluK2, but not GluK1, could also robustly coimmunoprecipitate KCC2 (Figure S1C; n = 3). Based on the interaction of KCC2 and GluK2 in these coimmunoprecipitation experiments, we hypothesized that these two proteins would colocalize in neurons. We tested this hypothesis by performing immunofluorescent staining of endogenous proteins using antibodies specific for KCC2 and GluK1/2 in cultured hippocampal neurons, followed by quantitative colocalization. Hippocampal neurons showed immunofluorescence for both endogenous KCC2 and KARs, with a partial colocalization of these two proteins (Figure 1C; n = 26). We then performed an intensity correlation analysis to quantitate the colocalization and calculated a Pearson’s correlation coefficient of 0.61 ± 0.03 between the GluK1/2 and KCC2 immunofluorescent signals, indicating ~60% colocalization of these proteins. Thus, we have discovered a surprising protein interaction between KCC2 and the predominant kainate receptor subunit, GluK2.
Figure 1. KCC2 Interacts with GluK2 KARs in Mouse Brain and in Heterologous Cells.
(A) Native KCC2 complexes from C12E9-solubilized whole-brain membrane fractions immunoprecipitated with anti-KCC2 and immunoblotted with the antibodies indicated at right (KCC2, GluK2/3, Neto2). Representative example of three independent replicates. IP, immunoprecipitate; I, input fraction (1% of IP); U. unbound fraction (1 % of IP); O. oligomer; M. monomer; also see Figure S1A.
(B) (Bi) Coimmunoprecipitation experiments performed in HEK293 cells transfected with KCC2 and KAR subunits, solubilized in RIPA buffer, immunoprecipitated with anti-KCC2, and immunoblotted with the antibodies indicated at right (KCC2, GluK2/3, myc); also see Figures S1B and S1C. Representative example of three to four independent biological replicates (Bii) Quantitation of the bound fractions to KCC2 was performed by measuring the band intensity of the immunoprecipitated fraction compared with total input (10%) using ImageJ software.
(C) Confocal images of DIV 12-14 cultured mouse hippocampal neurons immunostained for endogenous KCC2 (left, green) and GluK1/2 (middle, red), demonstrating that the two proteins are colocalized (right, yellow). Representative of confocal images obtained from 26 neurons over four independent experiments performed using eight coverslips. (Scale bars, 10 μm.) Bottom inset is a magnification from the primary dendrite indicated in the box.
All summary figures represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Native KCC2 Exists in a Hetero-Oligomeric Ensemble with Native KARs
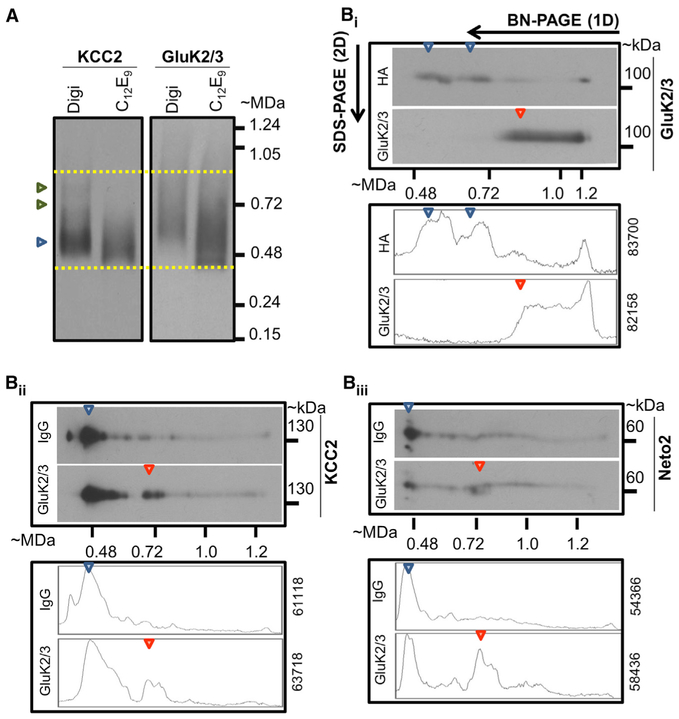
Functional KCC2 predominantly exists as an oligomer in the mature brain (Blaesse et al., 2006; Ivakine et al., 2013; Uvarov et al., 2009). Consistently, we observed that native KCC2 exist as a putative tetramer and in higher-order complexes above 400 kDa (Figures 2A and S2A; n = 3), in P30 whole-brain membrane lysates using a one-dimensional blue native polyacrylamide gel electrophoresis (1D-BN-PAGE). Similar to native KCC2, we also observed higher-order complexes of comparable molecular weights that contained native GluK2/3 (Figures 2A and S2A). Next, to determine whether native KCC2 exists in an ensemble with KAR subunits, we employed an antibody-shift assay coupled with two-dimensional blue native polyacrylamide gel electrophoresis (2D-BN-PAGE). The 2D-BN-PAGE strategy has been previously employed to examine the native assemblies of AMPA receptor multimeric complex (Schwenk et al., 2009).
Figure 2. Native KCC2 and KARs Exist in the Same Oligomeric Complex.
(A) One-dimensional BN-PAGE separation of native KCC2 and GluK2/3 complexes from P30 mouse brain solubilized with Digitonin or C12E9; gel separations were immunoblotted with the antibodies indicated above (KCC2, GluK2/3). This blot is a representative example of three independent biological replicates. Native KCC2 is present as a putative tetramer (blue arrow head), and as higher-order protein complexes (green arrow heads). Native GluK2/3 migrates in a similar molecular weight range as native KCC2 (dotted yellow lines); also see Figure S2A.
(B) (Bi) Antibody-shift assay followed by two-dimensional BN-PAGE separation using C12E9-solubilized whole-brain membrane fractions, incubated with antibodies for HA or GluK2/3; samples resolved first in a 5% 1D-BN-PAGE, and individual lanes from the first dimension were separated in a 6% 2D-SDS-PAGE); gel separations were immunoblotted with anti GluK2/3 antibody. (Bii and Biii) Similar to (Bi), but using digitonin-solubilized hippocampal membrane fractions and antibodies targeted to immunoglobulin G or GluK2/3. Samples resolved in a 4% 1D-BN-PAGE and 6% SDS-PAGE. Gel separations were immunoblotted with KCC2 and Neto2 antibodies respectively. Bottom panels in Bi, Bii, Biii represent densitometric profiles and area under the densitogram to indicate similar loading; red arrowheads denote antibody-induced shifts in the proteins indicated. Representative example of three independent biological replicates; also see Figures S2B–S2D.
Using this approach, we first verified that the addition of the GluK2/3 antibody could shift GluK2/3 to higher molecular weights (Figure 2Bi; n = 3). We observed that this antibody-induced shift in GluK2/3 also shifted a population of native KCC2 in hippocampal preparations (Figure 2Bii; n = 3). Using the same experimental strategy, we found that KCC2 antibodies could also shift a population of GluK2/3 in hippocampal preparations (Figure S2C; n = 3), a finding that we also observed in digitonin-solubilized cortical membrane preparations (Figure S2D; n = 2). As a positive control for this assay, we probed for Neto2, because this protein interacts with both KCC2 and GluK2 (Ivakine et al., 2013; Tang et al., 2011; Zhang et al., 2009). As expected, we found that Neto2 could be shifted with both GluK2/3 (Figure 2Biii and KCC2 antibodies (Figure S2D). We confirmed the specificity of these interactions in this assay by repeating the experiments using antibodies for the transferrin receptor and observed that antibodies to this receptor did not shift GluK2/3 (Figure S2B). Thus, we established that functional oligomeric KCC2 coexists in a hetero-oligomeric complex with the predominant KAR subunit GluK2 in hippocampus and cortex.
KARs Regulate the Assembly or Stability of Native KCC2 Oligomers
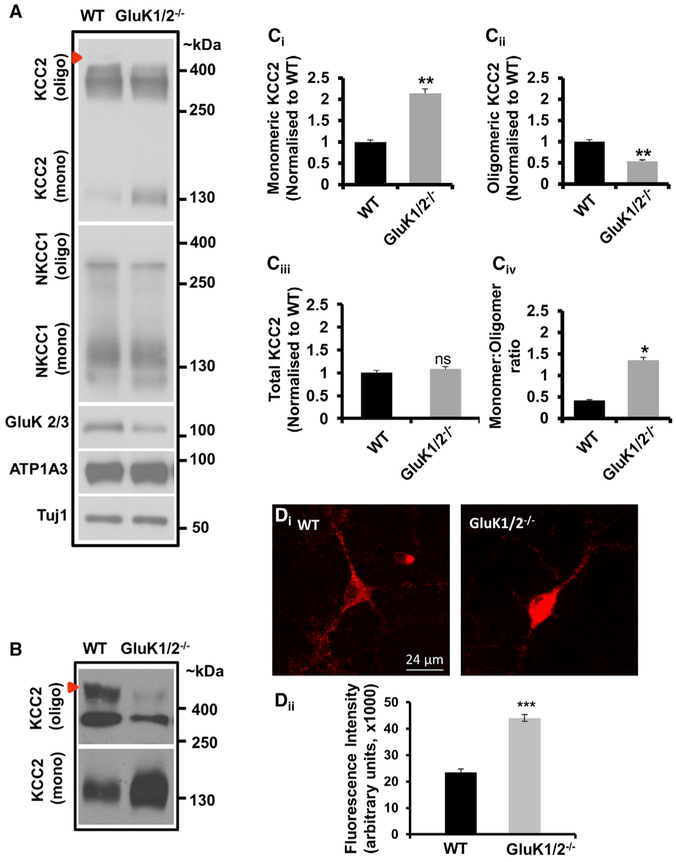
If oligomeric KCC2 exists in a complex with GluK2, and oligomeric KCC2 is the functional form of this transporter, this raises the possibility that KARs may play an important role in the regulation of KCC2 oligomers. To test this possibility, we examined the ratio of KCC2 monomers to oligomers in native membranes prepared from wild-type and GluK1/2-null hippocampal lysates under conditions preventing the formation of nonspecific disulphide bonds (Uvarov et al., 2009). In an SDS-PAGE, we observed an increase in monomeric KCC2 and a decrease in oligomeric KCC2 in GluK1/2-null hippocampi (Figures 3A and S3Ai; n = 3). We also examined the levels of the neuron-specific pump ATP1A3 and another KCC2-family member NKCC1, and observed no significant difference between wild-type and GluK1/2-null lysates (Figure 3A). We reasoned that the denaturing gel running conditions of SDS-PAGE could impede a robust quantification of KCC2 oligomeric levels, so we subsequently chose to resolve changes in the monomer:oligomer ratio using a previously established nondenaturing PFO-PAGE (Uvarov et al., 2009). Using these conditions, we observed a 2-fold increase in monomeric KCC2 in GluK1/2-null hippocampi (Figures 3B, 3Ci, and S3Aii; n = 3) as expected from the previous SDS-PAGE result. In addition, we also observed a significant decrease in oligomeric KCC2 levels above ~400 kDa in GluK1/2-null hippocampi compared with wild-type levels (Figures 3B, 3Cii, and S3Aii), and no significant change in total KCC2 levels under the same conditions (Figure 3Ciii. We verified that there is no change in total KCC2 levels by an additional standard approach by preparing the samples in the absence of iodoacetamide, and resolved them under strong denaturing conditions, indicating that there is no net change in total KCC2 levels (Figure S3Aiii; n = 3). Additionally, we verified that the increases in monomeric KCC2 levels were not accompanied by changes in KCC2 gene expression, by examining the relative KCC2 mRNA abundance using quantitative real-time PCR. We found no significant differences between wild-type and GluK1/2-null hippocampi prepared from postnatal day 30 mice (Figure S3B; n = 3). Put together, these results demonstrate that the presence of GluK1/2 determines the monomer:oligomer ratio of KCC2 (Figure 3B and 3Civ) at the posttranscriptional level. Moreover, by showing that loss of KAR subunits induces a significant reduction in KCC2 oligomers, particularly above ~400 kDa, we further strengthen our two major claims: (1) the existence of a KAR:KCC2 hetero-oligomeric complex in the hippocampus, and (2) KARs promote the assembly or the stability of KCC2 oligomers within the complex.
Figure 3. GluK1/2-Null Hippocampal Neurons Have an Increased Monomeric: Oligomeric KCC2 Ratio.
(A) Representative immunoblots of C12E9-solubilized native lysates prepared in the presence of 25 mM iodoacetamide to prevent the formation of nonspecific disulphide bonds between KCC2 monomers during membrane extraction, from wild-type and GluK1/2-null hippocampi; resolved in a standard 6% SDS-PAGE; immunoblotted with the antibodies indicated at left (KCC2, NKCC1, GluK2/3, ATP1A3, Tuj1). Also see Figure S3Ai.
(B) Samples obtained from the same preparation as in (A) were resolved in the absence of DTT in nondenaturing PFO-PAGE conditions. Red arrowhead indicates the oligomeric KCC2 band migrating above ~400 kDa that is predominantly reduced in GluK1/2−/− lysates; blots shown in (A) and (B) are representative of three independent biological replicates, see Figures S3Aii and S4A.
(C) Summary figures showing levels of (Ci) KCC2 monomers, (Cii) KCC2 oligomers, (Ciii) total KCC2, and (Civ) monomer:oligomer KCC2 ratio in GluK1/2-null hippocampal homogenates relative to that of wild-type.
(D) (Di) Example confocal microscopic immunofluorescent images of cultured hippocampal neurons from wild-type and GluK1/2-null mice stained with anti-KCC2 antibody (red; n = 49 neurons). (Scale bars, 24 μm.) (Dii) Summary of the average fluorescence intensity of somatic KCC2 in wild-type and GluK1/2-null neurons.
All summary figures represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
KARs Maintain KCC2 Surface Expression in Neurons
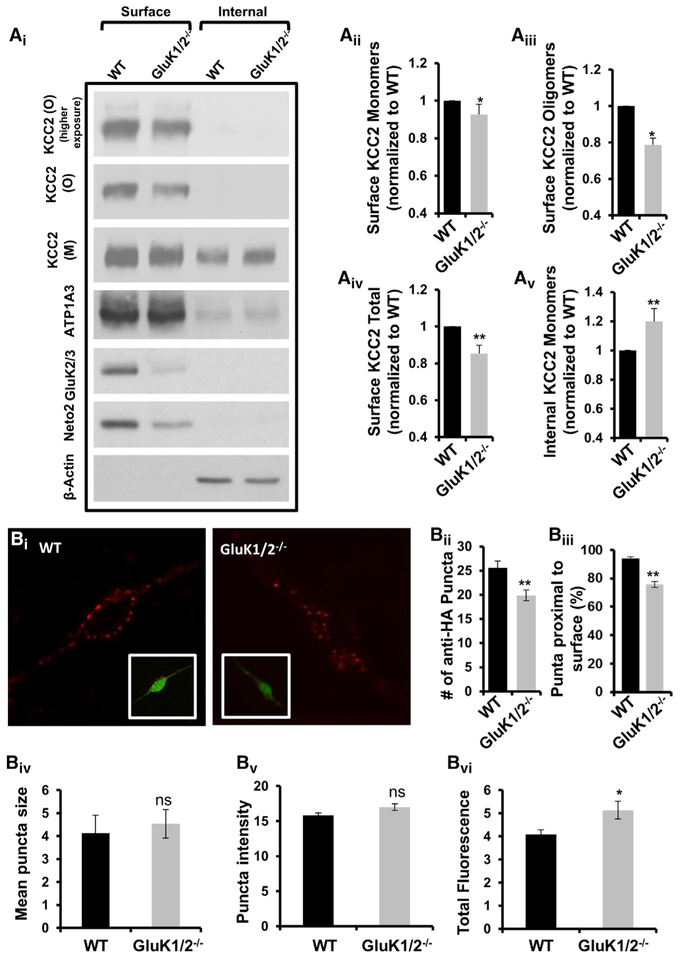
It has been previously demonstrated by several groups that immature neurons are characterized by predominantly monomeric KCC2 that exists intracellularly (reviewed in Chamma et al., 2012). Based on these previous demonstrations, we examined whether there were differences in the expression patterns of KCC2 between wild-type and GluK1/2−/− neurons. We first examined total KCC2 levels by performing immunofluorescent staining of cultured hippocampal neurons with anti-KCC2 antibody. We found a significant increase in endogenous KCC2 immunoreactivity in the soma of GluK1/2−/− neurons compared to wild-type neurons (wild-type, n = 47; GluM1/2−/−, n = 49; p < 0.001;Figure 3D). However, for KCC2 to be functional it needs to be expressed in the membrane. Next, we examined the membrane expression pattern of KCC2 by performing a surface biotinylation assay at 4°C. We found a significant decrease in monomeric (p = 0.029), oligomeric (p = 0.029), and total (p = 0.008) KCC2 levels in the surface of GluK1/2−/− neurons compared to wild-type neurons, with a corresponding increase in internal monomeric KCC2 (p = 0.008; n = 5; Figure 4A). To visualize KCC2 expression, we performed live immunofluorescence of KCC2 containing an extracellular HA tag under nonpermeabilizing conditions at 37°C. We have previously demonstrated that this KCC2-HA chimeric protein traffics to the membrane and is functional (Acton et al., 2012). We found that in GluK1/2−/− neurons there was a significant decrease the number of anti-HA puncta (Figure 4Bii; n = 23), with no significant differences in either the size or intensity of the puncta (Figures 4Biv and 4Bv; n = 23), indicating an overall decrease in the number of KCC2-HA puncta, with no difference in the puncta characteristics themselves. We then analyzed where the existing puncta were located and found that there was a significant decrease in the KCC2-HA puncta that were proximal to the membrane in GluK1/2−/− neurons (Figure 4Biii and S3; n = 23). We made two important observations from these biotinylation and immunostaining experiments: (1) overall there is an increased total KCC2 immunoreactivity in soma of GluK1/2−/− neurons (Figure 3D); and (2) GluK1/2−/− neurons have a decreased membrane expression (Figure 4A). Thus, in addition to promoting and/or stabilizing KCC2 oligomers, the presence of KAR subunits GluK1/2 also promotes and/or stabilizes surface KCC2 levels.
Figure 4. GluK1/2-Null Neurons Have Deceased KCC2 Expressed at the Surface.
(A) (Ai) Representative immunoblots of KCC2 monomers and oligomers from the surface and internal fraction of wild-type and GluK1/2-null neurons. The first two lanes correspond to biotinylated surface proteins (50 μg) recovered from the neutravidin beads. The last two lanes correspond to unbiotinylated internal proteins (5 μg) recovered from the supernatant. (Aii–v) Summary figures showing levels of surface KCC2 monomers, surface KCC2 oligomers, total surface KCC2 (normalized to surface levels of neuron-specific ATP1A3), and internal KCC2 (normalized to β-actin levels) in GluK1/2-null homogenates relative to that of wild-type (n = 5).
(B) (Bi Example confocal microscopic immunofluorescent images from a single confocal plane of live cultured hippocampal neurons overexpressing KCC2-HA from wild-type and GluK1/2-null mice (also see Figure S4B). Neuronal transfection is indicated by GFP fluorescence, KCC2-HA is indicated by red fluorescent signal. Summary of the (Bii) average number of anti-HA puncta/neuron, (Biii) percentage of anti-HA puncta proximal to the surface (Biv) mean puncta size (surface area, μm2), and (Bv) puncta intensity (in arbitrary units ***×1000) in wild-type and GluK1/2-null neurons. (Bvi) Total immunofluorescence of live imaging of wild-type and GluK1/2-null neurons. n = 23 neurons.
All summary figures represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Genetic Deletion and Acute Silencing of KAR Subunits Result in Depolarized EGABA
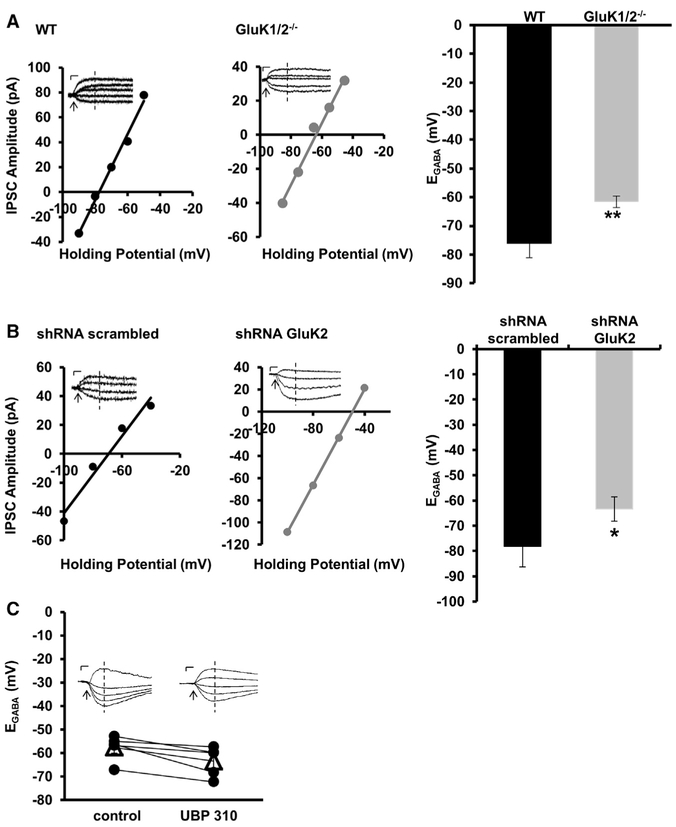
Because we observed a decrease in both oligomeric KCC2 and surface KCC2 in GluK1/2-null neurons, we hypothesized that these neurons would have aberrant KCC2-mediated Cl− homeostasis. To test this hypothesis, we measured KCC2 activity by recording the reversal potential for GABA (EGABA) using gramicidin-perforated patch clamp recordings. We found that EGABA was depolarized in cultured GluK1/2−/− hippocampal neurons (−65.2 ± 1.9 mV; n = 10) compared to wild-type neurons (−78.6 ± 3.5; n = 7; p = 0.003; Figure 5A), with no significant difference in either synaptic conductance (p = 0.5) or maximum current amplitude (p = 0.6; Table S2). We then took a two-step approach to rule out the possibility that the depolarization of EGABAin GluK1/2−/− neurons was due to differences in activity levels between the genotypes. First, we compared the spontaneous activity levels using Ca2+ imaging and found no differences between cultured hippocampal neurons prepared from wild-type (C57/Bl6 and 129SVE) and GluK1/2−/− mice (Figure S5A). Second, we used small hairpin RNA (shRNA) to acutely silence GluK2 in cultured hippocampal neurons; we verified GluK2 shRNA was effective at silencing GluK2 but not KCC2 using an in vitro assay (Figure S5B). We found that knocking down GluK2 in wild-type neurons (C57/Bl6) depolarized EGABA by 15.41 mV compared to neurons transfected with scrambled shRNA (Figures 5B and S5B; n = 11). Knocking down GluK2 in 129SVE wild-type neurons also significantly depolarized EGABA (n = 11; p < 0.05). Similar to our results from cultured GluK1/2−/− hippocampal neurons above, we found no significant difference in either synaptic conductance (p = 0.6) or maximum current amplitude (p = 0.1) for either genotype (Table S2). Last, we considered the possibility that the depolarization in EGABA we recorded following the genetic deletion and acute silencing of KAR subunits was not due to the loss of the protein, but rather was due to the loss of the GluK2-KAR current. We tested this possibility by recording EGABA in wild-type neurons in the presence and absence of the GluK2/5-KAR antagonist UBP 310 (5 μM) (Pinheiro et al., 2013). We found no significant difference in EGABA following this pharmacological blockade of these receptors (n = 5; p = 0.159; Figure 5C), allowing us to conclude that it is the protein interaction between GluK2-KARs and KCC2 that is required to maintain a high KCC2 function.
Figure 5. Both Genetic Deletion and Acute Knockdown of GluK2 KAR Subunits Depolarizes EGABA.
(A) Example IV curves measuring EGABA from cultured hippocampal neurons from wild-type (left) and GluK1/2−/− (middle) mice. Summary of EGABA obtained from all similar IV curves (right).
(B) Example IV curves measuring EGABA from cultured hippocampal neurons from wild-type transfected with scrambled shRNA (left) and shRNA for GluK2 (middle). Summary of EGABA obtained from all similar IV curves.
(C) Summary of EGABA recordings performed in the absence (control) and presence of the GluK2/5-KAR inhibitor (5 –M UBP 310). Solid circles are individual EGABA measurements, open triangles represent the mean ± SEM. Insets: are raw voltage clamp traces from example recordings.
Scale bars, 20 pA, 50 ms. Arrow indicates onset of GABA puff. Dashed vertical lines indicate where the current amplitudes were obtained for the generation of the IV curves. All summary figures represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
DISCUSSION
Overall, we have three compelling lines of evidence supporting the conclusion that KCC2 and KAR subunits coexist in a complex: (1) in vivo and in vitro coimmunoprecipitation, (2) antibody-shift assay coupled with 2D-BN-PAGE, and (3) immunofluorescence. Thus, the key K+-Cl−cotransporter required for fast synaptic inhibition binds to the predominant KAR-type ionotropic glutamate receptor subunit GluK2 in multiple brain regions including hippocampus. Furthermore, we find that the GluK2:KCC2 interaction occurs predominantly with oligomeric KCC2, and the importance of this preferential binding is to maintain the functional oligomeric KCC2 complex. Along with our evidence that both genetic deletion and acute knockdown of GluK2 result in aberrant KCC2-dependent neuronal Cl− extrusion, we have identified kainate receptors as an unexpected player during neuronal Cl− homeostasis.
Functional KCC2 Exists as a Hetero-Oligomeric Complex with KARs
Several studies have established that functional KCC2 predominantly exists as oligomers in mature neurons (Blaesse et al., 2006; Ivakine et al., 2013; Uvarov et al., 2009; Watanabe et al., 2009). Here, we report using BN-PAGE that the majority of oligomeric KCC2 in mature brain migrates above 400 kDa. The key finding of this study is that GluK2 is a member of this KCC2-heteromeric complex. This finding raises an important question: is this complex exclusive to KCC2 and GluK2, or do these complexes also include Neto2 and other proteins? This question is relevant because we recently identified that Neto2 associates with oligomeric KCC2 (Ivakine et al., 2013). Our current data demonstrate that, whereas KCC2 and Neto2 interact, Neto2 is not required for the GluK2:KCC2 interaction because GluK2 and KCC2 can interact in the absence of exogenous Neto2 in heterologous cells. Despite the fact that Neto2 is not required for the GluK2:KCC2 interaction, this does not discount the possibility that these three proteins commonly exist in a heteromeric complex. Moreover, there is another reason to believe that additional proteins may also coexist in this heteromeric complex. For example, the 4.1N protein has been identified as binding partners of both KCC2 (Li et al., 2007) and more recently GluK2 (Copits and Swanson, 2013). Because the 4.1 family of FERM domain proteins are abundant scaffolds between membrane and cytoskeletal proteins (Baines et al., 2013), it is possible to speculate that the GluK2:KCC2 interaction we have identified could be mediated/stabilized by neuronal FERM domain proteins. Interestingly, while this manuscript was in revision another group discovered that the dwell time of KCC2 in the vicinity of excitatory synapses is determined by its interactions with 4.1N (Chamma et al., 2013), further strengthening our claims about the putative participation of the 4.1N protein within the KCC2: KAR hetero-oligomeric complex.
KCC2 Oligomerization and Surface Expression Depend on GluK2-KARs
We found that GluK1/2-null hippocampal neurons have a decrease in KCC2 oligomers, particularly above 400 kDa. This suggests that GluK2 plays a role in either the formation of KCC2 oligomers and/or regulates their stability, but how might this happen? The answer may lie in the fact that GluK2-null hippocampi have an ~50% reduction in Neto2 protein levels (Figures 4A and S4A), and we know that Neto2 is required for KCC2 oligomerization and the efficacy of KCC2 transport (Ivakine et al., 2013). Does this mean that GluK2 regulates KCC2 via only Neto2, or can it directly regulate KCC2 function? Our current results suggest that GluK2 can also regulate KCC2 independently of Neto2. We found that in GluK1/2−/− neurons there was an increase in KCC2 monomers and a decrease in KCC2 oligomers, with no net change in total KCC2 levels. This is in contrast to our previous finding that Neto2-null neurons have an overall decrease in both monomeric and oligomeric KCC2, which results in a total decrease in KCC2 protein levels (Ivakine et al., 2013).
Results from our biotinylation experiments allowed us to conclude that GluK1/2−/− neurons have a decrease in cell-surface KCC2. Thus, in addition to promoting or stabilizing KCC2 oligomers GluK1/2-KARs also maintain and/or stabilize surface KCC2 levels. We supported these findings by immunostaining for KCC2; using standard fixed immunofluorescence, we found an increase in somatic KCC2 levels, whereas live immunofluorescence of KCC2-HA showed a decrease in the number of anti-KCC2-HA puncta. Although anti-KCC2-HA puncta must have been present on the surface at some point during the experiment, we cannot conclude that our puncta analysis represents only membrane expressed protein. At the temperature these experiments were performed (37°C), we would expect some proportion of KCC2-HA would be endocytosed, especially considering that KCC2 membrane turnover has been reporter to be relatively high (Lee et al., 2010, but see also Puskarjov et al., 2012). Thus, the KCC2 puncta we have quantified may represent a combination of KCC2-HA in the membrane and endocytotic vesicles. Thus, although we are able to conclude that the loss of GluK2-KARs decreases KCC2 membrane expression, future studies should address whether GluK2 also regulates KCC2 membrane turnover. Together, the increase in KCC2 monomers and decrease in membrane expression suggests that GluK1/2−/− neurons resemble immature neurons, which have an abundance of cytoplasmic KCC2 monomers (Gulyás et al., 2001). Our electrophysiological data support this observation of an immature Cl− homeostasis phenotype, where the genetic deletion of GluK1/2 is reminiscent of immature neurons with poor Cl− extrusion, suggesting that kainate receptors are an essential component of mature neuronal Cl− homeostasis.
Conclusions and Future Significance
The significance of our findings are manifold, but most importantly: (1) KCC2 and KARs exist in the same macromolecular complex, and (2) an ionotropic glutamate receptor can positively regulate the function of the predominant neuronal Cl− cotransporter KCC2. These findings have important implications for both normal physiological functions of neuronal networks and for pathophysiological conditions that result from dysfunction of KARs and KCC2. At the physiological level, we have demonstrated that both genetic deletion and acute knockdown of GluK2 weakens synaptic inhibition, suggesting that the coexistence of these proteins provides a nexus for the ongoing maintenance of the excitatory-inhibitory balance. At the pathophysiological levels, both KCC2 and KARs are strongly implicated in neurological disorders, including neuropathic pain (Bhangoo and Swanson, 2013), autism (Tyzio et al., 2014), and epilepsy (Woo et al., 2002). This raises the possibility that the disruption of the KCC2:KAR complex may underlie these neurophysiological disorders. Understanding the fundamental molecular pathways that regulate the cell intrinsic excitation, inhibition homeostasis is essential for designing of better therapeutic strategies for diseases.
EXPERIMENTAL PROCEDURES
Animals and Approvals
All experiments were performed in accordance with approval and guidelines from the University of Toronto Animal Care Committee and the Canadian Council on Animal Care. Animals of both sexes were used to prepare hippocampal cultures; all other experiments were performed on male mice. The following animal species were used:
Wild-type C57/Bl6 (Charles River Laboratories)
Wild-type 129/SV (Charles River Laboratories)
GluK1/2−/− maintained on a mixed 129SV/C57Bl6 background
KCC2+/− maintained on a mixed 129SV/C57Bl6 background
Antibodies
See Table S1 for complete details for all antibodies used in this study.
Biochemistry and Molecular Biology
See Supplemental Experimental Procedures for coimmunoprecipitation analysis, PFO-PAGE, BN-PAGE, antibody-shift assay, PCR, surface biotinylation.
Hippocampal Cultures and Electrophysiology
Low-density cultures of dissociated mouse hippocampal neurons were prepared as previously described (Acton et al., 2012). Experiments were performed after 10–13 days in culture. Gramicidin perforated patch clamp recordings were performed as previously described (Acton et al., 2012). See Supplemental Experimental Procedures for details on culturing and electrophysiology, shRNA, and neuronal transfection.
Immunostaining, Confocal Microscopy, Ca2+ Imaging
Live immunostaining was performed as described before (Acton et al., 2012). see Supplemental Experimental Proceduresfordetailson fixed and live immunostaining, confocal microscopy, and Ca2+ imaging.
Statistics
Results are given as mean ± SEM. See Supplemental Experimental Procedures for details on statistical tests used for individual figures.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Derek Bowie (McGill University) for the GluK2-GFP cDNA, Dr. Sari Lauri (University of Helsinki) for the GluK1b-myc cDNA, Dr. David B. Mount for KCC4 cDNA, anti-KCC4 antibody, and Dr. Chris McBain (NIH, Bethesda) for the GluK1/2−/− mice. We thank Dr. Mike Salter (University of Toronto) for helpful insight throughout the project. We thank Dr. Nivetha Ramachandran and Dr. Sakthi Devi Moorthy for technical advice. In addition, we thank Ella Czerwinska for excellent technical support. This study was supported the following funding agencies: Canadian Institutes of Health Research (CIHR) grant to M.A.W.; NIH grant (GM074771) to E.D.; The Academy of Finland grants to P.U. and M.S.A.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, five figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.05.022.
REFERENCES
- Acton BA, Mahadevan V, Mercado A, Uvarov P, Ding Y, Pressey J, Airaksinen MS, Mount DB, and Woodin MA (2012). Hyperpolarizing GABAergic transmission requires the KCC2 C-terminal ISO domain. J. Neurosci. 32, 8746–8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines AJ, Lu HC, and Bennett PM (2013). The Protein 4.1 family: Hub proteins in animals for organizing membrane proteins. Biochim. Biophys. Acta. 7838, 605–619. [DOI] [PubMed] [Google Scholar]
- Bhangoo SK, and Swanson GT (2013). Kainate receptor signaling in pain pathways. Mol. Pharmacol. 83, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Guillemin I, Schindler J, Schweizer M, Delpire E, Khiroug L, Friauf E, and Nothwang HG (2006). Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J. Neurosci. 26, 10407–10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, and Kaila K (2009). Cation-chloride cotransporters and neuronal function. Neuron 61, 820–838. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, and Nicoll RA (1997). Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388, 182–186. [DOI] [PubMed] [Google Scholar]
- Chamma I, Chevy Q, Poncer JC, and Lévi S (2012). Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front. Cell. Neurosci. 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamma I, Heubl M, Chevy Q, Renner M, Moutkine I, Eugène E, Poncer JC, and Lévi S (2013). Activity-dependent regulation of the K/Cl transporter KCC2 membrane diffusion, clustering, and function in hippocampal neurons. J. Neurosci. 33, 15488–15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor A, Swanson G, and Heinemann SF (2001). Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron 29, 209–216. [DOI] [PubMed] [Google Scholar]
- Copits BA, and Swanson GT (2013). Kainate receptor post-translational modifications differentially regulate association with 4.1N to control activity-dependent receptor endocytosis. J. Biol. Chem. 288, 8952–8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copits BA, Robbins JS, Frausto S, and Swanson GT (2011). Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (NETO) proteins. J. Neurosci. 31, 7334–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, and De Koninck Y (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021. [DOI] [PubMed] [Google Scholar]
- Fiumelli H, and Woodin MA (2007). Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr. Opin. Neurobiol. 17, 81–86. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Sík A, Payne JA, Kaila K, and Freund TF (2001). The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur. J. Neurosci. 13, 2205–2217. [DOI] [PubMed] [Google Scholar]
- Ivakine EA, Acton BA, Mahadevan V, Ormond J, Tang M, Pressey JC, Huang MY, Ng D, Delpire E, Salter MW, et al. (2013). Neto2 is a KCC2 interacting protein required for neuronal Cl- regulation in hippocampal neurons. Proc. Natl. Acad. Sci. USA 110, 3561–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, and Mount DB (2008). Roles of the cation-chloride cotransporters in neurological disease. Nat. Clin. Pract. Neurol. 4, 490–503. [DOI] [PubMed] [Google Scholar]
- Lamsa KP, Kullmann DM, and Woodin MA (2010). Spike-timing dependent plasticity in inhibitory circuits. Front. Synaptic Neurosci. 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Jurd R, and Moss SJ (2010). Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol. Cell. Neurosci. 45, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Deeb TZ, Walker JA, Davies PA, and Moss SJ (2011). NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat. Neurosci. 14, 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, and Marques JM (2013). Kainate receptors in health and disease. Neuron 80, 292–311. [DOI] [PubMed] [Google Scholar]
- Li H, Khirug S, Cai C, Ludwig A, Blaesse P, Kolikova J, Afzalov R, Coleman SK, Lauri S, Airaksinen MS, et al. (2007). KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron 56, 1019–1033. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Lanore F, Veran J, Artinian J, Blanchet C, Crépel V, Perrais D, and Mulle C (2013). Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb. Cortex 23, 323–331. [DOI] [PubMed] [Google Scholar]
- Puskarjov M, Ahmad F, Kaila K, and Blaesse P (2012). Activity-dependent cleavage of the K-Cl cotransporter KCC2 mediated by calcium-activated protease calpain. J. Neurosci. 32, 11356–11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, et al. (2009). Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell 138, 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, and Kaila K (1999). The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Herreras O, and Lerma J (1997). Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 19, 893–901. [DOI] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, and Klöcker N (2009). Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323, 1313–1319. [DOI] [PubMed] [Google Scholar]
- Tang M, Pelkey KA, Ng D, Ivakine E, McBain CJ, Salter MW, and Mclnnes RR (2011). Neto1 is an auxiliary subunit of native synaptic kainate receptors. J. Neurosci. 31, 10009–10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Ivakine E, Mahadevan V, Salter MW, and Mcinnes RR (2012). Neto2 interacts with the scaffolding protein GRIP and regulates synaptic abundance of kainate receptors. PLoS ONE 7, e51433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, et al. (2014). Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 343, 675–679. [DOI] [PubMed] [Google Scholar]
- Uvarov P, Ludwig A, Markkanen M, Soni S, Hübner CA, Rivera C, and Airaksinen MS (2009). Coexpression and heteromerization of two neuronal K-Cl cotransporter isoforms in neonatal brain. J. Biol. Chem. 284, 13696–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Wake H, Moorhouse AJ, and Nabekura J (2009). Clustering of neuronal K+-Cl- cotransporters in lipid rafts by tyrosine phosphorylation. J. Biol. Chem. 284, 27980–27988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NS, Lu J, England R, McClellan R, Dufour S, Mount DB, Deutch AY, Lovinger DM, and Delpire E (2002). Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus 12, 258–268. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, and Poo MM (2003). Coincident pre- and post-synaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron 39, 807–820. [DOI] [PubMed] [Google Scholar]
- Wyeth MS, Pelkey KA, Petralia RS, Salter MW, McInnes RR, and McBain CJ (2014). Neto auxiliary protein interactions regulate kainate and NMDA receptor subunit localization at mossy fiber-CA3 pyramidal cell synapses. J. Neurosci. 34, 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto- Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, and Tomita S (2009). A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron 61, 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.