Abstract
Background:
Mural Granulosa Cells (MGCs) and Cumulus Cells (CCs) are two specialized cell types that differentiate from a common progenitor during folliculogenesis. Although these two cell types have specialized functions and gene expression profiles, little is known about their microRNA (miRNA) expression patterns.
Objective:
To describe the miRNA profile of mural and cumulus granulosa cells from human pre-ovulatory follicles.
Methods:
Using small RNA sequencing, we defined the miRNA expression profiles of human primary MGCs and CCs, isolated from healthy women undergoing ovum pick-up for in vitro Fertilization (IVF).
Results:
Small RNA sequencing revealed the expression of several hundreds of miRNAs in MGCs and CCs with 53 miRNAs being significantly differentially expressed between MGCs and CCs. We validat-ed the differential expression of miR-146a-5p, miR-149-5p, miR-509-3p and miR-182-5p by RT-qPCR. Analysis of proven targets revealed 37 targets for miR-146a-5p, 43 for miR-182-5p, 2 for miR-509-3p and 9 for miR-149-5p. Gene Ontology (GO) analysis for these 4 target gene sets revealed enrichment of 12 GO terms for miR-146a-5p and 10 for miR-182-5p. The GO term ubiquitin-like protein conjugation was enriched within both miRNA target gene sets.
Conclusion:
We generated miRNA expression profiles for MGCs and CCs and identified several dif-ferentially expressed miRNAs.
Keywords: Cumulus cells, folliculogenesis, granulosa cells, microRNA, small RNA-seq, gene ontology
1. Introduction
Folliculogenesis is a complex and precisely regulated process essential for human reproduction. During growth and maturation of the ovarian follicle, granulosa cells divide and differentiate into a multilayer of Mural Granulosa Cells (MGCs) that line the follicular compartment and a cluster of Cumulus Cells (CCs) that tightly surround the oocyte [1]. Even though these two cell types have a common progenitor in the primordial follicle, their differentiation during folliculogenesis results in cells with specific functions and gene expression profiles [2, 3]. More precisely, CCs are specialized in nutritional support and trafficking of macromolecules [2, 4] while MGCs support the oocyte via endocrine and paracrine pathways [5].
MicroRNAs (miRNAs) are small non-coding RNAs that modulate gene expression through inhibition of protein translation and/or reducing mRNA stability. MiRNAs have been shown to regulate a wide variety of cellular processes [6]. Several protein-coding genes are differentially expressed between CCs and MGCs and their relevance for CC and MGC function has been previously demonstrated in the context of cell communication, extracellular matrix and signaling pathways [2, 7]. In contrast, the expression and function of miRNAs in CCs and MGCs are only scarcely investigated [8, 9]. Therefore, we aimed to determine the miRNA profile in CCs and MGCs and to identify differentially regulated miRNAs between these specialized cell types.
2. Materials and Methods
2.1. Patients and Cell Culture
Primary Granulosa Cells (PrGCs) were obtained from pre-ovulatory follicles from seven individual healthy women between 24-38 years old undergoing ovum pick-up for in vitro fertilization (IVF) for male factor infertility at the University Medical Centre Groningen, The Netherlands. Patients received human menopausal gonadotrophin 150-225 IU per day (Menopur®, Ferring Pharmaceuticals, The Netherlands) and a daily subcutaneous GnRH analog, triporeline 0.5 mg (Decapeptyl®, Ferring Pharmaceuticals, The Netherlands). Oocytes were collected 36 hours after treatment with 5000 IU human chorionic gonadotropin (hCG) (Pregnyl® Organon, Oss, The Netherlands) and cumulus aggregates were obtained by visual inspection and manual separation from the cumulus-oocyte complex followed by centrifugation in HBSS (Life technologies, USA) for 10 minutes at 400g. For MGCs isolation, the Follicular Fluid (FF) was centrifuged 5 minutes at 400g and 5 minutes at 500g, and the pellet was resuspended in HBSS. Blood cells were separated from the MGCs using a 40% PercollTM (GE Healthcare, Sweden) gradient and 10 minutes centrifugation at 550g. Cells from the interface were collected and washed in HBSS through 10 minutes centrifugation at 450g. For both CCs and MGCs the pelleted cells were resuspended in 1 ml trypsin followed by 3-4 minutes incubation at 37°C to disperse the clustered cells. CCs and MGCs from individual patients were cultured in DMEM/F12 medium supplemented with 10% FCS, 1% penicillin-streptomycin-amphotericin B at 37°C and 5% CO2 or directly harvested. Cultured cells were washed with HBSS every two days until the culture was devoid of contaminants upon visual inspection. For the MGCs/CCs sequencing and RT-qPCR validation experiments, cultured cells (2-4 days) were washed 2x with HBSS and incubated for 24 hours with serum-free DMEM/F12 supplemented with 1% penicillin-streptomycin-amphotericin-B and 10 uM androstenedione (AST) (Sigma, USA). Three patients were randomly selected for small RNA sequencing analysis and all seven were used for RT-qPCR experiments. From an additional three patients MGCs and CCs were directly harvested after isolation to compare the possible effect of short-term culturing on the miRNA expression of MGCs and CCs.
2.2. RNA Isolation and Quality Control
RNA was isolated using the miRNeasy micro kit (QIAGEN, Germany) according to the manufacturer’s instructions. For sequencing samples, RNA quality was assessed using Experion™ RNA StdSens Analysis Kit (Bio-Rad, USA).
2.3. Small RNA Sequencing
Small RNA libraries were generated from 200 ng total RNA using NEXTflex Small RNA Sequencing Kit v3 for Illumina Platforms (Bio Scientific, Austin, TX, USA). Libraries were purified with an automated agarose gel separation system (Labchip XT, PerkinElmer, Waltham, MA, USA) and sequenced on Illumina Nextseq500 (Illumina, San Diego, CA) using default parameters (single read 1x50bp). Briefly, 3′- and 5′-adaptor sequences were removed using the CLC Genomics Workbench (CLC Bio, Cambridge, MA). RNA reads were analyzed with miRDeep version 2.0 [10] and annotated against miRBASE version 21 (http://www.mirbase.org) allowing one mismatch. Total read counts were standardized to read counts per million (RPM). Read counts for miRNAs with the same mature sequence were merged. For statistical analysis, we included all unique miRNAs with an average read count of at least 20 in at least one out of the two cell types. Small RNA sequencing data are available at the Gene Expression Omnibus data repository, accession number GSE106776.
2.4. Reverse Transcriptase (RT)-Quantitative (q)PCR
RT-qPCR was performed using TaqMan miRNA quantitative PCR assays (Thermo Fisher Scientific Inc., USA). Per miRNA the most abundant mature miRNA isoform was identified from the small RNA sequencing data and used to select a pre- or custom- designed taqman assay (Supplementary Table 1 (159.7KB, pdf) ). cDNA synthesis was performed in a multiplex reaction as described previously [11]. Expression levels were normalized to RNU44. Cycle threshold (Ct) values were determined with the QuantStudio RT-PCR software version 1.2 (Applied Biosystems, USA). Relative expression levels of miRNAs were determined by using the 2−ΔCt method [12].
2.5. Proven miRNA Target Gene Interactions and Gene Ontology Analysis
Proven target genes of the validated miRNAs were retrieved from the miRTarBase database (http://mirtarbase. mbc.nctu.edu.tw/, release 7.0, Sept 15th, 2017). We only included genes that were validated with at least a reporter assay based method. Gene ontology analysis was performed with DAVID 6.8 bioinformatic database (https://david. ncifcrf.gov/) using an FDR cut-off of 0.001.
2.6. Statistical Analysis
For sequencing data, a paired t-test was used to analyze differential expression of individual miRNAs in MGC and CCs, considering p<0.05 as statistically significant, using GeneSpring GX software version 12.5 (Agilent Technologies, Santa Clara, CA). For the RT-qPCR validation, one-tailed paired t-test was used (GraphPad Prism 5.00 software). The heatmap was generated with Genesis software [13] and the hierarchical clustering was shown with median centering of the genes and Pearson correlation.
2.7. Institutional Review Board Approval
Institutional Review Board (IRB) approval was requested but waived, since in this study only anonymized material and data were used from patients who had signed the universal consent form for the anonymous usage of their data and who did not object to the usage of the waste material.
3. Results
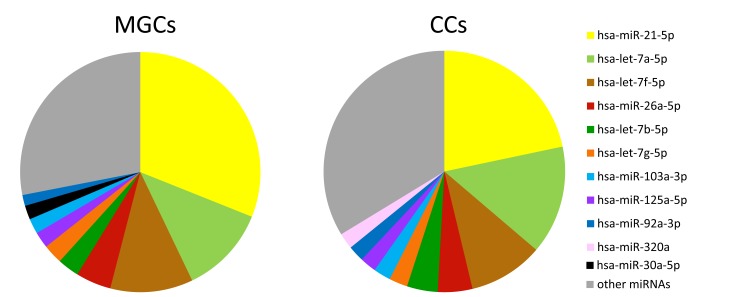
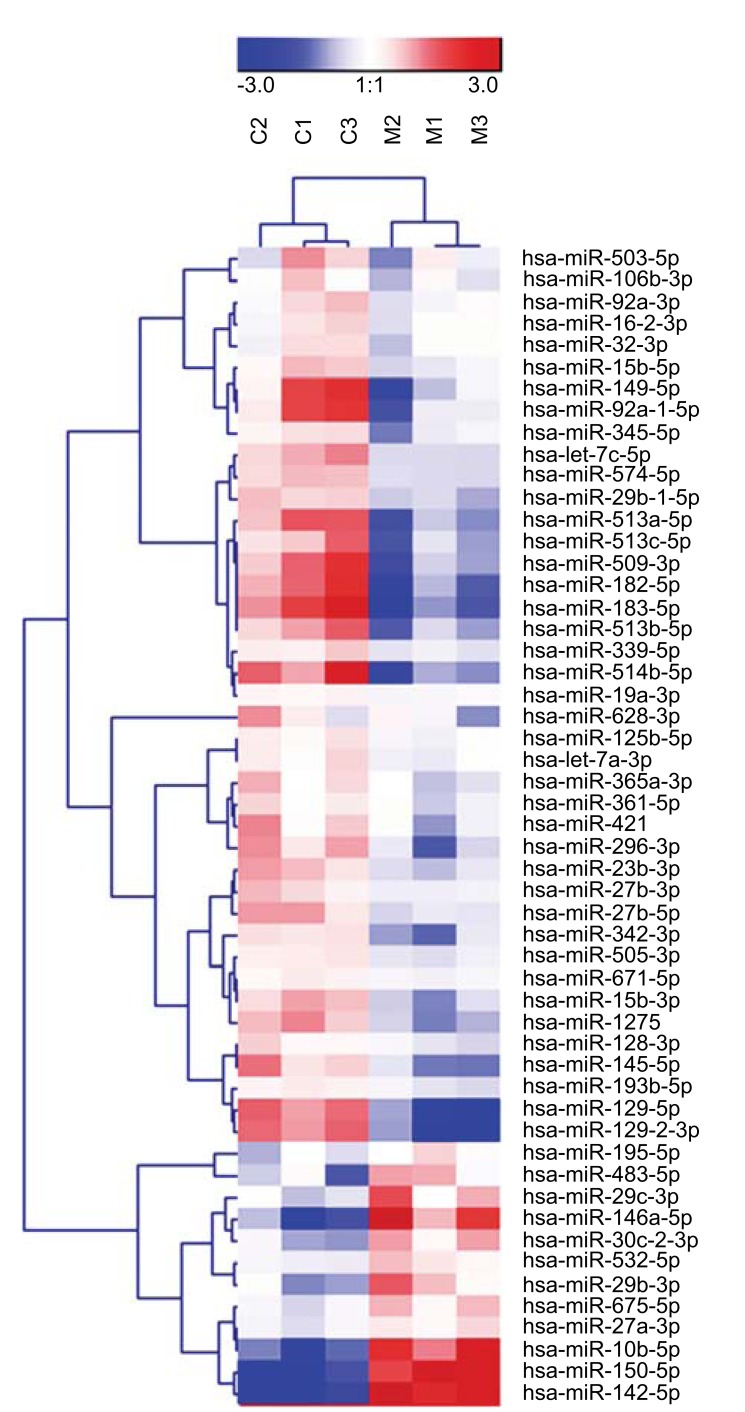
Small RNA sequencing was performed to establish the miRNA expression profile of MGCs and CCs. A comparison of the ten most abundantly expressed miRNAs in MGCs and CCs revealed that the majority of highly abundant miRNAs are shared between the two cell types with the highest expression levels for miR-21-5p, let-7a-5p and let-7f-5p (Fig. 1). MiR-30a-5p was uniquely present in the top-10 most abundantly expressed miRNAs of MGCs, while miR-320a was only present in the top-10 of CCs. Principal component analysis showed that 43% of the variation in miRNA expression within the MGCs and CCs could be explained by differences between cell types (first component) and 21% by differences between patients (second component). In total, 53 miRNAs were significantly differentially expressed between MGCs and CCs. Of those 12 showed higher expression levels in MGCs while 41 were more abundant in CCs (Fig. 2).
Fig. (1).
Pie chart showing the relative abundance of the top 10 most abundant miRNAs in mural (MGCs) and cumulus (CCs) granulosa cells as identified by small RNA sequencing analysis. n=3 individual patients.
Fig. (2).
Heatmap showing unsupervised clustering of 53 differentially expressed miRNAs between cumulus (CCs) and Mural (MGCs) Granulosa Cells. n=3 individual patients.
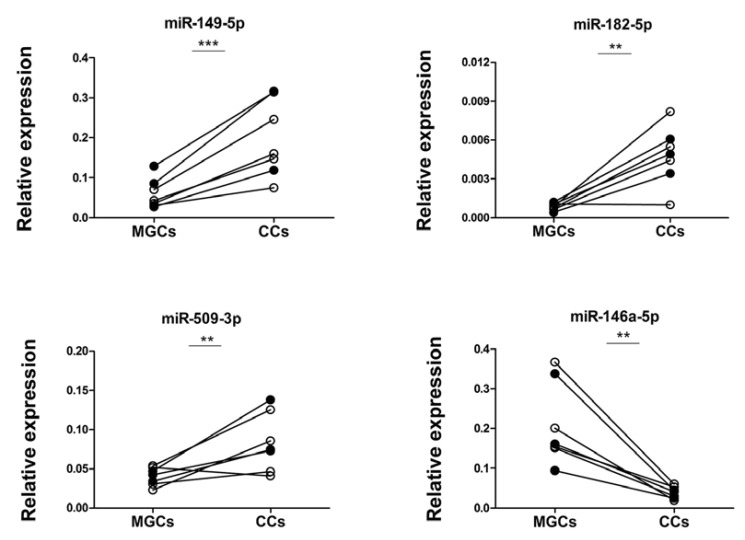
To confirm the small RNA sequencing results, we selected the five miRNAs with the highest fold change and a read count of 500 rpm or more in MGCs or CCs for RT-qPCR analysis (Table 1). Four of the selected candidates were increased in CC, i.e. miR-183-5p, miR-182-5p, miR-509-3p and miR-149-5p, while miR-146a-5p was increased in MGC. Validation of the differential expression patterns was performed on the same samples as used for small RNA sequencing (n=3 individual patients) as well as in an independent set of samples (n=4 individual patients). RT-qPCR analysis confirmed the expected significant differences for four out of five miRNAs (Fig. 3). For the fifth miRNA, i.e. miR-183-5p, no expression in MGCs or CCs was detected by RT-qPCR. To determine the possible effect of short-term culturing, miRNA levels were also studied in directly isolated MGCs and CCs (n=3). This revealed similar differences in miRNA expression as compared to the RT-qPCR and RNA-seq results using cultured cells (Fig. S1 (159.7KB, pdf) ).
Table 1.
Overview of all miRNAs differentially expressed between mural (MGCs) and cumulus (CCs) granulosa cells with >500 RPM in at least one of the two cell types.
| miRNA | Read Counts (RPM)* | FC | p-value | |
|---|---|---|---|---|
| CCs | MGCs | |||
| Increased in CCs | ||||
| hsa-miR-183-5p | 535 | 53 | 10.1 | 0.013 |
| hsa-miR-182-5p | 1123 | 159 | 7.1 | 0.021 |
| hsa-miR-509-3p | 1266 | 254 | 5.0 | 0.013 |
| hsa-miR-149-5p | 961 | 204 | 4.7 | 0.001 |
| hsa-miR-513a-5p | 717 | 153 | 4.7 | 0.002 |
| hsa-let-7c-5p | 18366 | 7628 | 2.4 | 0.017 |
| hsa-miR-342-3p | 820 | 348 | 2.4 | 0.039 |
| hsa-miR-23b-3p | 10957 | 4881 | 2.2 | 0.031 |
| hsa-miR-574-5p | 1739 | 811 | 2.1 | 0.005 |
| hsa-miR-15b-5p | 4059 | 2276 | 1.8 | 0.023 |
| hsa-miR-365a-3p | 1643 | 956 | 1.7 | 0.008 |
| hsa-miR-339-5p | 516 | 308 | 1.7 | 0.046 |
| hsa-miR-27b-3p | 8550 | 5132 | 1.7 | 0.045 |
| hsa-miR-505-3p | 569 | 355 | 1.6 | 0.005 |
| miRNA | Read Counts (RPM)* | FC | p-value | |
| CCs | MGCs | |||
| hsa-miR-503-5p | 1062 | 664 | 1.6 | 0.011 |
| hsa-miR-128-3p | 2734 | 1746 | 1.6 | 0.007 |
| hsa-miR-92a-3p | 20973 | 14469 | 1.4 | 0.030 |
| hsa-miR-361-5p | 2869 | 1987 | 1.4 | 0.005 |
| hsa-let-7a-3p | 504 | 358 | 1.4 | 0.011 |
| hsa-miR-125b-5p | 4482 | 3297 | 1.4 | 0.011 |
| Increased in MGCs | ||||
| hsa-miR-146a-5p | 48 | 608 | 12.6 | 0.002 |
| hsa-miR-675-5p | 527 | 921 | 1.7 | 0.006 |
| hsa-miR-195-5p | 426 | 663 | 1.6 | 0.019 |
| hsa-miR-27a-3p | 652 | 985 | 1.5 | 0.004 |
* Read counts is given as the geometric mean of the RPM values across all samples. miRNAs in bold are selected for RT-qPCR validation.
Fig. (3).
RT-qPCR validation of differentially expressed miRNAs between MGCs and CCs as identified by small RNA sequencing. Closed circles represent the three patients used for small RNA sequencing and open circles represent four additional patients. n= 7 individual patients in total. ** p <0.01, *** p < 0.001.
To obtain insight into the possible functions of the four validated miRNAs we retrieved known targets using miRTarBase [14]. This resulted in the identification of 91 genes in total without any overlap between the genes targeted per miRNA. 37 targets were identified for miR-146a-5p, 43 for miR-182-5p, 2 for miR-509-3p and 9 for miR-149-5p. Gene Ontology (GO) analysis revealed 12 enriched GO terms for miR-146a-5p and 10 for miR-182-5p (Table 2). No significantly enriched GO terms were found for miR-509-3p and miR-149-5p. The GO term for Ubiquitin-Like Protein (UBL) conjugation was enriched within miR-146a-5p as well as miR-182-5p proven target genes. Next, we performed GO analysis using the combined targets of the three miRNAs with increased abundance in CCs, i.e. miR-182-5p, miR-509-3p and miR-149-5p (54 target genes). This revealed 15 significantly enriched GO terms, including increased significance for UBL conjugation and regulation of transcription.
Table 2.
Gene Ontology (GO) analysis for miR-146a-5p, miR-149-5p, miR-509-3p and miR-182-5p.
| miR-146a-5p (up in MGCs) | GO Term | # genes | FDR |
|---|---|---|---|
| Pathways in cancer | 15 | 3.77E-07 | |
| Mutagenesis site | 20 | 8.54E-07 | |
| Cellular response to mechanical stimulus | 7 | 1.04E-05 | |
| Toll-like receptor signalling pathway | 9 | 1.62E-05 | |
| Inflammatory response | 10 | 8.93E-05 | |
| Positive regulation of smooth muscle cell proliferation | 6 | 2.26E-04 | |
| Chagas disease (American trypanosomiasis) | 8 | 3.27E-04 | |
| Ubl conjugation | 15 | 5.26E-04 | |
| Lipopolysaccharide-mediated signaling pathway | 5 | 8.39E-04 | |
| Leishmaniasis | 7 | 6.68E-04 | |
| Protein binding | 33 | 7.99E-04 | |
| Toxoplasmosis | 8 | 7.77E-04 | |
| miR-182-5p (up in CCs) | Tumor suppressor | 9 | 2.20E-06 |
| Ubl conjugation | 18 | 9.56E-06 | |
| Nucleus | 29 | 1.18E-05 | |
| Positive regulation of transcription, DNA-templated | 12 | 4.28E-05 | |
| Sequence-specific DNA binding | 12 | 3.36E-05 | |
| Prostate cancer | 8 | 4.66E-05 | |
| Negative regulation of apoptotic process | 11 | 1.57E-04 | |
| Nucleoplasm | 21 | 5.40E-04 | |
| PI3K-Akt signaling pathway | 11 | 5.67E-04 | |
| Cellular response to DNA damage stimulus | 8 | 9.77E-04 | |
| miR-182-5p, miR-509-3p, miR-149-5p (all up in CCs) | Ubl conjugation | 23 | 3.91E-08 |
| Positive regulation of transcription, DNA-templated | 15 | 5.22E-07 | |
| Sequence-specific DNA binding | 14 | 4.81E-06 | |
| Tumor suppressor | 9 | 1.65E-05 | |
| Prostate cancer | 9 | 1.69E-05 | |
| Nucleus | 33 | 4.97E-05 | |
| Negative regulation of apoptotic process | 12 | 1.79E-04 | |
| PI3K-Akt signaling pathway | 13 | 1.40E-04 | |
| Positive regulation of transcription from RNA polymerase II promoter | 16 | 2.59E-04 | |
| Cellular response to DNA damage stimulus | 9 | 3.59E-04 | |
| Phosphoprotein | 40 | 3.83E-04 | |
| Transcription regulation | 21 | 4.57E-04 | |
| Pathways in cancer | 13 | 5.79E-04 | |
| Transcription | 21 | 7.17E-04 | |
| Activator | 12 | 7.44E-04 |
FDR= false discovery rate
4. Discussion
Although MGCs and CCs are closely related cell types, clear differences in their functions and protein coding gene expression profiles have been described [2, 3]. In our study, we determined the miRNA expression profile of human MGCs and CCs using small RNA sequencing. Although the majority of the highly abundant miRNAs showed similar expression levels in both cell types, we were able to identify 53 significantly differentially expressed miRNAs and validated 4 of them using RT-qPCR. Identification of differentially expressed miRNAs is a first step to elucidate the potential contribution of miRNAs in granulosa cell function and cell type specialization of MGCs and CCs.
Velthut et al., previously described the differential expression of miRNAs in MGCs and CCs. We found nine significantly differentially expressed miRNAs in common with their study (upregulated in MGC: hsa-miR-146a-5p, hsa-miR-10b-5p, hsa-miR-29b-3p and hsa-miR-142-5p and upregulated in CC: hsa-let-7c-5p, hsa-miR-125b-5p, hsa-miR-1275, hsa-miR-129-5p and hsa-miR-129-2-3p). A large proportion of the previously identified differentially expressed miRNAs (50%) showed in our study less than 20 RPM in both groups. This suggests that these miRNAs are expressed at a relatively low level and that these are possibly of minor relevance to MGC and CC biology. The limited overlap between the two studies might be related to differences in experimental procedures and the data analysis strategy. We used short-term cultured MGCs and CCs, while Velthut et al. used directly isolated cells. However, for the four selected miRNAs, we observed similar expression patterns in cultured and non-cultured MGCs and CCs (Fig. S1 (159.7KB, pdf) ). Both current studies used MGCs and CCs from patients treated with gonadotropins. To exclude possible influences of this treatment on the miRNA expression profile it would be interesting to define the miRNA profile of MGCs and CCs from patients with physiological ovarian cycles without gonadotropin treatment in future studies.
Several of the significantly differentially expressed miRNAs have been implicated to positively or negatively affect steroidogenesis. For example, miR-149-5p inhibits estradiol release [8] and miR-509-3p promotes estradiol secretion by targeting MAP3K8 [15]. Moreover, miR-183-5p was described to inhibit the progesterone release [8]. For other miRNAs a role in regulating apoptosis and proliferation in PrGC has been reported, with miR-146a-5p promoting apoptosis of MGCs by directly targeting IRAK1 and TRAF6 [16] and miR-503-5p inhibiting proliferation by targeting CCND2 [17].
Analysis of previously validated targets for the four differentially expressed miRNAs revealed a total of 91 targets. GO analysis using this list of targets revealed 12 enriched GO terms for miR-146a-5p and 10 for miR-182-5p. One GO term, UBL conjugation, was shared between these two miRNAs. This term was even more enriched when targets of miR-149-5p, miR-182-5p and miR-509-3p (all miRNAs that were higher expressed in CCs) were combined in the GO analysis. Another term for which the significance increased in the combined analysis was positive regulation of transcription, indicating that genes related to this term and UBL conjugation are targeted by multiple miRNAs expressed at higher levels in CCs. In future studies, it would be interesting to further focus on the role of these miRNAs in MGCs and CCs in relation to the specific functions of these cells.
Conclusion
We have defined the miRNA profiles of MGCs and CCs and identified 53 differentially expressed miRNAs between these specialized cell types. Further studies may build on our data to elucidate the functions of these miRNAs in relation to folliculogenesis and their impact on oocyte quality.
ACKNOWLEDGEMENTS
Daniela Andrei and Roland A. Nagy contributed to the design of the study, performed experiments, analyzed and interpreted the data and wrote the manuscript. Aafke van Montfoort, Uwe Tietge, Annemieke Hoek, Joost Kluiver, Anke van den Berg, Rogier Donker conceived and designed the study, supervised experiments, data analyses and interpretation, and supervised writing of the paper. Martijn Terpstra and Klaas Kok performed bio-informatic analyses of sequencing data. All authors contributed in critical discussion of the data, revisions of the manuscript and all approved the final manuscript.
The authors thank B. van Rijkdom, D. van Brandenburg-Weening and P. van der Vlies from the Department of Genetics, University Medical Centre Groningen, University of Groningen, The Netherlands, for assistance in RNA quality control and small RNA sequencing experiments, and the laboratory technicians from the IVF laboratory at University Medical Centre Groningen, University of Groningen, The Netherlands, for assistance with primary cell isolations. We thank the UMCG Genomics Coordination Center, the UG Center for Information Technology and their sponsors BBMRI-NL & TarGet for storage and compute infrastructure. We would like to thank the Center for Information Technology of the University of Groningen for their support and for providing access to the Peregrine high performance computing cluster.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
Ethics Approval and Consent to Participate
Institutional Review Board (IRB) approval was requested but waived, since in this study only anonymized material and data were used.
Human and Animal Rights
No animals were used for studies that are the basis of this research. The reported experiments in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
Consent for Publication
Patients who had signed the universal consent form for the anonymous usage of their data and who did not object to the usage of the waste material.
conflict of interest
The study is supported by an unrestricted educational grant from Ferring Pharmaceuticals BV, The Netherlands, to the Department of Obstetrics and Gynecology of the University Medical Centre Groningen, The Netherlands.
References
- 1.Atwood C.S., Vadakkadath M.S. Meethal. The spatiotemporal hormonal orchestration of human folliculogenesis, early embryogenesis and blastocyst implantation. Mol. Cell. Endocrinol. 2016;430:33–48. doi: 10.1016/j.mce.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Grøndahl M.L., Andersen C.Y., Bogstad J., Borgbo T., Boujida V.H., Borup R. Specific genes are selectively expressed between cumulus and granulosa cells from individual human pre-ovulatory follicles. Mol. Hum. Reprod. 2012;12:572–584. doi: 10.1093/molehr/gas035. [DOI] [PubMed] [Google Scholar]
- 3.Khan D.R., Fournier E., Dufort I., Richard F.J., Singh J., Sirard M.A. Meta-analysis of gene expression profiles in granulosa cells during folliculogenesis. Reproduction. 2016;6:103–110. doi: 10.1530/REP-15-0594. [DOI] [PubMed] [Google Scholar]
- 4.Makabe S., Naguro T., Stallone T. Oocyte-follicle cell interactions during ovarian follicle development, as seen by high resolution scanning and transmission electron microscopy in humans. Microsc. Res. Tech. 2006;6:436–449. doi: 10.1002/jemt.20303. [DOI] [PubMed] [Google Scholar]
- 5.Chang H.M., Qiao J., Leung P.C. Oocyte-somatic cell interactions in the human ovary - novel role of bone morphogenetic proteins and growth differentiation factors. Hum. Reprod. 2016;1:1–18. doi: 10.1093/humupd/dmw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurtan A.M., Sharp P.A. The role of miRNAs in regulating gene expression networks. J. Mol. Biol. 2013;19:3582–3600. doi: 10.1016/j.jmb.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wigglesworth K., Lee K.B., Emori C., Sugiura K., Eppig J.J. Transcriptomic diversification of developing cumulus and mural granulosa cells in mouse ovarian follicles. Biol. Reprod. 2015;1:1–14. doi: 10.1095/biolreprod.114.121756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirotkin A.V., Ovcharenko D., Grossmann R., Laukova M., Mlynček M. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. Cell Physiol. 2015;2:415–420. doi: 10.1002/jcp.21689. [DOI] [PubMed] [Google Scholar]
- 9.Velthut A.M., Simm J., Tuuri T., Tapanainen S.J., Metsis M., Salumets A. Research resource: small RNA-seq of human granulosa cells reveals miRNAs in FSHR and aromatase genes. Mol. Endocrinol. 2013;7:1128–1141. doi: 10.1210/me.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedländer M.R., Chen W., Adamidi C., et al. Discovering microRNAs from deep sequencing data using miRDeep. Nat. Biotechnol. 2008;4:407–415. doi: 10.1038/nbt1394. [DOI] [PubMed] [Google Scholar]
- 11.Kluiver J., Slezak-Prochazka I., van den Berg A. 2013. Studying microRNAs in lymphoma. [DOI] [PubMed] [Google Scholar]
- 12.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Sturn A., Quackenbush J., Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;1:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 14.Hsu S.D., Lin F.M., Wu W.Y., et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011;39(Suppl. 1):D163–D9. doi: 10.1093/nar/gkq1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X., Liu C., Hao C., et al. Identification of altered microRNAs and mRNAs in the cumulus cells of PCOS patients: miRNA-509-3p promotes oestradiol secretion by targeting MAP3K8. Reproduction. 2015;4:565–572. doi: 10.1530/REP-16-0071. [DOI] [PubMed] [Google Scholar]
- 16.Chen X., Xie M., Liu D., Shi K. Downregulation of microRNA-146a inhibits ovarian granulosa cell apoptosis by simultaneously targeting interleukin-1 receptor-associated kinase and tumor necrosis factor receptor associated factor 6. Mol. Med. Rep. 2015;4:5155–5162. doi: 10.3892/mmr.2015.4036. [DOI] [PubMed] [Google Scholar]
- 17.Lei L., Jin S., Gonzalez G., Behringer R.R., Woodruff T.K. The regulatory role of dicer in folliculogenesis in mice. Mol. Cell. Endocrinol. 2010;1:63–73. doi: 10.1016/j.mce.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.