Abstract
Objective:
Aiming at the modified release of melatonin (MLT), electrospun-MLT loaded nano-fibers, filled into hard gelatin and DRcapsTM capsules, were used as formulants.
Methods:
Cellulose acetate, polyvinylpyrrolidinone and hydroxypropylmethylcellusose (HPMC 2910) were used for the preparation of the fiber matrices through electrospinning. The in vitro modified release profile of MLT from the fabricated matrices in gastrointestinal-like fluids was studied. At pH 1.2, the formulations CA1, CA2, PV1, HP1, HP2 and the composite formulations CAPV1-CAPV5 in hard gela-tin capsules exhibited fast MLT release.
Results:
In general, the same trend was observed at pH 6.8, with the exception of CAPV1 and CAPV2. These two composite formulations delivered 52.08% and 75.25% MLT, respectively at a slower pace (6 h) when encapsulated in DRcapsTM capsules. In all other cases, the release of MLT from DRcapsTM cap-sules filled with the MLT-loaded nanofibers reached 100% at 6h.
Conclusion:
These findings suggest that the MLT-loaded nanofibrous mats developed in this study ex-hibit a promising profile for treating sleep dysfunctions.
Keywords: Electrospun nanofibers, polymers, scanning electron microscopic images, melatonin, capsules, modified release
1. Introduction
Melatonin (N-acetyl-5-methoxytryptamine, MLT) is synthesized and secreted by the pineal gland. Its secretion is considered to be a reliable and predictable marker of the central pacemaker's circadian phase [1, 2], which is controlled through a multisynaptic pathway originating from the suprachiasmatic nucleus (SCN) [3]. In healthy individuals, the duration of MLT secretion is a faithful representation of the period of darkness [4] and is thought to influence the circadian clock reciprocally through feedback to MLT receptors in the SCN [5]. Although the exact role of endogenous MLT in humans is unclear [6], pharmacological administration of supraphysiological doses of MLT during discrete time windows has been shown to phase-shift the circadian clock and potentially promote sleep and help with various other sleep disorders, such as jet lag, sleep problems related to shift work, and some sleep disorders in children and the elderly [7]. It has also been shown to be helpful for a sleep dysfunction that causes changes in sleep and wake times of blind people.
In the recent years, we have developed new oral MLT delivery systems suitable for treating sleep onset and sleep maintenance problems [8-13]. We have especially focused on modified versus immediate release tablet formulations, because MLT is known to be more clinically useful in initiating and maintaining sleep in elderly insomniacs, compared with immediate release or conventional therapy [14].
In this report, we communicate our findings on the in vitro modified release characteristics of MLT, in aqueous media (pH 1.2 and 6.8), from hard gelatin and DRcapsTM capsules loaded with electrospun nanofiber matrices [15] incorporating MLT.
Fibrous nanomaterials have gained significant attention in the last years due to their unique properties allowing for a wide range of applications [16-20]. Electrospinning is a simple and versatile technique for the production of fibrous scaffolds with diameters from submicron down to nanometer scale. Under the application of a high voltage electric field, electrospun nonwoven matrices can be produced through electrically charged jets of polymer solution or melts on rotary or stationary collectors [21-23]. A broad spectrum of biocompatible and biodegradable natural or synthetic polymers can be used for the generation of tailor-made fibrous scaffolds with tunable morphology, high surface-to-volume area, high porosity and small pore size. Such electrospun nonwovens, possessing favorable micromechanical properties, can serve as filtration, sensor or energy storage media and may find applications in the biomedical sector as drug release, wound healing and tissue engineering systems [24-26]. In the field of drug delivery, electrospinning has received attention due to its ability to produce drug-loaded fibers with high loading capacity and high encapsulation efficiency [27-29]. Especially in oral drug delivery systems, electrospun nanofibers have been successfully used for the sustained and controlled release of various incorporated drugs, achieving desirable release profiles [30]. Electrospun nanofibers can be easily subjected to modifications for fine tuning of their release characteristics. Depending on the selected systems of natural and synthetic biodegradable polymers [15, 31-34] and the electrospinning parameters, nanofibrous systems can be designed to provide rapid, immediate, delayed or modified dissolution profiles [35, 36].
2. Materials and methods
2.1. Materials
Polyvinylpyrrolidone (PVP) (Mw 1,300,000), cellulose acetate (CA) (Mw 50,000), hypromellose 2910 (HPMC), polyethylene oxide (PEO) (Mw 900,000 and Mw 400,000), acetone, ethanol (EtOH), and dichloromethane (DCM) were obtained from Sigma–Aldrich (Darmstadt, Germany). Melatonin (MLT) (lot 402HH-IT) was purchased from the Tokyo Chemical Industry (Tokyo, Japan). All chemicals were of reagent grade and used directly without further purification.
2.2. Electrospinning
All spinning solutions were prepared at room temperature under stirring for 24 h to ensure their homogeneity. Electrospinning was conducted using a γ-High Voltage Research DC power supply generator (Gamma High Voltage Research, Ormond Beach, FL, USA) with a maximum voltage of 50 kV. The polymer solutions were loaded into 10-mL disposable syringes fitted with 23G tip-ground-to-flat needles, which were mounted on a horizontally positioned Harvard PHD 2000 programmable syringe pump (Harvard Apparatus, Holliston, MA, USA). The produced nanofibers were deposited on aluminum foil wrapped on an RC-6000 (NaBond Technologies, Hong Kong) rotating drum collector at a rotation speed of 400 rpm. Temperature and relative humidity were 20±2°C and 60±5%, respectively.
2.3. Preparation of CA-MLT Fiber Mats
Spinning solutions were prepared by dissolving CA at a concentration of 10% w/v in acetone. Subsequently, MLT was added to the polymer solutions in concentrations of 1.01% and 2.04% w/w (weight to polymer weight) to afford fiber mats with 1% and 2% w/w total concentration of MLT (CA1 and CA2, respectively). Applied voltage, tip-to-collector distance and solution feeding rate were fixed at 20 kV, 10 cm and 1 mL/h, respectively.
2.4. Preparation of PVP-MLT Fiber Mats
The spinning solution was prepared by dissolving PVP at a concentration of 10% w/v in EtOH. Subsequently, MLT was added to the polymer solution in the concentration of 1.01% w/w (weight to polymer weight) to afford fiber mats with 1% w/w total concentration of MLT (PV1). Applied voltage, tip-to-collector distance and solution feeding rate were fixed at 20 kV, 10 cm and 1 mL/h, respectively.
2.5. Preparation of HPMC(PEO)-MLT Fiber Mats
Spinning solutions were prepared by dissolving HPMC at a concentration of 1% w/v in a solvent system of EtOH:DCM (1:1). PEO of different molecular weight (Mw 900,000 and Mw 400,000) was added to each spinning solution at 0.05% w/v in order to facilitate the electrospinning of HPMC (ratio of HPMC to PEO 20:1). Subsequently, MLT was added to the polymer solutions in the concentration of 1.01% w/w (weight to polymer weight) to afford fiber mats with 1% w/w total concentration of MLT (HP1 and HP2, respectively). Applied voltage, tip-to-collector distance and solution feeding rate were fixed at 20 kV, 20 cm and 0.5 mL/h, respectively.
2.6. Preparation of CA/PVP-MLT Fiber Mats
Spinning solutions were prepared by dissolving CA at a concentration of 10% w/v in acetone and PVP at a concentration of 10% w/v in EtOH. Subsequently, MLT was added to polymer solutions in concentrations of 1.51%, 1.01%, 0.5% and 0% w/w (weight to polymer weight). For the blended fiber mats, electrospinning was conducted with the syringe needles mounted on an antiparallel set up to ensure a homogeneous blending of the CA and PVP polymer fibers. The polymer solutions of CA and PVP were co-spun in five combinations to afford blended fiber matrices with 1% w/w total concentration of MLT, distributed in different ratios into each polymer of the blended matrices (ratio of MLT in the blended fiber mats of CA to PVP 1:1, 3:1, 1:3 1:0 and 0:1 for the CAPV1, CAPV2, CAPV3, CAPV4 and CAPV5, respectively). Applied voltage, tip-to-collector distance and solution feeding rate were fixed at 20 kV, 10 cm and 1 mL/h, respectively.
2.7. Characterization
A PhenomWorld desktop scanning electron microscope (SEM - Eindhoven, The Netherlands) with tungsten filament (10 kV) and charge reduction sample holder was used for SEM analyses of the nanofibers. The diameters of 100 fibers from each SEM image were measured using the embedded image analysis software (Phenom Pro Suite/Fibermetric) and the average fiber diameter was determined. The obtained fiber mats were extracted with EtOH and the recovered residues were analyzed by 1H NMR spectroscopy on a Bruker DRX 400 MHz spectrometer to evaluate the chemical integrity of MLT after electrospinning.
2.8. Preparation of Formulations and Dissolution Tests
Nanofibers (100 mg) (Table 1) were inserted to hard gelatin capsules (Syndesmos SA, Athens, Greece) or DRcapsTM (Capsugel, Colmar, France) capsules No. 0. The in vitro dissolution tests of these capsules were performed using a USP Apparatus I (basket method) in triplicate. The concentration of MLT in the dissolution medium was measured at 278 nm by using a UV-Vis spectrophotometer. The dissolution medium was 450 ml of buffer solution (pH 1.2 or 6.8) maintained at 37 ± 0.5oC as described in the USP 28. The basket rotation speed was set at 50 rpm. The samples were collected at predetermined time intervals and replaced with an equal volume of fresh medium at each time point.
Table 1.
Formulants’ (%) in encapsulated fiber mats (values in mg). The amount of MLT dissolved in each polymer solution is given in parenthesis.
| Fiber Mat Formulation | CA1 | CA2 | PV1 | HP1 | HP2 | CAPV1 | CAPV2 | CAPV3 | CAPV4 | CAPV5 |
|---|---|---|---|---|---|---|---|---|---|---|
| MLT | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| CA | 99.0 (1 mg of MLT) |
98.0 (2 mg of MLT) |
- | - | - | 49.5 (0.5 mg of MLT) |
49.5 (0.75mg of MLT) |
49.5 (0.25 mg of MLT) |
49.5 (1 mg of MLT) |
49.5 (0 mg of MLT) |
| PVP | - | - | 99.0 (1 mg of MLT) |
- | - | 49.5 (0.5 mg of MLT) |
49.5 (0.25 mg of MLT) |
49.5 (0.75 mg of MLT) |
49.5 (0 mg of MLT) |
49.5 (1 mg of MLT) |
| HPMC 2910 | - | - | - | 94.05 (1 mg of MLT) |
94.05 (1 mg of MLT) |
- | - | - | - | - |
| PEO (MW 900,000) |
- | - | - | 4.95 (0 mg of MLT) |
- | - | - | - | - | - |
| PEO (MW 400,000) |
- | - | - | - | 4.95 (0 mg of MLT) |
- | - | - | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
3. Results and discussion
In the present study, MLT is reported for the first time to be successfully incorporated in micro/nanofiber nonwovens. The chemical integrity of MLT, following the electrospinning process under the specific conditions, was confirmed by 1H NMR spectroscopy of the recovered residues following extraction of the obtained fiber mats.
Analysis of the SEM images revealed the morphological characteristics of the produced micro/nanofibrous matrices. In all cases, uniform fiber mats were produced from all systems with well-shaped fibers without the presence of beads. The diameter distribution and the average diameter of the fibers for the different systems are shown in Figs. (1 and 2).
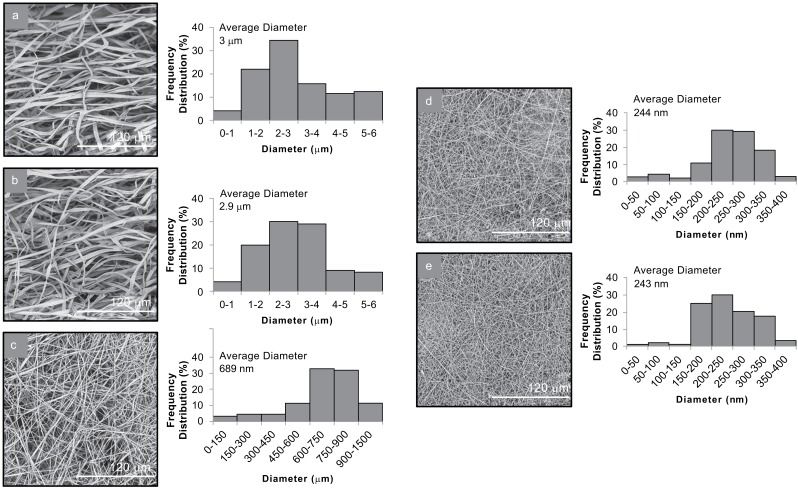
Fig. (1).
SEM images and average diameter distribution histograms of (a) CA1, (b) CA2, (c) PV1, (d) HP1 and (e) HP2.
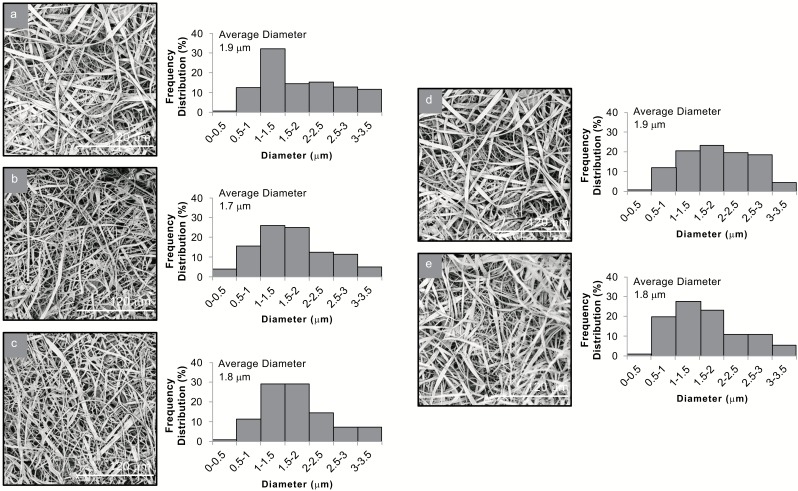
Fig. (2).
SEM images and average diameter distribution histograms of (a) CAPV1, (b) CAPV2, (c) CAPV3, (d) CAPV4 and (e) CAPV5.
In the case of CA-MLT fiber mats, ribbon-like shaped fibers were obtained with broad diameter distribution in the nano-micro scale. The different loading amount of MLT did not affect the morphology nor the size of the produced fibers. In CA1 mats, fiber diameters ranged from 689 nm to 6 μm, with an average diameter of 3 ± 0.67 μm, whereas in CA2 mats, fibers were measured from 637 nm to 5.8 μm, with an average diameter of 2.9 ± 0.62 μm. The produced PV1 fiber mats consisted of smooth fibers of cylindrical shape with diameters ranging from 110 to 990 nm and an average diameter of 689 ± 164 nm. Uniform, cylindrical fibers were also observed in both systems of HPMC(PEO)-MLT fiber mats (HP1 and HP2). The different molecular weights of PEO in the HP1 and HP2 did not result in significant differences in the average diameters of the two systems. In HP1 mats, fiber diameters ranged from 35 to 382 nm, with an average diameter 244 ± 70 nm. Similarly, the fibers of HP2 mats ranged from 50 to 413 nm, with an average diameter of 243 ± 81 nm (Fig. 1).
SEM observations of the blended mats of CA and PVP revealed the successful blending of the two polymers, as deciphered by the discrete presence of both the ribbon-like fibers of CA and the cylindrical fibers of PVP. In all systems, the diameters of the blended fibers were measured in a similar size range, from 430 nm to 3.7 μm, with an average diameter of 1.9 ± 0.44 μm for the CAPV1 system, 284 nm to 4.3 μm, with an average diameter of 1.7 ± 0.47 μm for the CAPV2 system, 336 nm to 4.6 μm, with an average diameter of 1.8 ± 0.44 μm for the CAPV3 system, 386 nm to 3.4 μm, with an average diameter of 1.9 ± 0.43 μm for the CAPV4 system and 325 nm to 5.2 μm, with an average diameter of 1.8 ± 0.42 μm for the CAPV5 system (Fig. 2).
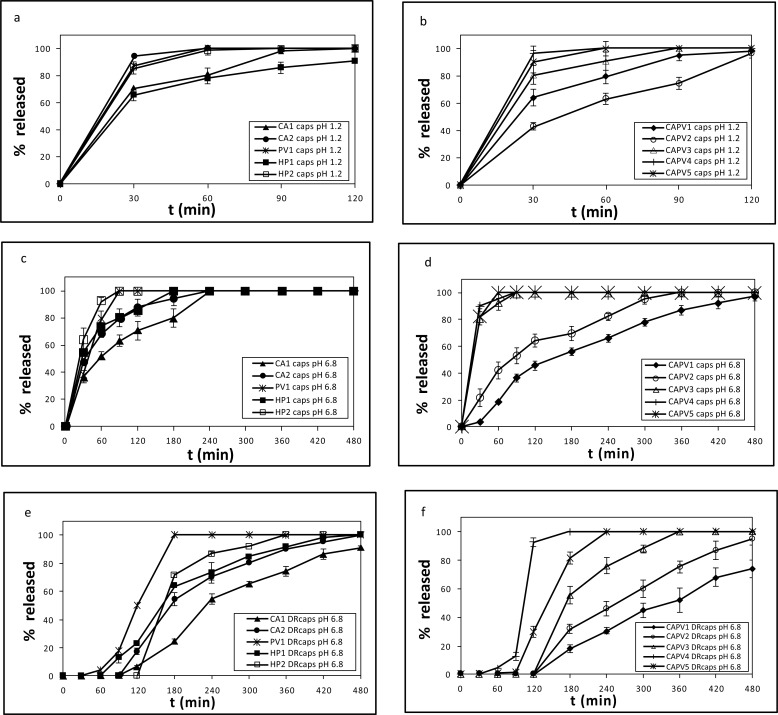
The developed nanofibrous systems were incorporated in both hard gelatin and DRcapsTM capsules and the in vitro dissolution of MLT was studied. The release profiles of MLT from the hard gelatin capsules at pH 1.2 are depicted in Figs. (3a and 3b) and at pH 6.8 in Figs. (3c and 3d), whereas the release profiles of MLT from the DRcapsTM capsules at pH 6.8 are shown in Figs. (3e and 3f).
Fig. (3).
% release of MLT vs. time from formulations (a) CA1, CA2, PV1, HP1, HP2 and (b) CAPV1-CAPV5 at pH 1.2, with hard gelatin capsules, (c) CA1, CA2, PV1, HP1, HP2 and (d) CAPV1-CAPV5 at pH 6.8, with hard gelatin capsules, (e) CA1, CA2, PV1, HP1, HP2 and (f) CAPV1-CAPV5 at pH 6.8, with DRcapsTM capsules.
The release of MLT at pH 1.2 from CA-MLT nanofibers depended on its quantity in the nanofiber matrix. Doubling the amount of MLT (from 1 to 2 mg) led to a substantial release increase (70.74% to 94.43%) in the same time period (30 min) (Fig. 3a). This difference in the percentage of released MLT is in agreement with the Higuchi equation, a theoretical model for studying the release of water-soluble and poorly soluble drugs from a variety of matrices, including semisolids and solids [37] (Eq. 1).
Q=(2ADCst)1/2 (1)
where, Q is the amount of drug per unit area released from a formulation system at time t; Cs is the solubility or saturation concentration of drug in the matrix; A is the total concentration (amount per unit volume), dissolved and undissolved, of drug in the matrix; D the diffusion coefficient of the drug in the matrix.
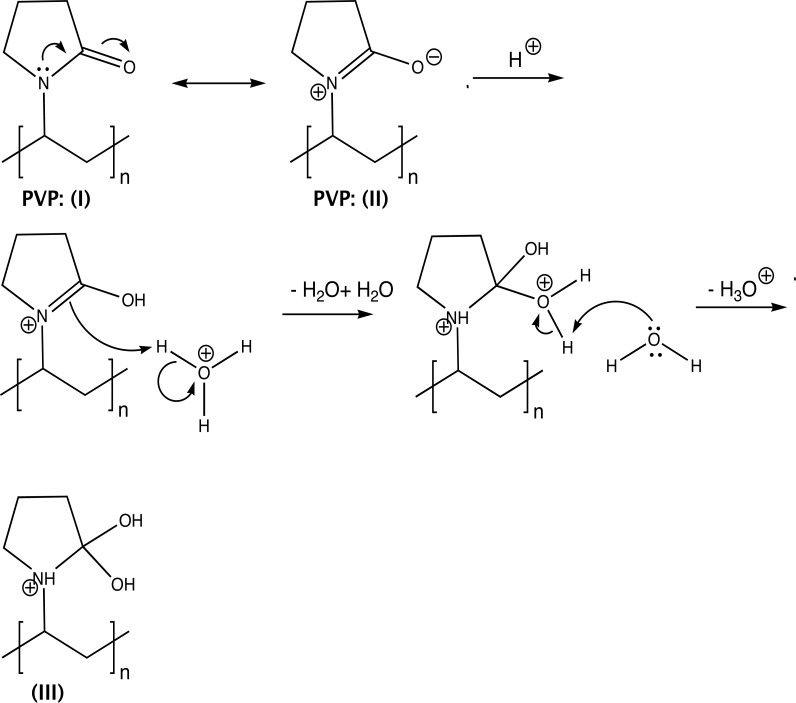
When PVP was used for the preparation of the nanofiber matrix, the release of MLT (1 mg) reached 94.43% in 30 min at pH 1.2 (Fig. 3a). The enhanced release of MLT in this case, compared to the CA1 formulation system (70.74%), can be attributed to the conversion of PVP (II) to the gem-diol (III) (Fig. 4), at pH 1.2 [12]. The -OHs of III participate in H-bond formation with MLT’s C5-oxygen atom and the NHCOCH3 group, thus facilitating its aqueous solubility. Interestingly, the release of melatonin at pH 1.2 from HP1 nanofibers follows an analogous pattern to the CA-based formulations. In the case of HP2 mats, MLT is released in higher quantity (87.59%) than from the HP1 system (65.76%) (Fig. 3a). This difference in the percentage of released MLT is possibly due to the intrinsic poor aqueous solubility of PEO [38], which decreases as its molecular weight increases. These findings correlate well with the average fiber diameters. With the exception of HP1, the lower the fiber diameter, the higher the release (%) of MLT (PV1 and HP2 versus CA1) [39].
Fig. (4).
PVP (I) conversion to the gem-diol (III).
When mixtures of CA and PVP were used for the preparation of MLT containing electrospun nanofibers, the release of MLT at pH 1.2, in the case of formulation CAPV4 (MLT is present in the CA fibers), reached 100% at t = 30 min (Fig. 3b). Likewise, MLT’s release from the CAPV5 formulation (MLT is present only in the PVP fibers), reached 90.55%, at t= 30 min (Fig. 3b). It seems that in these cases, the release of MLT is independent of its sole presence in either the CA or the PVP fibers. These findings are corroborated by the average diameter of these systems determined by SEM (formulation CAPV4: 1.9 μm; formulation CAPV5: 1.8 μm, Fig. 2). This trend in the release of MLT was seen in almost all the CA/PVP-MLT composites compared to the respective CA formulations, where CA was the only polymer of the nanofiber mats. This can be attributed to the fact that PVP, as already mentioned, enhances the dissolution of MLT at pH 1.2, due to its conversion to the gem-diol form III (Fig. 4).
At pH 6.8 the release profile of MLT from CA1 and CA2 formulations was analogous to that observed in the corresponding cases at pH 1.2. The release of MLT at pH 6.8 from formulations CA1 and PV1 follows initially (30 min) the same pattern (Fig. 3c). At t > 30 min, the release of MLT from PV1 nanofibers becomes faster, compared to CA1, possibly due to the fact that the average diameter in the former case is much smaller (PV1: 689 nm; CA1: 3 μm, Fig. 1) resulting to a higher S/V ratio (surface area / volume) and thus to a dissolution rate increase [40, 41]. In general, at pH 6.8, MLT is released slower from the CA-MLT and PVP-MLT formulations than from the CA/PVP-MLT composite formulations, at t = 30 min (Fig. 3c and 3d).
The release of MLT from the developed systems incorporated in DRcapsTM capsules, resembles, in general, the profile seen when hard gelatin capsules with the respective nanofiber mats were used (Fig. 3e and 3f). The differences noticed, in this case, in the %release of MLT with respect to time, are due to the lag period. The DRcapsTM capsules dissolve slower because of the different material used for their preparation, compared to that employed in the formation of hard gelatin capsules. DRcapsTM capsules, made with an innovative hypromellose (HPMC) formulation, a gelling agent (Gellan gum) and water, are known to display delayed release, which occurs at about 20 min after the onset of release in the intestine [42, 43].
Conclusion
This is the first report on the preparation and characterization of MLT-loaded nanofibrous systems based on CA, PVP and HPMC. The electrospun nanofiber mats, loaded in hard gelatin capsules, exhibited variable MLT release in the gastric-like fluids, ranging from 30 min (PV1 and HP2) to 120 min (CAPV1 and CAPV2). In contrast, formulations in DRcapsTM capsules filled with HP2 and CAPV3 nanofiber matrices delivered MLT at a slower pace (6 h). Thus, it becomes apparent that nanofibrous matrices loaded with MLT have a significant potential for the development of tailor-made systems for treating sleep dysfunctions.
Acknowledgements
Declared none.
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No Animals/Humans were used for studies that are the basis of this research.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Korf H.W., von Gall C. Mice, melatonin and the circadian system. Mol. Cell. Endocrinol. 2006;252:57–68. doi: 10.1016/j.mce.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Vlachou M., Eikosipentaki A., Xenogiorgis V. Pineal hormone melatonin: Solubilization studies in model aqueous gastrointestinal environments. Curr. Drug Deliv. 2006;3:255–265. doi: 10.2174/156720106777731073. [DOI] [PubMed] [Google Scholar]
- 3.Kalsbeek A., Buijs R.M. Output pathways of the mammalian suprachiasmatic nucleus: Coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- 4.Brzezinski A. Melatonin in humans. N. Engl. J. Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 5.Dubocovich M.L., Rivera-Bermudez M.A., Gerdin M.J., Masana M.I. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front. Biosci. 2003;8:1093–1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- 6.Skene D.J., Arendt J. Human circadian rhythms: Physiological and therapeutic relevance of light and melatonin. Ann. Clin. Biochem. 2006;43:344–353. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt J.K., Dijk D.J., Ritz De C.A., Ronda J.M. Czeisler. C.A. Sleep-facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–618. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 8.Vlachou M., Ioannidou V., Vertzoni M., Tsotinis A., Afroudakis P., Sugden D. Controlled release from solid pharmaceutical formulations of two nalkanoyl-4-methoxybicyclo [4.2.0]octa-1,3,5-trien-7-ethanamines with melatoninergic activity. Lett. Drug Des. Discov. 2015;12:259–262. [Google Scholar]
- 9.Vlachou M., Siamidi A., Pareli I., Zampakola A., Konstantinidou S. An account of modified release of melatonin from compression-coated, uncoated and bilayer tablets. J. Pharm. Pharm. Sci. 2016;1:10–14. [Google Scholar]
- 10.Vlachou M., Siamidi A., Konstantinidou S., Dotsikas Y. Optimization of controlled release matrix formulation of melatonin via experimental design. J. Pharm. Drug Deliv. Res. 2016;5:1–5. [Google Scholar]
- 11.Vlachou M., Papamichael M., Siamidi A., Fragouli I., Afroudakis P.A., Kompogennitaki R., Dotsikas Y. Comparative in vitro controlled release studies on the chronobiotic hormone melatonin from cyclodextrins-containing matrices and cyclodextrin: Melatonin complexes. Int. J. Mol. Sci. 2017;18:1641. doi: 10.3390/ijms18081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlachou M., Tragou T., Siamidi A., Kikionis S., Chatzianagnostou A.L., Mitsopoulos A., Ioannou E., Roussis V., Tsotinis A. Modified in vitro release of the chronobiotic hormone melatonin from matrix tablets based on the marine sulfated polysaccharide ulvan. J. Drug Deliv. Sci. Technol. 2018;44:41–48. [Google Scholar]
- 13.Zampakola A., Siamidi A., Pippa N., Demetzos C., Vlachou M. Chronobiotic hormone melatonin: Comparative in vitro release studies from matrix tablets and liposomal formulations. Lett. Drug Des. Discov. 2017;14:476–480. [Google Scholar]
- 14.Kumar A., Agarwal S.P., Khanna R. Modified release bi-layered tablet of melatonin using β-cyclodextrin. Pharmazie. 2008;58:642–644. [PubMed] [Google Scholar]
- 15.Kikionis S., Ioannou E., Toskas G., Roussis V. Electrospun biocomposite nanofibers of ulvan/PCL and ulvan/PEO. J. Appl. Polym. Sci. 2015;132:42153. [Google Scholar]
- 16.Al-Enizi A.M., Zagho M.M., Elzatahry A.A. Polymer-based electrospun nanofibers for biomedical applications. Nanomaterials (Basel) 2018;8:259. doi: 10.3390/nano8040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng H., Yang X., Che X., Yang M., Zhai G. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C. 2018;90:750–763. doi: 10.1016/j.msec.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Kenry Lim. C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017;70:1–17. [Google Scholar]
- 19.Chang L., Hu J., Chen F., Chen Z., Shi J., Yang Z., Li Y., Lee L.J. Nanoscale bio-platforms for living cell interrogation: Current status and future perspectives. Nanoscale. 2016;8:3181–3206. doi: 10.1039/c5nr06694h. [DOI] [PubMed] [Google Scholar]
- 20.Gallego-Perez D., Chang L., Shi J., Ma J., Kim S-H., Zhao X., Malkoc V., Wang X., Minata M., Kwak K.J., Wu Y., Lafyatis G.P., Lu W., Hansford D.J., Nakano I., Lee L.J. On-chip clonal analysis of glioma-stem-cell motility and therapy resistance. Nano Lett. 2016;16:5326–5332. doi: 10.1021/acs.nanolett.6b00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhardwaj N., Kundu S. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Greiner A., Wendorff J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007;46:5670–5703. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 23.Teo W.E., Ramakrishna S.A. Review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17:89–106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal S., Wendorff J.H., Greiner A. Use of electrospinning technique for biomedical applications. Polymer (Guildf.) 2008;49:5603–5621. [Google Scholar]
- 25.Chakraborty S., Liao I.C., Adler A., Leong K.W. A facile technique to fabricate drug delivery systems. Adv. Drug Deliv. Rev. 2009;61:1043–1054. doi: 10.1016/j.addr.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramakrishna S., Fujihara K., Teo W.E., Yong T., Ma Z., Ramaseshan R. Electrospun nanofibers: solving global issues. Mater. Today. 2006;9:40–50. [Google Scholar]
- 27.Hu X., Liu S., Zhou G., Huang Y., Xie Z., Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Joshi D., Garg T., Goyal A.K., Rath G. Development and characterization of novel medicated nanofibers against periodontitis. Curr. Drug Deliv. 2015;5:564–577. doi: 10.2174/1567201812666141205131331. [DOI] [PubMed] [Google Scholar]
- 29.Singh A., Rath G., Singh R., Goyal A.K. Nanofibers: An effective tool for controlled and sustained drug delivery. Curr. Drug Deliv. 2018;2:155–166. doi: 10.2174/1567201814666171002115230. [DOI] [PubMed] [Google Scholar]
- 30.Akhgari A., Shakib Z., Sanati S. Review on electrospun nanofibers for oral drug delivery. Nanomed. J. 2017;4:197–207. [Google Scholar]
- 31.Kikionis S., Ioannou E., Andrén O.C.J., Chronakis I., Fahmi A., Malkoch M., Toskas G., Roussis V. Nanofibrous nonwovens based on dendritic-linear-dendritic poly (ethylene glycol) hybrids. J. Appl. Polym. Sci. 2017;135:45949. [Google Scholar]
- 32.Kikionis S., Ioannou E., Konstantopoulou M., Roussis V. Electrospun micro/nanofibers as controlled release systems for pheromones of Bactrocera oleae and Prays oleae. J. Chem. Ecol. 2017;43:254–262. doi: 10.1007/s10886-017-0831-2. [DOI] [PubMed] [Google Scholar]
- 33.Toskas G., Hund R.D., Laourine E., Cherif C., Smyrniotopoulos V., Roussis V. Nanofibers based on polysaccharides from the green seaweed Ulva rigida. Carbohydr. Polym. 2011;84:1093–1102. [Google Scholar]
- 34.Toskas G., Heinemann S., Heinemann C., Cherif C., Hund R.D., Roussis V., Hanke T. Ulvan and ulvan/chitosan polyelectrolyte nanofibrous membranes as a potential substrate material for the cultivation of osteoblasts. Carbohydr. Polym. 2012;89:997–1002. doi: 10.1016/j.carbpol.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 35.Ignatious F., Sun L., Lee C-P., Baldoni J. Electrospun nanofibers in oral drug delivery. Pharm. Res. 2010;27:576–588. doi: 10.1007/s11095-010-0061-6. [DOI] [PubMed] [Google Scholar]
- 36.Thakkar S., Misra M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017;107:148–167. doi: 10.1016/j.ejps.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Sinko P.J., Singh Y. Martin’s Physical Pharmacy and Pharmaceutical Sciences. 6th ed. Baltimore: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 38.Balogh A., Farkas B., Verreck G., Mensch J., Borbás E., Nagy B., Marosi G., Nagy Z.K. AC and DC electrospinning of hydroxypropylmethylcellulose with polyethylene oxides as secondary polymer for improved drug dissolution. Int. J. Pharm. 2016;30:159–166. doi: 10.1016/j.ijpharm.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Paaver U., Heinämäki J., Laidmäe I., Lust A., Kozlova J., Sillaste E., Kirsimäe K., Veski P., Kogermann K. Electrospun nanofibers as a potential controlled-release solid dispersion system for poorly water-soluble drugs. Int. J. Pharm. 2015;479:252–260. doi: 10.1016/j.ijpharm.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 40.Verreck G., Chun I., Peeters J., Rosenblatt J., Brewster M.E. Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm. Res. 2003;20:810–817. doi: 10.1023/a:1023450006281. [DOI] [PubMed] [Google Scholar]
- 41.Skoug J.W., Borin M.T., Fleishaker J.C., Cooper A.M. In vitro and in vivo evaluation of whole and half tablets of sustained-release adinazolam mesylate. Pharm. Res. 1991;8:1482–1488. doi: 10.1023/a:1015834114359. [DOI] [PubMed] [Google Scholar]
- 42.Cole E.T., Scott R.A., Connor. A.L., Wilding B.R., Petereit H.U., Schminke C., Beckert T., Cadé D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharm. 2002;231:83–95. doi: 10.1016/s0378-5173(01)00871-7. [DOI] [PubMed] [Google Scholar]
- 43.Al-Tabakha M.M., Arida A.I., Fahelelbom K.M.S., Sadek B., Jarad R.A.A. Performances of new generation of delayed release capsules. J. Young Pharm. 2015;7:36–44. [Google Scholar]