Abstract
Background:
Dysbiosis of intestinal microbiota in the elderly can cause a leaky gut, which may result in silent systemic inflammation and promote neuroinflammation - a relevant pathomechanism in the early course of Alzheimer’s disease.
Objective:
The rebalancing of the microbiome could benefically impact on gut inflammation and immune activation.
Methods:
In this study, routine laboratory tests in twenty outpatients (9 females, 11 males, aged 76.7 ± 9.6 years) with Alzheimer’s disease were investigated. The mean Mini Mental State Examination score was 18.5 ± 7.7. Biomarkers of immune activation – serum neopterin and tryptophan breakdown - as well as gut inflammation markers and microbiota composition in fecal specimens were analyzed in 18 patients be-fore and after probiotic supplementation for 4 weeks.
Results:
After treatment a decline of fecal zonulin concentrations and an increase in Faecalibacterium prausnitzii compared to baseline were observed. At the same time, serum kynurenine concentrations in-creased (p <0.05). Delta values (before - after) of neopterin and the kynurenine to tryptophan ratios (Kyn/Trp) correlated significantly (p <0.05).
Conclusion:
Results show that the supplementation of Alzheimer’s disease patients with a multispecies probiotic influences gut bacteria composition as well as tryptophan metabolism in serum. The correlation between Kyn/Trp and neopterin concentrations points to the activation of macrophages and/or dendritic cells. Further studies are warranted to dissect the potential consequences of Probiotic supplementation in the course of Alzheimer’s disease.
Keywords: Gut microbiota, Faecalibacterium prausnitzii, probiotics, neopterin, brain-gut axis, neuroinflammation, Alzheimer’s disease, dementia
1. INTRODUCTION
Dementia is an increasing health problem in the aging population. Without effective prevention and treatment, it will become a steadily increasing socioeconomic burden [1, 2]. Age-related changes in the brain can be seen decades before the first symptoms of cognitive decline appear [3] and data accumulates that they are also related to signs of an activated immune system [4]. Nowadays, the gastrointestinal tract with its commensal microbiota is of increasing interest in different neurodegenerative diseases [5]. The microbiome influences on the regulation of immunity, inflammatory response, and neuromodulatory mechanisms. Thus, a healthy microbiota composition can be of immense benefit for the systemic immune defense and brain function and homeostasis.
Gut microbes are able to produce short chain fatty acids e.g. from complex carbohydrates such as dietary fiber, which are important for intestinal and blood brain barrier integrity [6]. Some of these metabolites are neuromodulators, moreover microbiota are able to generate neurotransmitters and precursors such as noradrenalin and tryptophan or γ-gamma-aminobutyric acid (GABA) [7, 8]. These neuroactive metabolites are important for the neuropsychiatric status, especially in the elderly [6, 9].
On the contrary, the production of mixtures of immunogenic lipopolysaccharide and the secretion of amyloid from the outer membranes of microbial cells is also suspected to be linked to the progression of neurodegeneration [10]. Thus, the gastrointestinal barrier function and gut microbiota seem to be among the key factors of mental health and represent another piece to the puzzle in the pathophysiology of Alzheimer’s disease (AD) [11].
The administration of probiotics is suggested to promote suppression of pathogen proliferation, and to stimulate epithelial cell proliferation and differentiation. Thereby, the intestinal barrier becomes fortified and modulates the immune system, enabling an environment in which beneficial microbes can proliferate easier. This can be enhanced further in combination with prebiotics [12]. Supplementation of both were postulated to provide synergistic effects especially important in preventing dementia, as there is an age-related decrease in the diversity of gut microbiota [13]. However, studies that dissect the potential benefit of probiotic supplements in the course of AD are still scarce.
In this study, we analyzed serum and feces specimens of AD patients before and after intake of a probiotic preparation. The concentrations of biomarkers of immune activation neopterin and the ratio of kynurenine to tryptophan were analyzed. Results were compared to fecal bacteria compositions and to their change under supplementation with probiotic.
The conversion to kynurenine is the key initiating step of tryptophan breakdown via indoleamine 2,3-dioxygenase (IDO-1). Enhanced production of immune system biomarker neopterin [14-16] and accelerated breakdown of tryptophan have been reported to correlate with the cognitive decline in patients suffering from AD and other forms of dementia. These findings link activated macrophages and an activated immune system to the pathogenesis of AD.
2. PATIENTS AND METHODS
After written informed consent, twenty consecutive outpatients from the Department of Gerontology of the Neuromed Campus at the Kepler University Clinic Upper Austria with symptoms of dementia (9 females, 11 males, aged 76.7 ± 9.7 years) were included in the study between October 2017 and February 2018. The patients fulfilled the International Classification of Diseases (ICD)-10 criteria of AD (F 00.1). Diagnosis was proofed by magnetic resonance tomography (MRT) scans and routine laboratory tests (electrolytes, blood cell counts, serum enzymes, electrolytes, urine analysis, blood glucose, hemoglobin A1c, bilirubin, creatinine, blood urea nitrogen, C-reactive protein (CRP), total serum protein, folic acid, vitamin B12, thyroid parameters). The Mini Mental State Examination (MMSE) and the Clock Drawing Test (CDT) scores were examined for determination of the patients’ cognitive status.
Before and after 28 days of supplementation with a multispecies probiotic, two gramms of fecal samples, were collected from the patients and stored immediately at -20°C for later analysis of microbiota.
Microbiota were quantified with commercially available assays (Immundiagnostik, Bensheim, Germany) according to the manufacturer´s instructions. Briefly, DNA was extracted from faecal samples utilizing the MagNA Pure LC DNA Isolation Kit - (Roche Life Science, Mannheim, Germany). Quantitative polymerase chain reaction (qPCR) was performed using the MutaPLATE qPCR Assays (Immundiagnostik, Bensheim, Germany) for the designated microbial targets Akkermansia muciniphila, Faecalibacterium prausnitzii and Clostridium cluster I, for detection and amplification in a RotorGene cycler (Qiagen, Hilden, Germany). For quantification, the cycle threshold of each sample was compared with a standard curve according to the standards supplied with each Kit, respectively.
Measurements of fecal inflammation markers calprotectin, α1-antitrypsin and zonulin were performed using the respective enzyme-linked immonosorbent assays (EIAs from Immundiagnostik, Bensheim, Germany).
Additionally, the following parameters were measured in serum specimens collected in parallel: neopterin, vitamin D and brain derived nerve growth factor (BDNF) by ELISA (BRAHMS, Hennigsdorf, Germany and R&D Systems, Inc., Minneapolis, MN), and aromatic amino acids by HPLC. For tryptophan and kynurenine, the kynurenine to tryptophan ratio (Kyn/Trp) was calculated as an index of tryptophan breakdown [19]. The phenylalanine to tyrosine ratio (Phe/Tyr) is an index of phenylalanine hydroxylase (PAH) activity [20]. Nitrite concentrations were determined with Griess reagent and allows indirect conclusions about the potential role of nitric oxide synthase [21]. Serum specimens were kept at -18°C until measurements that were performed in one single run at the end of the study.
Aqueous suspensions of the probiotic Omnibiotic Stress Repair (Allergosan, Graz, Austria) were administered daily in the morning before breakfast for 28 days. The content of one sachet was suspended and kept for 15 min in 1/8 L lukewarm water. Then the suspensions was stirred again and drunk. The preparation consisted of Lactobacillus casei W56, Lactococcus lactis W19, Lactobacillus acidophilus W22, Bifidobacterium lactis W52, Lactobacillus paracasei W20, Lactobacillus plantarum W62, Bifidobacterium lactis W51, Bifidobacterium bifidum W23 and Lactobacillus salivarius W24. Any additional medication including vitamin D supplementation remained constant throughout probiotic treatment.
Data were analyzed using the Statistical Package for the Social Sciences (version 19, SPSS, Chicago, IL, USA). To take into account that not all collected data followed a normal distribution, non-parametric Friedman and Wilcoxon signed-rank test were applied. To test for associations between variables, Spearman rank correlation analysis was performed, p values below 0.05 (two-sided) were considered to indicate statistical significance.
3. RESULTS
3.1. Baseline
The routine laboratory tests excluded all secondary forms of a dementing process. In MRT scans of all patients, global cerebral atrophy with emphasis of the temporobasal region without a circumscribed vascular lesion was observed. The MMSE in patients was mean ± SD 18.5 ± 7.7, and CDT was 4.3 ± 2.7. There were no clinical signs of an acute infection, and the average serum concentration of CRP was 1.6 ± 2.3 mg/dl at the time of specimen collection. However, one patient presented with CRP = 5.5 mg/dl without any clinical explanation. He remained to be included in the data set for statistical analyses.
The average concentrations and ranges of neopterin, tryptophan, kynurenine, Kyn/Trp, tyrosine, phenylalanine, Phe/Tyr and of vitamin D as well as BDNF are summarized in Table 1. Close associations existed between serum concentrations of neopterin and tryptophan (rs = -0.572, p <0.01) and Kyn/Trp (rs = 0.626, p <0.001).
Table 1.
Baseline characteristics and concentrations (mean + SD, ranges in brackets) of serum immune activation and inflammation markers, of serum neurotransmitter precursor amino acids, of vitamin D and of brain-derived neurotrophic factor (BDNF) in 20 patients with Alzheimer’s dementia at study entry.
| Age [y] | 76.7 ± 9.7 | (60 - 93) |
|---|---|---|
| Sex | 9 females, 11 males | |
| MMSE | 17.9 ± 7.9 | (0 - 30) |
| Clock drawing test | 4.1 ± 2.8 | (0 - 9) |
| Neopterin [nmol/L] | 10.0 ± 5.2 | (2.0 - 23.7) |
| Kyn/Trp [µmol/mmol] | 38.6 ± 15.1 | (6.8 - 80.3) |
| Kynurenine [µmol/L] | 1.86 ± 0.59 | (1.1 - 7.0) |
| Tryptophan [µmol/L] | 53.3 ± 13.6 | (22.6 - 85.5) |
| Phe/Tyr [µmol/µmol] | 0.68 ± 0.16 | (0.34 - 0.90) |
| Tyrosine [µmol/L] | 145 ± 28.3 | (93.7 - 204) |
| Phenylalanine [µmol/L] | 97.7 ± 25.5 | (32.1 - 141) |
| Nitrite [µmol/L] | 43.9 ± 30.9 | (5.3 - 105) |
| C-reactive protein [mg/L] | 1.6 ± 2.3 | (0 – 5.5) |
| Vitamin D [µg/L] | 25.7 ± 9.3 | (9.6 - 44.1) |
| BDNF [pg/ml] | 154 ± 144 | (15.6 - 457) |
The concentrations of the fecal biomarkers are given in Table 2. Fecal S100A12 correlated with fecal α1-antitrypsin (rs = 0.789, p <0.001). Moreover, fecal S100A12 correlated inversely with CDT (rs = -0.674, p <0.01), but not significantly with MMSE.
Table 2.
Concentrations of fecal inflammation markers and fecal bacterial strains (mean + SD, range in brackets) in patients with Alzheimer’s dementia (n = 18) before and after 28 days of a multi-specific probiotic supplementation.
| Before | After | |
|---|---|---|
| Mean + SD (Range) | Mean + SD (Range) | |
| α1-Antitrypsin [mg/g] | 37.9 ± 23.7 (9.6 - 282) | 44.7 ± 35.8 (6.2 - 520) |
| Calprotectin [mg/L] | 84.7 ± 71 (2.8 - 112) | 119 ± 131 (3.8 - 123) |
| Zonulin [µg/L] | 93.1 ± 56.3 (16 - 220) | 66.6 ± 54.2 (19 - 213)** |
| S100A12 [µg/L] | 3.5 + 6.0 (0 - 23) | not available |
| Clostridium cluster I* | 7.76 ± 1.10 (5.46 - 9.26) | 7.92 ± 1.28 (5.34 - 10.2) |
| Faecalibacterium prausnitzii* | 8.25 ± 1.47 (5.15 – 10.1) | 9.04 ± 1.43 (5.83 - 10.9) *** |
| Akkermansia muciniphila* | 8.61 ± 1.67 (6.00 – 10.9) | 8.47 ± 1.85 (6.00 - 10.8) |
(*RNA copy/g feces, log10; **U = 2.461, p = 0.01; ***U = 3.375, p <0.001).
The results of the determination of fecal bacteria strains are shown in Table 2. Clostridium cluster I correlated inversely with zonulin (rs = -0.557; p <0.05) and positively but only weakly with calprotectin (rs = 0.461) and with CDT (rs = 0.457, both p <0. 1). Faecalibacterium prausnitzii correlated with Akkermansia muciniphila (rs = 0.619; p <0.01).
Zonulin correlated with cognitive parameters CDT (rs =-0.542;p <0.05), and MMSE (rs=-0.531; p <0.05).
BNDF correlated with vitamin D concentrations (rs = 0.767; p <0.001) and inversely with nitrite levels (rs = -0.575; p <0.05). No significant correlation was seen between the other parameters investigated.
3.2. Influence of Supplementation with Probiotics
The intake of probiotics was well tolerated and no side effects were reported. Paired serum specimens before and after supplementation were available of 15 patients (7 males, 8 females). Paired stool specimens before and after supplementation were available of 18 patients (9 males, 9 females). No significant change was observed with all cognitive parameters after probiotics supplementation for 28 days, likewise there was no significant change in the concentrations of tryptophan, phenylalanine and tyrosine. However, a significant increase of kynurenine serum levels (p <0.05) was observed after probiotic supplementation (see Table 3), but this did not lead to a significant change of the Kyn/Trp concentrations. A trend of an increase of neopterin and nitrite concentrations (p <0.1) was seen. A significant correlation existed between the delta values (before - after) of neopterin and Kyn/Trp concentrations (rs = 0.611, p <0.05).
Table 3.
Concentrations of serum inflammation markers (mean + SD and range) and of serum neurotransmitter precursor amino acids before and after 28 days of a multi-specific probiotic supplementation in 15 patients with Alzheimer’s dementia for whom pre- and follow-up data were available.
| Before: | After: | |||
|---|---|---|---|---|
| Mean + SD | Range | Mean + SD | Range | |
| Neopterin [nmol/L] | 9.8 ± 4.9 | 6.0 - 23.7 | 12.8 ± 10.1 | 6.6 - 44.5 |
| Kyn/Trp [µmol/mmol] | 38.2 ± 13.8 | 17.8 - 80.3 | 39.4 ± 10.5 | 23.5 - 61.0 |
| Kynurenine [µmol/L] | 1.82 ± 0.29 | 1.4 - 2.5 | 2.06 ± 0.42* | 1.3 - 2.6 |
| Tryptophan [µmol/L] | 51.9 ± 15.0 | 22.6 - 85.5 | 54.1 ± 11.6 | 37.8 - 82.2 |
| Phe/Tyr [µmol/µmol] | 0.77 ± 0.30 | 0.3 - 1.6 | 0.82 ± 0.24 | 0.5 - 1.26 |
| Tyrosine [µmol/L] | 133 ± 33.5 | 86.6 - 199 | 146 ± 65.0 | 71 - 280 |
| Phenylalanine [µmol/L] | 98.5 ± 29.1 | 32.1 - 143 | 111 ± 33.0 | 54.0 – 174 |
| Nitrite [µmol/L] | 238 ± 421 | 5.5 – 1050 | 442 ± 514 | 20.0 - 1050 |
*U = 2.481, p <0.05.
There was no difference of the BDNF levels before and after 4 weeks of supplementation with probiotics (before mean ± SD 209 ± 126, range: 15.6 - 457 pg/ml); after: 201 ± 121, range: 30.9 - 476 pg/ml).
The RNA content of fecal bacteria strain Faecalibacterium prausnitzii significantly increased between baseline and after 4 weeks of supplementation with probiotics, whereas contents of Clostridium cluster I and Akkermansia muciniphila did not change (Table 2). Zonulin concentrations declined between the two time points before and after 4 weeks of supplementation with the probiotic.
4. DISCUSSION
The pathology of AD is complex and dementia syndromes are a growing global challenge. Importantly, recent epidemiological data provide also some aspects, which point towards endogenous protective mechanisms [22]. Neuroinflammation is a characteristic feature in the early course of the disease. Investigations on the role of the human microbiota in immune-mediated inflammatory diseases including neurodegeneration and dementia are a topic of great relevance [23]. Moreover, the “superorgan“ of intestinal microbiota bears the potential for the discovery of new diagnostic biomarkers and therapeutic targets of AD [24].
In earlier studies, we have reported on associations between the composition of the gut microbiome and certain biomarkers of immune activation and inflammation in patients with cognitive impairment (AD and mild cognitive impairment [25]). Moreover, probiotics have been shown in earlier studies to influence tryptophan metabolism and immunological regulatory circuits with no obvious side effects in, e.g., patients with liver cirrhosis [26]. In addition, in training athletes probiotic treatment significantly reduced the number of infections [27].
In the present study, biomarkers of immune activation and inflammation were studied in serum and stool specimens of 20 AD patients. Compared to serum concentrations reported for healthy blood donors [28], the average concentrations of neopterin and Kyn/Trp were increased, while tryptophan concentrations were decreased in the patients. Moreover, strong associations between neopterin production and tryptophan breakdown were observed, indicating ongoing immune activation and inflammation. Interestingly, both tyrosine and phenylalanine concentrations were higher in patients compared to the concentrations determined in specimens of healthy blood donors [28].
For a subgroup of patients, data were available for a comparison of the situation before and after 28-days of supplementation with the probiotic, and signs of a further increase of immune activation were found. Similar results were described in earlier studies in patients with liver cirrhosis and in athletes during/after physical exercise [27]. Though the probiotic treatment and composition was different in those studies, results indicated a role of tryptophan signaling at different levels of the microbiota-gut-brain-axis [29].
Stool inflammation markers and microbiome composition were measured at baseline. Calprotectin is a complex of S100 calcium binding protein A8 (S100A8) and S100A9 [30]. Earlier studies described increased S100A9 expression in brains of AD mice and AD patients [31]. S100A12, another inflammation marker of this family, was measured in the present study at baseline and a clear correlation was found between stool S100A12 and results of CDT. This could indicate coincident low grade gut and systemic inflammation, which is consecutively followed by neuroinflammation and worse cognition, as has been suggested earlier [32]. These findings again point towards a probable role of silent inflammation in the different target tissue compartments as a cofactor in the pathophysiology of cognitive deterioration and dementia. In an earlier study [33], the potential role of pro-inflammatory S100A9 and S100A12 proteins in the pathogenesis of AD was described. In addition, circulating CRP, which is known to affect cognition negatively, was found elevated in these patients and represents a further sign of low grade inflammation.
The Clostridium cluster I correlated inversely with the zonulin levels, Akkermansia muciniphila correlated with Faecalibacterium prausnitzii. The inverse correlation between fecal calprotectin and both F. prausnitzii and L. paracasei suggests that intestinal inflammation may be related to a reduction in these bacteria [34]. All mentioned microbiota are known to be involved in competitive pro- and anti-inflammatory mechanisms, and thus could be relevant when being modulated in the progressive course of cognitive decline. In a recent study, a possible causal relation between gut microbiota-related inflammation and amyloidosis was suspected [35]. Age-related dysbiosis including diminished presence of the suggestible anti-inflammatory Faecalibacterium prausnitzii might be associated with cerebral accumulation of ß-amyloid in AD [36]. Gut microbiota is able to release ß-amyloid and lipopolysaccharides, which are inactivated by a healthy immune system, but when these mechanism fail, the consecutive overproduction of pro-inflammatory cytokines is suggested to be related to the pathogenesis of AD [37].
Gold et al. reported on the anti-inflammatory action of α1-antiptrypsin on microglial-mediated neuroinflammation in vitro [38]. In the present study, anti-inflammatory α1-antitrypsin strongly correlated with pro-inflammatory S100A12. α1-Antitrypsin also correlated with zonulin, which plays a critical role in increased age-related gut permeability, known as the “leaky gut” [39]. In addition, zonulin correlated inversely with CDT and MMSE in the patients. Also the finding of an association between CDT and Faecalibacterium prausnitzii could be of greater relevance pointing to the role of specific bacteria composition in the course of AD. These findings further indicate changes in the microbiota-gut-brain-axis resulting in neuroinflammation, an early event in the pathogenesis of AD [40].
In a recent animal study, the importance of host microbiota for microglia function was described for the brain’s innate immune system and for its possible role in different neuropsychiatric diseases and neurodegeneration [41]. Activation of immune cells by bacteria-derived products like lipopolysaccharides results in local synthesis of 1,25(OH)2 vitamin D in the gut. Alternatively, the use of probiotics may regulate the responsiveness of immune cells to vitamin D by enhancing the expression of vitamin D receptor thus reducing tissue inflammation [42].
Probiotic supplementation caused a significant increase of serum kynurenine, which could indicate the stimulation of the immune system, leading to the modulation of the tryptophan pathway involving indoleamine 2,3-dioxygenase-1 [43, 44]. The correlation observed between ΔKyn/Trp and Δneopterin concentrations further supports this conclusion.
However, our study is clearly limited by the small number and by the lack of an untreated control group. Moreover, it lacks a detailed dietary questionnaire. Further, deeper investigations on the production of tryptophan catabolites kynurenic acid and quinolinic acid was not in the focus of the present study. These pathways are of greater relevance inside the brain only, because both compounds do not cross blood-brain-barrier easily [45, 46]. Kyn/Trp was used as index for tryptophan breakdown because it reflects the first step of cytokine-induced tryptophan breakdown on which all other down-stream metabolites depend on. If tryptophan becomes low, the brain supply is lowered and it is directly linked to the production of down-stream catabolites.
Although the progression of AD is associated with an increase of immune activation, which is reflected by increasing neopterin [15, 16] and kynurenine concentrations [17, 18], this immunobiological strategy could represent a way how the Th1-type immune system tries to compensate mechanism that drives the pathogenesis of AD and involve events that relate to Th2 type immunity (Fig. 1). Similar observations were made in a recent study in patients with liver cirrhosis [26]. In the same directions are results of an animal study with aged rats in which probiotic treatment caused changes in genes controlling inflammatory and neuronal plasticity processes and consecutively an increase in expression of BDNF and synapsin in hippocampal tissue [47]. In our, BDNF correlated with vitamin D and inversely with nitrite, but no change could be seen in serum BDNF levels after probiotic supplementation.
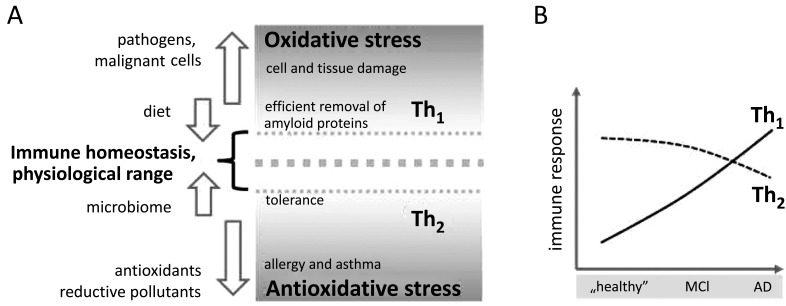
Fig. (1).
The proposed role of immune activation arms in Alzheimer’s disease (AD). (A) Oxidative stress is associated with Th1-type immunity and suppresses the Th2-arm, and vice versa. Both, the composition of diet and the microbiome, influence immune homeostasis in a physiological range. While the establishment of tolerance requires more Th2-like conditions, a deviation to pro-oxidative Th1 is necessary for an efficient removal of pathogens, tumor cells and amyloid proteins. The preponderance of a strong reductive milieu, e.g., due to excessive intake of food antioxidants and likewise the presence of chronic oxidative stress are crucial in the progression of a variety of disorders. (B) Corresponding hypothesis: It is suggested that in early stages of AD there is an inefficient clearance from amyloid precursors, potentially due to overwhelming Th2 conditions which in the course of disease development from mild cognitive impairment (MCI) to AD promote an activation of counteracting Th1-type immune mechanisms that also lead to neuronal damage.
The increases of kynurenine concentrations correlating with neopterin could indicate a worsening of the underlying pathogenic mechanism. However, it could also represent a protective adjustment to escape the inflammation process. This is becoming more and more clear regarding tryptophan breakdown which on the one hand is part of the antiproliferative activity of the immune system, but on the other has also an immunosuppressive component when tryptophan starvation hampers the functioning of the immune system because the kynurenine produced triggers regulatory T-cells, which dampen the inflammation process. Recently tryptophan restriction was found to increase microbial diversity in a murine model [48].
The role of prebiotics and diet as additional drivers of gut microbiota in modulating dementia [11, 49, 50] has to be studied in the future. Analysis of microbiota in correlation to genetic factors as the Apo E status and additional genetic mutations like TREM 2, a microglia receptor involved in ß-amyloid clearance [51], could give additional information in terms of prevention of cognitive decline and dementia [52, 53].
CONCLUSION
An increase of serum kynurenine levels was observed after probiotic supplementation most probably caused by macrophage activation similar to recent findings in patients with liver cirrhosis [26] and after physical exercise [27] receiving probiotic treatment. The results of the here presented study can only be regarded as preliminary due to the small sample size. Another limitation is that the study is not placebo controlled. Double blind placebo-controlled measurements of the inflammation markers before and after probiotic supplementation in larger populations including cognitively normal subjects are warranted to show whether probiotic supplements are able to modulate cognitive deterioration and dementia.
Nevertheless, the here reported changes point towards the activation of immunologic processes. On the one side, the stimulation of anergic immune cells could trigger mechanisms that are helpful in removing amyloid aggregates and damaged cells, on the other side, too intensive activating events could negatively impact gut barrier function and further stimulate neurodegenerative processes.
Acknowledgements
Declared none.
Author’S contributions
Substantial contributions to the conception or design of the work, the acquisition, analysis, or interpretation of data for the work; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: FL, KS, BS, DF and JMG. Drafting the work or revising it critically for important intellectual content: FL, DF and JMG.
ETHICS APROVAL AND CONSENT TO PARTICIPATE
The study was approved by the local ethics committee dated 2017/05/11 by the Ethikkommittee des Landes Oberösterreich, Studie Nr. I-24-16.
Human and Animal Rights
No animals were used in this research. Human specimen were collected in accordance with the criteria of the Declaration of Helsinki.
Consent for Publication
All participants gave their informed consent to take part in the study.
CONFLICT OF INTEREST
Author Burkhard Schuetz was employed by company Biovis Diagnostik MVZ GmbH. All other authors declare no competing interests. The study received no funding.
REFERENCES
- 1.Fratiglioni L., De Ronchi D., Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15(5):365–375. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Barnes D.E., Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangen K.J., Clark A.L., Edmonds E.C., Evangelista N.D., Werhane M.L., Thomas K.R., et al. Cerebral blood flow and amyloid-ß interact to affect memory performance in Cognitively normal older adults. Front. Aging Neurosci. 2017;9:181. doi: 10.3389/fnagi.2017.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGeer P.L., McGeer E.G. Inflammation, autotoxicity and Alzheimer disease. Neurobiol. Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 5.Westfall S., Lomis N., Kahouli I., Dia S.Y., Singh S.P., Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell. Mol. Life Sci. 2017;74(20):3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampson T.R., Mazmanian S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17(5):565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 8.Sandrini S., Aldriwesh M., Alruways M., Freestone P. Microbial endocrinology: host-bacteria communication within the gut microbiome. J. Endocrinol. 2015;225(2):R21–R34. doi: 10.1530/JOE-14-0615. [DOI] [PubMed] [Google Scholar]
- 9.Leblhuber F., Geisler S., Steiner K., Fuchs D., Schütz B. Elevated fecal calprotectin in patients with Alzheimer’s dementia indicates leaky gut. J. Neural Transm. (Vienna) 2015;122(9):1319–1322. doi: 10.1007/s00702-015-1381-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Dua P., Lukiw W.J. Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer’s disease (AD). J. Alzheimers Dis. Parkinsonism. 2015;5(1):177. doi: 10.4172/2161-0460.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The central nervous system and the gut microbiome. Cell. 2016;167(4):915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract. Res. Clin. Gastroenterol. 2016;30(1):17–25. doi: 10.1016/j.bpg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Jackson M.A., Jeffery I.B., Beaumont M., Bell J.T., Clark A.G., Ley R.E., et al. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8:8. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leblhuber F., Walli J., Demel U., Tilz G.P., Widner B., Fuchs D. Increased serum neopterin concentrations in patients with Alzheimer’s disease. Clin. Chem. Lab. Med. 1999;37:429–431. doi: 10.1515/CCLM.1999.070. [DOI] [PubMed] [Google Scholar]
- 15.Blasko I., Knaus G., Weiss E., Kemmler G., Winkler C., Falkensammer G., et al. Cognitive deterioration in Alzheimer’s disease is accompanied by increase of plasma neopterin. J Psych Res. 2007;41:694–701. doi: 10.1016/j.jpsychires.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Parker D.C., Mielke M.M., Yu Q., Rosenberg P.B., Jain A., Lyketsos C.G., et al. Plasma neopterin level as a marker of peripheral immune activation in amnestic mild cognitive impairment and Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 2013;28:149–154. doi: 10.1002/gps.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widner B., Leblhuber F., Walli J., Tilz G.P., Demel U., Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. J. Neural Transm. (Vienna) 2000;107:343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 18.Giil L.M., Midttun Ø., Refsum H., Ulvik A., Advani R., Smith A.D., et al. Kynurenine pathway metabolites in Alzheimer’s disease. J. Alzheimers Dis. 2017;60:495–504. doi: 10.3233/JAD-170485. [DOI] [PubMed] [Google Scholar]
- 19.Widner B., Werner E.R., Schennach H., Wachter H., Fuchs D. Simultaneous measurements of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997;43(12):2424–2426. [PubMed] [Google Scholar]
- 20.Neurauter G., Scholl-Bürgi S., Haara A., Geisler S., Mayersbach P., Schennach H., et al. Simultaneous measurement of phenylalanine and tyrosine by high performance liquid chromatography (HPLC) with fluorescence detection. Clin. Biochem. 2013;46(18):1848–1851. doi: 10.1016/j.clinbiochem.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Kleinbongard P., Dejam A., Lauer T., Jax T., Kerber S., Gharini P., et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006;40(2):295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Windblad B., Amouyel P., Andrieu S., Ballard C., Brayne C., Brodaty H., et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15(5):455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 23.Forbes J.D., Van Domselaar G., Bernstein C.N. The gut microbiota in immune mediated inflammatory diseases. Front. Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen L., Liu L., Ji H.F. Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J. Alzheimers Dis. 2017;56(1):385–390. doi: 10.3233/JAD-160884. [DOI] [PubMed] [Google Scholar]
- 25.Leblhuber F., Strasser B., Steiner K., Gostner J., Schuetz B., Fuchs D. On the role of intestinal microbiota in patients with cognitive decline. J. Pharm. Pharmacol. 2017;5:648–653. [Google Scholar]
- 26.Horvath A., Leber B., Schmerboek B., Tawdrous M., Zettel G., Hartl A., et al. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 2016;44(9):926–935. doi: 10.1111/apt.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strasser B., Geiger D., Schauer M., Gostner J.M., Gatterer H., Burtscher M., et al. Probiotic supplements beneficially affect tryptophan-kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double blinded, placebo-controlled trial. Nutrients. 2016;8(11):752. doi: 10.3390/nu8110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisler S., Mayersbach P., Becker K., Schennach H., Fuchs D., Gostner J.M. Serum tryptophan, kynurenine, phenylalanine, tyrosine and neopterin concentrations in 100 healthy blood donors. Pteridines. 2015;26:31–36. [Google Scholar]
- 29.O’Mashony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Gisbert J.P., McNicholl A.G. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig. Liver Dis. 2009;41:56–66. doi: 10.1016/j.dld.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Chang K.A., Kim J.H., Suth Y.H. The role of S100A9 in the pathogenesis of Alzheimer’s disease, the therapeutic effect of S100 A9 knockdown or knockout. Neurodegener. Dis. 2012;10(1-4):27–29. doi: 10.1159/000333781. [DOI] [PubMed] [Google Scholar]
- 32.Caracciolo B., Xu W., Collins S., Fratiglioni L. Cognitive decline, dietary factors and gut-brain interactions. Mech. Ageing Dev. 2014;136-137:59–69. doi: 10.1016/j.mad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Shepherd C.E., Goyette J., Utter V., Rahimi F., Yang Z., Geczy C.L., et al. Inflammatory S100A9 and S100A12 proteins in Alzheimer’s disease. Neurobiol. Aging. 2006;27(11):1554–1563. doi: 10.1016/j.neurobiolaging.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 34.de Freitas M.B., Moreira E.A.M., Tomio C., Moreno Y.M.F., Daltoe F.P., Barbosa E., et al. Altered intestinal microbiota composition, antibiotic therapy and intestinal inflammation in children and adolescents with cystic fibrosis. PLoS One. 2018;13(6):e0198457. doi: 10.1371/journal.pone.0198457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frasca D., Blomberg B.B. Inflammaging decreases adaptive and immune responses in mice and humans. Biogerontology. 2016;17(1):7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., et al. INDIA-FBP Group Association of brain amyloidosis with pro-inflammatory gut bacteria taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Pistollato F., Sumalla Cano S., Ello I., Masias Vergara M., Giampieri F., et al. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016;74(10):624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 38.Gold M., Dolga A.M., Koepke J., Mengel D., Culmsee C., Dodel R., et al. α1-antitrypsin modulates microglial-mediated neuroinflammation and protects microglial cells from amyloid-ß-induced toxicity. J. Neuroinflammation. 2014;11:165–176. doi: 10.1186/s12974-014-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi J., Goel R., Kim S., Richards E.M., Carter C.S., Pepine C.J., et al. Intestinal permeability biomarker zonulin is elevated in healthy aging. J. Am. Med. Dir. Assoc. 2017;18(9):810.e1–810.e4. doi: 10.1016/j.jamda.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fermandez-Perez E.J., Peters C., Aquayo L.G. Membrane damage induced by amyloid beta and a potential link with neuroinflammation. Curr. Pharm. Des. 2016;22(10):1295–1304. doi: 10.2174/138161282210160304111702. [DOI] [PubMed] [Google Scholar]
- 41.Erny D., Hrabe de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas R.M., Gorman S., Geldnhuys S., Hart P.H. Vitamin D and immunity. F1000Prime Rep. 2014;6:118. doi: 10.12703/P6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Widner B., Leblhuber F., Walli J., Tilz G.P., Demel U., Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. J. Neural Transm. (Vienna) 2000;107(3):343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 44.Widner B., Ledochowski M., Fuchs D. Interferon-gamma-induced tryptophan degradation: neuropsychiatric and immunological consequences. Curr. Drug Metab. 2000;1:193–204. doi: 10.2174/1389200003339063. [DOI] [PubMed] [Google Scholar]
- 45.Fukui S., Schwarcz R., Rapoport S.I., Takada Y., Smith Q.R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 1991;56(6):2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 46.Agudelo L.Z., Femenía T., Orhan F., Porsmyr-Palmertz M., Goiny M., Martinez-Redondo V., et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 47.Distrutti E., O’Reilly J.A., Mc Donald C., Cipriani S., Renga B., Lynch M.A., et al. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age related deficit in LTP. PLoS One. 2014;9(9):e106503. doi: 10.1371/journal.pone.0106503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Beek A.A., Hugenholtz F., Meijer B., Sovran B., Perdijk O., Vermeij W.P., et al. Tryptophan restriction arrests B cell development and enhances microbial diversity in WT and prematurely aging Ercc1-/Δ7 mice. J. Leukoc. Biol. 2017;101(4):811–821. doi: 10.1189/jlb.1HI0216-062RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daulatzai M.A. Non celiac gluten sensitivity triggers gut dysbiosis, neuroinflammation, gut brain axis dysfunction, and vulnerability for dementia. CNS Disord Drug Targets. 2015;14(1):110–131. doi: 10.2174/1871527314666150202152436. [DOI] [PubMed] [Google Scholar]
- 50.Alkasir R., Li J., Li X., Jin M., Zhu B. Human gut microbiota: the links with dementia development. Protein Cell. 2017;8(2):90–102. doi: 10.1007/s13238-016-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S., et al. Alzheimer’s disease. Lancet. 2016;388(100043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 52.Rune I, Rolin B, Larsen C, Nielsen DS, Kanter JE, Bornfeldt KE, et al. Modulating the gut microbiota improves glucose tolerance, lipoprotein profile and atherosclerotic plaque development in ApoE deficient mice. PLoS One . 2016;22(11(1)e0146439) doi: 10.1371/journal.pone.0146439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Lin S., Vanhutte P.M., Woo C.W., Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing endotoxemia induced inflammation in ApoE -/- mice. Circulation. 2016;133(24):2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]