Abstract
Background:
Conventional therapeutic strategies for tumors have had limited success, and innovative and more effective approaches to treatment are urgently required. The ancient idea that various biological, bacterial, yeast, viral, and para-sitic agents can be used as cancer therapeutics has gradually attracted considerable interest. Certain parasites have been widely discussed in association with human and animal tumors. The purpose of this review was to examine previous literatures which investigates the relations between Trichinella spiralis (T. spiralis) and tumors.
Methods:
Using PubMed, articles published before 2018 in the whole world have been searched and comprehensively re-viewed.
Results:
Many researches have provided proofs that T. spiralis possesses antitumor activities. The antitumor effect of T. spi-ralis was first described in the 1970s. However, its research has been inconsistent, and little progress has been made in this field. Therefore, the mechanisms underlying these inhibitory effects are still unclear, and convincing evidence of the links be-tween T. spiralis and the prevention or treatment of tumors from clinical trials is absent. Meanwhile, some other researches al-so suggested that T. spiralis may cause or contribute to coinfection with a tumors.
Conclusion:
The review has highlighted the scientific literature focussing on evidence for T. spiralis to act as a pro- or anti-tumorigenic agent is summarized and discussed, in hope of contributing to a better understanding of the relations between T. spiralis and tumors
Keywords: Trichinella spiralis, tumors, antitumor, tumorigenic, nurse cells, parasite
1. INTRODUCTION
According to the GLOBOCAN 2008 statistics, approximately 14.1 million cancer cases and 8.2 million cancer deaths worldwide were reported in 2012 [1]. Traditional treatments, such as surgery, radiotherapy and chemotherapy, focus on the efforts to eradicate the tumor cells. Not only do these treatments achieve only limited success, but also problems with tumor cell metastasis, the killing of normal cells, damages to the host’s immune system, hematopoietics, drug-toxicity, and drug-resistance remain unresolved. The fourth modality of tumor treatment, tumor biotherapy, plays a significant role in the broad spectrum of human and animal malignancies [2]. Tumor biotherapy has become the novel targeted therapeutic strategy for tumor treatment since Biological Response Modifiers (BRM) were first described by Oldham in 1984 [3] and represents a new concept of tumor therapy, which uses diverse mechanisms to suppress or eliminate tumors with new techniques in biotechnology and the use of biological agents. The mechanisms may be mobilized in the host’s defenses, increase the individual’s antitumor responses and the ability of normal cells to tolerate damage, increase differentiation or maturation of the tumor cells, directly kill tumor cells, decrease tumor metastasis, and suppress growth-promoting factor [1, 2]. Strategies of tumor biotherapy in clinical practice include the use of vaccines, cytokines, interferons, monoclonal antibody, immunotoxins, drug immunoconjugates, targeted radionuclide therapy, stem cells, cellular immunotherapy, growth and differentiation factors, granulocyte colony-stimulating factors, granulocyte-macrophage colony- stimulating factors, cancer gene therapy, and antitumor bioactive substances [3].
In addition to aforementioned treatments, many plants and marine microorganisms, bacterial, yeast, viral and parasitic agents have been used and shown promising and significant potency in inhibiting or eradicating tumors. These agents include the Bacillus Calmette-Guerin vaccine, Bifidobacterium, Clostridium, Salmonella, adenovirus, Hantaan virus, hepatitis A virus, herpes simplex virus, influenza A virus, poliovirus, rabies virus, measles virus, Newcastle disease virus, respiratory syncytial virus, bluetongue virus, amoeba, and Plasmodium [3-5]. More precisely, many microorganisms including Trichinella spiralis (T. spiralis) have been closely correlated with tumors, whether act as a positive or negative effect [5]. Epidemiological investigations and laboratory studies have independently demonstrated that T. spiralis can be positively or negatively associated with tumors. However, our limited understanding of the underlying mechanism makes it difficult to identify the progression of events whether T. spiralis may cause, coincide with, or be used to treat tumors.
2. PARASITES AND TUMORS
Beginning in the late 19th century, microorganisms and their products that suppressed tumor growth without seriously harming normal cells were considered as potential therapeutic agents for cancer [6]. Increasingly, scientific experimental evidence has indicated that parasitic infections enhance resistance against certain types of tumors, especially protozoa [7]. Toxoplasma gondii (T. gondii) Lysate Antigen (TLA) and Toxocara canis egg antigens were shown to inhibite tumor growth in the tumor-bearing mouse model [8], and induce a significant increase of antiangiogenic soluble factors [9], and also lowered the incidence of colon cancer induced by 1,2-dimethylhydrazine in chronically infected Wistar rats with Trypanosoma cruzi (T. cruzi) [10].
One common feature of all the aforementioned infections is that they trigger significant antitumor activity depending primarily on cell-mediated innate immunity, but humoral immunity; in particular, the induction of Th1 immune responses of recent interest [11, 12]. Interestingly, a highly genetically attenuated strain of the T. cruzi parasite was able to elicit vigorous and long-lasting T cell-mediated immunity and could be used as an oral vaccine or as a vehicle for gene therapy for tumors [13].
In addition, parasite-derived molecules could be correlated with the induction of antitumor properties in vivo in adult mice and in vitro in several types of tumor cells that are good potential targets for tumor immunotherapy and efficient immunological adjuvants [14]. One system included T. cruzi calreticulin (TcCRT) and enzyme Hypoxanthine-Guanine Phosphoribosyltransferase (HGPRT), and others involved tumor-associated antigens obtained from parasites (TF, Tk, Tn and sialyl-Tn) [15].
Most parasite infections do not cause severe acute symptoms. However, the proven carcinogenicities of several parasitic species, including Schistosoma haematobium (S. haematobium), S. mansoni, S. japonicum, Opisthorchis viverrini (O. viverrini), and Clonorchis sinensis (C. sinensis), were described in humans and in domesticated and laboratory animals [16]. Schistosomiasis caused by S. haematobium, S. mansoni and Schistosomiasis japonica resides in various types of malignancy, such as carcinomas of the liver, biliary tract, intestine, bladder and other pelvic organs and follicular lymphoma of the spleen [17] Liver flukes, O. viverrini, C. sinensis and O. felineus, were associated with an increased incidence of cholangiocarcinoma [18]. T. gondii could potentially increase the risk of adult brain cancer [19]. One possibility of the development of neoplasia was chronic inflammation leading to DNA damage, angiogenesis, and immunocompromise due to the establishment of a long-standing relation of the parasitic infections with the host [20].
In addition to the aforementioned parasites, a number of epidemiological investigations and animal experiments have shown that other parasitic diseases appear in association with the development of cancer [21].
3. T. SPIRALIS AND THE PREVENTION OR TREATMENT OF TUMORS
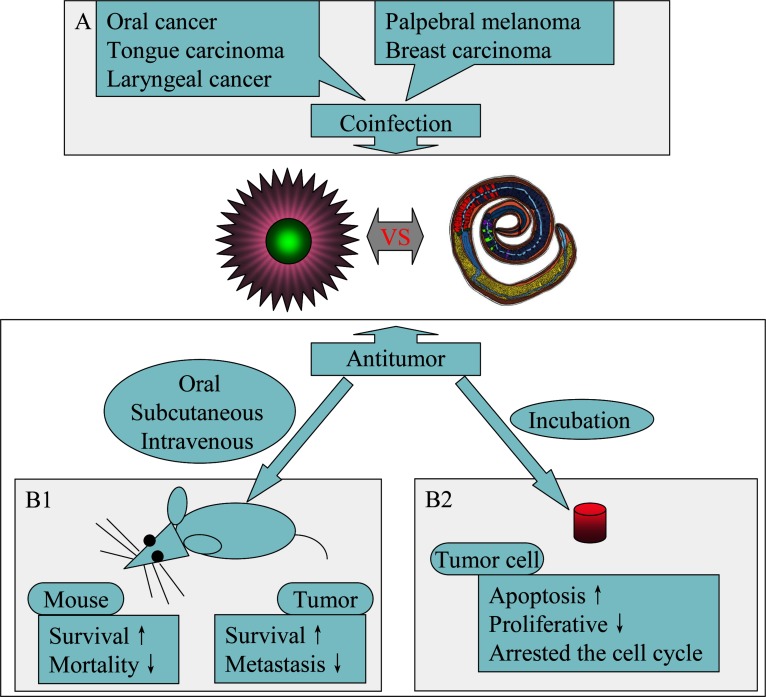
T. spiralis, which causes a globalized zoonotic parasitic disease known as trichinosis, is the most frequent food-transmitted helminth zoonosis and can infect humans and more than 150 types of other animals. However, death after infection with T. spiralis has an unusual presentation. Interestingly enough, T. spiralis has the capacity to inhibit tumor growth and prolong host survival time. In 1970, Weatherly first found that the survival rates of Swiss mice given sublethal infections of T. spiralis muscle larvae increased in mice breast tumor, and mortality was significantly reduced (Table 1) [22]. In this study, mice were given breast cancer cells or solid tumors through subcutaneous inoculation at the same time or the 7th day after experimental infection with T. spiralis. To gain better understanding, many other studies have also been carried out, and many aspects of the inhibition were discovered and studied since then. The growth of the tumor cell was inhibited, and the tumor volume was significantly decreased on the 30th day after inoculation. The suppression of tumor growth was also observed in Malignant Fibrous Histiocytoma (MFH) [23]. The extent of the decrease in the tumor growth was different due to inoculation at different times after infection with T. spiralis. Infection with T. spiralis in HaM/ICR mice for the 28 days preceding intraperitoneal inoculation with Sarcoma 180 (S-180) tumor cells produced a slight gentle increase in the length of the survival time; however, when tumor cells were inoculated into the mice after 56 days of the parasitic infection, no detectable effect on the resistance to tumors was noted [24]. Another study, unlike the one previously described, showed the control mice developed B16 melanoma cells by day 28 following tumor challenge and died within 60 days, whereas none of the T. spiralis -infected animals developed detectable neoplasms [25]. Only recently, T. spiralis-infected mice reduced tumor growth and metastasis through a complex transition in cytokine regulation profiles of an in vivo model system for B16-F10 melanoma (Fig. 1) [26].
Table 1.
Outline of study on the antitumor effect of T. spiralis in vivo and in vitro during recent years.
| Year | Tumor | Animal | Material | Method | Detection Time | Effect | Author |
|---|---|---|---|---|---|---|---|
| 1970 | Breast tumor | Swiss mice | T. spiralis | Subcutaneous | - | Survival↑ Mortality↓ |
Weatherly |
| 1975 | Sarcoma 180 | HaM/ICR mice | T. spiralis | Oral | 28 days 56 days |
Survival↑ No effect |
Lubiniecki |
| 1977 | B-16 melanoma | B6D2F1/J mice | T. spiralis | Oral | 176 days | No signs of neoplasia | Molinari |
| 1979 | Sarcoma 180 | ICR/CD-1 mice | T. spiralis | Oral | 2 weeks 6, 8 or 34 weeks |
Protection No Protection |
Molinari |
| 1982 | B16 melanoma | C57BL/6 mice | T. spiralis | Oral | 2 months | Tumour-free | Pocock |
| 2002 | Malignant fibrous histiocytoma | Rat | T. spiralis | Oral | - | Suppression | Apanasevich |
| 2008 | A549 lung cancer cells | BALB/c mice | T. spiralis | Oral | - | Suppression | Gong |
| 2008 | Human Colorectal Caricnoma HCT-8 | BALB/c mice | T. spiralis | Oral | - | Suppression | Li |
| 2008 | C6 glioma | BALB/c mice | T. spiralis | Oral | - | Suppression | Liu |
| 2009 | Murine Forestomach Carcinoma (MFC), Sarcoma 180, murine ascitic hepatoma (H22) | ICR mice | crude T. spiralis extract | Intravenous | - | Suppression | Wang |
| 2009 | MFC, H22, Sarcoma S180, human hepatoma (H7402), human chronic myeloid leukemia (K562) | In vitro | crude T. spiralis extract | Incubation | - | Anti-proliferative Arrested the cell cycle |
Wang |
| 2009 | Hepatoma carcinoma cell Hepa1-6 | C57BL/6 mice | T. spiralis | Oral | - | Suppression | Zhang |
| 2013 | SP2/0 myeloma | BALB/c mice | T. spiralis | Oral | 11 days | Suppression | Deng |
| 2013 | B16-F10 melanoma | C57BL/6 mice | T. spiralis | Oral | - | Suppression Metastasis↓ |
Kang |
| 2013 | H7402 | In vitro |
A200711 (T. spiralis protein) |
Incubation | - | Tumor apoptosis | Wang |
| 2015 | B16 melanoma | C57BL/6 mice | T. spiralis | Oral | 40 days | Suppression | Vasilev |
| 2015 | B16 melanoma | In vitro | Excretory-secretory product | Incubation | - | Tumor apoptosis | Vasilev |
Fig. (1).
Relations between T. spiralis and tumors. (A): T. spiralis act as a protumorigenic agent. (B): T. spiralis has the capacity to inhibit tumor growth and prolong host survival time in vivo (B1) and induct tumor apoptosis in vitro (B2).
The antitumor effects of T. spiralis observed with A549 lung cancer cells inoculated into animals after the 11th day of T. spiralis infection were better than that of the 7 days of infection [27]. Their results indicate that the inhibition effect is rather because of preventative control than the effect of treatment on tumors. The results are consistent with those obtained in studies of nerve neurospongioma C6 cells and human colorectal carcinoma HCT-8 cells [28]. Compared to the rate in animals inoculated with tumor cells before infection with T. spiralis, the enhancement rate of the host immune function activated by T. spiralis is less in animals inoculated after infection with T. spiralis due to tumor cell proliferation activity.
The degree of tumor inhibition depends on the magnitude of the infection in term of the number of infecting larvae. Compared to the size and weight of tumors in mice infected with 50, 300, or 400 T. spiralis muscle larvae and the control group, the growth of S180 tumors was diminished in the 100 and 200 parasite groups [29]. Meanwhile, in mice given 5×104 S180 sarcoma cells by peritoneal inoculation at 2 weeks after infection with T. spiralis, the subsequent tumor growth was not under control at 6, 8 and 34 weeks [29]. These studies indicate that the suppressive effect, which fully suppressed or reduced the growth of the tumors, was different due to the selection of tumor cell type. The antineoplastic effects of T. spiralis infection have been reported in murine ascitic hepatoma cell H22, murine forestomach carcinoma cell MFC and hepatoma carcinoma cell Hepa1-6 [30].
3.1. T. Spiralis and Immune Response
The exact mechanism of how T. spiralis inhibits tumor growth has remained vague. However, since T. spiralis, a complicated multicellular organism with a unique ability to trigger a Th2 immune response at the intestinal phase, produces proteins, lipids and metabolites that can be recognized by the host immune system [31], thus infection of T. spiralis may stimulate the activation, differentiation, and proliferation of macrophages, NK cells, K cells, and CTL cells, and, then, may promote the secretion of cytokines such as tumor necrosis factor, interleukin, interferon, colony stimulating factor, and transfer growth factor [32, 33]. This possible mechanism is also supported by the studies with other microorganisms. More than 20 years ago, Stewart et al. [34] reported that T. pseudospiralis was likely involved in immunomodulation. The nonspecific immune functions of animals and humans have also been enhanced through the activation of natural immune cells and the production of cytokines by Trichuris suis, and these indicators have been used to identify the severity of various immunity-related diseases [35]. These diseases include autoimmune Type I diabetes, experimental colitis, autoimmune encephalomyelitis, Inflammatory Bowel Disease (IBD) and airway allergic inflammation [36, 37]. In addition to classic Th2 cytokines, the proinflammatory mediator IL-17 is also generated, which is important to the maintenance of chronic intestinal inflammation [38]. The specific and/or nonspecific immune function was enhanced by the activation of the immune network system to generate antitumor effects [39].
The tumor cell killing effect is mediated by macrophages activated in vivo by directly killing tumor cells and producing effector molecules of oncolytic activity. Peritoneal macrophages, obtained from albino mice infected with T. spiralis at one week, had a strong cytotoxic effect on the R1 leukemia cells in vitro [40]. A striking increase in the number of peritoneal exudate cells, as high as eight times that of normal mice, obtained from C57BL/6 mice infected orally with T. spiralis larvae was observed from 3 to 37 days and overlapped the evacuation of adult worms from the gut [41]. The results showed that the in vitro syngeneic EL-4 tumor cells or allogeneic P815 tumor cells were significantly inhibited through the hindered incorporation of a [3H]-TdR effect on DNA synthesis. Indicating the inhibition of DNA synthesis was an especially powerful cytotocix effect killed the EL-4 tumor cells [41].
NK cells, the first line of defense against tumorigenesis, play a meaningful role in the prevention of the development of a neoplasm, inhibiting the proliferation and metastasis of tumors [42]. NK cell-mediated cytotoxicity reaction in vivo is provoked during the migration and early muscle phases of the infection with T. spiralis in mice [43]. Pulmonary NK cells in the lungs of B6C3F1 mice infected with 200 and 500 T. spiralis larvae showed considerably boosted cytotoxic activity against the semisyngeneic tumor cells on days 20 and 30 after challenge, and the effect was more significant as the parasitic infection increased. In contrast, Large Granular Lymphocytes (LGLs) from the spleens of the infected mice increased simultaneously with parasitic infection as well as the number of surface asialo-GM1 molecules [43].
The increase of IL-4 and TNF-β observed in mast cells is directly activated by the T. spiralis larvae 1 (TSL-1) antigens [44]. IL-3 might strengthen the Th2 immune response during the early stages of parasite infection. The release of IL-4 was significantly augmented in spleen cells isolated from mice injected with recombinant IL-3 and infected with T. spiralis. The expression of surface markers Thy1, CD4 and CD8 was also augmented [45], which was observed on day 30 but not day 20 after the administration of larvae [43], along with Monocyte Chemotactic Protein-1 (MCP-1) and TNF-α in serum [46] and IL-5 and IFN-γ in the intestinal lymph [33].
3.2. Antitumor Bioactive Substances
The antitumor effect of T. spiralis may depend not only on enhanced innate immune function but also on Excretory-Secretory (ES) products as some antitumor bioactive substances that may exert an antitumor effect indirectly by changing the expression of a tumor gene or by affecting the antitumor directly. ES products in T. spiralis were suggested participating in the amelioration of autoimmune, allergic, and malignant diseases in vitro and in animal models [47].
The intravenous injection of mixed crude extracts of T. spiralis adult and new born larvae dramatically inhibited the growth of MFC, H22 and S180 tumors in ICR mice, and a significant dose-dependent growth inhibition of the tumor cells in vitro was observed in our study [48]. No difference was observed among the extracts from T. spiralis adults, new born larvae or muscle larvae. The antitumor activity of the cytotoxic factor was also induced through apoptosis in mice thymocytes by T. spiralis ES products, but was much lower than in the mice treated with larval parasites. Moreover, the effect of intraperitoneal injection outweighed that of intramuscular injection.
Recently, studies have consistently indicated that the Translationally Controlled Tumor Protein (TCTP) is highly conserved and abundantly expressed across a wide range of eukaryotic organisms. This protein is most noticeably downregulated during tumor reversion, and its function has been associated with cell growth, including cell-cycle progression, malignant transformation, calcium-binding proteins, histamine-releasing factors, antiapoptotic and immunomodulatory activities [49]. TCTP has been identified as being briefly present in Trichinella, trematodes, Plasmodium subspecies, and other parasitic worms [50].
Caveolin-1 (cav-1) is an essential protein component of the caveolae, which are flask-shaped invaginations of the plasma membrane [51]. The cav-1 protein is reported to function as a tumor suppressor by inducing cell cycle arrest and apoptosis, thereby inhibiting primary tumor growth during the early stages of some tumors [52]. Recently, the cav-1 gene was cloned from T. spiralis as an adult-specific antigen by the Suppression Subtractive Hybridization (SSH) technique and was shown to be expressed and to gradually accumulate on the surface of maturing T. spiralis oocytes and embryos [53].
Heat shock proteins (hsps), highly homologous to the TCTP, play a significant role in the folding stability, intracellular disposition and proteolytic turnover of many of the key regulators of cell growth, differentiation and survival [54] and are also regarded as potent immunoadjuvants that can engender more powerful antitumor effects [55]. Previously, heat-inducible proteins, such as sHSP, HSP60, HSP70, histone H3, and histone H2B, have been isolated from somatic extracts and ES products from T. spiralis [56]. In T. spiralis, HSPs are important for maintaining homeostasis and preventing cell death [57].
Ribosomal proteins are involved in DNA repair, cell growth regulation, cell differentiation and are overexpressed in gastric cancer, colorectal cancer, esophageal cancer and liver cancer. In 1968, Rigby documented the successful use of an allograft for the treatment of tumor-bearing animals by immune RNA (iRNA) [58], and in the next 30 years, the use of iRNA was expeditiously applied to many types of tumors and viruses [59]. Unfortunately, the study of iRNA therapy gradually declined as other new cancer immunotherapies emerged and because iRNA therapy was not as effective as initially imagined; therefore, the mechanism of iRNA therapy has remained unelucidated. Previous investigations have shown that Trichinella iRNA showed a significant reduction in tumor growth in the SP2/0 tumor model of BALB/c mice, and two ribosomal proteins, S24 and S24e, were identified in T. spiralis [60].
Tropomyosins (Tms) are the core components of microfilaments (or the actin filaments), which are the thinnest filaments of the cytoskeleton. Tms are conserved acidic proteins found in a diverse array of eukaryotic organisms including yeasts, worms, flies, crustaceans, frogs, birds, and mammals [61]. A number of studies have documented that Tms possess tumor suppressor activity in a variety of human tumors, including breast, bladder, astrocytoma, central nervous system, and colon cancer [62]. The myeloma-associated antigen of T. spiralis has been shown to have antitumor activity in the myeloma cell line SP2/0 [63] and may play a role in eliciting cross-protective immunity [64]. The retinoblastoma(RB) gene involved in inhibiting breast cancer MCF 7 cell growth was obtained from T. spiralis by the SSH technique.
3.3. Nurse Cells and Apoptosis
Extensive research in the past few years has focused on the molecules of cell signaling pathways involved in the regulation of tumor cell growth, proliferation, adherence, differentiation, apoptosis and immunoregulation to allow the development of new pharmacologic approaches to tumor prevention and target therapy [65]. These signaling pathways include BMP, EGF, EGFR, HIF, Jak/STAT, MAPK, mTOR, NF-κB, p53, PI3K/Akt, Ras/ERK, Rho, TGFβ, Notch, and Wnt/β-catenin [66]. The antitumor agents being developed to target these molecules may positively (i.e., trigger or promote) or negatively (i.e., suppress or inhibit) affect tumors or even play conflicting roles as tumor suppressors and tumor promoters [67].
Apoptosis, also referred to as programmed cell death, plays an important physiological role in maintaining the balance of tissue homeostasis [68]. The above-mentioned molecules have a profound impact on apoptosis pathways [69]. The targeted activation of apoptosis pathways, including the intrinsic (mitochondrial) and the extrinsic (death receptors) pathways, is a subject of tumor therapy that is worthy of study and research [70]. In addition to apoptosis, the arrest of the cell cycle, which consists of four distinct phases designated G1, S, G2 and M, could be suggested as another novel avenue for the development of a potent antitumor agent for the management of tumors [71]. Cell cycle arrest most frequently focuses on the G1/S or G2/M boundaries [72]. Subsequent apoptosis occurs in the arrested cells [73].
One study showed that an infected myocyte could be transformed to its nurse cell form using secreted signaling molecules, the so-called “Parakines”, after new born larvae entered the skeletal muscle [74]. The expression of mitochondrial apoptosis-related genes (Bcl-2 associated protein X, BAX; apoptotic protease activating factor 1, Apaf-1; Caspase 9 and serine/threonine Protein Kinase, PK), the key regulators of differentiation, proliferation and apoptosis of the muscle cells, are upregulated in T. spiralis-infected muscles during encapsulation [75]. In addition, TNF-α, TNF receptor 1 (TNFR-1), TNF receptor-associated death-domain (TRADD), caspase 3, caspase 8, TNF receptor associated factor-2 (TRAF2) and Receptor Interactive Protein (RIP) are also upregulated [49, 76]. These responses suggest that the mitochondrial apoptosis signaling pathway and the TNF-α/TNFR-1 signaling pathway are involved in nurse cell formation. Furthermore, the two apoptosis pathways may be activated by antitumor genes or antitumor bioactive substances in T. spiralis to suppress proliferation and invasion or apoptosis of the tumor cells [48]. In our research group, recombinant A200711 (L-aminoadipate-semialdehyde dehydrogenase- phosphopantetheinyl transferase), a T. spiralis protein, has been shown to be involved in the induction of apoptosis in human hepatoma H7402 cells [77].
T. spiralis infection causes a variety of changes in the skeletal muscle cells and the long-term suspension of the infected cell in the cell cycle at G2/M [78]. Cyclin-dependent kinase inhibitor p21 has been reported to play an important role in blocking cell-cycle expression during the cyst formation of T. spiralis, similar to the roles of tumor suppressor genes p53 and mouse double minute 2 [75]. Studies have shown that p53 contributes to the G1/G0 phase arrest in the cell cycle [79]. Cell cycle distribution analysis show that crude extracts of T. spiralis arrested the growth of the human chronic myeloid leukemia cell line (K562) at the G1 phase and the hepatoma cell line (H7402) at the S phase [48]. The increased expression of the c-Ski for the TGF-b signaling pathway factor indicates that the c-Ski protein is involved in nurse cell formation and cell cycle blocking through the TGF-β signaling pathway [76].
The tumoricidal activity of eosinophils has been described for several tumors [80] and is indicated by a pronounced eosinophilia that coincides with the establishment of T. spiralis larval stages in skeletal muscle [81]. In addition, tumor growth may be suppressed due to fever induced by parasite migration as well as via heat therapy.
The expression of apoptosis and anti-apoptotic proteins of the infected host muscle cells and satellite cells is regulated toward nurse cell formation through a parakine-mediated, host-cell, death-receptor, apoptotic pathway; the mitochondrial apoptotic pathway; and the TGF-β and Insulin-like of Growth Factor (IGF) signaling pathway [82]. Furthermore, the infected muscle cells are arrested in the cell cycle at the G2/M phase when the basophils of the nurse cell cytoplasm are changing into eosinophils. Satellite cells transform into normal muscle cells, which are dissipated with further regulation of the proliferation and differentiation of the satellite cell by apoptosis and the anti-apoptotic proteins, MyoD, myogenin, Myfs and MRF4. However, satellite cells transformed into nurse cells when they are parasitized by T. spiralis larvae. The regulatory mechanism of nurse cell formation is similar to the mechanism for tumor cell apoptosis signal regulation.
Apoptosis, which can be mediated by several antitumor drugs, is one of the most important signaling pathways and controls the cell fate and is a common death model of tumor cells [83]. The induction of tumor cell apoptosis has been suggested as a new strategy for modern tumor therapy [84]. Therefore, the “Parakines” of T. spiralis not only guide the transformation of infected cells into nurse cells but are also involved in the regulation of tumor cell apoptosis mediated by death receptors and mitochondria [85]. Furthermore, the sequential identification of macrophage Migration Inhibiting Factor (MIF), serine protease family protein and inhibition factor also supports this hypothesis [86]. Recently, chronic infection with T. spiralis has been experimentally demonstrated to display the capacity to inhibit tumor growth in a mouse model receiving B16 melanoma cells possibly through the mechanism of enhancing apoptosis and/ or necrosis [87].
4. T. SPIRALIS IS ASSOCIATED WITH A COINCIDENTAL OR PROMOTABLE ROLE
Coinfection with different pathogens is a common occurrence, which can influence the progression of disease [37]. Human T. spiralis coinfection with cancer was studied before the antitumor effects of T. spiralis were studied in the human and animal cases [88].
The growth of the cancer in cases of coinfection was previously considered as possibly being caused by the irritation from the chronic inflammation of the muscle that had been occupied long term by T. spiralis cysts [89, 90]. The risk of developing duodenal and gastric ulcer disease and gastric cancer, for example, is significantly increased after infection with Helicobacter pylori [91]. Interestingly, the masticatory muscles have been shown to be preferentially infected with T. spiralis, and the coinfection has frequently occurred in oral organ cancer, including oral cancer, tongue carcinoma, and laryngeal cancer [89, 90, 92, 93] and mainly involves squamous cell carcinoma, which is the most common neoplastic process of the oral cavity [94, 95]. However, in some cases, coinfection occurred with palpebral melanoma and breast carcinoma [96] In addition to the chronic irritation, the potential causes are that the obvious preferential muscle tropism and encystment of the T. spiralis larvae may enhance the circulation of neoplastic neovascularization [95], and T. spiralis infection alone may not have induced the tumor. Instead, coinfection may have served only as a cocarcinogen and played an auxiliary function in the development of the tumors [93].
T helper cells play an essential role in the regulation and maintenance of cellular immunity and humoral immunity. Th precursors can be differentiated into the following four distinct subsets: Th1, Th2, Th17, and Treg cells. Under normal conditions, Th1/Th2 cytokines cross-regulate each other's development in the body and are in a dynamic balance, which is important in the regulation of immune function and inflammatory response; and a disturbance in this balance may contribute to a host of functional disorders [97]. The imbalance of the Th1/Th2 is involved in the pathological process of many diseases. When a Th1 shift occurs, chronic inflammatory and autoimmune diseases, including Rheumatoid Arthritis (RA), Multiple Sclerosis (MS), and Type 1 diabetes normally occur. In contrast, a Th2-polarization leads to host susceptibility to microbial infections, tumorigenesis and progression, allergic reactions, and graft rejection reactions, including allergy, asthma, hay fever, urticaria, atopic dermatitis, chronic graft-versus-host disease, progressive systemic sclerosis, and systemic lupus erythematosus [98].
Cellular immune function mediated by Th1 cells, which can suppress proliferation of malignant cells and angiogenesis, plays an important role in antitumor responses [99], whereas Th2 cells inhibit antitumor responses [100]. No antitumor protection was observed during S. mansoni infection that induce a Th2-predominated response [12] . Infection with T. spiralis in human and animals is associated with a predominant Th2 type immune response [101]. The powerful expression of Th2 cytokines may inhibit Th1 cells and prevent the secretion of the IL-2, IL-12, and INF-γ that are mainly responsible for the development and persistence of cytotoxic T cells that emerge during a Th1→Th2 switch in which the Th1 cell activity declines and the Th2 activity increases [102]. The predominant expression of Th2-type cytokines in tumor cells may be related to development in cancer patients and the escape of tumor cells from immune surveillance [103]. Reduced Th1 immune responses have been considered an immunological link between Echinococcus granulosus infection and cancer metastasis in the liver [104]. Thus, Th2-polarized responses triggered by T. spiralis infection may not have an antitumor effect and, instead, may promote tumorigenesis or tumor recurrence.
CONCLUSION
Progress in the research on the relations between T. spiralis and tumors has greatly increased our understanding of its role in antitumor. Although evidences suggest that T. spiralis may be a powerful agent for tumor biotherapy, many questions remain unanswered, such as whether T. spiralis can be really used to prevent or cure tumors? If so through which mechanism? Even if it can be used to prevent or cure cancers, challenges remain, such as viable parasite infection or oral eggs cannot be administered easily to patients because these treatments could result in a persistent parasitic infection in humans and animals, and the large-scale application of T. spiralis during the parasite’s life cycle for tumor treatment is infeasible. Can an imbalance in Th1 and Th2 cells caused by T. spiralis promote tumorigenesis and progression? Is coinfection with tumors and Trichinellosis a coincidence or are the tumors caused by Trichinellosis? Thus, to elucidate the mechanisms and explain the coinfection of Trichinellosis and exploit the opportunities for tumor biotherapy more rapidly and efficiently in the future, more experimental and epidemiological investigations must be conducted.
Acknowledgements
The authors would like to thank Pan Liu and Jing Du for their critical comments, which improved this manuscript. This was supported by the National Natural Science Foundation of China (81201302 and 31572489), Key Project of Henan Province for Scientific Research Higher Education of China Colleges and Universities (17A230009), PhD Start-up Fund of Henan University of Science and Technology (13480071), Young Scientist Fund of Henan University of Science and Technology (2015QN033), Henan Science and Technology Key Project (152102110078).
list of ABBREVIATIONS
- Apaf-1
Apoptotic Protease Activating Factor 1
- BAX
Bcl-2 Associated Protein X
- BRM
Biological Response Modifiers
- cav-1
Caveolin-1
- ES
Excretory-Secretory
- HGPRT
Hypoxanthine-Guanine Phosphoribosyltransferase
- hsps
Heat Shock Proteins
- IBD
Inflammatory Bowel Disease
- IGF
Insulin-like of Growth Factor
- iRNA
Immune RNA
- LGLs
Large Granular Lymphocytes
- MCP-1
Monocyte Chemotactic Protein-1
- MFH
Malignant Fibrous Histiocytoma
- MIF
Migration Inhibiting Factor
- MS
Multiple Sclerosis
- PK
Protein Kinase
- RA
Rheumatoid Arthritis
- RB
Retinoblastoma
- RIP
Receptor Interactive Protein
- SSH
Suppression Subtractive Hybridization
- TcCRT
Trypanosoma Cruzi Calreticulin
- TCTP
Translationally Controlled Tumor Protein
- TLA
Lysate Antigen
- Tms
Tropomyosins
- TNFR-1
TNF Receptor 1
- TRADD
TNF Receptor-Associated Death-Domain
- TRAF2
TNF Receptor Associated Factor-2
- TSL-1
Trichinella spiralis Larvae 1
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Oldham R.K., Dillman R.O. Principles of Cancer Biotherapy. 5th ed. Dordrecht: Springer; 2009. pp. 1–22. [Google Scholar]
- 3.Oldham R.K. Biologicals and biological response modifiers: Fourth modality of cancer treatment. Cancer Treat. Rep. 1984;68(1):221–232. [PubMed] [Google Scholar]
- 4.Morrissey D., O’Sullivan G.C., Tangney M. Tumour targeting with systemically administered bacteria. Curr. Gene Ther. 2010;10(1):3–14. doi: 10.2174/156652310790945575. [DOI] [PubMed] [Google Scholar]
- 5.Chen L.L., He Z.X., Qin L., Li Q.Y., Shi X.B., Zhao S.T., Chen L., Zhong N.S., Chen X.P. Antitumor effect of malaria parasite infection in a murine lewis lung cancer model through induction of innate and adaptive immunity. PLoS One. 2011;6(9):e24407. doi: 10.1371/journal.pone.0024407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coley W.B. A preliminary note on the treatment of inoperable sarcoma by the toxic products of erysipelas. Post-Graduate. 1893;8:278–286. [Google Scholar]
- 7.Hibbs J.B., Jr, Lambert L.H., Jr, Remington J.S. Resistance to murine tumors conferred by chronic infection with intracellular protozoa, Toxoplasma gondii and Besnoitia jellisoni. J. Infect. Dis. 1971;124(6):587–592. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- 8.Darani H.Y., Shirzad H., Mansoori F., Zabardast N., Mahmoodzadeh M. Effects of Toxoplasma gondii and Toxocara canis antigens on WEHI-164 fibrosarcoma growth in a mouse model. Korean J. Parasitol. 2009;47(2):175–177. doi: 10.3347/kjp.2009.47.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pyo K.H., Jung B.K., Chai J.Y., Shin E.H. Suppressed CD31 expression in sarcoma-180 tumors after injection with Toxoplasma gondii lysate antigen in BALB/c mice. Korean J. Parasitol. 2010;48(2):171–174. doi: 10.3347/kjp.2010.48.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira E.C., Leite M.S., Miranda J.A., Andrade A.L., Garcia S.B., Luquetti A.O., Moreira H. Chronic Trypanosoma cruzi infection associated with low incidence of 1,2-dimethylhydrazine-induced colon cancer in rats. Carcinogenesis. 2001;22(5):737–740. doi: 10.1093/carcin/22.5.737. [DOI] [PubMed] [Google Scholar]
- 11.Sacks D., Sher A. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 2002;3(11):1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 12.Rankin E.B., Yu D., Jiang J., Shen H., Pearce E.J., Goldschmidt M.H., Levy D.E., Golovkina T.V., Hunter C.A., Thomas-Tikhonenko A. An essential role of Th1 responses and interferon gamma in infection-mediated suppression of neoplastic growth. Cancer Biol. Ther. 2003;2(6):687–693. [PubMed] [Google Scholar]
- 13.Junqueira C., Santos L.I., Galvao B., Teixeira S.M., Rodrigues F.G., DaRocha W.D., Chiari E., Jungbluth A.A., Ritter G., Gnjatic S., Old L.J., Gazzinelli R.T. Trypanosoma cruzi as an effective cancer antigen delivery vector. Proc. Natl. Acad. Sci. USA. 2011;108(49):19695–19700. doi: 10.1073/pnas.1110030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Junqueira C., Guerrero A.T., Galvao-Filho B., Andrade W.A., Salgado A.P., Cunha T.M., Ropert C., Campos M.A., Penido M.L., Mendonca-Previato L., Previato J.O., Ritter G., Cunha F.Q., Gazzinelli R.T. Trypanosoma cruzi adjuvants potentiate T cell-mediated immunity induced by a NY-ESO-1 based antitumor vaccine. PLoS One. 2012;7(5):e36245. doi: 10.1371/journal.pone.0036245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez G., Valck C., Ferreira V.P., Lopez N., Ferreira A. Extracellular Trypanosoma cruzi calreticulin in the host-parasite interplay. Trends Parasitol. 2011;27(3):115–122. doi: 10.1016/j.pt.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Fried B., Reddy A., Mayer D. Helminths in human carcinogenesis. Cancer Lett. 2010;305(2):239–249. doi: 10.1016/j.canlet.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Salim H.O.E., Hamid H.K., Mekki S.O., Suleiman S.H., Ibrahim S.Z. Colorectal carcinoma associated with schistosomiasis: a possible causal relationship. World J. Surg. Oncol. 2010;8:68. doi: 10.1186/1477-7819-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sripa B., Kaewkes S., Sithithaworn P., Mairiang E., Laha T., Smout M., Pairojkul C., Bhudhisawasdi V., Tesana S., Thinkamrop B., Bethony J.M., Loukas A., Brindley P.J. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4(7):e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas F., Lafferty K.D., Brodeur J., Elguero E., Gauthier-Clerc M., Misse D. Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biol. Lett. 2012;8(1):101–103. doi: 10.1098/rsbl.2011.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison W.B. Inflammation and cancer: A comparative view. J. Vet. Intern. Med. 2011;26(1):18–31. doi: 10.1111/j.1939-1676.2011.00836.x. [DOI] [PubMed] [Google Scholar]
- 21.Afzan M.Y., Suresh K. Pseudocyst forms of Trichomonas vaginalis from cervical neoplasia. Parasitol. Res. 2012;111(1):371–381. doi: 10.1007/s00436-012-2848-3. [DOI] [PubMed] [Google Scholar]
- 22.Weatherly N.F. Increased survival of Swiss mice given sublethal infections of Trichinella spiralis. J. Parasitol. 1970;56(4):748–752. [PubMed] [Google Scholar]
- 23.Apanasevich V.I., Britov V.A. Zban’ Iu, V. Antitumor cross-resistance of trichinosis. Vopr. Onkol. 2002;48(2):223–226. [PubMed] [Google Scholar]
- 24.Lubiniecki A.S., Cypess R.H. Quantitative study of the effect of previous Trichinella spiralis infection on sarcoma 180 ascitic tumor formation in mice. Tropenmed. Parasitol. 1975;26(3):329–334. [PubMed] [Google Scholar]
- 25.Molinari J.A., Ebersole J.L. Antineoplastic effects of long-term Trichinella spiralis infection on B-16 melanoma. Int. Arch. Allergy Appl. Immunol. 1977;55(1-6):444–448. doi: 10.1159/000231956. [DOI] [PubMed] [Google Scholar]
- 26.Kang Y.J., Jo J.O., Cho M.K., Yu H.S., Leem S.H., Song K.S., Ock M.S., Cha H.J. Trichinella spiralis infection reduces tumor growth and metastasis of B16-F10 melanoma cells. Vet. Parasitol. 2013;196(1-2):106–113. doi: 10.1016/j.vetpar.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Gong P.T., Zhang X.C., Li J.H., Zhang G.C., Yang J., Cao L.L., Zhang J.Z. Observation of anti-tumer effect of Trichinella spirialis in mice on A549 lung cancer cell. J. Pathogen Biol. 2008;3:200–202. [Google Scholar]
- 28.Liu J., Sun J.H., Liu L.D. Observation of trichinella on C6 glioma in BALB/c mice. J. Apoplexy Nerv. Dis. 2008;6:722–724. [Google Scholar]
- 29.Molinari J.A., Carrick L., Jr, Lubiniecki A.S. Influence of Trichinella spiralis infection on development of sarcoma-180 ascites tumors. Tropenmed. Parasitol. 1979;30(4):429–433. [PubMed] [Google Scholar]
- 30.Zhang Y.Y., Gong P.T., Zhang X.C., Li J.H., Yang J., Zhang G.C. Anti-tumor effect of Trichinella spirialis on Hepal-6 hepatoma carcinoma cell in the C57BL/6 mice. J. Pathogen Biol. 2009;4:24–26. [Google Scholar]
- 31.Dvoroznakova E., Hurnikova Z., Kolodziej-Sobocinska M. Development of cellular immune response of mice to infection with low doses of Trichinella spiralis, Trichinella britovi and Trichinella pseudospiralis larvae. Parasitol. Res. 2011;108(1):169–176. doi: 10.1007/s00436-010-2049-x. [DOI] [PubMed] [Google Scholar]
- 32.Frydas S., Karagouni E., Dotsika E., Reale M., Barbacane R.C., Vlemmas I., Anogianakis G., Trakatellis A., Conti P. Generation of TNF alpha, IFN gamma, IL-6, IL-4 and IL-10 in mouse serum from trichinellosis: Effect of the anti-inflammatory compound 4-deoxypyridoxine (4-DPD). Immunol. Lett. 1996;49(3):179–184. doi: 10.1016/0165-2478(96)02501-1. [DOI] [PubMed] [Google Scholar]
- 33.Ramaswamy K., Negrao-Correa D., Bell R. Local intestinal immune responses to infections with Trichinella spiralis. Real-time, continuous assay of cytokines in the intestinal (afferent) and efferent thoracic duct lymph of rats. J. Immunol. 1996;156(11):4328–4337. [PubMed] [Google Scholar]
- 34.Stewart G.L., Wood B., Boley R.B. Modulation of host response by Trichinella pseudospiralis. Parasite Immunol. 1985;7(3):223–233. doi: 10.1111/j.1365-3024.1985.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 35.Summers R.W., Elliott D.E., Qadir K., Urban J.F., Thompson R., Weinstock J.V. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 2003;98(9):2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 36.Khan W.I., Blennerhasset P.A., Varghese A.K., Chowdhury S.K., Omsted P., Deng Y., Collins S.M. Intestinal nematode infection ameliorates experimental colitis in mice. Infect. Immun. 2002;70(11):5931–5937. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furze R.C., Hussell T., Selkirk M.E. Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infect. Immun. 2006;74(3):1924–1932. doi: 10.1128/IAI.74.3.1924-1932.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu Y., Wang W., Tong J., Pan Q., Long Y., Qian W., Hou X. Th17: A new participant in gut dysfunction in mice infected with Trichinella spiralis. Mediators Inflamm. 2009;2009:517052. doi: 10.1155/2009/517052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parmentier H.K., Ruitenberg E.J., De-Weger R.A., Van-Loveren H. Mechanisms of T-cell-mediated hypersensitivity in mice infected with the intestinal helminth Trichinella spiralis. Tijdschr. Diergeneeskd. 1990;115(23):1085–1091. [PubMed] [Google Scholar]
- 40.Meerovitch E., Bomford R. Macrophage potentiation by Trichinella spiralis. Ann. Trop. Med. Parasitol. 1977;71(2):245–248. doi: 10.1080/00034983.1977.11687187. [DOI] [PubMed] [Google Scholar]
- 41.Wing E.J., Krahenbuhl J.L., Remington J.S. Studies of macrophage function during Trichinella spiralis infection in mice. Immunology. 1979;36(3):479–485. [PMC free article] [PubMed] [Google Scholar]
- 42.Hansson M., Karre K., Kiessling R., Roder J., Andersson B., Hayry P. Natural NK-cell targets in the mouse thymus: Characteristics of the sensitive cell population. J. Immunol. 1979;123(2):765–771. [PubMed] [Google Scholar]
- 43.Bany J., Janiak M.K., Budzynski W. Activity of natural killer (NK) cells in the course of experimental trichinellosis in mice. Wiad. Parazytol. 1992;38(3-4):117–126. [PubMed] [Google Scholar]
- 44.Niborski V., Vallee I., Fonseca-Linan R., Boireau P., Enciso A., Ortega-Pierres G., Yepez-Mulia L. Trichinella spiralis: Stimulation of mast cells by TSL-1 antigens trigger cytokine mRNA expression and release of IL-4 and TNF through an Ig-independent pathway. Exp. Parasitol. 2004;108(3-4):101–108. doi: 10.1016/j.exppara.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Korenaga M., Akimaru Y., Hashiguchi Y. Exogenous interleukin-3 enhances IL-4 production by splenic CD4+ cells during the early stages of a Trichinella spiralis infection. Int. Arch. Allergy Immunol. 1998;117(2):131–137. doi: 10.1159/000024000. [DOI] [PubMed] [Google Scholar]
- 46.Reale M., Frydas S., Barbacane R.C., Placido F.C., Cataldo I., Vacalis D., Trakatellis A., Anogianakis G., Felaco M., Di Gioacchino M., Conti P. Induction of monocyte chemotactic protein-1 (MCP-1) and TNF alpha by Trichinella spiralis in serum of mice in vivo. Mol. Cell. Biochem. 1998;179(1-2):1–5. doi: 10.1023/a:1006875429323. [DOI] [PubMed] [Google Scholar]
- 47.Sofronic-Milosavljevic L., Ilic N., Pinelli E., Gruden-Movsesijan A. Secretory products of Trichinella spiralis muscle larvae and immunomodulation: Implication for autoimmune diseases, allergies, and malignancies. J. Immunol. Res. 2015;2015:523875. doi: 10.1155/2015/523875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X.L., Fu B.Q., Yang S.J., Wu X.P., Cui G.Z., Liu M.F., Zhao Y., Yu Y.L., Liu X.Y., Deng H.K., Chen Q.J., Liu M.Y. Trichinella spiralis--a potential anti-tumor agent. Vet. Parasitol. 2009;159(3-4):249–252. doi: 10.1016/j.vetpar.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 49.Bazile F., Pascal A., Arnal I., Le Clainche C., Chesnel F., Kubiak J.Z. Complex relationship between TCTP, microtubules and actin microfilaments regulates cell shape in normal and cancer cells. Carcinogenesis. 2009;30(4):555–565. doi: 10.1093/carcin/bgp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mak C.H., Poon M.W., Lun H.M., Kwok P.Y., Ko R.C. Heat-inducible translationally controlled tumor protein of Trichinella pseudospiralis: Cloning and regulation of gene expression. Parasitol. Res. 2007;100(5):1105–1011. doi: 10.1007/s00436-006-0373-y. [DOI] [PubMed] [Google Scholar]
- 51.Parton R.G., Simons K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007;8(3):185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 52.Goetz J.G., Lajoie P., Wiseman S.M., Nabi I.R. Caveolin-1 in tumor progression: The good, the bad and the ugly. Cancer Metastasis Rev. 2008;27(4):715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 53.Hernandez-Bello R., Bermudez-Cruz R.M., Fonseca-Linan R., Garcia-Reyna P., Le Guerhier F., Boireau P., Ortega-Pierres G. Identification, molecular characterisation and differential expression of caveolin-1 in Trichinella spiralis maturing oocytes and embryos. Int. J. Parasitol. 2008;38(2):191–202. doi: 10.1016/j.ijpara.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Gomez-Monterrey I., Sala M., Musella S., Campiglia P. Heat Shock Protein 90 Inhibitors as Therapeutic Agents. Rec. Pat. Anti-Canc. 2012;7(3):313–336. doi: 10.2174/157489212801820066. [DOI] [PubMed] [Google Scholar]
- 55.Wang X.P., Lin H.P., Wang Q.X., Gu Y. Specific antitumor immunity induced by cross-linking complex heat shock protein 72 and alpha-fetoprotein. Cancer Biother. Radiopharm. 2012;27(3):189–197. doi: 10.1089/cbr.2011.1135. [DOI] [PubMed] [Google Scholar]
- 56.Wang S., Zhu X., Yang Y., Yang J., Gu Y., Wei J., Hao R., Boireau P., Cui S. Molecular cloning and characterization of heat shock protein 70 from Trichinella spiralis. Acta Trop. 2009;110(1):46–51. doi: 10.1016/j.actatropica.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Martinez J., Perez-Serrano J., Bernadina W.E., Rincon I., Rodriguez-Caabeiro F. Heat shock protein synthesis over time in infective Trichinella spiralis larvae raised in suboptimal culture conditions. J. Helminthol. 2004;78(3):243–247. doi: 10.1079/joh2003225. [DOI] [PubMed] [Google Scholar]
- 58.Rigby P.G. Restoration of allograft immunity in tumor-bearing animals by “immune” RNA. J. Lab. Clin. Med. 1968;71(5):775–783. [PubMed] [Google Scholar]
- 59.De Lucca F.L., Sawan F.M., Watanabe M.A., de Souza L.R. Effect of the calcium phosphate-mediated RNA uptake on the transfer of cellular immunity of a synthetic peptide of HIV-1 to human lymphocytes by exogenous RNA. Mol. Cell. Biochem. 2001;228(1-2):9–14. doi: 10.1023/a:1013305708539. [DOI] [PubMed] [Google Scholar]
- 60.Duan L., Li J., Cheng B., Lv Q., Gong P.T., Su L.B., Cai Y., Zhang X. Identification of a novel gene product expressed by Trichinella spiralis that binds antiserum to Sp2/0 myeloma cells. Vet. Parasitol. 2013;194(2-4):183–185. doi: 10.1016/j.vetpar.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 61.Choi C., Kim D., Kim S., Jeong S., Song E., Helfman D.M. From skeletal muscle to cancer: Insights learned elucidating the function of tropomyosin. J. Struct. Biol. 2012;177(1):63–69. doi: 10.1016/j.jsb.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Pawlak G., McGarvey T.W., Nguyen T.B., Tomaszewski J.E., Puthiyaveettil R., Malkowicz S.B., Helfman D.M. Alterations in tropomyosin isoform expression in human transitional cell carcinoma of the urinary bladder. Int. J. Cancer. 2004;110(3):368–373. doi: 10.1002/ijc.20151. [DOI] [PubMed] [Google Scholar]
- 63.Deng B., Gong P., Li J., Cheng B., Ren W., Yang J., Li H., Zhang G., Zhang X. Identification of the differentially expressed genes in SP2/0 myeloma cells from Balb/c mice infected with Trichinella spiralis. Vet. Parasitol. 2013;194(2-4):179–182. doi: 10.1016/j.vetpar.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 64.Gong P., Zhang J., Cao L., Nan Z., Li J., Yang J., Fang H., Jiao H., Jiang T., Su L., Zhang X. Identification and characterization of myeloma-associated antigens in Trichinella spiralis. Exp. Parasitol. 2011;127(4):784–788. doi: 10.1016/j.exppara.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Mukherjee T.K., Paul K., Mukhopadhyay S. Cell signaling molecules as drug targets in lung cancer: An overview. Curr. Opin. Pulm. Med. 2011;17(4):286–291. doi: 10.1097/MCP.0b013e328347bda6. [DOI] [PubMed] [Google Scholar]
- 66.Wang R., Sun Q., Wang P., Liu M., Xiong S., Luo J., Huang H., Du Q., Geller D.A., Cheng B. Notch and Wnt/beta-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7(5):5754–5768. doi: 10.18632/oncotarget.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolos V., Grego-Bessa J., de la Pompa J.L. Notch signaling in development and cancer. Endocr. Rev. 2007;28(3):339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 68.Ghobrial I.M., Witzig T.E., Adjei A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005;55(3):178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 69.Yang M., Wu S., Su X., May W.S. JAZ mediates G1 cell-cycle arrest and apoptosis by positively regulating p53 transcriptional activity. Blood. 2006;108(13):4136–4145. doi: 10.1182/blood-2006-06-029645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang T.Y., Chang W.C., Wang M.Y., Yang Y.R., Hsu Y.C. Effect of sulforaphane on growth inhibition in human brain malignant glioma GBM 8401 cells by means of mitochondrial- and MEK/ERK-mediated apoptosis pathway. Cell Biochem. Biophys. 2012;63(3):247–259. doi: 10.1007/s12013-012-9360-3. [DOI] [PubMed] [Google Scholar]
- 71.Hahm E.R., Singh S.V. Honokiol causes G0-G1 phase cell cycle arrest in human prostate cancer cells in association with suppression of retinoblastoma protein level/phosphorylation and inhibition of E2F1 transcriptional activity. Mol. Cancer Ther. 2007;6(10):2686–2695. doi: 10.1158/1535-7163.MCT-07-0217. [DOI] [PubMed] [Google Scholar]
- 72.Oh J.J., Razfar A., Delgado I., Reed R.A., Malkina A., Boctor B., Slamon D.J. 3p21.3 tumor suppressor gene H37/Luca15/RBM5 inhibits growth of human lung cancer cells through cell cycle arrest and apoptosis. Cancer Res. 2006;66(7):3419–3427. doi: 10.1158/0008-5472.CAN-05-1667. [DOI] [PubMed] [Google Scholar]
- 73.Moore M.J., Wang Q., Kennedy C.J., Silver P.A. An alternative splicing network links cell-cycle control to apoptosis. Cell. 2010;142(4):625–636. doi: 10.1016/j.cell.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Despommier D.D. How does Trichinella spiralis make itself at home? Parasitol. Today. 1998;14(8):318–323. doi: 10.1016/s0169-4758(98)01287-3. [DOI] [PubMed] [Google Scholar]
- 75.Boonmars T., Wu Z., Nagano I., Takahashi Y. What is the role of p53 during the cyst formation of Trichinella spiralis? A comparable study between knockout mice and wild type mice. Parasitology. 2005;131(Pt 5):705–712. doi: 10.1017/S0031182005008036. [DOI] [PubMed] [Google Scholar]
- 76.Wu Z., Nagano I., Boonmars T., Takahashi Y. A spectrum of functional genes mobilized after Trichinella spiralis infection in skeletal muscle. Parasitology. 2005;130(Pt 5):561–573. doi: 10.1017/s0031182004006912. [DOI] [PubMed] [Google Scholar]
- 77.Wang X.L., Liu M.Y., Sun S.M., Liu X.L., Yu L., Wang X.R., Chu L.X., Rosenthal B., Shi H.N., Boireau P., Wang F., Zhao Y., Wu X.P. An anti-tumor protein produced by Trichinella spiralis induces apoptosis in human hepatoma H7402 cells. Vet. Parasitol. 2013;194(2-4):186–188. doi: 10.1016/j.vetpar.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 78.Jasmer D.P. Trichinella spiralis infected skeletal muscle cells arrest in G2/M and cease muscle gene expression. J. Cell Biol. 1993;121(4):785–793. doi: 10.1083/jcb.121.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X.H., Zhao C., Seleznev K., Song K., Manfredi J.J., Ma Z.A. Disruption of G1-phase phospholipid turnover by inhibition of Ca2+-independent phospholipase A2 induces a p53-dependent cell-cycle arrest in G1 phase. J. Cell Sci. 2006;119(Pt 6):1005–1015. doi: 10.1242/jcs.02821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Legrand F., Driss V., Delbeke M., Loiseau S., Hermann E., Dombrowicz D., Capron M. Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J. Immunol. 2010;185(12):7443–7451. doi: 10.4049/jimmunol.1000446. [DOI] [PubMed] [Google Scholar]
- 81.Gebreselassie N.G., Moorhead A.R., Fabre V., Gagliardo L.F., Lee N.A., Lee J.J., Appleton J.A. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J. Immunol. 2012;188(1):417–245. doi: 10.4049/jimmunol.1101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu Z., Nagano I., Boonmars T., Takahashi Y. Tumor necrosis factor receptor-mediated apoptosis in Trichinella spiralis-infected muscle cells. Parasitology. 2005;131(Pt 3):373–381. doi: 10.1017/s0031182005007663. [DOI] [PubMed] [Google Scholar]
- 83.Sharaf-el-dein O., Mayola E., Chopineau J., Brenner C. The adenine nucleotide translocase 2, a mitochondrial target for anticancer biotherapy. Curr. Drug Targets. 2011;12(6):894–901. doi: 10.2174/138945011795529047. [DOI] [PubMed] [Google Scholar]
- 84.Kurose H., Shibata M.A., Iinuma M., Otsuki Y. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with alpha-mangostin extracted from mangosteen pericarp. J. Biomed. Biotechnol. 2012;2012:672428. doi: 10.1155/2012/672428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo J., Yu L.I., Xie G., Li D., Su M., Zhao X., Du L. Study on the mitochondrial apoptosis pathways of small cell lung cancer H446 cells induced by Trichinella spiralis muscle larvae ESPs. Parasitology. 2017;11:1–8. doi: 10.1017/S0031182016002535. [DOI] [PubMed] [Google Scholar]
- 86.Nagano I., Wu Z., Takahashi Y. Functional genes and proteins of Trichinella spp. Parasitol. Res. 2009;104(2):197–207. doi: 10.1007/s00436-008-1248-1. [DOI] [PubMed] [Google Scholar]
- 87.Vasilev S., Ilic N., Gruden-Movsesijan A., Vasilijic S., Bosic M., Sofronic-Milosavljevic L. Necrosis and apoptosis in Trichinella spiralis-mediated tumour reduction. Cent. Eur. J. Immunol. 2015;40(1):42–53. doi: 10.5114/ceji.2015.50832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewy R.B. Carcinoma of Larynx and Trichinosis. Arch. Otolaryngol. 1964;80:320–321. doi: 10.1001/archotol.1964.00750040330015. [DOI] [PubMed] [Google Scholar]
- 89.Kean H. Cancer and trichinosis of the larynx. Laryngoscope. 1966;76(11):1766–1768. doi: 10.1288/00005537-196611000-00002. [DOI] [PubMed] [Google Scholar]
- 90.Simaskos N., Palaiologos Y., Eliopoulos P.N. Trichinosis and cancer of the larynx. J. Laryngol. Otol. 1992;106(2):171–172. doi: 10.1017/s0022215100119000. [DOI] [PubMed] [Google Scholar]
- 91.Wroblewski L.E., Peek R.M., Jr, Wilson K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010;23(4):713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheung L.K., Yeung R.W., Leung S.Y., Samman N. Trichinosis associated with carcinoma of the tongue: Case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997;84(1):32–34. doi: 10.1016/s1079-2104(97)90290-0. [DOI] [PubMed] [Google Scholar]
- 93.Cvorovic L., Milutinovic Z., Kiurski M. Trichinella spiralis and laryngeal carcinoma: A case report. Eur. Arch. Otorhinolaryngol. 2005;262(6):456–458. doi: 10.1007/s00405-004-0850-9. [DOI] [PubMed] [Google Scholar]
- 94.Josephson J.S., Josephson G.D., Dennis N.N. Pathologic quiz case 2. Laryngeal trichinosis with simultaneous squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 1989;115(11):1384–1385, 1387. [PubMed] [Google Scholar]
- 95.Moisan P.G., Lorenz M.D., Stromberg P.C., Simmons H.A. Concurrent trichinosis and oral squamous cell carcinoma in a cat. J. Vet. Diagn. Invest. 1998;10(2):199–202. doi: 10.1177/104063879801000220. [DOI] [PubMed] [Google Scholar]
- 96.Kristek J., Marjanovic K., Dmitrovic B., Krajinovic Z., Sakic K. Trichinella spiralis and breast carcinoma--a case report. Coll. Antropol. 2005;29(2):775–777. [PubMed] [Google Scholar]
- 97.Zhao J., Xie Y., Qian C., Li L., Jiang R., Kan H., Chen R., Song W. Imbalance of Th1 and Th2 cells in cardiac injury induced by ambient fine particles. Toxicol. Lett. 2012;208(3):225–231. doi: 10.1016/j.toxlet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 98.Kidd P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003;8(3):223–246. [PubMed] [Google Scholar]
- 99.Disis M.L., Park K.H. Immunomodulation of breast cancer via tumor antigen specific Th1. Cancer Res. Treat. 2009;41(3):117–121. doi: 10.4143/crt.2009.41.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wada H., Seki S., Takahashi T., Kawarabayashi N., Higuchi H., Habu Y., Sugahara S., Kazama T. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. 2007;106(3):499–506. doi: 10.1097/00000542-200703000-00014. [DOI] [PubMed] [Google Scholar]
- 101.Jackson J.A., Friberg I.M., Little S., Bradley J.E. Review series on helminths, immune modulation and the hygiene hypothesis: Immunity against helminths and immunological phenomena in modern human populations: Coevolutionary legacies? Immunology. 2009;126(1):18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Becker Y. The changes in the T helper 1 (Th1) and T helper 2 (Th2) cytokine balance during HIV-1 infection are indicative of an allergic response to viral proteins that may be reversed by Th2 cytokine inhibitors and immune response modifiers--a review and hypothesis. Virus Genes. 2004;28(1):5–18. doi: 10.1023/B:VIRU.0000012260.32578.72. [DOI] [PubMed] [Google Scholar]
- 103.Tabata T., Hazama S., Yoshino S., Oka M. Th2 subset dominance among peripheral blood T lymphocytes in patients with digestive cancers. Am. J. Surg. 1999;177(3):203–208. doi: 10.1016/s0002-9610(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 104.Turhan N., Esendagli G., Ozkayar O., Tunali G., Sokmensuer C., Abbasoglu O. Co-existence of Echinococcus granulosus infection and cancer metastasis in the liver correlates with reduced Th1 immune responses. Parasite Immunol. 2015;37(1):16–22. doi: 10.1111/pim.12152. [DOI] [PubMed] [Google Scholar]