Abstract
Toxoplasmosis and neosporosis are closely related protozoan diseases that lead to important economic impacts in farm ruminants. Toxoplasma gondii infection mainly causes reproductive failure in small ruminants and is a widespread zoonosis, whereas Neospora caninum infection is one of the most important causes of abortion in cattle worldwide. Vaccination has been considered the most economic measure for controlling these diseases. However, despite vaccine development efforts, only a live-attenuated T. gondii vaccine has been licensed for veterinary use, and no promising vaccines against ne-osporosis have been developed; therefore, vaccine development remains a key goal. Additionally, drug therapy could be a valuable strategy for disease control in farm ruminants, as several drugs that limit T. gondii and N. caninum proliferation and dissemination have been evaluated. This approach may also be relevant to performing an initial drug screening for potential human therapy for zoonotic parasites. Treat-ments can be applied against infections in adult ruminants to minimize the outcomes of a primo-infection or the reactivation of a chronic infection during gestation or in newborn ruminants to avoid infection chronification. In this review, the current status of drug development against toxoplasmosis and neosporo-sis in farm ruminants is presented, and in an effort to promote additional treatment options, prospective drugs that have shown efficacy in vitro and in laboratory animal models of toxoplasmosis and neosporosis are examined
Keywords: Toxoplasma, Neospora, Cattle, Sheep, Goats, Chemotherapy, Chemoprophylaxis
1. Introduction
The cosmopolitan obligate intracellular protozoan parasites Toxoplasma gondii and Neospora caninum are considered major causes of reproductive failure in small ruminants and cattle, respectively [1-3]. Thereby, toxoplasmosis and neosporosis deserve special attention since they lead to severe economic losses to ruminant livestock producers [3-5]. In fact, the negative economic effects are more devastating in the case of epidemic abortion storms than in herds/flocks with endemic cases [3, 6]. In addition, despite the undoubtedly under-detection and underreporting of cases [7, 8], toxoplasmosis affects 25-50% of the world population [9-11] and it is recognized as parasitic zoonosis with the highest human incidence by the European Food Safety Authority (EFSA) [7].
T. gondii and N. caninum are closely related apicomplexan parasites, sharing many morphological and biological features and showing remarkably conserved genomes [12-14]. Moreover, they present similar facultative heteroxenous coccidian life cycles, including three invasive stages: i) the rapidly replicating and abortion-causing tachyzoites, ii) the slowly replicating or quiescent bradyzoites harboured in tissue cysts, especially those within the brain and skeletal muscle, and iii) the sporulated oocysts bearing sporozoites [15-17]. However, large differences are found between them in relation to the host range of definitive and intermediate hosts, the zoonotic potential, which has been proven for only T. gondii, and the importance of horizontal and vertical routes of transmission in maintaining natural infections in the ruminant populations [17, 18].
2. Parasites and diseases
2.1. Host Range and Transmission
According to current knowledge, the members of the family Felidae, especially domestic cats (Felis catus), are the definitive hosts of T. gondii. They commonly become infected after eating wild rodents and birds containing T. gondii tissue cysts (bradyzoites) and, to a lesser extent, by ingesting oocysts shed by other cats [4, 19]. In turn, any warm-blooded mammal, including humans and birds, can act as an intermediate host [4].
Most infections in sheep and goat flocks occur postnatally, mainly by the horizontal route after the ingestion of water and food contaminated with viable sporulated oocysts shed by cats [2, 4]. However, the vertical transmission of T. gondii (from the dam to the foetus during gestation) can also occur in these two small ruminant species by both ‘Exogenous Transplacental Transmission’ (ExTT) and ‘Endogenous Transplacental Transmission’ (EnTT) mechanisms. The former mechanism, which is the most frequent, occurs during pregnancy as a consequence of an exogenous source while the latter, whose importance remains controversial [4], occurs after the recrudescence of a latent infection acquired in utero by the reactivation of bradyzoites and their reconversion to tachyzoites. In human hosts, the consumption of raw or undercooked meat with T. gondii tissue cysts containing the bradyzoite stage is commonly considered the main source of infections in developed countries [20], although water is increasingly being investigated as a risk factor. In fact, oocyst contamination has been demonstrated to be an important source of infection in tropical and subtropical countries, and also in some European countries [21, 22].
The domestic dog (Canis lupus familiaris) and wild canids, such as American coyote (Canis latrans), Australian dingo (Canis lupus dingo) and Eurasian grey wolf (Canis lupus lupus) are the definitive hosts of N. caninum, while farm ruminants (cattle and sheep), horses and wildlife white-tailed deer and water buffalo are assumed to be intermediate hosts in the life cycle of N. caninum based on its successful isolation [18].
Dogs are usually infected through consumption of N. caninum-infected bovine tissues (mainly foetuses and placentas). Cattle can become infected through the oral uptake of N. caninum sporulated oocysts that are shed by dogs from the farm environment [23, 24], but the relative frequency with which cows are infected postnatally compared to that of cows infected by transplacental transmission remains unknown [25]. Highly efficient transplacental infections (ExTT and EnTT) through tachyzoites are responsible for the majority of natural neosporosis outbreaks in livestock [26-28]. As mentioned above for toxoplasmosis, ExTT of N. caninum is associated with dams that are oocyst-derived primo-infected during gestation, while EnTT of N. caninum is related to the reactivation and recrudescence of a previous latent infection during gestation. The efficiency of transplacental infection has been estimated to range from 44% [29] to over 95% [26], according to precolostral serology in calves born to seropositive dams. In fact, this route of infection is crucial to maintaining the infection within cattle herds, especially in herds with a high prevalence of seropositive cows [29]. Nevertheless, it has been proposed that transplacental transmission alone is not enough to maintain the infection within the herds, and thus horizontal transmission is required [30, 31].
2.2. Pathogenesis: Outcome of Infection in Pregnant Ruminants
The complex pathogenesis of both reproductive ruminant diseases is not fully understood [1, 32], and there is still much to know concerning the role of parasite multiplication in the target tissues, maternal-foetal immunity [33] and other essential factors, such as variations in virulence and parasite stages. However, it is well documented in the literature that the outcome of toxoplasmosis, as well as the effects of neosporosis in pregnant sheep, goats and cattle, are strongly associated with the period of gestation at the time of primary infection [34-38].
Ewes infected prior to mating will develop an anti-T. gondii immune response, enhancing protection against abortion in subsequent pregnancies [4], whereas repeated abortion in the next pregnancies has been described in goats [39]. In particular, if T. gondii tachyzoites reach a relatively immunologically immature foetus during the early stages of pregnancy, the infection usually results in foetal death. In turn, the birth of a stillborn or weak lamb, which is sometimes accompanied by a small, mummified foetus, is highlighted at mid-gestation stages, whereas the birth of healthy and clinically normal lambs that are congenitally infected is the main finding for ewes with late T. gondii infections or after the recrudescence of an endogenous infection [27].
Typically, N. caninum infection leads to a latent and asymptomatic course in non-pregnant cows. Conversely, bovine neosporosis in pregnant cows is associated with repeated abortion and the birth of clinically healthy, but persistently infected, calves [33]. The likelihood of abortion by N. caninum also greatly depends on the period of gestation in which the infection occurs. Although N. caninum-infected cows of any age may abort from 3 months gestation to term, most abortions are concentrated in the mid-gestation stages, between 5 to 7 months of gestation [36, 40, 41]. The outcome of infection at the late stages of gestation is consistent with most foetuses going-on-to-term, and thus these infections lead to the birth of congenitally infected calves that are clinically normal, but persistently infected [42, 43]. A limited number of calves may show neuromuscular disorders [44]. It is also possible that congenitally infected cows may abort in successive gestations. In fact, around one in twenty cows that aborted once due to an N. caninum infection will abort again [28, 45].
2.3. Control
For the control of toxoplasmosis and neosporosis, different measures have been suggested; however, the combination of different approaches is known to be the optimal strategy [1, 2]. The implementation of farm biosecurity protocols, hygienic measures and management practices should be adopted in all farms, for reducing the level of environmental contamination with T. gondii oocysts via cat faeces or N. caninum oocysts via dog faeces (if present) and for avoiding novel infections through the introduction of infected animals to the herd. Among the biosafety practices that should be conducted to achieve this aim, the following are highlighted: limit cat and dog access to ruminant areas, especially to those housing pregnant ruminants, or to the areas for food storage and water supplies; promptly remove of placentas or foetal materials; appropriately dispose of dead livestock; and establish rodent control. Reproductive practices considering the artificial insemination of seropositive dams or the use of embryo transfer and test and cull strategies based on serological diagnosis are also recommended for the control of N. caninum infections [1, 46, 47]. Despite being properly designed and meticulously practised, globally, these control measures alone are not cost-viable or completely effective in eliminating toxoplasmosis and neosporosis from a herd, and it is necessary to complement them with an immune-chemotherapeutical approach [47-49].
In particular, vaccination has been considered a promising and economically viable solution to prevent the transmission of both infectious agents, despite its two current principal limitations related to efficacy and safety [4, 50]. In fact, to date, the control of ovine toxoplasmosis is primarily based on preventing its horizontal transmission and on the establishment of a vaccination programme with a live attenuated S48 strain (Toxovax™, MSD). The benefits are associated with protection against abortions induced by T. gondii during pregnancy and a decrease in tissue cyst development [4, 51]. Although the protective immunity against T. gondii can persist over one year, vaccination does not imply latent infections, and its use has two main drawbacks: a short shelf-life and a risk of infection to the humans who are handling live vaccines [4, 51]. In terms of efficacy, the design of successful vaccines requires blocking the stages of the life cycle (e.g., cell invasion, intracellular replication) and simulating the natural immune responses of the hosts [48]. Therefore, vaccine development against T. gondii infection in humans and food-producing animals represents an adequate and global challenge that could greatly improve disease control. Regarding bovine neosporosis and vaccination, economic analyses suggest that vaccination may be a more cost-effective and largely preferred approach than a test and cull programme [52]. In the United States, a commercial killed vaccine for pregnant cows was available until 2009 (C548 NeoGuard™, Intervet), but it showed variable efficacy. At this time, no commercial vaccine is available, which precisely represents a major handicap that needs to be solved [50]. Live-attenuated vaccines appear to be the most promising approach, although subunit vaccines have also been investigated in relation to two functional points of view: the physical interaction between the parasite and its host cell during invasion or tachyzoite-to-bradyzoite stage conversion [49], and these vaccines have emerged as the best way forward [50].
Alternatively, several drugs appear to have a potentially beneficial effect on controlling these infections. Effective chemotherapy has been identified as an economically promising option in the literature [53, 54]. However, the disadvantage of using pharmacological treatments against toxoplasmosis and neosporosis is related to the timing of their administration; there is no evidence of infection until abortion cases are observed, and this timing could be indicative of a stage that is too late for treatment administration [55]. An oral administration of drugs is expected to be effective against the T. gondii and N. caninum sporozoites released from oocysts. In addition, the drugs should be effective against the tachyzoite stage, which is associated with acute disease, and the bradyzoite stage, which occurs in the chronic phase of infection [56]. The ideal treatment would be parasiticidal against sporozoites and bradyzoites, but a parasitostatic capability against the tachyzoite stage, since tachyzoites have greater difficulty resisting the host cell’s adaptive immune response. Additionally, a suitable drug should have low toxicity and should be able to cross the blood-brain barrier to effectively eliminate parasitic brain cysts [11].
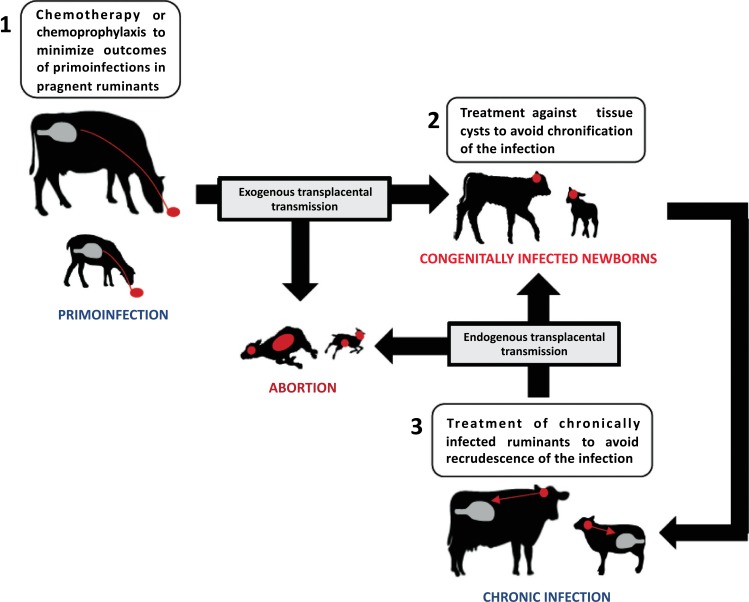
In practical terms, the treatment of ruminant toxoplasmosis and neosporosis should be focused on 3 main levels: i) chemotherapy or chemoprophylaxis in infected cows, ewes and goats (Fig. 1), ii) treatment of newborn congenitally infected calves, lambs and kids (Fig. 1) and iii) treatment of definitive hosts in order to reduce environmental contamination with T. gondii and N. caninum oocysts.
Fig. (1).
Intervention points for chemotherapy and chemoprophylaxis in toxoplasmosis and neosporosis in farm ruminants.
The comprehensive immune-chemotherapeutical approach with a combination of protective immunity generated by vaccines (applied before mating) and the capability of bioactive compounds (applied in the gestation period where abortions are more likely to occur) to limit the proliferation and dissemination of tachyzoites or to abrogate tissue cysts would be a great option for controlling toxoplasmosis and neosporosis in farm ruminants [49]. In a context where vaccination against both parasites needs to be improved, the objective of this review is to summarize relevant studies on the treatment of toxoplasmosis and neosporosis in farm ruminants that could serve as chemotherapeutical guidelines. Furthermore, in order to address novel therapeutic approaches, considerations on promising drugs will be described.
3. Available drug treatments for ruminant toxoplasmosis and neosporosis
Published research, including ruminant studies on drug therapy against toxoplasmosis (Table 1) and neosporosis (Table 2), are reviewed. Each pharmacological group, with its chemical structure, main uses for therapy, mode/s of action, and in vitro and in vivo activities are summarized.
Table 1.
Summary of published chemoprophylactic and chemotherapeutic studies against T. gondii infection in farm ruminants.
| Drug | Experimental Design | Main Results | References | |||
|---|---|---|---|---|---|---|
| Class | Compounds | Animal | Infection | Treatment | ||
| Macrolide antibiotics | Spiramycin | 85-100-days pregnant ewes (n=19) | 1500 RH strain tachyzoites/kg body weight | 100 mg/kg PO from 3 weeks after infection until parturition | Reduction in the humoral immune response in pregnant ewes and T. gondii seropositive lambs | [73] |
| Polyether ionophore antibiotics | Monensin | 80-days pregnant ewes (n=14) | 100 M1 strain tissue cysts SC at day 90 of pregnancy | 15 mg/day PO from day 80 of pregnancy until parturition | 44% increase in live lambs and 43% enhancement in survival 72 hours after birth | [92] |
| 80-days pregnant ewes (n=69) | 2000-12000 M1 strain oocysts PO at day 90 of pregnancy | 15-30 mg/day PO from day 80 of pregnancy until parturition | Reduction in febrile response, foetal mortality (46% increase in live lambs) and humoral immune response in dams and foetuses | [91] | ||
| Lasalocid | 55-days pregnant ewes (n=33) | 100 TS-1 strain oocysts PO at day 60 of pregnancy | 30 g/day PO from day 55 of pregnancy until parturition | No effect in reducing humoral immune responses, lesions or abortions | [99] | |
| Folate inhibitors | Sulphamezathine and pyrimethamine | 89-days pregnant ewes (n=65) | 2000 M3 isolate oocysts PO at 89 days of pregnancy | Sulphamezathine (83-166 mg/ kg SC). Pyrimethamine (1-2 mg/kg IP) on days 100, 115 and 130 of gestation, each of three consecutive days | No foetal mortality from 130 days of gestation. Reduction in antibody titres of lambs with less severe placental lesions | [109] |
| Sulphadimidine | 110-130-days pregnant ewes (n=200) | Field conditions: high abortion rate flock | Sulphadimidine 20-33 mg/kg IM, 4 times every 48 hours | Reduced abortion rate: 60% (before treatment) and 25% or 7% (after treatment) | [110] | |
| Quinolones | Decoquinate | 90-days pregnant ewes (n=98) | 200 M3 isolate oocysts orally PO at 90 days of pregnancy | 1-2 mg/kg PO from day 80 of pregnancy until parturition | Reduced febrile and humoral immune responses with increase of viable lambs up to 61.8%, increased weight of the lambs up to 33.3% and mean gestation period increased by 5 days | [125] |
| Triazinones | Toltrazuril | Lambs (n=33) | 9x104 IP and 1x104 IM ME-49 oocysts | 20-40 mg/kg PO twice, once every week, beginning 15 days pi | Reduction in serum antibody titres at 90 days after infection and 44.4% reduction in tissue cysts | [139] |
PO: per os. Oral administration.
SC: subcutaneously.
IP: intraperitoneally.
IM: intramuscularly.
pi: post-infection.
Table 2.
Summary of published chemoprophylactic and chemotherapeutic studies against N. caninum infection in farm ruminants.
| Drug | Experimental Design | Main Results | References | |||
|---|---|---|---|---|---|---|
| Class | Compounds | Animal | Infection | Treatment | ||
| Polyether ionophore antibiotics | Monensin | Non-pregnant dairy cows (n=27) | 5x106 Nc-1 tachyzoites SC | 335 mg/day, bolus PO. From 21 days before challenge until 3 months pi | Lower humoral immune response on week 4 pi | [101] |
| Quinolones | Decoquinate | 1.5-months pregnant heifers (n=77) | Chronically infected or primo-infected heifers | 2 mg/kg PO from 1.5 until 8 months of pregnancy | Tendency to reduce abortions (44% in chronically infected heifers and 64% in primo-infected heifers) and infection in calves at birth (25% in chronically infected heifers and 40% in primo-infected heifers) | [126] |
| Triazinones | Ponazuril | Calves (n=19) | 1x108 IV and 1x108 SC Nc-1 tachyzoites | 20 mg/kg PO, applied 24 hours pi once or six consecutive days | Reduction of symptoms, lower antibody response and abrogation of parasite detection in organs | [142] |
| Toltrazuril | Calves (n=72) | Congenitally infected calves | 20 mg/kg PO, three times, every second day, within 7 days after birth | Strong antibody reactivity four to six months after birth | [140] | |
| Lambs (n=38) | Congenitally infected lambs | 20 mg/kg PO on days 0, 7, 14 and 21 after birth | Lower antibody levels, but no reduction of presence or severity of histopathological lesions | [141] | ||
| Triazinones + Folate inhibitors | Toltrazuril + Sulphadiazine and Trimethoprim | Dairy cows (n=936) | Field conditions: high abortion rate herds | Toltrazuril 20 mg/kg/day IV for 3 consecutive days to newborn calves during the first week. Sulphadiazine/trimethoprim at 20 mg/kg IV once a year from 3 months of age |
Reduction of abortions (from 188 to 9) | [111] |
PO: per os. Oral administration.
SC: subcutaneously.
IV: intravenously.
pi: post-infection.
3.1. Macrolide Antibiotics
Macrolide antibiotics, which were discovered in the early 1950s, are a well-established class of antimicrobial agents containing 12- to 16-membered lactone rings that have been substituted with one or more sugar residues, some of which may be amino sugars [57]. All macrolides inhibit bacterial protein synthesis to varying extents. The macrolides bind to the 50S ribosomal subunit with a specific target in the 23S ribosomal RNA molecule and various ribosomal proteins. The most recent hypothesis suggests that all macrolides stimulate the dissociation of peptidyl-tRNA from the ribosomes during the elongation phase, leading to the inhibition of protein synthesis [58, 59]. The spectrum of activity includes many common pathogens, such as gram-positive bacteria (e.g., Streptococcus pyogenes, Corynebacterium diphtheria and Staphylococcus aureus), gram-negative bacteria (e.g., Neisseria gonorrhoeae, Moraxella catarrhalis and Bordetella pertussis) and protozoan parasites, including T. gondii, Plasmodium falciparum and Entamoeba histolytica [60].
Spiramycin (2-[(4R,5S,6S,7R,9R,10R,11E,13E,16R)-6-[5-(4,5-dihydroxy-4,6-dimethyloxan-2-yl)oxy-4-(dimethyl-amino)-3-hydroxy-6-methyloxan-2-yl]oxy-10-[5-(dimethyl-amino)-6-methyloxan-2-yl]oxy-4-hydroxy-5-methoxy-9,16-dimethyl-2-oxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetal-dehyde), a 16-membered macrolide derived from Streptomyces ambofaciens, has been used for the treatment of acute toxoplasmosis in pregnant women for decades because it is a safe and well-tolerated drug with no known adverse effects on the foetus [61-63]; however, its effectiveness and benefits are still unclear [64-66]. Spiramycin showed limited in vitro activity against T. gondii, with a 50% inhibitory concentration (IC50) of 20.16 µg/ml and an inhibitory, rather than a curative, effect [67, 68]. Despite its low in vitro efficacy, spiramycin was the first macrolide to demonstrate activity against acute toxoplasmosis in mice [69]. Spiramycin improves mouse survival after an acute T. gondii infection and reduces brain cyst burdens in both acute and chronic toxoplasmosis [70, 71]. These paradoxical results are explained by spiramycin’s ability to achieve intra-cellular and tissue concentrations that exceed its serum concentrations. Furthermore, spiramycin clearance is low, resulting in sustained tissue and intracellular concentrations [72].
Spiramycin has also been tested in pregnant ewes infected with T. gondii (RH strain) between the 85th and 100th days of pregnancy and treated orally (PO) with 100 mg/kg spiramycin from 3 weeks after infection until parturition. All ewes gave birth, with only one stillbirth in the untreated group. Analysis of the placental tissues did not show differences neither in the histopathological lesions, nor in the presence of T. gondii between the treated and untreated groups. However, the humoral immune response in pregnant ewes decreased in the treated group compared to that in the untreated group. Additionally, a lower number of lambs were seropositive to T. gondii in the treated group than in the untreated one. It was concluded that spiramycin treatment in ewes during the mid-stage of gestation exhibited a reduction in the humoral immune response in dams and in T. gondii seropositive lambs [73].
3.2. Polyether Ionophore Antibiotics
Polyether ionophore antibiotics, whose structures involve an alkyl-rich, lipid-soluble exterior and a cage-like interior, are produced by Streptomyces spp. and form complexes with metal cations, transporting these complexes across hydrophobic membranes [74]. Polyether antibiotics form mainly neutral complexes with monovalent cations (i.e., monensin, salinomycin) or divalent metal cations (i.e., lasalocid, calcimycin) and with organic bases (i.e., lasalocid) [75-77]. Polyether antibiotics induce ionic gradient perturbations, acting on different metabolic pathways [78], and show broad spectrum bioactivity, including antibacterial, antifungal, antiparasitic and antiviral effects, as well as tumour cell cytotoxicity [77]. Monensin (4-[2-[5-ethyl-5-[5-[6-hydroxy-6-(hydroxymethyl)-3,5-dimethyl-oxan-2-yl]-3-methyl oxolan-2-yl]oxolan-2-yl]- 9-hydroxy-2,8-dimethyl-1,6-dioxasp iro [4.5]dec-7-yl]-3-methoxy-2-methyl-pentanoic acid) was shown to have coccidiostatic effects [79, 80] and growth promoting properties [81] by favouring ruminal propionic acid production [82]. However, European Union regulations on the additives for use in animal nutrition banned the use of growth promoting agents as feed additives in animals [83].
In vitro studies showed that polyether ionophore antibiotics affect all endogenous apicomplexan parasite forms, whether they are dividing or not [78]. Lasalocid (6-[(3R,4S,5S,7R)-7-[(2S,3S,5S)-5-ethyl-5-[(2R,5R,6S)-5-ethyl-5-hydroxy-6-methyloxan-2-yl]-3-methyloxolan-2-yl]-4-hydroxy-3,5-dimethyl-6-oxononyl]-2-hydroxy-3-methyl-benzoic acid) at 0.05 µg/ml is directly toxic to extracellular T. gondii tachyzoites and inhibits cell penetration and intracellular multiplication [84]. Monensin at 0.001 µg/ml has also shown in vitro efficacy (IC50) against T. gondii tachyzoites [77, 85]. Working against the T. gondii cyst form, monensin inhibits the infectivity and viability of bradyzoites at low concentrations (0.1 ng/ml) [78]. Recently, it has been demonstrated that monensin, through TgMSH-1 protein, disrupts the mitochondrial function of T. gondii, triggering a disruption of cell cycle progression that precedes the events that resemble an autophagy-like death process [86, 87]. In addition, the disruption of mitochondrial function results in the generation of reactive oxygen species [88]. T. gondii monensin resistance has been described after chemical mutagenesis in vitro [85] or as a result of a disruption of TgMSH-1 [89]. Furthermore, lasalocid and monensin at 0.001 ng/ml showed a 95% reduction of N. caninum tachyzoites in cell cultures [90].
The efficacy of monensin against ovine toxoplasmosis was evaluated in experimentally infected pregnant sheep. Ninety-days pregnant ewes were experimentally infected PO with 2,000 and 12,000 T. gondii (M1 strain) oocysts and were treated PO with monensin (15 or 30 mg) daily from day 80 of gestation until parturition [91]. Ewes receiving monensin showed a reduction in foetal mortality compared with the non-treated ewes (83.3% vs 44.8% alive lambs, re-spectively). In this study, monensin seems to act earlier in the infection, possibly by effects on the sporozoites released from infectious oocysts within the intestinal lumen. Ewes infected and treated with monensin showed a lesser febrile response. In addition, ewes receiving monensin showed lower anti-T. gondii IgG levels. However, when monensin administration ceased after lambing, circulating specific IgG antibodies against T. gondii increased over three months to reach values similar to those observed in infected and non-treated ewes. This observation suggests that monensin could also have a systemic effect, possibly acting on the tachyzoites present in the pregnant uterus. In addition, lambs born from ewes receiving monensin had higher live weights, and less of these animals showed evidence of infection and pathological changes in foetal or placental tissues than the lambs born from infected and non-treated ewes.
The possible systemic effect of monensin after T. gondii infection could be of value, as the time of infection during natural outbreaks of toxoplasmosis can only rarely be clearly defined. To avoid intestinal infection, and hence any intestinal effect of monensin PO at day 90 of pregnancy, the pregnant ewes were challenged Subcutaneously (SC) with 100 T. gondii (M1 strain) tissue cysts [92]. No differences in febrile and serological responses were found between the treated and non-treated groups. However, the treated ewes produced more viable lambs (less premature and greater live weight) than the untreated ewes (75% vs 42%, respectively). In addition, 58% of the lambs from treated ewes survived 72 hours after birth, whereas only 33% survived from the untreated ewes. Based on this evidence, it would appear that monensin, while being most effective in the gut lumen, does have a lesser systemic action, presumably by suppressing the multiplication of T. gondii in the placentome.
The accidental poisoning of domestic animals with monensin PO has been reported in cattle, horses, poultry and dogs [93, 94]. The clinical findings in sheep include lethargy, stiffness, muscular weakness, a stilted gait and recumbency, followed by a decrease in the muscle volume of the rump and thigh. The post-mortem lesions in the skeletal muscles consisted of pale streaking, with atrophy in the chronic stages. In lambs younger than one month old, diffuse gastrointestinal haemorrhage was the only finding [95]. Later, another researcher reported an outbreak of monensin poisoning in sheep and highlighted the need for a licensed, safe product [96]. An option to avoid monensin poisoning in sheep would be to use blocks containing monensin or slow-release boluses. In addition, this option would overcome the practical problems of monensin delivery to grazing sheep [97, 98].
Lasalocid has also been tested for its efficacy against ovine toxoplasmosis [99]. Ewes were treated PO with lasalocid (30 g/day) daily from day 55 of pregnancy until lambing and were PO inoculated with 100 T. gondii (TS-1 strain) oocysts 5 days after beginning lasalocid administration. Similar specific antibody titres and histopathological lesions were found in the ewes and foetuses, and there were no differences in the rate of abortion and neonatal mortality in both the treated and untreated ewes. These results suggest that lasalocid was not effective in preventing T. gondii abortion in sheep.
Concerning N. caninum infection in cattle, a risk factor analysis in dairy farms showed that cows receiving monensin as a feed additive were 1.5 times less likely to be infected with this parasite than the cows that did not receive monensin [100]. Thus, the effect of monensin was tested against experimental neosporosis in cattle [101]. Non-pregnant cows were treated with a slow-release bolus PO that delivered 100 days of monensin (335 mg/day) and were challenged with 5x106 N. caninum (Nc-1 strain) tachyzoites by the SC route three weeks after bolus administration. The cows treated with monensin showed a significantly lower humoral immune response than those treated with a placebo at week 4 post-challenge. Before recommendations on monensin use for neosporosis could be made, further research on other larger populations of cattle, preferably pregnant, must be explored. Furthermore, trials on monensin’s effectiveness in more natural modes of N. caninum transmission (e.g., bradyzoite recrudescence leading to transplacental transmission, or oocyst ingestion) would also be needed.
In summary, PO administered monensin was shown to be partially effective in the control of ovine toxoplasmosis, but it is not licensed for use in the EU and may be toxic at high dosages. In contrast, PO dosed lasalocid did not exhibit efficacy in reducing the ovine toxoplasmosis outcome. In N. caninum-infected non-pregnant cows, monensin was associated with a lower specific humoral immune response, but further research on the outcome of infection in pregnant animals is needed.
3.3. Folate Inhibitors
Folate inhibitors, such as sulphonamides and pyrimethamine, and their mechanism of action were identified years ago [102]. Sulphonamides are para-aminobenzoic acid analogues that competitively inhibit parasite Dihydropteroate Synthetase (DPHS). Pyrimethamine is a folate analogue that competitively inhibits parasite Dihydrofolate Reductase (DHFR). DHPS is one of the enzymes responsible for folate compound synthesis while DHFR maintains folate in a reduced state. Folate compounds are essential for parasite metabolism, including nucleoside biosynthesis and therefore nucleic acid formation. Sulphonamides, in combination with pyrimethamine, are a mainstay of chemotherapy in human toxoplasmosis [9].
Sulphonamides, such as sulphadiazine (IC50 of 2.5 µg/ml), were found to have important inhibitory effects on T. gondii. This inhibitory effect on parasite growth was associated with a reduction in the number of parasitized cells and intracellular parasites that were morphologically normal [103]. In contrast, sulphonamides demonstrate little activity against N. caninum tachyzoites at 100 µg/ml [90]. With DHFR inhibitors, a strong inhibition of T. gondii growth was observed with pyrimethamine (IC50 of 0.04 µg/ml) and trimethoprim (IC50 of 2.3 µg/ml), along with striking morphological changes in the parasites. Additionally, trimethoprim is effective against N. caninum tachyzoites at 10 µg/ml [90]. When sulphonamides and DHFR inhibitors were used in combination, a remarkable synergistic activity against the replicating forms of T. gondii and N. caninum was observed [103, 104]. Laboratory-induced resistance to antifolates has been described for T. gondii and N. caninum [104, 105]. In addition, it is also known that sulphonamide resistance in T. gondii can be associated with nucleotide polymorphism in DHPS [106]. When pyrimethamine and sulphadiazine were administered in combination to non-pregnant mice for 10 days from day 1 after intraperitoneal (IP) infection with T. gondii tachyzoites, 100% of the mice survived, and they were considered cured because the parasites remained undetectable [107]. Likewise, sulphadiazine is effective in preventing deaths and clinical disease in N. caninum infected mice [108].
Experimentally induced toxoplasmosis in pregnant ewes with 2,000 oocysts (M3 strain) at 89 days of pregnancy was treated with a combination of sulphamezathine and pyrimethamine sulphate for three consecutive days on days 100, 115 and 130 of gestation. Sulphamezathine (1 g per 3 ml of solution) was injected SC at an initial dose of 5 ml/10 kg on the first day, with subsequent doses on the following two days of 2.5 ml/10 kg. Pyrimethamine sulphate (10 mg/ml) was injected IP at 2 mg/kg on the first treatment day and at 1 mg/kg on the two subsequent days. After 130 days of gestation, 30% of foetuses from untreated ewes died, whereas all the lambs from the treated ewes were born and were viable. In addition, the gestational period of infected and untreated ewes was shorter than that observed in the infected and treated ewes. At birth, all the lambs showed a specific antibody response to the protozoa, indicating that the infection was in utero. However, the mean IFAT titres of lambs born from the infected and treated ewes were lower than those of the lambs born from the infected and untreated ewes (1/256 vs 1/730 for IgG and 1/665 vs 1/5206 for IgM). Histopathological examination showed less severe placental lesions in the lambs born from the treated ewes [109].
In field conditions, the therapeutic efficacy of sulphonamides in an ovine toxoplasmosis outbreak has been evaluated. Before treatment, 60% of the ewes in the flock had aborted, and treatment with sulphadimine at 20 mg/kg Intramuscularly (IM), 4 times every 48 hours, reduced the abortion rate to 25%; the rest of the ewes gave birth normally, but 61.1% of their lambs were stillbirths and did not survive. A higher dosage at 33 mg/kg IM, 4 times every 48 hours, reduced the abortion rate to 7%, and 75% of the lambs survived [110]. Folate inhibitors have also been evaluated in field conditions for the treatment of neosporosis in cattle. A combination of toltrazuril given Intravenously (IV) at 20 mg/kg/day for 3 consecutive days to newborn calves during the first week of age and sulphadiazine/trimethoprim given IV at 20 mg/kg to cattle once a year from 3 months of age along with dog treatment with toltrazuril and periodic disinfection of environment resulted in a significant reduction of abortion (from 188 to 9) [111].
In brief, parenteral administration of sulphonamides, in combination with pyrimethamine, could be a valuable option for chemotherapy of ovine toxoplasmosis in the third term of gestation, as a reduction in abortion rates, but not in the percentage of transplacental transmission, is observed. There is some evidence in field experiments that folate inhibitor administration can reduce abortions in ruminants, although more research is needed.
3.4. Quinolones
Quinolones were derived from quinine [112]. Decoquinate (6-ethyl-(decycloxy)-7-ethoxy-4-hydroxy-3-quinoli-necarboxylate) is a quinolone derivative coccidiostat that was initially developed as an anticoccidial for poultry in 1967 [113]. Decoquinate’s effects are mainly observed earlier in the life cycle of coccidia by acting on the sporozoites that are released from the ingested sporulated oocysts during the first day of their life cycle [114, 115]. Decoquinate significantly inhibits mitochondrial respiration and electron transport in Eimeria [116].
It has been used for over 20 years in the control of coccidiosis in domestic ruminants [117, 118]. Decoquinate is registered and commercialized for use in ruminants in multiple countries worldwide, including the USA and several countries in Latin America, Europe and the Middle East [119]. Decoquinate is poorly absorbed by the target species when administered PO and is largely eliminated unchanged in faeces. A recent study in cattle showed that only small quantities of decoquinate become systemically available and that the drug is rapidly eliminated with negligible residues in milk [120]. Therefore, decoquinate is in Annex II of the EU Council Regulation No.2377 ⁄ 90 [121], which means that it is not subject to maximum residue levels, with a meat withdrawal time of zero days in cattle and sheep in Europe [122].
Research has shown decoquinate to have in vitro activity against T. gondii tachyzoites with an IC50 of 0.005 µg/ml [85], although chemical mutagenesis revealed T. gondii resistant mutants [85, 123]. Likewise, intracellular N. caninum tachyzoites were killed quickly at 0.1 µg/ml [124].
In a study, sheep were challenged PO with 200 T. gondii (M3 strain) oocysts at 90 days of gestation, and decoquinate was administered PO at 1 or 2 mg⁄kg⁄day from 10 days prior to oocyst challenge until lambing [125]. The administration of decoquinate at the higher rate of 2 mg⁄kg⁄day was associated with a delayed onset of the febrile response to infection, reduction in the overall severity of fever and a delay in the production of specific antibodies to the parasite. This treatment also reduced the placental damage caused by the protozoa, lengthened the mean gestation period by five days and increased the proportion of viable lambs (up 61.8%) and the mean weight of the lambs (up 33.3%) in comparison with ewes that were not treated with decoquinate but were challenged with T. gondii oocysts.
In addition, it is necessary to note that the decoquinate-medicated feed distributed to pregnant heifers that are chronically infected or primo-infected with N. caninum at the dose of 2 mg/kg from 1.5 months of gestation until the end of the 8th month of pregnancy tends to reduce the associated abortions (from 38% to 21% in chronically infected heifers and from 17% to 6% in primo-infected heifers) and neonatal infections, as more seronegative calves were born (28% in the treated group vs 21% in the untreated group for chronically infected heifers and 59% in the treated group vs 35% in the untreated group for primo-infected heifers) [126].
To summarize, PO administration of decoquinate reduces the effect of experimentally induced toxoplasmosis in pregnant ewes. These results support the indication of decoquinate as an aid in the prevention of abortion due to ovine toxoplasmosis if used during mid and late pregnancy. Decoquinate may have an application in the treatment of bovine neosporosis.
3.5. Triazinones
The triazine structure is a heterocyclic ring, analogous to the six-membered benzene ring, but with three carbons replaced by nitrogen. The three isomers of triazine are distinguished from each other by the positions of their nitrogen atoms and are referred to 1,2,3-triazine, 1,2,4-triazine and 1,3,5-triazine [127].
Toltrazuril, a symmetric 1,3,5-triazine derivative, is metabolized into an active metabolite, toltrazuril sulphone (ponazuril). Both toltrazuril and ponazuril are effective against a broad spectrum of cyst-forming and non-cyst-forming apicomplexan protozoa [128]. Several studies have shown that toltrazuril and ponazuril affect the respiratory chain through the reduction of some enzymes of the respiratory chain, such as succinate-cytochrome C reductase, NADH oxidase and succinate oxidase. Second, the DHFR involved in pyrimidine synthesis is also affected, but the effect is weaker than that observed with pyrimethamine [129]. In addition, nuclear division is affected, mitochondrial activity is impaired, and the endoplasmic reticulum is vacuolized [130]. Additionally, differential phenotypic changes between the tachyzoites of N. caninum and T. gondii were observed after ponazuril treatment [131].
In vitro, toltrazuril has positive effects against T. gondii with an IC50 of 0.4 µg/ml [85, 132, 133]. Toltrazuril also shows in vitro efficacy against N. caninum, although a concentration of 30 µg/ml for 14 days is required to eliminate parasites [134], and no NcGRA2 expression is found, showing a lack of parasite viability after treatment [135]. Ponazuril administered prophylactically or after treatment in mice is effective in preventing acute toxoplasmosis based on a lack of mortality [133]. Toltrazuril and ponazuril administered in N. caninum-infected non-pregnant mice abrogate the formation of cerebral lesions, and 90% of the treated mice had N. caninum-DNA-free brains [136]. In addition, toltrazuril reduces foetal losses and transplacental transmission in N. caninum-infected pregnant mice [137], and it has an impact on the course of infection in congenitally N. caninum-infected newborn mice [138].
It has been emphasized that the production of T. gondii free lamb, sheep or goat meat for human consumption is critically important for public health [20]. The in vivo therapeutic efficacy of toltrazuril on T. gondii tissue cysts in experimentally infected lambs has been studied after a chronic infection in newborn lambs through a parenteral inoculation of 105 T. gondii (ME-49 strain) oocysts [139]. Beginning at the 15th day after inoculation, the lambs were treated with toltrazuril PO 2 times, once every week at a dose of 20 mg/kg and 40 mg/kg. Following toltrazuril treatment, at day 90 after inoculation, the specific immune humoral response in lambs of both treatment groups was lower. On day 90 after inoculation, the lambs were necropsied. The histopathological findings in the toltrazuril-treated lambs include morphologic and structural changes of the tissue cysts in the musculature, which were characterized by initial degenerative changes in the cyst wall and a minimal inflammatory cell response. The presence of T. gondii DNA in heart, brain and semitendinosus muscle from the treated groups was lower than that in tissues of the non-treated lambs. Moreover, in the treated groups, 4 out of 9 (44.4%) lambs did not contain any tissue cysts in the examined tissues, but untreated animals showed T. gondii tissue cysts at least in one of the sampled muscles. The administration of toltrazuril seems to be associated with a reduction of T. gondii cysts in the musculature.
It is doubtful that encysted N. caninum bradyzoites were susceptible to toltrazuril treatment. Congenitally infected calves born from Neospora-seropositive cows were PO treated with toltrazuril at 20 mg/kg three times at 48-hour intervals within 7 days after birth, following a humoral immune response [140]. Four to six months after birth, a stronger antibody reactivity was found in the treated animals than in the untreated calves. Conversely, in a study to evaluate whether the treatment of congenitally infected lambs with toltrazuril PO at 20 mg/kg on days 0, 7, 14 and 21 after birth eliminated N. caninum, toltrazuril did not show any effect on the reduction of N. caninum presence or on the severity of histopathological lesions, and the lambs were all seropositive, although they had significantly lower specific antibody levels than those in the untreated animals, suggesting higher antigenic stimulation in the non-treated lambs than in the treated lambs [141].
The efficacy of ponazuril has been tested in calves that were experimentally infected with N. caninum at 2x108 tachyzoites (Nc-1 strain) [142]. Medication was performed PO with 20 mg/kg of ponazuril. The first medication dose was applied 24 hours after infection, and if repeated, it was administered every subsequent 24 hours for six days. Ponazuril allows a complete abrogation of parasite DNA detection by PCR in the brain and other organs. Regarding the non-medicated calves, it is noteworthy that there was a relatively lower susceptibility of calves to experimental infection, since only 50% of the calves became PCR-positive in the brain and muscles. The efficacy of a six-day treatment was also suggested by the significantly lower anti-N. caninum antibody response and later seroconversion than those in the infected and non-medicated calves.
In addition, as explained above, toltrazuril administration to newborn calves, along with sulphadiazine/trimethoprim administration, resulted in reduced abortions and N. caninum seroprevalence in naturally infected cattle herds [111].
In summary, toltrazuril PO administered in lambs would be a valuable strategy to minimize human exposure to T. gondii tissue cysts from the consumption of raw or undercooked mutton. Triazinon derivatives are directed against an N. caninum tachyzoite challenge, whereas treatment of congenitally infected young ruminants remains an elusive goal. In addition, it would likely result in considerable unacceptable milk or meat residues or withdrawal periods [1].
4. Present approaches in drug development for ruminant toxoplasmosis and neosporosis
Experimental studies have revealed that several compounds have potentially interesting effects on T. gondii and N. caninum in vitro, but only a few drugs have been tested in laboratory animal models in vivo. The most interesting drugs tested for toxoplasmosis and neosporosis were derived from screenings in other intracellular protozoan parasites, including Plasmodium, Trypanosoma and Leishmania species, and some drugs exhibited broad-spectrum anti-parasitic activity against various protozoan and helminth parasites. However, it is notable that T. gondii was the least responsive to these sets of drugs, suggesting that it may be more difficult to target chemotherapeutically than the aforementioned parasites [143]. In addition, other approaches identified compounds that inhibited targets that were conserved almost exclusively within the group of apicomplexan parasites; thus, drug repurposing is a valuable option [49, 144]. The main prospective drug classes with in vitro and in vivo activity in small animal models against T. gondii and N. caninum are reviewed below (Table 3), although more chemotherapeutic options for toxoplasmosis and neosporosis have been described [11, 49].
Table 3.
Summary of published research, including in vitro and in vivo laboratory animal studies, on prospective drugs against toxoplasmosis and neosporosis.
| Drug | In Vitro Results | References | In Vivo Results from Small Animal Studies | References | |
|---|---|---|---|---|---|
| Class | Compounds | ||||
| Thiazolides | Nitazoxanide and nitazoxanide derivatives | Nitazoxanide and RM4847 showed activity against N. caninum. RM4847 is effective against T. gondii | [149, 150] | Nitazoxanide in mice PO is not effective in a mouse model of neosporosis and IP shows high toxicity | [56] |
| Diamidines | Di-cationic arylimidamides | DB786, DB750 and DB745 inhibit proliferation of T. gondii and N. caninum | [156-158] | DB750 and DB745 administered IP reduce mortality and brain parasite load in mouse neosporosis. | [56, 158] |
| Artemisinins | Artemisinin derivatives | Amongst all the tested artemisinin derivatives, artemisone and artemiside are the most potent against T. gondii. In addition, artemisone is also effective against N. caninum | [162, 164, 165, 166, 171, 172, 173, 174] | Artesunate-dihydroartemisinin (PO) showed a reduction in the burden of brain cysts and artemisone and artemiside (SC) are effective against acute, chronic and reactivated toxoplasmosis. Controversial results are found on the efficacy of artemisone in gerbil (IP) and mouse (PO) models of neosporosis |
[162, 166, 173, 175] |
| Naphthoquinones | Atovaquone | Active against T. gondii tachyzoites and, at high concentrations, also bradyzoites | [185-189] | Atovaquone PO is efficient for the treatment of acute and chronic toxoplasmosis. Additionally, atovaquone IV and PO treatment is effective against reactivated mouse toxoplasmosis | [185, 188, 189, 190, 191] |
| Buparvaquone | Slow inhibition of N. caninum tachyzoites and adaptation to high levels of buparvaquone | [192] | Buparvaquone PO or IP is effective in non-pregnant and pregnant mouse model of neosporosis | [175, 192] | |
| Anticancer drugs | Miltefosine | Efficacious against T. gondii extracellular tachyzoites and N. caninum tachyzoites proliferation | [200, 201] | Miltefosine PO reduces brain cyst burden and pathological lesions in chronic mouse toxoplasmosis. Moreover, miltefosine PO improves survival and reduces brain parasite burden in a mouse model of neosporosis | [200, 201] |
| Organometallic ruthenium complexes | Efficacious against T. gondii and N. caninum in the nanomolar range | [208, 209] | No studies available | ||
| Endochin-like quinolones | 4-(1H)-quinolone derivatives | Nanomolar concentrations of ELQ-271 and ELQ-316 against T. gondii and ELQ-400 against N. caninum were effective | [214, 217] | ELQ-271 and ELQ-316 were effective against acute toxoplasmosis in mice when administered PO and against the cyst form of T. gondii in mice when administered IP. ELQ-400 was effective PO in experimentally N. caninum infected non-pregnant mice | [214, 217] |
| Calcium-dependent protein kinase inhibitors | Bumped kinase inhibitors (BKIs) | BKI-1517, BKI-1294 and BKI-1553 showed efficacy against N. caninum and BKI-1294 is effective against T. gondii. | [230, 233, 234] | BKI-1294 and BKI-1517 PO led to good protection against vertical transmission in a pregnant mouse model of neosporosis. Additionally, BKI-1294 reduces T. gondii tachyzoites in an acute mouse model of toxoplasmosis and is effective in a murine vertical transmission model of T. gondii | [230-234] |
PO: per os. Oral administration.
SC: subcutaneously.
IP: intraperitoneally.
IV: intravenously.
4.1. Thiazolides
Nitazoxanide (2-acetolyloxy-N-(5-nitro 2-thiazolyl) benzamide), the mother compound of this class, is essentially composed of a nitrothiazole-ring and a salicylic acid moiety, which are linked together through an amide bond [145, 146]. Since nitazoxanide non-selectivity can lead to undesired side effects in both human and animals, nitazoxanide derivatives were designed without the undesirable nitro group [147]. The nitazoxanide derivative RM4847 produces the upregulation of NQO1 (quinone reductase) expression by N. caninum but not by T. gondii. This fact may reflect differences between these parasites in terms of the mechanisms of circumventing host cell apoptosis [148]. Thiazolides have favourable effects against N. caninum in vitro, with an IC50 of 4.23 µM and 13.68 µM for nitazoxanide and RM4847, respectively. Host cell invasion of extracellular N. caninum tachyzoites is inhibited by RM4847, but not by nitazoxanide [149], and RM4847 was shown to be much more effective against T. gondii tachyzoites, with an IC50 of 0.2 µM [150]. For the treatment of N. caninum in mice, nitazoxanide fails when it is applied PO, or it is even toxic when applied IP [56]. In calves, the use of nitazoxanide to treat Cryptosporidium infection showed acute diarrhoea in non-infected animals, which indicates that this compound severely affects the intestinal flora [151]. In conclusion, thiazolides might be interesting tools to study the biology of N. caninum and possibly T. gondii, but they are useless as drugs since they exert acute toxicity in small and large animals.
4.2. Diamidines
Diamidines represent a class of broad-spectrum antimicrobial compounds in which pentamidine and its analogues exhibit activity against intracellular and extracellular protozoan parasites [152-154]. Pentamidine displays in vitro activity against T. gondii by inhibiting the replication of the parasites in cell cultures [155].
Pentamidine derivatives, and the more recently synthesised di-cationic arylimidamides, which have more favourable pharmacokinetic profiles, improved bioavailability, lowered host toxicity and had a higher chance of passing the blood-brain barrier to exert its activity by binding to AT-rich sites in the DNA minor groove, thus inhibiting transcription or the interaction with DNA-binding enzymes, such as topoisomerases or nucleases [154]. This result indicates that these compounds could influence gene expression, and thus many diverse cellular functions could be affected. Di-cationic arylimidamides, such as DB786, DB750 and DB745, showed in vitro activity against T. gondii tachyzoites, with an IC50 of 0.22 µM, 0.16 µM and 0.03 µM, respectively [156, 157]. In contrast to DB750, DB745 also had a profound negative impact on extracellular T. gondii tachyzoites. In addition, a lower adaptation of T. gondii tachyzoites to DB745 was observed [157]. The lowest IC50s against N. caninum tachyzoites were found for DB786, DB750 and DB745 (0.21 µM, 0.23 µM, 0.08 µM, respectively), which caused damage to the parasite’s ultrastructure. The activities of DB750 and DB786 are limited to intracellular N. caninum tachyzoites; however, DB745 had an impact on both host cell invasion and intracellular proliferation [156, 158]. Di-cationic arylimidamides have also been found to be effective against neosporosis in mice. DB750 that was administered IP prior to infection or 14 days after infection reduced the severity of clinical signs and the cerebral parasite load, while PO application resulted in weight loss, indicating toxicity [56, 158]. In addition, DB745 IP treatment initiated 14 days after infection had similar positive effects on the percentage of surviving mice and the parasite load in the brain [158]. Arylimidamide treatments in mice beginning 14 days post-infection, after the N. caninum tachyzoites had crossed the blood-brain border and invaded the Central Nervous System (CNS) [159], indicated that DB745, just like DB750, most likely crossed the blood–brain barrier and also exerted its action within the cerebral tissues [56, 158]. Potentially, features of DB745 could open the door for testing this compound against neosporosis in ruminants.
4.3. Artemisinins
Chinese herbal extracts are thought to possess the desired properties of potency and low toxicity, and they show promise for the identification of new therapeutic agents. Artemisinin, a sesquiterpene lactone with an unusual endoperoxide bond in a unique 1,2,4-trioxane heterocycle [3R,5aS,6R,8aS, 9R,12S,12aR)-octahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10(3H)-one] is present in the leaves and flowers of the sweet wormwood (Artemisia annua) [160], and its derivatives are highly potent antimalarial drugs. This safe drug class is also effective against apicomplexan parasites causing abortions in ruminants such as T. gondii and N. caninum. The mechanism of action of artemisinin and its derivatives is dependent upon the presence of the endoperoxide bridge [161], although the difference in potencies between P. falciparum and T. gondii suggests that different targets may be affected in these two organisms [162]. Additionally, it is known that artemisinin perturbs calcium homeostasis in T. gondii, supporting the idea that Ca2-ATPases (SERCA) are potential drug targets in parasites [163]. Artemisinin, when administered in vitro at 0.4 µg/ml for 5 days or 1.3 µg/ml for 14 days, completely eliminated T. gondii [164]. Artesunate showed an IC50 of 0.075 μM [165]. Artesunate and its active metabolite, dihydroartemisinin, resulted in approximately 40% and 70% growth inhibition in vitro, respectively, and the combination resulted in approximately 65% inhibition. As they are able to cross the blood-brain barrier, in vivo experiments with low virulence T. gondii (DUR strain) challenge causing a chronic infection in mice, different from previous artemisinin derivatives studies with RH strain, showed a 40% reduction in the number of T. gondii tissue cysts found in the brain of mice treated 5 days with artesunate-dihydroartemisinin and also modifications in the microscopic aspect of the cysts [166]. Artemisone, a second-generation semi-synthetic artemisinin derivative, and artemiside, the thiomorpholine precursor of artemisone, with an improved half-live, oral bioavailability, metabolic stability in various animal systems [167], tolerance in vivo [168] and a lack of detectable neurotoxic potential [169, 170], are the most potent artemisinin analogues to date in terms of inhibiting the growth of T. gondii in vitro, with an IC50 of 0.12 µM and 0.10 µM for artemisone and artemiside, respectively. Both of these compounds (artemiside and artemisone) were effective in reducing mortality during an acute challenge (60% of artemiside-treated mice and more than 50% of artemisone-treated mice survived) and during the reactivation of chronic infection in a mouse model (80% of artemiside-treated mice and 60% of artemisone-treated mice). Furthermore, there was an accompanying reduction in the chronic burden of tissue cysts in the CNS [162].
Against N. caninum, artemisinin reduces the intracellular multiplication of tachyzoites at ≥0.1 µg/ml for 30 hours or 1 µg/ml for 14 days [171], and artemether exhibited activity against tachyzoite replication with an IC50 of 1.0 µg/ml [172]. Additionally, artemisone, when added prior to infection or in established infection, reduced the number of N. caninum-infected cells [173] with an IC50 of 3 nM, exerting their activity against intracellular parasites, causing ultrastructural alterations and switched aberrant gene expression, including bradyzoite markers [174]. In a gerbil model for acute neosporosis, artemisone increased survival, as 1 out of 8 mice died in the artemisone-treated group, while 8 out 9 mice succumbed in the control group [173]. However, in a mouse model of neosporosis, artemiside and artemisone had no effect on parasite loads in the brain or in the lungs [175]. In short, artemiside seems to be useful against T. gondii in vitro and in mice models whereas artemisone is less likely to be promising for neosporosis in ruminants.
4.4. Naphthoquinones
Naphthoquinone is a class of organic compounds derived from naphthalene. Buparvaquone (2-((4-tert-butylcyclohexyl)methyl)-3-hydroxy-1,4-naphthoquinone) and atovaquone (trans-2[4-(4-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthalenedione) are hydroxynaphthoquinones related to parvaquone and were originally developed as anti-malarial compounds [176, 177].
Buparvaquone is currently commercially available for use in endemic regions against theileriosis in cattle [178]; however, in other regions of the world, e.g., the EU, it is not registered. A focus on the parasite mitochondrion as an anti-parasitic target has been a priority in the drug development field for decades [179, 180]. Hydroxynaphthoquinones are structurally similar to the inner mitochondrial protein ubiquinone (also called coenzyme Q), which is an integral component of electron flow in aerobic respiration. Ubiquinone accepts electrons from the dehydrogenase enzymes and passes them to the electron transport cytochromes [181]. The passage of electrons from ubiquinone to the cytochrome bc1 (complex III) requires the binding of coenzyme Q complex III at the Qo cytochrome domain; it is this step that is inhibited by hydroxynaphthoquinones [123, 182, 183]. The consequence of this inhibition is the collapse of the mitochondrial membrane potential [184]. On the one hand, atovaquone inhibited the replication of T. gondii tachyzoites in vitro with an IC50 of 64 nM [185, 186]. Moreover, atovaquone is active in vitro against the cyst stage of T. gondii [187], although high concentrations of it are required [188, 189]. In a mouse model of acute toxoplasmosis, atovaquone administration resulted in prolonged survival, with a reduction of parasite burdens in the blood and tissues during the course of treatment [185, 188]. In a mouse model of chronic toxoplasmosis, atovaquone showed a decline in the number of tissue cysts and a decrease in the inflammatory response in the brain [188-190]. Indeed, atovaquone is effective against reactivation, as it protected mice against reactivated toxoplasmic encephalitis [191].
In short term studies, buparvaquone efficiently inhibited the replication of N. caninum tachyzoites with an IC50 of 4.9 nM exerting parasiticidal activity after 9 days of culture in 0.5 μM or 6 days in 1 μM buparvaquone. However, in the long-term studies, the tachyzoites reached an adaption to high levels of buparvaquone. Additionally, ultrastructural changes confirm that buparvaquone acted rather slowly [192]. In a non-pregnant mouse model of neosporosis, buparvaquone reduced the mortality and parasite load in the lungs, while the brain parasite load was higher than in untreated mice [192]; the brain parasite load was lower when a 20-times reduced challenge dose was used [175]. In addition, in a pregnant mouse model of neosporosis, pup mortality and the transmission of N. caninum to offspring were strongly reduced by buparvaquone treatment of the dams [175]. In light of these results, atovaquone represents a valuable candidate to be tested for toxoplasmosis in ruminants and, although further studies are required to improve the efficacy in a mouse model, buparvaquone represents an obvious candidate to be tested against neosporosis in ruminants.
4.5. Anticancer Agents
Another example of prospective compounds that provide interesting results is anticancer drugs, as parasites have several of the common characteristics of malignant tumours [193, 194], sharing a crucial feature of living and multiplying in a host organism. Tumour cells are defined by their independence from exogenous growth factors, their resistance to programmed cell death (apoptosis), and their infinite proliferative capacity. Unlimited proliferation and independence of growth factors are also characteristics of many parasites. Although it remains controversial whether apoptosis occurs in unicellular parasites [195], it seems to be clear that intracellular parasites interfere with the programmed host cell apoptosis [196]. Parasite and cancer cells disseminate in immune compromised tissues in order to escape host immune responses. Anticancer drugs may affect parasite survival at two completely different levels. Firstly, they might kill the parasite directly, if the target molecules of parasite and cancer cell are sufficiently similar. In this case, the original cancer drugs may serve as leader compounds and can be modified accordingly to specifically inhibit the parasite homologue. Secondly, to kill intracellular parasites successfully, the drug might also act on a host cell signalling pathway, which is essential for the parasite's survival. The advantage here is that the drug need not be modified, since it is already directed against the target molecule [194].
Miltefosine (2-[hexadecoxy-oxido-phosphinoyl] oxy-ethyl-trimethyl-ammonium), an alkyl phospholipid and an analogue of the ubiquitous compound phosphatidyl choline found in eukaryotic cell membranes, was initially developed as an anticancer agent [197] and widely used for Leishmania therapy [198]. It is highly active against extracellular T. gondii tachyzoites and exerts its activity by triggering apoptosis [199]. Miltefosine has no effect on a mouse model of acute toxoplasmosis after 5 days of treatment, but it shows activity in a 15-day treatment against chronic experimental toxoplasmosis, with a 78% reduction in the brain cyst burden. Pathological findings showed that the tissue cysts were smaller in size upon microscopical examination and that there were ultrastructural changes in the remaining cysts, suggesting that miltefosine effectively penetrates the blood-brain barrier [200]. Against N. caninum, miltefosine showed an IC50 of 5.2 μM in vitro with a parasitostatic effect at 25 μM for 10 hours and parasiticidal activity after 20 hours. In addition, N. caninum tachyzoites revealed ultrastructural changes after miltefosine exposure. In a mouse model of neosporosis, miltefosine improved mouse survival and reduced the cerebral parasite burden [201].
Recently, organometallic ruthenium complexes are object of great attention as antitumor agents with acceptable toxicity [202, 203]. They also have antibacterial activity against some bacteria and parasites [204, 205]. Earlier studies indicated that ruthenium compounds interact with DNA [206], but more recent investigations showed that ruthenium compounds bind more strongly to proteins [207]. Ruthenium compounds (compounds 16 and 18) were reported to exhibit IC50 values of 6-12 nM for N.caninum and 18-41 nM for T.gondii [208]. Furthermore, dinuclear thiolato-bridged arene ruthenium complexes (complex 9, complex 1 and complex 2) showed promising activities against T. gondii with IC50 values of 1.2 nM, 34 nM and 62 nM [209]. Therefore, anticancer drug such as miltefosine could be considered as an appropriate drug to be evaluated in ruminant model for toxoplasmosis and neosporosis whereas ruthenium complexes need to be evaluated in mice models before contemplate the option of testing in ruminants.
4.6. Endochin-like Quinolones
Endochin is a 4-(1H)-quinolone initially investigated as an antimalarial drug [210]. Subsequently, endochin was active against avian and murine toxoplasmosis [211]. Recent 4-(1H)-quinolone derivatives, Endochin-Like Quinolones (ELQ), compounds that are analogs of ubiquinone, have been developed and tested against apicomplexan parasites such as P. falciparum and T. gondii, as well as other protozoa such as Leishmania parasites [212]. ELQs exert their activity by inhibit cytochrome c reduction by the cytochrome bc1 complex [213, 214]. The cytochrome bc1 complex is a membrane-bound enzyme complex located in the inner mitochondrial membrane that contributes to pyrimidine biosynthesis and oxidative phosphorylation [215]. 3-alkyl-2-methyl-4(1H)-quinolones exhibited excellent in vitro activity against P. falciparum [216]. 4(1H)-quinolone-3-diarylethers, with improved stable and solubility properties, have been tested against T. gondii and ELQ-271 and ELQ-316 showed in vitro IC50 values of 0.1 nM and 0.007 nM, respectively. ELQ-271 and ELQ-316 were also efficacious against acute toxoplasmosis in mice when administered orally and against the cyst form of T. gondii in mice when administered intraperitoneally [214]. Against N. caninum, ELQ-400 showed an IC50 value below 10 nM, had an impact on intracellular proliferation of tachyzoites and transmission electron microscopy showed that the primary target of ELQ-400 was the mitochondrion. In experimentally infected non-pregnant mice, ELQ-400 orally showed a reduction in the number of animals with lung and brain infection, as well as a reduction in the humoral immune response against N. caninum [217]. Thus, ELQ-316 is a promising starting point for the development of a future toxoplasmosis therapy in ruminants, and ELQ-400 showed hopeful results against N. caninum, but further studies are needed to assess efficacy in pregnant animal models.
4.7. Calcium-dependent Protein Kinase Inhibitors
Calcium signalling is a very important pathway that regulates diverse cellular processes [218]. In apicomplexan parasites, this signalling pathway directs motility, cell invasion and egression [219]. Members of the family of Calcium Dependent Protein Kinases (CDPK’s) are abundant in certain pathogenic parasites and absent in mammalian cells making them strong drug target candidates. Genetic disruption of CDPKs has shown they control a wide range of phenotypes in T. gondii including egress (TgCDPK1 and TgCDPK3) [219-222], microneme secretion (TgCDPK1) [219], motility (TgCDPK1) [220], or cell division (TgCDPK7) [223]. Other CDPKs in T. gondii (CDPK4, CDPK4A, CDPK5, CDPK6, CDPK8, and CDPK9) were described non essential for the lytic cycle [224]. CDPKs may are also involved in other functions in T. gondii, for example, CDPK2 is essential for bradyzoite development [225] and CDPK4A, CDPK6 and CDPK7A may play roles during sporozoite formation or transmission via oocysts [226].
Bumped kinase inhibitors (BKIs), a particular class of pyrazolopyrimidine inhibitors of CDPK1, have bulky C3 aryl moieties entering a hydrophobic pocket in the ATP binding site. BKIs selectively inhibit CDPK1 from apicomplexans in a good structure-activity relationship [227], but they do not inhibit mammalian kinases because they have larger amino acid residues adjacent to the hydrophobic pocket, thereby blocking the entry of the bulky C3 aryl group. CDPK1 is found in most apicomplexans, and the highly conserved nature of the ATP binding domain shared by apicomplexan CDPK homologues could be exploited, to some extent, in the development of potential broad-spectrum inhibitors [227]. BKIs were originally developed to combat malaria [228], but they have been tested for many apicomplexan parasites [229]. BKI-1294 acted with an IC50 of 20-220 nM in vitro for different strains of T. gondii [230]. BKI-1294 was highly effective against acute toxoplasmosis in mice, decreasing the numbers of T. gondii tachyzoites in the peritoneal lavage fluid [231] and in a murine vertical transmission model of T. gondii [232].
BKI-1517 showed a 3-times lower IC50 than BKI-1553 and BKI-1294 against N. caninum in vitro [233, 234]. In a pregnant mouse model, BKI-1294, BKI-1517 and, less clearly, BKI-1553 achieved protection against the vertical transmission of N. caninum, but BKI-1553 and more markedly BKI-1517 showed detrimental effects on fertility [230, 234]. Hence, BKI-1294 could be considered a promising drug to be tested in ruminant models for toxoplasmosis and neosporosis.
Since BKIs showed parasitostatic rather than parasiticidal effects, they are appropriated for a combined immunization plus treatment protocol. These compounds triggered the formation of relatively long-lived multinucleated complexes where parasites are blocked in the process of cytokinesis and remain trapped within the host cell but are still viable. These multinucleated complexes Express Specific Antigen (SAG1) and the Bradyzoite Marker (BAG1) [230, 234]. Thereby, these antigens may be presented to the immune system eliciting stable immune responses against tachyzoite as well as bradyzoite stages.
Apart from CDPK1, the rarity of kinases containing small gatekeeper residues in the apicomplexan genome reduces the chance of off-target effects, although intermediate sensitivity is expected for kinases containing A, S, or T in the gatekeeper position, thus potentially limiting this approach in some cases. However, an interesting strategy based on the exploitation of the kinase gatekeeper residue was recently developed to identify parasite CDPKs substrates [235].
CONCLUSION
Although vaccination has been considered the better control option for ovine and caprine toxoplasmosis and bovine neosporosis, drug therapy should be a viable approach, possibly when combined in an immune-chemotherapy strategy. A large number of experimental efficacy drug studies against T. gondii and N. caninum infections have been carried out so far in mouse models, but studies in farm ruminants are scarce, showing a lack of efficacy. Regarding toxoplasmosis, several drug treatments that were applied before (spiramycin or sulphonamides and pyrimethamine) or after infection (monensin or decoquinate) have been carried out in pregnant ewes to reduce the outcome of ExTT after experimental infection of the ewes at the mid-stage of pregnancy. Sulphonamides are also useful in field conditions to reduce T. gondii-related abortions, and toltrazuril is applied to chronically infected lambs to reduce tissue cysts. Concerning neosporosis, the triazinones administered in infected newborn calves or lambs modulate the humoral immune response and reduce the clinical signs and N. caninum detection in organs. In addition, triazinones, along with folate inhibitors, can reduce abortions due to neosporosis under natural conditions. Finally, monensin seems to reduce the humoral immune response in non-pregnant cattle, and decoquinate could decrease abortions and transplacental transmission in chronically and primo-infected pregnant heifers. Despite these efforts, no safe and effective drug is available for toxoplasmosis and neosporosis in farm ruminants at present, so new approaches in drug development are needed. These new developments should also focus on the therapy of toxoplasmosis in humans. Highly promising future drugs against T. gondii and N. caninum have been tested in vitro and in small animal models; most of them target tachyzoites efficiently, but they are missing information regarding their efficacy against the bradyzoite stage. Best results have been reported with artemiside, atovaquone, miltefosine, ELQ-316 and BKI-1294 against T. gondii and with DB745, buparvaquone, miltefosine, ELQ-400 and BKI-1294 against N. caninum.
ACKNOWLEDGEMENTS
Roberto Sánchez Sánchez is supported by a fellowship from the Spanish Ministry of Education, Culture and Sports (MECD), as a part of the Program of Training of University Teaching Staff (FPU, grant number FPU13/03438). Patricia Vázquez has a Juan de la Cierva-Formación post-doctoral contract (FJCI-2014-20982) from the Spanish Ministry of Economy and Competitiveness (MINECO). Also, Public Health Service, National Institutes of Health, Bethesda, MD (grants R01 AI 111341 and R01 HD 080670), the U.S. Department of Agriculture (grant 2014-67015-22106) and the Community of Madrid, Spain (PLATESA, S2013/ABI2906) are acknowledged for their financial support.
Consent for Publication
Not applicable.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest. This work was supported by the National Institutes of Child Health and Human Development (NICHD, grants R01 AI 111341 and R01 HD 080670), the United States Department of Agriculture (USDA, grant 2014-67015-22106), the Community of Madrid (PLATESA grant No. S2013/ABI2906) and the Iberoamerican Science and Technology Programme for Development (CYTED, Grant No. 113RT0469). Roberto Sánchez Sánchez was financially supported through a grant from the Spanish Ministry of Education, Culture and Sports (grant No. FPU13/03438). Patricia Vázquez was supported by the postdoctoral Juan de la Cierva-Formación program of the Spanish Ministry of Economy and Competitiveness (grant No. FJCI-2014-20982).
REFERENCES
- 1.Dubey J.P., Schares G., Ortega-Mora L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007;20:323–367. doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey J. Toxoplasmosis in sheep-the last 20 years. Vet. Parasitol. 2009;163:1–14. doi: 10.1016/j.vetpar.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Reichel M.P., Ayanegui-Alcérreca M.A., Gondim L.F.P., Ellis J.T. What is the global economic impact of Neospora caninum in cattle – The billion dollar question. Int. J. Parasitol. 2013;43:133–142. doi: 10.1016/j.ijpara.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Innes E.A., Bartley P.M., Buxton D., Katzer F. Ovine toxoplasmosis. Parasitology. 2009;136:1887–1894. doi: 10.1017/S0031182009991636. [DOI] [PubMed] [Google Scholar]
- 5.Trees A.J., Davison H.C., Innes E.A., Wastling J.M. Towards evaluating the economic impact of bovine neosporosis. Int. J. Parasitol. 1999;29:1195–1200. doi: 10.1016/s0020-7519(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 6.Castaño P., Fuertes M., Regidor-Cerrillo J., Ferre I., Fernández M., Ferreras M.C., Moreno-Gonzalo J., González-Lanza C., Pereira-Bueno J., Katzer F. Experimental ovine toxoplasmosis: Influence of the gestational stage on the clinical course, lesion development and parasite distribution. Vet. Res. (Faisalabad) 2016;47:1. doi: 10.1186/s13567-016-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivier A., Herbert B., Sava B., Pierre C., John D., Aline D. Surveillance and monitoring of Toxoplasma in humans, food and animals: A scientific opinion of the panel on biological hazards. EFSA. 2007;583:1–64. [Google Scholar]
- 8.Halsby K., Walsh A., Smith R., Said B., Kirkbride H., Smyth B., Browning L., Larkin L., Morgan D. The health burden of orphan zoonotic disease in the United Kingdom, 2005-2009. Zoonoses Public Health. 2014;61:39–47. doi: 10.1111/zph.12040. [DOI] [PubMed] [Google Scholar]
- 9.Montoya J.G., Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 10.Flegr J., Prandota J., Sovičková M., Israili Z.H. Toxoplasmosis–a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One. 2014;9:e90203. doi: 10.1371/journal.pone.0090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neville A.J., Zach S.J., Wang X., Larson J.J., Judge A.K., Davis L.A., Vennerstrom J.L., Davis P.H. Clinically available medicines demonstrating anti-toxoplasma activity. Antimicrob. Agents Chemother. 2015;59:7161–7169. doi: 10.1128/AAC.02009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mugridge N.B., Morrison D.A., Heckeroth A.R., Johnson A.M., Tenter A.M. Phylogenetic analysis based on full-length large subunit ribosomal RNA gene sequence comparison reveals that Neospora caninum is more closely related to Hammondia heydorni than to Toxoplasma gondii. Int. J. Parasitol. 1999;29:1545–1556. doi: 10.1016/s0020-7519(99)00150-2. [DOI] [PubMed] [Google Scholar]
- 13.Speer C.A., Dubey J.P., McAllister M.M., Blixt J.A. Comparative ultrastructure of tachyzoites, bradyzoites, and tissue cysts of Neospora caninum and Toxoplasma gondii. Int. J. Parasitol. 1999;29:1509–1519. doi: 10.1016/s0020-7519(99)00132-0. [DOI] [PubMed] [Google Scholar]
- 14.Reid A.J., Vermont S.J., Cotton J.A., Harris D., Hill-Cawthorne G.A., Konen-Waisman S., Latham S.M., Mourier T., Norton R., Quail M.A., Sanders M., Shanmugam D., Sohal A., Wasmuth J.D., Brunk B., Grigg M.E., Howard J.C., Parkinson J., Roos D.S., Trees A.J., Berriman M., Pain A., Wastling J.M. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog. 2012;8:e1002567. doi: 10.1371/journal.ppat.1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubey J.P., Lindsay D.S., Speer C.A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemphill A., Gottstein B. Neospora caninum and neosporosis - recent achievements in host and parasite cell biology and treatment. Acta Parasitol. 2006;51:15–25. [Google Scholar]
- 17.Robert-Gangneux F., Darde M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012;25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubey J., Schares G. Neosporosis in animals-the last five years. Vet. Parasitol. 2011;180:90–108. doi: 10.1016/j.vetpar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Dubey J. 1993.
- 20.Kijlstra A., Jongert E. Toxoplasma-safe meat: Close to reality? Trends Parasitol. 2009;25:18–22. doi: 10.1016/j.pt.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Jones J., Dubey J. Waterborne toxoplasmosis–recent developments. Exp. Parasitol. 2010;124:10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Hussain M.A., Stitt V., Szabo E.A., Nelan B. Toxoplasma gondii in the Food Supply. Pathogens. 2017;6:21. doi: 10.3390/pathogens6020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall C.A., Reichel M.P., Ellis J.T. Neospora abortions in dairy cattle: Diagnosis, mode of transmission and control. Vet. Parasitol. 2005;128:231–241. doi: 10.1016/j.vetpar.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 24.McCann C.M., McAllister M.M., Gondim L.F., Smith R.F., Cripps P.J., Kipar A., Williams D.J., Trees A.J. Neospora caninum in cattle: Experimental infection with oocysts can result in exogenous transplacental infection, but not endogenous transplacental infection in the subsequent pregnancy. Int. J. Parasitol. 2007;37:1631–1639. doi: 10.1016/j.ijpara.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Williams D.J., Hartley C.S., Bjorkman C., Trees A.J. Endogenous and exogenous transplacental transmission of Neospora caninum - how the route of transmission impacts on epidemiology and control of disease. Parasitology. 2009;136:1895–1900. doi: 10.1017/S0031182009990588. [DOI] [PubMed] [Google Scholar]
- 26.Davison H.C., Otter A., Trees A.J. Significance of Neospora caninum in British dairy cattle determined by estimation of seroprevalence in normally calving cattle and aborting cattle. Int. J. Parasitol. 1999;29:1189–1194. doi: 10.1016/s0020-7519(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 27.Trees A.J., Williams D.J.L. Endogenous and exogenous transplacental infection in Neospora caninum and Toxoplasma gondii. Trends Parasitol. 2005;21:558–561. doi: 10.1016/j.pt.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Ortega-Mora L.M., Fernández-García A., Gómez-Bautista M. Diagnosis of bovine neosporosis: Recent advances and perspectives. Acta Parasitol. 2006;51:1–14. [Google Scholar]
- 29.Bergeron N., Fecteau G., Pare J., Martineau R., Villeneuve A. Vertical and horizontal transmission of Neospora caninum in dairy herds in Quebec. Can. Vet. J. 2000;41:464–467. [PMC free article] [PubMed] [Google Scholar]
- 30.French N.P., Clancy D., Davison H.C., Trees A.J. Mathematical models of Neospora caninum infection in dairy cattle: Transmission and options for control. Int. J. Parasitol. 1999;29:1691–1704. doi: 10.1016/s0020-7519(99)00131-9. [DOI] [PubMed] [Google Scholar]
- 31.Bartels C.J., Huinink I., Beiboer M.L. van, S.G.; Wouda, W.; Dijkstra, T.; Stegeman, A. Quantification of vertical and horizontal transmission of Neospora caninum infection in Dutch dairy herds. Vet. Parasitol. 2007;148:83–92. doi: 10.1016/j.vetpar.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Dubey J.P., Buxton D., Wouda W. Pathogenesis of bovine neosporosis. J. Comp. Pathol. 2006;134:267–289. doi: 10.1016/j.jcpa.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Buxton D., McAllister M.M., Dubey J.P. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002;18:546–552. doi: 10.1016/s1471-4922(02)02414-5. [DOI] [PubMed] [Google Scholar]
- 34.Buxton D., Finlayson J. Experimental infection of pregnant sheep with Toxoplasma gondii: Pathological and immunological observations on the placenta and foetus. J. Comp. Pathol. 1986;96:319–333. doi: 10.1016/0021-9975(86)90052-6. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay D.S., Rippey N.S., Powe T.A., Sartin E.A., Dubey J.P., Blagburn B.L. Abortions, fetal death, and stillbirths in pregnant pygmy goats inoculated with tachyzoites of Neospora caninum. Am. J. Vet. Res. 1995;56:1176–1180. [PubMed] [Google Scholar]
- 36.Collantes-Fernández E., Rodríguez-Bertos A., Arnaiz-Seco I., Moreno B., Aduriz G., Ortega-Mora L.M. Influence of the stage of pregnancy on Neospora caninum distribution, parasite loads and lesions in aborted bovine foetuses. Theriogenology. 2006;65:629–641. doi: 10.1016/j.theriogenology.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Arranz-Solis D., Benavides J., Regidor-Cerrillo J., Fuertes M., Ferre I., Ferreras Mdel C., Collantes-Fernandez E., Hemphill A., Perez V., Ortega-Mora L.M. Influence of the gestational stage on the clinical course, lesional development and parasite distribution in experimental ovine neosporosis. Vet. Res. (Faisalabad) 2015;46:19. doi: 10.1186/s13567-014-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porto W.J.N., Regidor-Cerrillo J., Kim P.C.P., Benavides J., Silva A.C.S., Horcajo P., Oliveira A.A.F., Ferre I., Mota R.A., Ortega-Mora L.M. Experimental caprine neosporosis: The influence of gestational stage on the outcome of infection. Vet. Res. (Faisalabad) 2016;47:1. doi: 10.1186/s13567-016-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubey J.P. Repeat transplacental transfer of Toxoplasma gondii in dairy goats. J. Am. Vet. Med. Assoc. 1982;180:1220–1221. [PubMed] [Google Scholar]
- 40.Thurmond M.C., Hietala S.K. Effect of congenitally acquired Neospora caninum infection on risk of abortion and subsequent abortions in dairy cattle. Am. J. Vet. Res. 1997;58:1381–1385. [PubMed] [Google Scholar]
- 41.Pereira-Bueno J., Quintanilla-Gozalo A., Pérez-Pérez V., Espi-Felgueroso A., Álvarez G.G., Collantes-Fernández E., Ortega-Mora L.M. Evaluation by different diagnostic techniques of bovine abortion associated with Neospora caninum in Spain. Vet. Parasitol. 2003;111:143–152. doi: 10.1016/s0304-4017(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 42.Quintanilla-Gozalo A., Pereira-Bueno J., Seijas-Carballedo A., Costas E., Ortega Mora L.M. Observational studies in Neospora caninum infected dairy cattle: Relationship infection-abortion and gestational antibody fluctuations. Int. J. Parasitol. 2000;30:900–906. [Google Scholar]
- 43.Pereira-Bueno J., Quintanilla-Gozalo A., Seijas-Carballedo A., Costas E., Ortega Mora L.M. Observational studies in Neospora caninum infected dairy cattle: Pattern of transmission and age-related antibody fluctuations. Int. J. Parasitol. 2000;30:906–909. [Google Scholar]
- 44.De M.F., Focant C., Detry J., Rettigner C., Cassart D., Losson B. Clinical, pathological and diagnostic aspects of congenital neosporosis in a series of naturally infected calves. Vet. Rec. 2005;157:115–118. doi: 10.1136/vr.157.4.115. [DOI] [PubMed] [Google Scholar]
- 45.Thurmond M.C., Hietala S.K. Culling associated with Neospora caninum infection in dairy cows. Am. J. Vet. Res. 1996;57:1559–1562. [PubMed] [Google Scholar]
- 46.Reichel M.P., McAllister M.M., Pomroy W.E., Campero C., Ortega-Mora L.M., Ellis J.T. Control options for Neospora caninum-is there anything new or are we going backwards? Parasitology. 2014;141:1455–1470. doi: 10.1017/S0031182014000158. [DOI] [PubMed] [Google Scholar]
- 47.McAllister M.M. Diagnosis and Control of Bovine Neosporosis. Vet. Clin. North Am. Food Anim. Pract. 2016;32(2):443–463. doi: 10.1016/j.cvfa.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Zhang N., Chen J., Wang M., Petersen E., Zhu X. Vaccines against Toxoplasma gondii: New developments and perspectives. Expert Rev. Vaccines. 2013;12:1287–1299. doi: 10.1586/14760584.2013.844652. [DOI] [PubMed] [Google Scholar]
- 49.Hemphill A., Aguado-Martínez A., Müler J. Approaches for the vaccination and treatment of Neospora caninum infections in mice and ruminants models. Parasitology. 2016;143(2):245–259. doi: 10.1017/S0031182015001596. [DOI] [PubMed] [Google Scholar]
- 50.Horcajo P., Regidor C.J., Aguado M.A., Hemphill A., Ortega Mora L.M. Vaccines for bovine neosporosis: Current status and key aspects for development. Parasite Immunol. 2016;38:709–723. doi: 10.1111/pim.12342. [DOI] [PubMed] [Google Scholar]
- 51.Verma R., Khanna P. Development of Toxoplasma gondii vaccine: A global challenge. Hum. Vaccin. Immunother. 2013;9:291–293. doi: 10.4161/hv.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reichel M.P., Ellis J.T. If control of Neospora caninum infection is technically feasible does it make economic sense? Vet. Parasitol. 2006;142:23–34. doi: 10.1016/j.vetpar.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 53.Häsler B., Regula G., Stark K.D., Sager H., Gottstein B., Reist M. Financial analysis of various strategies for the control of Neospora caninum in dairy cattle in Switzerland. Prev. Vet. Med. 2006;77:230–253. doi: 10.1016/j.prevetmed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Häsler B., Stark K.D., Sager H., Gottstein B., Reist M. Simulating the impact of four control strategies on the population dynamics of Neospora caninum infection in Swiss dairy cattle. Prev. Vet. Med. 2006;77:254–283. doi: 10.1016/j.prevetmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Benavides J., Collantes-Fernandez E., Ferre I., Perez V., Campero C., Mota R., Innes E., Ortega-Mora L.M. Experimental ruminant models for bovine neosporosis: What is known and what is needed. Parasitology. 2014;141:1471–1488. doi: 10.1017/S0031182014000638. [DOI] [PubMed] [Google Scholar]
- 56.Debache K., Guionaud C., Kropf C., Boykin D., Stephens C.E., Hemphill A. Experimental treatment of Neospora caninum-infected mice with the arylimidamide DB750 and the thiazolide nitazoxanide. Exp. Parasitol. 2011;129:95–100. doi: 10.1016/j.exppara.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Schönfeld W., Kirst H.A. Macrolide antibiotics. Berlin, Germany: Springer Science & Business Media; 2002. [Google Scholar]
- 58.Brisson-Noël A., Trieu-Cuot P., Courvalin P. Mechanism of action of spiramycin and other macrolides. J. Antimicrob. Chemother. 1988;22:13–23. doi: 10.1093/jac/22.supplement_b.13. [DOI] [PubMed] [Google Scholar]
- 59.Mazzei T., Mini E., Novelli A., Periti P. Chemistry and mode of action of macrolides. J. Antimicrob. Chemother. 1993;31(Suppl.):C–1-9. doi: 10.1093/jac/31.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- 60.Root R.K. Clinical infectious diseases: a practical approach. USA: Oxford University Press; 1999. [Google Scholar]
- 61.Desmonts G., Couvreur J. Congenital toxoplasmosis: A prospective study of 378 pregnancies. N. Engl. J. Med. 1974;290:1110–1116. doi: 10.1056/NEJM197405162902003. [DOI] [PubMed] [Google Scholar]
- 62.Daffos F., Forestier F., Capella-Pavlovsky M., Thulliez P., Aufrant C., Valenti D., Cox W.L. Prenatal management of 746 pregnancies at risk for congenital toxoplasmosis. N. Engl. J. Med. 1988;318:271–275. doi: 10.1056/NEJM198802043180502. [DOI] [PubMed] [Google Scholar]
- 63.McCabe R. Antitoxoplasma chemotherapy. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 64.Wallon M., Liou C., Garner P., Peyron F. Congenital toxoplasmosis: Systematic review of evidence of efficacy of treatment in pregnancy. BMJ. 1999;318:1511–1514. doi: 10.1136/bmj.318.7197.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serranti D., Buonsenso D., Valentini P. Congenital toxoplasmosis treatment. Eur. Rev. Med. Pharmacol. Sci. 2011;15:193–198. [PubMed] [Google Scholar]
- 66.Rodrigues I.M., Costa T.L., Avelar J.B., Amaral W.N., Castro A.M., Avelino M.M. Assessment of laboratory methods used in the diagnosis of congenital toxoplasmosis after maternal treatment with spiramycin in pregnancy. 2014. [DOI] [PMC free article] [PubMed]
- 67.Chang H.R., Pechere J.C. In vitro effects of four macrolides (roxithromycin, spiramycin, azithromycin [CP-62,993], and A-56268) on Toxoplasma gondii. Antimicrob. Agents Chemother. 1988;32:524–529. doi: 10.1128/aac.32.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chamberland S., Kirst H.A., Current W.L. Comparative activity of macrolides against Toxoplasma gondii demonstrating utility of an in vitro microassay. Antimicrob. Agents Chemother. 1991;35:903–909. doi: 10.1128/aac.35.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garin J., Eyles D. Treatment of experimental toxoplasmosis in the mouse with spiramycin. Presse Med. 1958;66:957. [PubMed] [Google Scholar]
- 70.Grujić J., Djurković-Djaković O., Nikolić A., Klun I., Bobić B. Effectiveness of spiramycin in murine models of acute and chronic toxoplasmosis. Int. J. Antimicrob. Agents. 2005;25:226–230. doi: 10.1016/j.ijantimicag.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Chew W.K., Segarra I., Ambu S., Mak J.W. Significant reduction of brain cysts caused by Toxoplasma gondii after treatment with spiramycin coadministered with metronidazole in a mouse model of chronic toxoplasmosis. Antimicrob. Agents Chemother. 2012;56:1762–1768. doi: 10.1128/AAC.05183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith C.R. The spiramycin paradox. J. Antimicrob. Chemother. 1988;22:141–144. doi: 10.1093/jac/22.supplement_b.141. [DOI] [PubMed] [Google Scholar]
- 73.Dubreuil G. Trial of spiramycin in the prevention of experimental toxoplasmosis. 1972. [Google Scholar]
- 74.Wang J., MacNeil J.D., Kay J.F. Chemical analysis of antibiotic residues in food. New Jersey: John Wiley & Sons; 2011. [Google Scholar]
- 75.Westley J.W. 1982. [Google Scholar]
- 76.Bakker E., Bühlmann P., Pretsch E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997;97:3083–3132. doi: 10.1021/cr940394a. [DOI] [PubMed] [Google Scholar]
- 77.Rutkowski J., Brzezinski B. Structures and properties of naturally occurring polyether antibiotics. BioMed Res. Int. 2013;2013:162513. doi: 10.1155/2013/162513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Couzinet S., Dubremetz J., Buzoni-Gatel D., Jeminet G., Prensier G. In vitro activity of the polyether ionophorous antibiotic monensin against the cyst form of Toxoplasma gondii. Parasitology. 2000;121:359–365. doi: 10.1017/s0031182099006605. [DOI] [PubMed] [Google Scholar]
- 79.Bergstrom R.C., Maki L.R. Coccidiostatic action of monensin fed to lambs: Body weight gains and feed conversion efficacy. Am. J. Vet. Res. 1976;37:79–81. [PubMed] [Google Scholar]
- 80.McDougald L.R., Dunn W.J. Efficacy of monensin against coccidiosis in lambs. Am. J. Vet. Res. 1978;39:1459–1462. [PubMed] [Google Scholar]
- 81.Herberg R., Manthey J., Richardson L., Cooley C., Donoho A. Excretion and tissue distribution of [14C] monensin in cattle. J. Agric. Food Chem. 1978;26:1087–1090. doi: 10.1021/jf60219a004. [DOI] [PubMed] [Google Scholar]
- 82.Richardson L., Raun A., Potter E., Cooley C., Rathmacher R. Effect of monensin on rumen fermentation in vitro and in vivo. J. Anim. Sci. 1976;43:657–664. [Google Scholar]
- 83.European Commission . Ban on antibiotics as growth promoters in animal feed enters into effect (1831/2003/EC). Brussels: Europa; 2005. [Google Scholar]
- 84.Melton M.L., Sheffield H.G. Activity of the anticoccidial compound, lasalocid, against Toxoplasma gondii in cultured cells. J. Parasitol. 1975:713–717. [PubMed] [Google Scholar]
- 85.Ricketts A.P., Pfefferkorn E.R. Toxoplasma gondii: Susceptibility and development of resistance to anticoccidial drugs in vitro. Antimicrob. Agents Chemother. 1993;37:2358–2363. doi: 10.1128/aac.37.11.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lavine M.D., Arrizabalaga G. The antibiotic monensin causes cell cycle disruption of Toxoplasma gondii mediated through the DNA repair enzyme TgMSH-1. Antimicrob. Agents Chemother. 2011;55:745–755. doi: 10.1128/AAC.01092-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lavine M.D., Arrizabalaga G. Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death. PLoS One. 2012;7:e42107. doi: 10.1371/journal.pone.0042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charvat R.A., Arrizabalaga G. Oxidative stress generated during monensin treatment contributes to altered Toxoplasma gondii mitochondrial function. Sci. Rep. 2016;6:22997. doi: 10.1038/srep22997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garrison E.M., Arrizabalaga G. Disruption of a mitochondrial MutS DNA repair enzyme homologue confers drug resistance in the parasite Toxoplasma gondii. Mol. Microbiol. 2009;72:425–441. doi: 10.1111/j.1365-2958.2009.06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lindsay D.S., Rippey N.S., Cole R.A., Parsons L.C., Dubey J.P., Tidwell R.R., Blagburn B.L. Examination of the activities of 43 chemotherapeutic agents against Neospora caninum tachyzoites in cultured cells. Am. J. Vet. Res. 1994;55:976–981. [PubMed] [Google Scholar]
- 91.Buxton D., Blewett D., Trees A., McColgan C., Finlayson J. Further studies in the use of monensin in the control of experimental ovine toxoplasmosis. J. Comp. Pathol. 1988;98:225–236. doi: 10.1016/0021-9975(88)90021-7. [DOI] [PubMed] [Google Scholar]
- 92.Buxton D., Donald K.M., Finlayson J. Monensin and the control of experimental ovine toxoplasmosis: A systemic effect. Vet. Rec. 1987;120:618–619. doi: 10.1136/vr.120.26.618. [DOI] [PubMed] [Google Scholar]
- 93.Beck B.E., Harries W.N. Proceedings of annual meeting-American Association of Veterinary Laboratory Diagnosticians. USA: American Association of Veterinary Laboratory Diagnosticians; 1979. The diagnosis of monensin toxicosis: A report on outbreaks in horses, cattle and chickens. [Google Scholar]
- 94.Wilson J.S. Toxic myopathy in a dog associated with the presence of monensin in dry food. Can. Vet. J. 1980;21:30–31. [PMC free article] [PubMed] [Google Scholar]
- 95.Nation P.N., Crowe S.P., Harries W.N. Clinical signs and pathology of accidental monensin poisoning in sheep. Can. Vet. J. 1982;23:323–326. [PMC free article] [PubMed] [Google Scholar]
- 96.Synge B.A. Monensin poisoning in sheep. Vet. Rec. 1989;124:410–411. doi: 10.1136/vr.124.15.410. [DOI] [PubMed] [Google Scholar]
- 97.Trees A.J. Monensin and toxoplasmosis in sheep. Vet. Rec. 1989;125:116. doi: 10.1136/vr.125.5.116-c. [DOI] [PubMed] [Google Scholar]
- 98.Ellis K.J., Costigan P. Monensin and ovine toxoplasmosis. Vet. Rec. 1990;126:92–93. [PubMed] [Google Scholar]
- 99.Kirkbride C.A., Dubey J., Libal M.C. Effect of feeding lasalocid to pregnant ewes experimentally infected with Toxoplasma gondii. Vet. Parasitol. 1992;44:299–303. doi: 10.1016/0304-4017(92)90126-t. [DOI] [PubMed] [Google Scholar]
- 100.Vanleeuwen J.A., Haddad J.P., Dohoo I.R., Keefe G.P., Tiwari A., Scott H.M. Risk factors associated with Neospora caninum seropositivity in randomly sampled Canadian dairy cows and herds. Prev. Vet. Med. 2010;93:129–138. doi: 10.1016/j.prevetmed.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 101.Vanleeuwen J.A., Greenwood S., Clark F., Acorn A., Markham F., McCarron J., O’Handley R. Monensin use against Neospora caninum challenge in dairy cattle. Vet. Parasitol. 2011;175:372–376. doi: 10.1016/j.vetpar.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 102.Hitchings G.H., Burchall J.J. Inhibition of folate biosynthesis and function as a basis for chemotherapy. Adv. Enzymol. Relat. Areas Mol. Biol. 1965;27:417–468. doi: 10.1002/9780470122723.ch9. [DOI] [PubMed] [Google Scholar]
- 103.Derouin F., Chastang C. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob. Agents Chemother. 1989;33:1753–1759. doi: 10.1128/aac.33.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lindsay D.S., Butler J.M., Rippey N.S., Blagburn B.L. Demonstration of synergistic effects of sulfonamides and dihydrofolate reductase/thymidylate synthase inhibitors against Neospora caninum tachyzoites in cultured cells, and characterization of mutants resistant to pyrimethamine. Am. J. Vet. Res. 1996;57:68–72. [PubMed] [Google Scholar]
- 105.Pfefferkorn E., Borotz S.E., Nothnagel R.F. Toxoplasma gondii: Characterization of a mutant resistant to sulfonamides. Exp. Parasitol. 1992;74:261–270. doi: 10.1016/0014-4894(92)90149-5. [DOI] [PubMed] [Google Scholar]
- 106.Aspinall T.V., Joynson D.H., Guy E., Hyde J.E., Sims P.F. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J. Infect. Dis. 2002;185:1637–1643. doi: 10.1086/340577. [DOI] [PubMed] [Google Scholar]
- 107.Piketty C., Derouin F., Rouveix B., Pocidalo J.J. In vivo assessment of antimicrobial agents against Toxoplasma gondii by quantification of parasites in the blood, lungs, and brain of infected mice. Antimicrob. Agents Chemother. 1990;34:1467–1472. doi: 10.1128/aac.34.8.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lindsay D.S., Dubey J.P. Effects of sulfadiazine and amprolium on Neospora caninum (Protozoa: Apicomplexa) infections in mice. J. Parasitol. 1990;76:177–179. [PubMed] [Google Scholar]
- 109.Buxton D., Thomson K.M., Maley S. Treatment of ovine toxoplasmosis with a combination of sulphamezathine and pyrimethamine. Vet. Rec. 1993;132:409–411. doi: 10.1136/vr.132.16.409. [DOI] [PubMed] [Google Scholar]
- 110.Giadinis N.D., Terpsidis K., Diakou A., Siarkou V., Loukopoulos P., Osman R., Karatzias H., Papazahariadou M. Massive Toxoplasma abortions in a dairy sheep flock and therapeutic approach with different doses of sulfadimidine. Turk. J. Vet. Anim. Sci. 2011;35:207–211. [Google Scholar]
- 111.Cuteri V., Nisoli L., Preziuso S., Attili A., Guerra C., Lulla D., Traldi G. Application of a new therapeutic protocol against Neospora caninum-induced abortion in cattle: A field study. J. Anim. Vet. Adv. 2005;4:510–514. [Google Scholar]
- 112.Andersson M.I., MacGowan A.P. Development of the quinolones. J. Antimicrob. Chemother. 2003;51(Suppl. 1):1–11. doi: 10.1093/jac/dkg212. [DOI] [PubMed] [Google Scholar]
- 113.Williams R. Tracing the emergence of drug-resistance in coccidia (Eimeria spp.) of commercial broiler flocks medicated with decoquinate for the first time in the United Kingdom. Vet. Parasitol. 2006;135:1–14. doi: 10.1016/j.vetpar.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 114.Joyner L.P., Norton C.C. The recording and analysis of coccidiostatic activity; quinolone and pyridone compounds. Res. Vet. Sci. 1971;12:80–88. [PubMed] [Google Scholar]
- 115.Fitzgerald P.R., Mansfield M.E. Effect of decoquinate on the control of coccidiosis in young ruminating calves. Am. J. Vet. Res. 1986;47:130–133. [PubMed] [Google Scholar]
- 116.Fry M., Williams R.B. Effects of decoquinate and clopidol on electron transport in mitochondria of Eimeria tenella (Apicomplexa: Coccidia). Biochem. Pharmacol. 1984;33:229–240. doi: 10.1016/0006-2952(84)90480-5. [DOI] [PubMed] [Google Scholar]
- 117.Laval A., Remy D. Decoquinate. Practical utilisation in the control of coccidiosis in mammals. Recueil de Medecine Veterinaire (France) 1994;170(12):811–821. [Google Scholar]
- 118.Daugschies A., Najdrowski M. Eimeriosis in cattle: Current understanding. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2005;52:417–427. doi: 10.1111/j.1439-0450.2005.00894.x. [DOI] [PubMed] [Google Scholar]
- 119.Taylor M., Bartram D. The history of decoquinate in the control of coccidial infections in ruminants. J. Vet. Pharmacol. Ther. 2012;35:417–427. doi: 10.1111/j.1365-2885.2012.01421.x. [DOI] [PubMed] [Google Scholar]
- 120.Quintero-de Leonardo J., Rosiles R., Bautista J., González-Monsón N., Sumano H. Oral pharmacokinetics and milk residues of decoquinate in milking cows. J. Vet. Pharmacol. Ther. 2009;32:403–406. doi: 10.1111/j.1365-2885.2008.01049.x. [DOI] [PubMed] [Google Scholar]
- 121.European Commission Regulation, C. No. 2377/90 laying down a community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin, amending regulation no. 1430/94 of 22 June 1994, Off. J. Eur. Commun. L. 1994;156:23. [Google Scholar]
- 122.EMEA (European Medicines Evaluation Agency) Http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500013578.pdf
- 123.Pfefferkorn E., Borotz S.E., Nothnagel R.F. Mutants of Toxoplasma gondii resistant to atovaquone (566C80) or decoquinate. J. Parasitol. 1993:559–564. [PubMed] [Google Scholar]
- 124.Lindsay D.S., Butler J.M., Blagburn B.L. Efficacy of decoquinate against Neospora caninum tachyzoites in cell cultures. Vet. Parasitol. 1997;68:35–40. doi: 10.1016/s0304-4017(96)01054-0. [DOI] [PubMed] [Google Scholar]
- 125.Buxton D., Brebner J., Wright S., Maley S.W., Thomson K.M., Millard K. Decoquinate and the control of experimental ovine toxoplasmosis. Vet. Rec. 1996;138:434–436. doi: 10.1136/vr.138.18.434. [DOI] [PubMed] [Google Scholar]
- 126.Journel C., Chatagnon G., Martin D., Richard A., Tainturier D. Proceedings of the XXII World Buiatrics Congress; Hannover, Germany. 2002. [Google Scholar]
- 127.Arshad M., Khan T.A., Khan M.A. 1, 2, 4 Triazine Derivatives: Synthesis and Biological Applications. Int. J. Pharm. Sci. Res. 2015;5(4):149–162. [Google Scholar]
- 128.Haberkorn A. Chemotherapy of human and animal coccidioses: State and perspectives. Parasitol. Res. 1996;82:193–199. doi: 10.1007/s004360050094. [DOI] [PubMed] [Google Scholar]
- 129.Harder A., Haberkorn A. Possible mode of action of toltrazuril: Studies on two Eimeria species and mammalian and Ascaris suum enzymes. Parasitol. Res. 1989;76:8–12. doi: 10.1007/BF00931064. [DOI] [PubMed] [Google Scholar]
- 130.Mehlhorn H., Ortmann-Falkenstein G., Haberkorn A. The effects of sym. triazinones on developmental stages of Eimeria tenella, E. maxima and E. acervulina: A light and electron microscopical study. Z. Parasitenkd. 1984;70:173–182. doi: 10.1007/BF00942219. [DOI] [PubMed] [Google Scholar]
- 131.Mitchell S.M., Zajac A.M., Davis W.L., Kennedy T.J., Lindsay D.S. The effects of ponazuril on development of apicomplexans in vitro. J. Eukaryot. Microbiol. 2005;52:231–235. doi: 10.1111/j.1550-7408.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 132.Greif G., Harder A., Haberkorn A. Chemotherapeutic approaches to protozoa: Coccidiae--current level of knowledge and outlook. Parasitol. Res. 2001;87:973–975. doi: 10.1007/s004360100403. [DOI] [PubMed] [Google Scholar]
- 133.Mitchell S.M., Zajac A.M., Davis W.L., Lindsay D.S. Efficacy of ponazuril in vitro and in preventing and treating Toxoplasma gondii infections in mice. J. Parasitol. 2004;90:639–642. doi: 10.1645/GE-250R. [DOI] [PubMed] [Google Scholar]
- 134.Darius A.K., Mehlhorn H., Heydorn A.O. Effects of toltrazuril and ponazuril on the fine structure and multiplication of tachyzoites of the NC-1 strain of Neospora caninum (a synonym of Hammondia heydorni) in cell cultures. Parasitol. Res. 2004;92(6):453–458. doi: 10.1007/s00436-003-1063-7. [DOI] [PubMed] [Google Scholar]
- 135.Strohbusch M., Muller N., Hemphill A., Greif G., Gottstein B. NcGRA2 as a molecular target to assess the parasiticidal activity of toltrazuril against Neospora caninum. Parasitology. 2008;135(9):1065–1073. doi: 10.1017/S0031182008004599. [DOI] [PubMed] [Google Scholar]
- 136.Gottstein B., Eperon S., Dai W.J., Cannas A., Hemphill A., Greif G. Efficacy of toltrazuril and ponazuril against experimental Neospora caninum infection in mice. Parasitol. Res. 2001;87:43–48. doi: 10.1007/s004360000306. [DOI] [PubMed] [Google Scholar]
- 137.Gottstein B., Razmi G.R., Ammann P., Sager H., Muller N. Toltrazuril treatment to control diaplacental Neospora caninum transmission in experimentally infected pregnant mice. Parasitology. 2005;130:41–48. doi: 10.1017/s0031182004006365. [DOI] [PubMed] [Google Scholar]
- 138.Strohbusch M., Muller N., Hemphill A., Krebber R., Greif G., Gottstein B. Toltrazuril treatment of congenitally acquired Neospora caninum infection in newborn mice. Parasitol. Res. 2009;104(6):1335–1343. doi: 10.1007/s00436-009-1328-x. [DOI] [PubMed] [Google Scholar]
- 139.Kul O., Yildiz K., Ocal N., Freyre A., Deniz A., Karahan S., Atmaca H.T., Gokpinar S., Dincel G.C., Uzunalioğlu T. In-vivo efficacy of toltrazuril on experimentally induced Toxoplasma gondii tissue cysts in lambs: A novel strategy for prevention of human exposure to meat-borne toxoplasmosis. Res. Vet. Sci. 2013;94:269–276. doi: 10.1016/j.rvsc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 140.Haerdi C., Haessig M., Sager H., Greif G., Staubli D., Gottstein B. Humoral immune reaction of newborn calves congenitally infected with Neospora caninum and experimentally treated with toltrazuril. Parasitol. Res. 2006;99:534–540. doi: 10.1007/s00436-006-0199-7. [DOI] [PubMed] [Google Scholar]
- 141.Syed-Hussain S., Howe L., Pomroy W., West D., Hardcastle M., Williamson N. Study on the use of toltrazuril to eliminate Neospora caninum in congenitally infected lambs born from experimentally infected ewes. Vet. Parasitol. 2015;210:141–144. doi: 10.1016/j.vetpar.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 142.Kritzner S., Sager H., Blum J., Krebber R., Greif G., Gottstein B. An explorative study to assess the efficacy of Toltrazuril-sulfone (Ponazuril) in calves experimentally infected with Neospora caninum. Ann. Clin. Microbiol. Antimicrob. 2002;1:4. doi: 10.1186/1476-0711-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guiguemde W.A., Shelat A.A., Bouck D., Duffy S., Crowther G.J., Davis P.H., Smithson D.C., Connelly M., Clark J., Zhu F. Chemical genetics of Plasmodium falciparum. Nature. 2010;465:311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brown D., Superti-Furga G. Rediscovering the sweet spot in drug discovery. Drug Discov. Today. 2003;8:1067–1077. doi: 10.1016/s1359-6446(03)02902-7. [DOI] [PubMed] [Google Scholar]
- 145.Hemphill A., Müller J., Esposito M. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert Opin. Pharmacother. 2006;7:953–964. doi: 10.1517/14656566.7.7.953. [DOI] [PubMed] [Google Scholar]
- 146.Hemphill A., Müller N., Müller J. Structure-function relationship of thiazolides, a novel class of anti-parasitic drugs, investigated in intracellular and extracellular protozoan parasites and larval-stage cestodes. Antiinfect. Agents Med. Chem. 2007;6:273–282. [Google Scholar]
- 147.Esposito M., Muller N., Hemphill A. Structure-activity relationships from in vitro efficacies of the thiazolide series against the intracellular apicomplexan protozoan Neospora caninum. Int. J. Parasitol. 2007;37:183–190. doi: 10.1016/j.ijpara.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 148.Müller J., Hemphill A. Identification of a host cell target for the thiazolide class of broad-spectrum anti-parasitic drugs. Exp. Parasitol. 2011;128:145–150. doi: 10.1016/j.exppara.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 149.Esposito M., Moores S., Naguleswaran A., Müller J., Hemphill A. Induction of tachyzoite egress from cells infected with the protozoan Neospora caninum by nitro- and bromo-thiazolides, a class of broad-spectrum anti-parasitic drugs. Int. J. Parasitol. 2007;37:1143–1152. doi: 10.1016/j.ijpara.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 150.Müller J., Limban C., Stadelmann B., Missir A.V., Chirita I.C., Chifiriuc M.C., Nitulescu G.M., Hemphill A. Thioureides of 2-(phenoxymethyl) benzoic acid 4-R substituted: A novel class of anti-parasitic compounds. Parasitol. Int. 2009;58:128–135. doi: 10.1016/j.parint.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 151.Schnyder M., Kohler L., Hemphill A., Deplazes P. Prophylactic and therapeutic efficacy of nitazoxanide against Cryptosporidium parvum in experimentally challenged neonatal calves. Vet. Parasitol. 2009;160:149–154. doi: 10.1016/j.vetpar.2008.10.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Buckner F.S., Navabi N. Advances in Chagas disease drug development: 2009-2010. Curr. Opin. Infect. Dis. 2010;23:609–616. doi: 10.1097/QCO.0b013e3283402956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Soeiro M.N., De Souza E., Stephens C.E., Boykin D. Aromatic diamidines as antiparasitic agents. Expert Opin. Investig. Drugs. 2005;14:957–972. doi: 10.1517/13543784.14.8.957. [DOI] [PubMed] [Google Scholar]
- 154.Wilson W.D., Tanious F.A., Mathis A., Tevis D., Hall J.E., Boykin D.W. Antiparasitic compounds that target DNA. Biochimie. 2008;90:999–1014. doi: 10.1016/j.biochi.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lindsay D.S., Blagburn B.L., Hall J.E., Tidwell R.R. Activity of pentamidine and pentamidine analogs against Toxoplasma gondii in cell cultures. Antimicrob. Agents Chemother. 1991;35:1914–1916. doi: 10.1128/aac.35.9.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leepin A., Studli A., Brun R., Stephens C.E., Boykin D.W., Hemphill A. Host cells participate in the in vitro effects of novel diamidine analogues against tachyzoites of the intracellular apicomplexan parasites Neospora caninum and Toxoplasma gondii. Antimicrob. Agents Chemother. 2008;52:1999–2008. doi: 10.1128/AAC.01236-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kropf C., Debache K., Rampa C., Barna F., Schorer M., Stephens C.E., Ismail M.A., Boykin D.W., Hemphill A. The adaptive potential of a survival artist: Characterization of the in vitro interactions of Toxoplasma gondii tachyzoites with di-cationic compounds in human fibroblast cell cultures. Parasitology. 2012;139:208–220. doi: 10.1017/S0031182011001776. [DOI] [PubMed] [Google Scholar]
- 158.Schorer M., Debache K., Barna F., Monney T., Muller J., Boykin D.W., Stephens C.E., Hemphill A. Di-cationic arylimidamides act against Neospora caninum tachyzoites by interference in membrane structure and nucleolar integrity and are active against challenge infection in mice. Int. J. Parasitol. Drugs Drug Resist. 2012;2:109–120. doi: 10.1016/j.ijpddr.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Collantes-Fernández E., López-Pérez I., Álvarez-García G., Ortega-Mora L.M. Temporal distribution and parasite load kinetics in blood and tissues during Neospora caninum infection in mice. Infect. Immun. 2006;74:2491–2494. doi: 10.1128/IAI.74.4.2491-2494.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bilia A., de Malgalhaes P.M., Bergonzi M., Vincieri F. Simultaneous analysis of artemisinin and flavonoids of several extracts of Artemisia annua L. obtained from a commercial sample and a selected cultivar. Phytomedicine. 2006;13:487–493. doi: 10.1016/j.phymed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 161.Haynes R.K., Krishna S. Artemisinins: Activities and actions. Microbes Infect. 2004;6:1339–1346. doi: 10.1016/j.micinf.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 162.Dunay I.R., Chan W.C., Haynes R.K., Sibley L.D. Artemisone and artemiside control acute and reactivated toxoplasmosis in a murine model. Antimicrob. Agents Chemother. 2009;53:4450–4456. doi: 10.1128/AAC.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nagamune K., Beatty W.L., Sibley L.D. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot. Cell. 2007;6:2147–2156. doi: 10.1128/EC.00262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ke O.Y., Krug E.C., Marr J.J., Berens R.L. Inhibition of growth of Toxoplasma gondii by qinghaosu and derivatives. Antimicrob. Agents Chemother. 1990;34:1961–1965. doi: 10.1128/aac.34.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gomes T.C. Andrade Júnior, Heitor Franco de; Lescano, S.A.Z.; Amato-Neto, V. In vitro action of antiparasitic drugs, especially artesunate, against Toxoplasma gondii. Rev. Soc. Bras. Med. Trop. 2012;45:485–490. doi: 10.1590/s0037-86822012000400014. [DOI] [PubMed] [Google Scholar]
- 166.Sarciron M.E., Saccharin C., Petavy A.F., Peyron F. Effects of artesunate, dihydroartemisinin, and an artesunate-dihydroartemisinin combination against Toxoplasma gondii. Am. J. Trop. Med. Hyg. 2000;62:73–76. doi: 10.4269/ajtmh.2000.62.73. [DOI] [PubMed] [Google Scholar]
- 167.Haynes R.K., Fugmann B., Stetter J., Rieckmann K., Heilmann H., Chan H., Cheung M., Lam W., Wong H., Croft S.L. Artemisone—a highly active antimalarial drug of the artemisinin class. Angew. Chem. Int. Ed. 2006;118:2136–2142. doi: 10.1002/anie.200503071. [DOI] [PubMed] [Google Scholar]
- 168.Nagelschmitz J., Voith B., Wensing G., Roemer A., Fugmann B., Haynes R.K., Kotecka B.M., Rieckmann K.H., Edstein M.D. First assessment in humans of the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of the new artemisinin derivative artemisone. Antimicrob. Agents Chemother. 2008;52:3085–3091. doi: 10.1128/AAC.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nontprasert A., Nosten-Bertrand M., Pukrittayakamee S., Vanijanonta S., Angus B.J., White N.J. Assessment of the neurotoxicity of parenteral artemisinin derivatives in mice. Am. J. Trop. Med. Hyg. 1998;59:519–522. doi: 10.4269/ajtmh.1998.59.519. [DOI] [PubMed] [Google Scholar]
- 170.Schmuck G., Klaus A., Krötlinger F., Langewische F. Developmental and reproductive toxicity studies on artemisone. Birth Defects Res. B Dev. Reprod. Toxicol. 2009;86:131–143. doi: 10.1002/bdrb.20192. [DOI] [PubMed] [Google Scholar]
- 171.Kim J.T., Park J.Y., Seo H.S., Oh H.G., Noh J.W., Kim J.H., Kim D.Y., Youn H.J. In vitro antiprotozoal effects of artemisinin on Neospora caninum. Vet. Parasitol. 2002;103:53–63. doi: 10.1016/s0304-4017(01)00580-5. [DOI] [PubMed] [Google Scholar]
- 172.Qian W., Wang H., Shan D., Li B., Liu J., Liu Q. Activity of several kinds of drugs against Neospora caninum. Parasitol. Int. 2015;64:597–602. doi: 10.1016/j.parint.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 173.Mazuz M.L., Haynes R., Shkap V., Fish L., Wollkomirsky R., Leibovich B., Molad T., Savitsky I., Golenser J. Neospora caninum: In vivo and in vitro treatment with artemisone. Vet. Parasitol. 2012;187:99–104. doi: 10.1016/j.vetpar.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 174.Müller J., Balmer V., Winzer P., Rahman M., Manser V., Haynes R.K., Hemphill A. In vitro effects of new artemisinin derivatives in Neospora caninum-infected human fibroblasts. Int. J. Antimicrob. Agents. 2015;46:88–93. doi: 10.1016/j.ijantimicag.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 175.Müller J., Aguado-Martínez A., Manser V., Wong H.N., Haynes R.K., Hemphill A. Repurposing of antiparasitic drugs: The hydroxy-naphthoquinone buparvaquone inhibits vertical transmission in the pregnant neosporosis mouse model. Vet. Res. (Faisalabad) 2016;47:1. doi: 10.1186/s13567-016-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hudson A., Randall A., Fry M., Ginger C., Hill B., Latter V., McHardy N., Williams R. Novel anti-malarial hydroxynaphthoquinones with potent broad spectrum anti-protozoal activity. Parasitology. 1985;90:45–55. doi: 10.1017/s0031182000049003. [DOI] [PubMed] [Google Scholar]
- 177.Hudson A.T., Dickins M., Ginger C.D., Gutteridge W.E., Holdich T., Hutchinson D.B., Pudney M., Randall A.W., Latter V.S. 566C80: A potent broad spectrum anti-infective agent with activity against malaria and opportunistic infections in AIDS patients. Drugs Exp. Clin. Res. 1991;17:427–435. [PubMed] [Google Scholar]
- 178.Wilkie G., Brown C., Kirvar E., Thomas M., Williamson S., Bell-Sakyi L., Sparagano O. Chemoprophylaxis of Theileria annulata and Theileria parva infections of calves with buparvaquone. Vet. Parasitol. 1998;78:1–12. doi: 10.1016/s0304-4017(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 179.Sen N., Majumder H.K. Mitochondrion of protozoan parasite emerges as potent therapeutic target: Exciting drugs are on the horizon. Curr. Pharm. Des. 2008;14:839–846. doi: 10.2174/138161208784041024. [DOI] [PubMed] [Google Scholar]
- 180.Mather M.W., Vaidya A.B. Mitochondria in malaria and related parasites: Ancient, diverse and streamlined. J. Bioenerg. Biomembr. 2008;40:425–433. doi: 10.1007/s10863-008-9176-4. [DOI] [PubMed] [Google Scholar]
- 181.Sun I.L., Sun E.E., Crane F.L., Morre D.J., Lindgren A., Low H. Requirement for coenzyme Q in plasma membrane electron transport. Proc. Natl. Acad. Sci. USA. 1992;89:11126–11130. doi: 10.1073/pnas.89.23.11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fry M., Pudney M. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1, 4-naphthoquinone (566C80). Biochem. Pharmacol. 1992;43:1545–1553. doi: 10.1016/0006-2952(92)90213-3. [DOI] [PubMed] [Google Scholar]
- 183.McFadden D.C., Tomavo S., Berry E.A., Boothroyd J.C. Characterization of cytochrome b from Toxoplasma gondii and Q(o) domain mutations as a mechanism of atovaquone-resistance. Mol. Biochem. Parasitol. 2000;108:1–12. doi: 10.1016/s0166-6851(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 184.Srivastava I.K., Rottenberg H., Vaidya A.B. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J. Biol. Chem. 1997;272:3961–3966. doi: 10.1074/jbc.272.7.3961. [DOI] [PubMed] [Google Scholar]
- 185.Romand S., Pudney M., Derouin F. In vitro and in vivo activities of the hydroxynaphthoquinone atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, or minocycline against Toxoplasma gondii. Antimicrob. Agents Chemother. 1993;37:2371–2378. doi: 10.1128/aac.37.11.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Meneceur P., Bouldouyre M.A., Aubert D., Villena I., Menotti J., Sauvage V., Garin J.F., Derouin F. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob. Agents Chemother. 2008;52:1269–1277. doi: 10.1128/AAC.01203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huskinson-Mark J., Araujo F.G., Remington J.S. Evaluation of the effect of drugs on the cyst form of Toxoplasma gondii. J. Infect. Dis. 1991;164:170–171. doi: 10.1093/infdis/164.1.170. [DOI] [PubMed] [Google Scholar]
- 188.Araujo F.G., Huskinson J., Remington J.S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob. Agents Chemother. 1991;35:293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Araujo F.G., Huskinson-Mark J., Gutteridge W.E., Remington J.S. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob. Agents Chemother. 1992;36:326–330. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ferguson D.J., Huskinson-Mark J., Araujo F.G., Remington J.S. An ultrastructural study of the effect of treatment with atovaquone in brains of mice chronically infected with the ME49 strain of Toxoplasma gondii. Int. J. Exp. Pathol. 1994;75:111–116. [PMC free article] [PubMed] [Google Scholar]
- 191.Dunay I.R., Heimesaat M.M., Bushrab F.N., Muller R.H., Stocker H., Arasteh K., Kurowski M., Fitzner R., Borner K., Liesenfeld O. Atovaquone maintenance therapy prevents reactivation of toxoplasmic encephalitis in a murine model of reactivated toxoplasmosis. Antimicrob. Agents Chemother. 2004;48:4848–4854. doi: 10.1128/AAC.48.12.4848-4854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Müller J., Aguado-Martinez A., Manser V., Balmer V., Winzer P., Ritler D., Hostettler I., Arranz-Solis D., Ortega-Mora L., Hemphill A. Buparvaquone is active against Neospora caninum in vitro and in experimentally infected mice. Int. J. Parasitol. Drugs Drug Resist. 2015;5:16–25. doi: 10.1016/j.ijpddr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dissous C., Grevelding C.G. Piggy-backing the concept of cancer drugs for schistosomiasis treatment: A tangible perspective? Trends Parasitol. 2011;27:59–66. doi: 10.1016/j.pt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 194.Klinkert M., Heussler V. The use of anticancer drugs in antiparasitic chemotherapy. Mini Rev. Med. Chem. 2006;6:131–143. doi: 10.2174/138955706775475939. [DOI] [PubMed] [Google Scholar]
- 195.Debrabant A., Lee N., Bertholet S., Duncan R., Nakhasi H.L. Programmed cell death in trypanosomatids and other unicellular organisms. Int. J. Parasitol. 2003;33:257–267. doi: 10.1016/s0020-7519(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 196.Lüder C.G., Gross U., Lopes M.F. Intracellular protozoan parasites and apoptosis: Diverse strategies to modulate parasite–host interactions. Trends Parasitol. 2001;17:480–486. doi: 10.1016/s1471-4922(01)02016-5. [DOI] [PubMed] [Google Scholar]
- 197.Wieder T., Reutter W., Orfanos C.E., Geilen C.C. Mechanisms of action of phospholipid analogs as anticancer compounds. Prog. Lipid Res. 1999;38:249–259. doi: 10.1016/s0163-7827(99)00004-1. [DOI] [PubMed] [Google Scholar]
- 198.Solano-Gallego L., Miró G., Koutinas A., Cardoso L., Pennisi M.G., Ferrer L., Bourdeau P., Oliva G., Baneth G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors. 2011;4:86. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nyoman A.D.N., Lüder C.G. Apoptosis-like cell death pathways in the unicellular parasite Toxoplasma gondii following treatment with apoptosis inducers and chemotherapeutic agents: A proof-of-concept study. Apoptosis. 2013;18:664–680. doi: 10.1007/s10495-013-0832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Eissa M.M., Barakat A.M., Amer E.I., Younis L.K. Could miltefosine be used as a therapy for toxoplasmosis? Exp. Parasitol. 2015;157:12–22. doi: 10.1016/j.exppara.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 201.Debache K., Hemphill A. Effects of miltefosine treatment in fibroblast cell cultures and in mice experimentally infected with Neospora caninum tachyzoites. Parasitology. 2012;139:934–944. doi: 10.1017/S0031182012000066. [DOI] [PubMed] [Google Scholar]
- 202.Kostova I. Platinum complexes as anticancer agents. Recent Patents Anticancer Drug Discov. 2006;1:1–22. doi: 10.2174/157489206775246458. [DOI] [PubMed] [Google Scholar]
- 203.Bergamo A., Sava G. Ruthenium anticancer compounds: Myths and realities of the emerging metal-based drugs. Dalton Trans. 2011;40:7817–7823. doi: 10.1039/c0dt01816c. [DOI] [PubMed] [Google Scholar]
- 204.Beckford F., Dourth D., Shaloski M., Didion J., Thessing J., Woods J., Crowell V., Gerasimchuk N., Gonzalez-Sarrías A., Seeram N.P. Half-sandwich ruthenium–arene complexes with thiosemicarbazones: Synthesis and biological evaluation of [(η 6-p-cymene) Ru (piperonal thiosemicarbazones) Cl]Cl complexes. J. Inorg. Biochem. 2011;105:1019–1029. doi: 10.1016/j.jinorgbio.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Caroli A., Simeoni S., Lepore R., Tramontano A., Via A. Investigation of a potential mechanism for the inhibition of SmTGR by Auranofin and its implications for Plasmodium falciparum inhibition. Biochem. Biophys. Res. Commun. 2012;417:576–581. doi: 10.1016/j.bbrc.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 206.Schwietert C.W., McCue J.P. Coordination compounds in medicinal chemistry. Coord. Chem. Rev. 1999;184:67–89. [Google Scholar]
- 207.Ravera M., Baracco S., Cassino C., Colangelo D., Bagni G., Sava G., Osella D. Electrochemical measurements confirm the preferential bonding of the antimetastatic complex [ImH][RuCl 4 (DMSO)(Im)](NAMI-A) with proteins and the weak interaction with nucleobases. J. Inorg. Biochem. 2004;98:984–990. doi: 10.1016/j.jinorgbio.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 208.Barna F., Debache K., Vock C.A., Kuster T., Hemphill A. In vitro effects of novel ruthenium complexes in Neospora caninum and Toxoplasma gondii tachyzoites. Antimicrob. Agents Chemother. 2013;57:5747–5754. doi: 10.1128/AAC.02446-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Basto A.P., Muller J., Rubbiani R., Stibal D., Giannini F., Suss-Fink G., Balmer V., Hemphill A., Gasser G., Furrer J. Characterization of the activities of dinuclear thiolato-bridged arene ruthenium complexes against Toxoplasma gondii. Antimicrob. Agents Chemother. 2017;61:e01031–e17. doi: 10.1128/AAC.01031-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Salzer W., Timmler H., Andersag H. A new type of compounds active against avian malaria. Chem. Ber. 1948;81:12–19. [Google Scholar]
- 211.Gingrich W.D., Darrow E.M. The effect of endochin on experimental toxoplasmosis. Am. J. Trop. Med. Hyg. 1951;1:12–17. doi: 10.4269/ajtmh.1951.s1-31.12. [DOI] [PubMed] [Google Scholar]
- 212.Ortiz D., Forquer I., Boitz J., Soysa R., Elya C., Fulwiler A., Nilsen A., Polley T., Riscoe M.K., Ullman B., Landfear S.M. Targeting the cytochrome bc1 complex of Leishmania parasites for discovery of novel drugs. Antimicrob. Agents Chemother. 2016;60:4972–4982. doi: 10.1128/AAC.00850-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Winter R.W., Kelly J.X., Smilkstein M.J., Dodean R., Hinrichs D., Riscoe M.K. Antimalarial quinolones: Synthesis, potency, and mechanistic studies. Exp. Parasitol. 2008;118:487–497. doi: 10.1016/j.exppara.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Doggett J.S., Nilsen A., Forquer I., Wegmann K.W., Jones-Brando L., Yolken R.H., Bordon C., Charman S.A., Katneni K., Schultz T., Burrows J.N., Hinrichs D.J., Meunier B., Carruthers V.B., Riscoe M.K. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc. Natl. Acad. Sci. USA. 2012;109:15936–15941. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vercesi A.E., Rodrigues C.O., Uyemura S.A., Zhong L., Moreno S.N. Respiration and oxidative phosphorylation in the apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 1998;273:31040–31047. doi: 10.1074/jbc.273.47.31040. [DOI] [PubMed] [Google Scholar]
- 216.Winter R., Kelly J.X., Smilkstein M.J., Hinrichs D., Koop D.R., Riscoe M.K. Optimization of endochin-like quinolones for antimalarial activity. Exp. Parasitol. 2011;127:545–551. doi: 10.1016/j.exppara.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Müller J., Aguado A., Laleu B., Balmer V., Ritler D., Hemphill A. In vitro screening of the open source Pathogen Box identifies novel compounds with profound activities against Neospora caninum. Int. J. Parasitol. 2017;47:801–809. doi: 10.1016/j.ijpara.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 218.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 219.Lourido S., Tang K., Sibley L.D. Distinct signalling pathways control Toxoplasma egress and host-cell invasion. EMBO J. 2012;31:4524–4534. doi: 10.1038/emboj.2012.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lourido S., Shuman J., Zhang C., Shokat K.M., Hui R., Sibley L.D. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Garrison E., Treeck M., Ehret E., Butz H., Garbuz T., Oswald B.P., Settles M., Boothroyd J., Arrizabalaga G. A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog. 2012;8:e1003049. doi: 10.1371/journal.ppat.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.McCoy J.M., Whitehead L., Van Dooren G.G., Tonkin C.J. TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells. PLoS Pathog. 2012;8:e1003066. doi: 10.1371/journal.ppat.1003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Morlon‐Guyot J., Berry L., Chen C., Gubbels M., Lebrun M., Daher W. The Toxoplasma gondii calcium‐dependent protein kinase 7 is involved in early steps of parasite division and is crucial for parasite survival. Cell. Microbiol. 2014;16:95–114. doi: 10.1111/cmi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang J., Huang S., Li T., Chen K., Ning H., Zhu X. Evaluation of the basic functions of six calcium-dependent protein kinases in Toxoplasma gondii using CRISPR-Cas9 system. Parasitol. Res. 2016;115:697–702. doi: 10.1007/s00436-015-4791-6. [DOI] [PubMed] [Google Scholar]
- 225.Uboldi A.D., McCoy J.M., Blume M., Gerlic M., Ferguson D.J., Dagley L.F., Beahan C.T., Stapleton D.I., Gooley P.R., Bacic A. Regulation of starch stores by a Ca 2 -dependent protein kinase is essential for viable cyst development in Toxoplasma gondii. Cell Host Microbe. 2015;18:670–681. doi: 10.1016/j.chom.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 226.Long S., Wang Q., Sibley L.D. Analysis of Noncanonical Calcium-Dependent Protein Kinases in Toxoplasma gondii by Targeted Gene Deletion Using CRISPR/Cas9. Infect. Immun. 2016;84:1262–1273. doi: 10.1128/IAI.01173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Keyloun K.R., Reid M.C., Choi R., Song Y., Fox A.M., Hillesland H.K., Zhang Z., Vidadala R., Merritt E.A., Lau A.O., Maly D.J., Fan E., Barrett L.K., Voorhis V.A.N. W.C.; Ojo, K.K. The gatekeeper residue and beyond: Homologous calcium-dependent protein kinases as drug development targets for veterinarian Apicomplexa parasites. Parasitology. 2014;141:1499–1509. doi: 10.1017/S0031182014000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ojo K.K., Pfander C., Mueller N.R., Burstroem C., Larson E.T., Bryan C.M., Fox A.M., Reid M.C., Johnson S.M., Murphy R.C., Kennedy M., Mann H., Leibly D.J., Hewitt S.N., Verlinde C.L., Kappe S., Merritt E.A., Maly D.J., Billker O., Van Voorhis W.C. Transmission of malaria to mosquitoes blocked by bumped kinase inhibitors. J. Clin. Invest. 2012;122:2301–2305. doi: 10.1172/JCI61822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Van Voorhis W.C., Doggett J.S., Parsons M., Hulverson M.A., Choi R., Arnold S., Riggs M.W., Hemphill A., Howe D.K., Mealey R.H. Extended-spectrum antiprotozoal bumped kinase inhibitors: A review. Exp. Parasitol. 2017;180:71–83. doi: 10.1016/j.exppara.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Winzer P., Muller J., Aguado-Martinez A., Rahman M., Balmer V., Manser V., Ortega-Mora L.M., Ojo K.K., Fan E., Maly D.J., Van Voorhis W.C., Hemphill A. In vitro and in vivo effects of the bumped kinase inhibitor 1294 in the related cyst-forming apicomplexans Toxoplasma gondii and Neospora caninum. Antimicrob. Agents Chemother. 2015;59:6361–6374. doi: 10.1128/AAC.01236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Doggett J.S., Ojo K.K., Fan E., Maly D.J., Van Voorhis W.C. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob. Agents Chemother. 2014;58:3547–3549. doi: 10.1128/AAC.01823-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Müller J., Aguado-Martínez A., Ortega-Mora L., Moreno-Gonzalo J., Ferre I., Hulverson M.A., Choi R., McCloskey M.C., Barrett L.K., Maly D.J. Development of a murine vertical transmission model for Toxoplasma gondii oocyst infection and studies on the efficacy of bumped kinase inhibitor (BKI)-1294 and the naphthoquinone buparvaquone against congenital toxoplasmosis. J. Antimicrob. Chemother. 2017;72(8):2334–2341. doi: 10.1093/jac/dkx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ojo K.K., Reid M.C., Kallur Siddaramaiah L., Muller J., Winzer P., Zhang Z., Keyloun K.R., Vidadala R.S., Merritt E.A., Hol W.G., Maly D.J., Fan E., Van Voorhis W.C., Hemphill A. Neospora caninum calcium-dependent protein kinase 1 is an effective drug target for neosporosis therapy. PLoS One. 2014;9:e92929. doi: 10.1371/journal.pone.0092929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Müller J., Aguado-Martínez A., Balmer V., Maly D.J., Fan E., Ortega-Mora L., Ojo K.K., Van Voorhis W.C., Hemphill A. Two novel calcium-dependent kinase 1-inhibitors interfere with vertical transmission in mice infected with Neospora caninum tachyzoites. Antimicrob. Agents Chemother. 2017;61(4):02324–16. doi: 10.1128/AAC.02324-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lourido S., Jeschke G.R., Turk B.E., Sibley L.D. Exploiting the unique ATP-binding pocket of Toxoplasma calcium-dependent protein kinase 1 to identify its substrates. ACS Chem. Biol. 2013;8:1155–1162. doi: 10.1021/cb400115y. [DOI] [PMC free article] [PubMed] [Google Scholar]