Abstract
The activities of individual cells must be tightly coordinated in order to build and maintain complex 3-dimensional body structures during embryogenesis and regeneration. Thus, one way to view cancer is within systems biology as a network disorder affecting the ability of cells to properly interact with a morphodynamic field of instructive signals that keeps proliferation and migration orchestrated toward the anatomical needs of the host or-ganism. One layer of this set of instructive microenvironmental cues is bioelectrical. Voltage gradients among all somatic cells (not just excitable nerve and muscle) control cell behavior, and the ionic coupling of cells into networks via electrochemical synapses allows them to implement tissue-level patterning decisions. These gradients have been increasingly impli-cated in the induction and suppression of tumorigenesis and metastasis, in the emerging links between developmental bioelectricity to the cancer problem. Consistent with the well-known role of neurotransmitter molecules in transducing electrical activity to downstream cascades in the brain, serotonergic signaling has likewise been implicated in cancer. Here, we review these recent data and propose new approaches for manipulating bioelectric and neurotransmitter pathways in cancer biology based on a bioelectric view of cancer. To sup-port this methodology, we present new data on the effects of the SSRI Prozac and its analog (ZINC ID = ZINC06811610) on survival of both cancer (MCF7) and normal (MCF10A) breast cells exposed to these compounds. We found an IC50 concentration (25 μM for Pro-zac and 100 μM for the Prozac analog) at which these compounds inhibited tumor cell sur-vival and proliferation. Additionally, at these concentrations, we did not observe alterations in a non-tumoral cell line. This constitutes a proof-of-concept demonstration for our hy-pothesis that the use of both existing and novel drugs as electroceuticals could serve as an alternative to highly toxic chemotherapy strategies replacing or augmenting them with less toxic alternatives. We believe this new approach forms an exciting roadmap for future bio-medical advances.
Keywords: Ion channels, serotonin, neurotransmitter, bioelectricity, biophysics, resting potential, Prozac, SSRI
1. Introduction: cancer as a developmental disorder
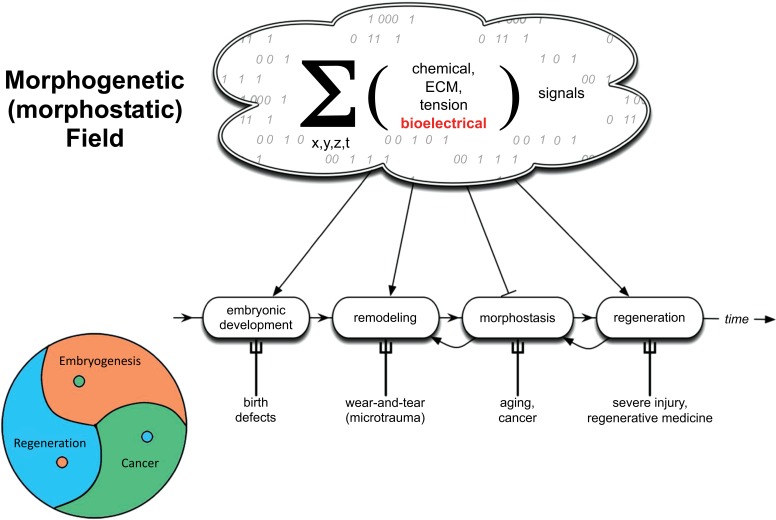
A defining feature of multicellular life is pattern homeostasis: the establishment and maintenance of a complex bodyplan during embryogenesis, and its upkeep during remodeling, wound healing, and aging. Some animals have developed this property to a remarkable degree, such as salamanders that fully regenerate amputated limbs, eyes, jaws, and spinal cords [1-3]. The single thread that connects all of these processes is the need to subjugate single cell activities to the anatomical needs of the host organism. Cell proliferation, differentiation, migration, apoptosis, and other functions are normally harnessed to large-scale morphogenesis by a complex system of instructive cues that functionally determines cell behavior. This morphodynamic field [4] is the sum total of signals that impinge upon cells in vivo, carrying information from distant regions that is necessary for cells to implement coordinated patterning at a large scale (Fig. 1). It has long been recognized that such a process necessarily runs a risk of cells becoming unable to interact properly with the somatic bodyplan [5-7]. This would result in cells reverting to a unicellular mode in which they survive and multiply as any life form tries to do, at the expense of the environment within which it lives [8, 9]. Thus, one view of cancer is as a developmental disorder [7, 10-13]: a network disease of the complex signaling pathways that normally keep cells away from tumorigenesis and toward correct anatomical structure. Supporting this view is an evidence that regenerative and embryonic environments, which feature very strong instructive patterning influences applied to cells, are well-known to be able to normalize tumor cells (Fig. 2, [14-16] and [17]).
Fig. (1).
Morphogenetic/morphostatic fields
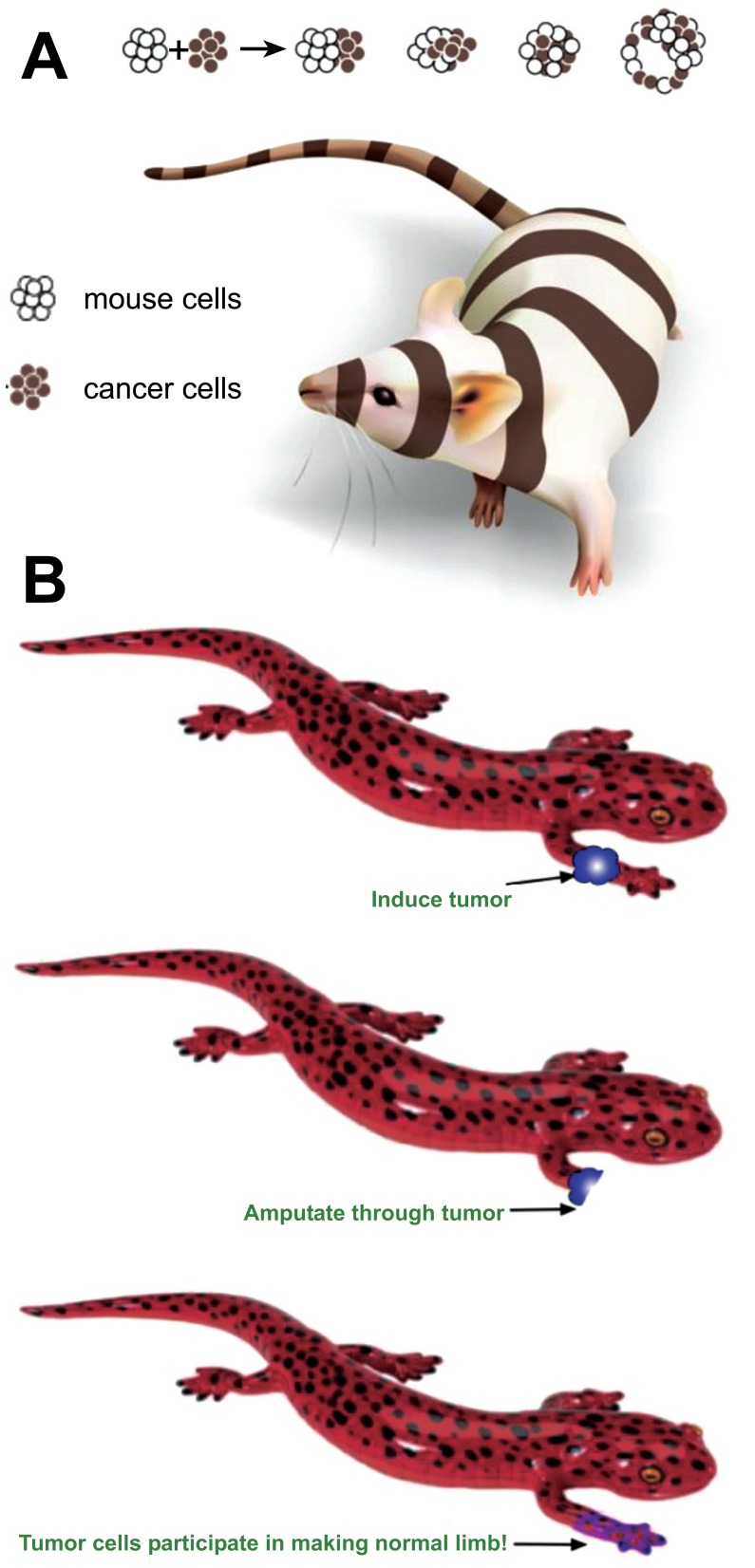
Fig. (2).
Normalization of cancer by actively patterning environments.
(A) Cancer cells introduced into embryonic environments are able to participate in normal development and contribute to healthy organs [104, 130]. (B) Similarly, tumors induced on limbs of regenerative species become normalized if the process of regeneration is induced [131-134]. These examples reveal that at least in some cases, cancer is not irrevocable cell-autonomous damage but a reversible network disorder – an error of communication and geometry [135]. Images courtesy of Jeremy Guay of Peregrine Creative.
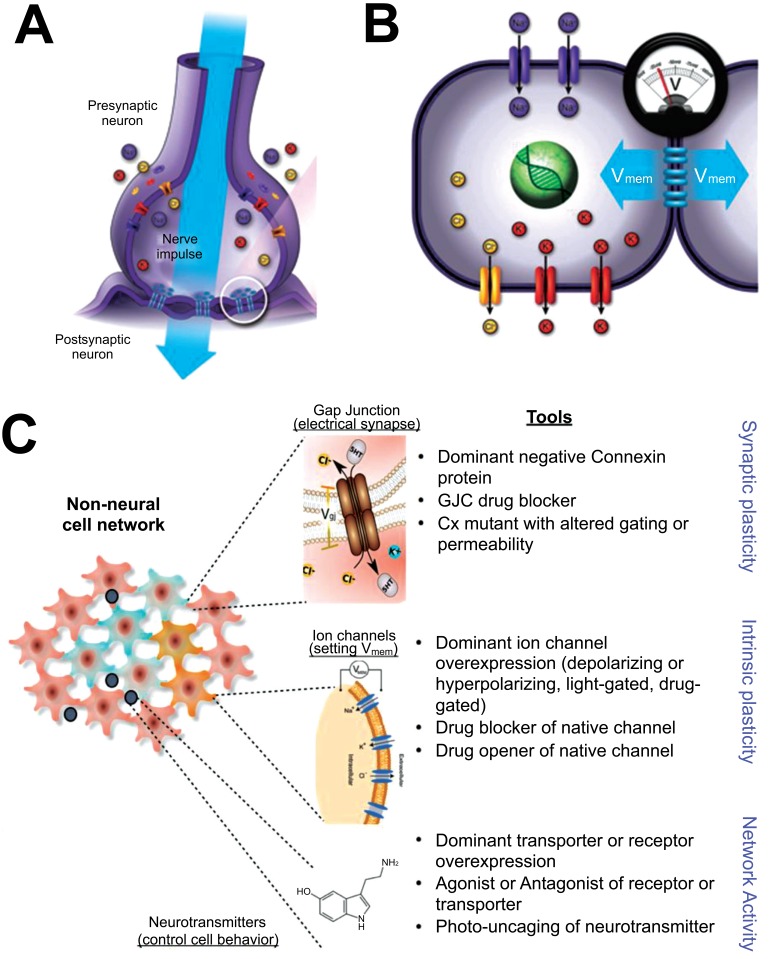
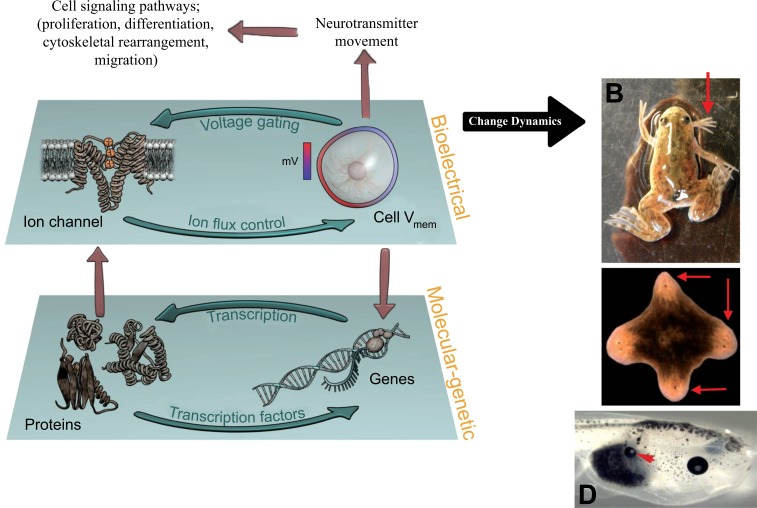
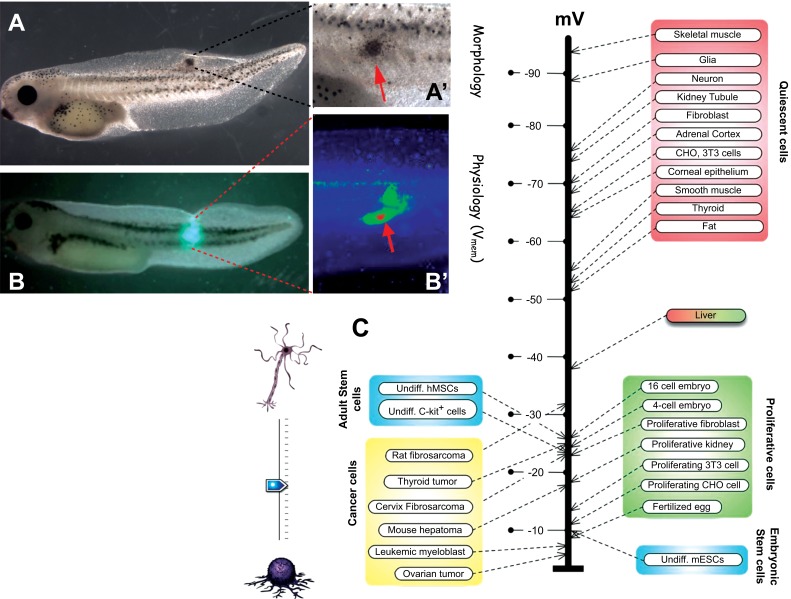
Here, we focus on the role of one signaling modality that underlies this multiscale pattern regulation: non-neural bioelectricity [18-21]. It is perhaps not widely appreciated that the use of electrical signaling by the brain is not an original invention: nervous systems evolved by exploiting ionic signaling that was ancient, and used by many kinds of cells to coordinate physiological signaling, aneural behavior patterns, and morphogenesis [22, 23]. Ion channels and electrical synapses (gap junctions, which function alongside the more familiar chemical synapses, especially outside the CNS) (Fig. 3) are widely-expressed throughout metazoan bodies, and the resulting electrical dynamics are an important regulatory modality for cell number, cell shape, differentiation, and morphogenesis [24]. Differences in cells’ resting potentials (Vmem) across anatomical distances (Fig. 4) result in instructive physiological prepatterns that determine gene expression domains and subsequent morphogenesis, for example in craniofacial patterning [25, 26] and axial polarity during regeneration [27, 28]. Crucially (Fig. 5), bioelectric signals play a role in long-range coordination of growth and form, regulating the size of brains [29], appendages [30], and even kickstarting the appearance of whole organs [31, 32]. Thus, many of the endpoints that go awry in cancer, including overproliferation, dedifferentiation, vascular patterns, chromatin changes, gene expression, and migratory behavior are all known to be downstream of the activity of bioelectric circuits during normal pattern regulation [18, 21].
Fig. (3).
The basics of bioelectricity.
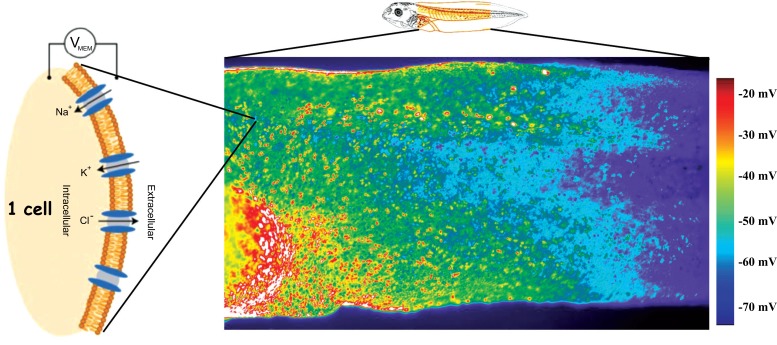
Fig. (4).
Bioelectric gradients.
Fig. (5).
Feedback between bioelectrics and gene regulatory networks regulates patterning.
Each cell in vivo exists within a complex field of information imposed on it by the host organism. This set of signals, impinging on cells from 3 dimensions and varying in time, is mediated by a number of physical properties, including gradients of diffusible chemical messengers, extracellular matrix properties (ECM), tensile/pressure forces, and bioelectric states. The sum total of these instructive cues are responsible for establishment of normal somatic pattern during embryogenesis, and for harnessing cell activity toward the anatomical needs of the host organism throughout its life. It guides cell behaviors during regeneration, remodeling, and on-going pattern homeostasis; the interactions of cells with this guidance system reveals a key common feature among development, regeneration, and cancer, all of which are fundamentally problems of spatio-temporal organization and the imposition of global order on local cell activity. It may be that cancer can be efficiently understood as a disorder of cells’ communication with this field (either because the signals become absent from a region of the environment, or because cells become inattentive to its informational influence); in turn, re-establishing correct morphogenetic cues and forcing cell communication with this set of cues may be a path forward for tumor normalization/reprogramming.
Changes in the biophysical parameter Vmem can be transduced into downstream transcriptional responses by the voltage-regulated movement of neurotransmitter molecules across cell membranes and between cells through gap junctions. These electrical synapses, like ion channels, are ancient and widely present outside the nervous system [33, 34]. Molecules such as serotonin have pre-neural roles, such as in gastrulation, determination of left-right patterning, craniofacial development, and cardiac morphogenesis [35-42]. Together, ion channels, gap junctions, and neurotransmitter signaling machinery form somatic bioelectric circuits that coordinate cell behaviors toward prescribed and limited tissue-level outcomes. One of the key aspects of this control system is that it is fundamentally epigenetic, operating at the level of ion flows and channel gating and invisible in fixed samples or from profiling of transcriptional or translational states. In some cases, this physiological layer dominates genomic defaults; appropriate bioelectric manipulation can rescue normal brain patterning despite defects in important neurogenesis genes such as Notch [29], induce regeneration of spinal cord in non-regenerative animals [43, 44], convert normally non-permissive tissue such as gut into complete eyes [32], and alter the shape [27] and number [45, 46] of heads in regenerating animals.
These data on multi-cellular reprogramming suggest that targeting physiology, not only genetics, may be an effective and dominant strategy for diagnostics and control of cancer. The ability to trigger coherent change in thecell fields into a whole organ [32] or starting the growth of a complex appendage with a simple gradient change [43, 44] suggests that it may be possible to trigger modular patterning: exert organ-level control over a body region without having to micromanage each cell. Long-term, this may be part of a strategy to mimic the ability of regeneration or development to provide patterning cues that dominate individual cell fate and normalize tumors. Thus, we proposed the hypothesis that exploiting the bioelectrical system by which cells coordinate their constraints on growth could lead to advances in cancer biology.
2. Bioelectrics and cancer
Interestingly, while the importance of steady-state Vmem is only recently becoming understood, true neural-like excitability in cancer cells was discovered over 20 years ago [47, 48]. Aside from spiking, steady-state resting potentials are an important regulatory factor (Fig. 6): tumor cells tend to be more depolarized than their normal counterparts (complicated by the fact that Vmem tends to fluctuate somewhat during the mitotic phases, reviewed in [49]), and experimental modulation of resting potential can functionally normalize cells and prevent or reverse tumorigenesis [50-52]. Recent work has extended this observation into testing roles of individual ion channels as bona fide oncogenes (Table 1), ion channel expression profiles as markers [53, 54], and drug approaches targeting specific channels for cancer therapeutics [55]. Role of gap junctions in cancer has been covered extensively [56, 57], and these too are currently an exciting target for cancer therapies [58, 59].
Fig. (6).
Resting potential and cancer signatures
Table 1.
list of ion channel oncogenes.
| Ion Translocator Protein | Species | References | Cancer-relevant role |
|---|---|---|---|
| NaV1.5 sodium channel | Human | [109, 110] | Oncogene |
| ERG potassium channels | Human | [111-113] | Oncogene |
| KCNK9 potassium channel | Mouse | [114] | Oncogene |
| Ductin (proton V-ATPase component) | Mouse | [115] | Oncogene |
| SLC5A8 sodium/butyrate transporter | Human | [116] | Oncogene |
| KCNE2 potassium channel | Mouse | [117] | Oncogene |
| KCNQ1 potassium channel | Human, mouse | [118-120] | Oncogene |
| SCN5A voltage-gated sodium channel | Human | [121] | Oncogene |
| Metabotropic glutamate receptor | Mouse, Human | [122-124] | Oncogene |
| CFTR chloride channel | Human | [125, 126] | Tumor suppressor |
| Connexin43 | Human | [127] | Tumor suppressor |
| BKCa | Human | [128] | Oncogene |
| Muscarinic Acetylcholine receptor | Human, mouse | [129] | Tumor suppressor |
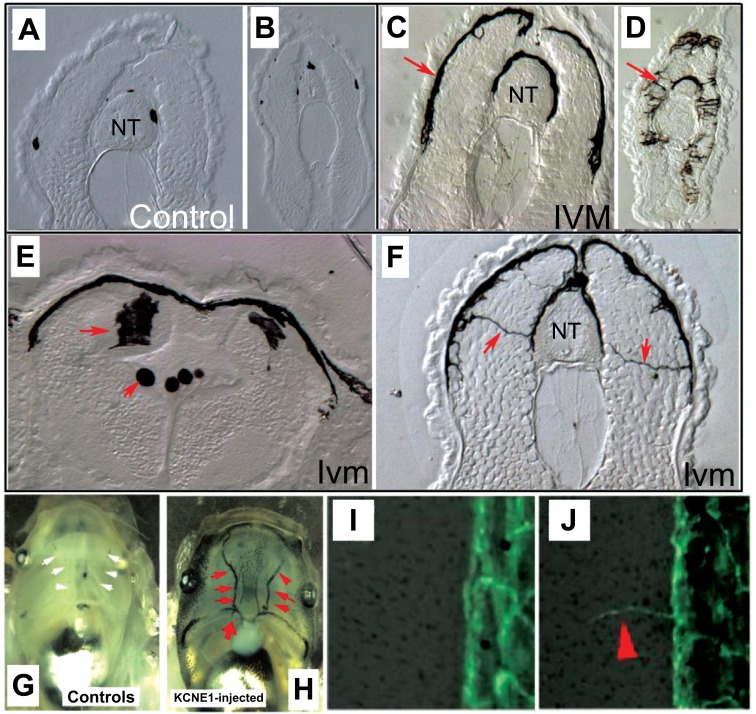
Subsequent experiments suggested roles of resting potential per se (generated by one of any number of electrogenic proteins) in activating or suppressing cancer-relevant processes in vivo. Most of these data remain to be validated in mammalian models, being first derived in the frog Xenopus laevis [17]. Artificial depolarization of a selected cell population of cells was sufficient to induce a melanoma-like phenotype in normal animals’ pigment cell population [60, 61]. This metastatic conversion (Fig. 7) takes place in a wild-type background, not exposed to any mutagen or carcinogen; it features stochastic outcomes within a cohort of animals exposed to the same manipulation [62], and may be a model for the variability of cancer incidence in clinical settings.
Fig. (7).
Metastasis induced by depolarization.
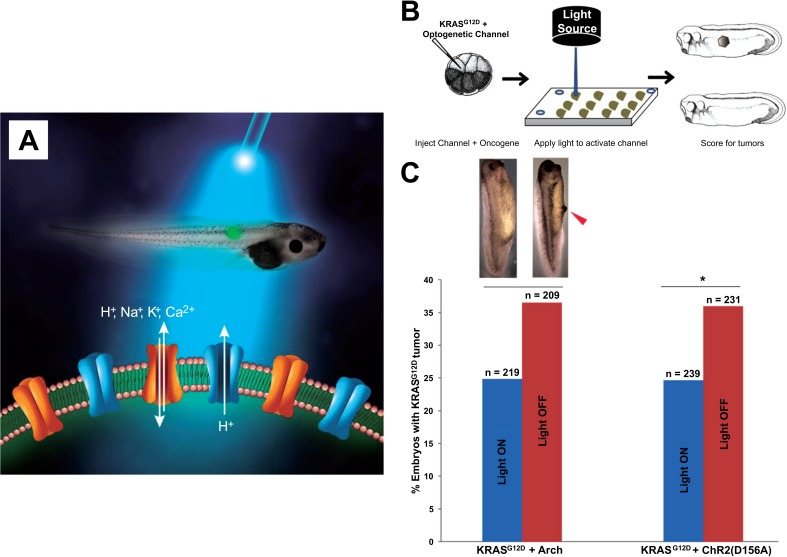
Conversely, tumorigenesis induced by expression of human oncogenes in frog larvae can be over-ridden by appropriate modulation of resting potential [63]. This also works at considerable distance via a gap junction-dependent mechanism [64, 65], and tumors can be prevented or reverted to normal by pharmacological, genetic, or even light-based stimuli (Fig. 8) that induce hyperpolarization [65, 66]. This was another example of the over-riding of genetic state by physiological parameters, as the mutant oncogene was strongly present in the regions in which tumorigenesis was prevented. In normal cases, the appearance of tumor structures can be observed very early, by fluorescent signals from a voltage reporting dye [60].
Fig. (8).
Non-cell-autonomous signaling via serotonin underlies voltage-mediated conversion to metastasis.
A few overall lessons emerged from this most recent body of work. First, that it is imperative to focus on the physiological state (Vmem and cellular connectivity), not individual channel genes, since channels open and close post-translationally, while Vmem is the sum of numerous channels’ activity. Thus, the same effect can be obtained via the action of any number of channels that contribute to overall resting potential, and Vmem can change in the absence of detectable changes of channel mRNA or protein levels. Thus, tracking individual channels or using profiling of fixed (non-living) tissue can give confusing results due to the lack of one-to-one correspondence between genetic and physiological states. Second, the effects are often non-local: metastatic or tumorigenic outcomes can be a function of bioelectric states at considerable distances – the signaling is definitely not cell-autonomous, although the maximum distance (size of the “microenvironment”) that might be involved in mammals is unknown. Finally, that the physiological signaling of the environment is a key determinant of outcome which cannot be predicted from molecular profiling alone.
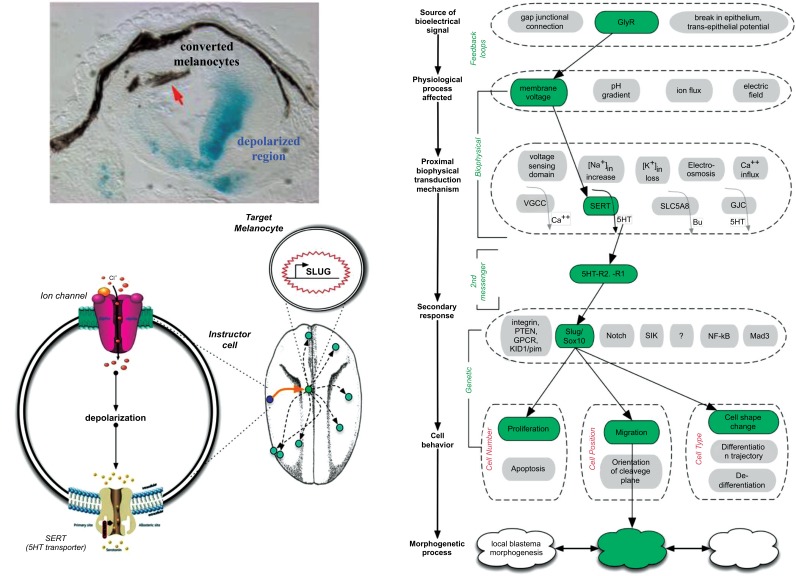
We suggest that the multitude of ion channel drugs, many of which are already approved for human use (as antiepileptics, antiarrhythmic agents, etc.) and some of which are kept on pharmaceutical company shelves, form a powerful toolkit. These compounds are potential electroceuticals; while such approaches have so far been focused on neural targets [67, 68], the existence of ion channel-regulated bioelectric signaling in many cell types [69-73] and the plethora of ion channel drugs suggest that the next advances in this field will include expanding the use of electroceuticals to harness them in modulating cancer processes. Work dissecting the mechanisms by which bioelectric signals regulate melanocyte conversion implicated serotonergic signaling (Fig. 9) [17, 60-62]. Depolarized cells produce a serotonin signal that causes melanocytes to undergo a change toward highly metastatic behavior. The characterization of this pathway suggested that blocking serotonin movement across cell membranes could be a promising strategy for the suppression of metastases. Luckily, human-compatible drugs already exist that do precisely this: the class of widely-used serotonin reuptake inhibitors (SSRIs) [74].
Fig. (9).
Optogenetic approaches to cancer: light gating of electrical cues.
3. Neurotransmitters and cancer
In the nervous system, electrical activity of neurons is coupled to important changes in cell behavior via the movement of neurotransmitter molecules. Voltage changes alter the import/secretion of neurotransmitters across membranes. Neurotransmitters such as serotonin are potent mitogens and have many other effects on transcription, cytoskeleton, and cell metabolism. In this way, neurotransmitter dynamics cross cell membranes are a crucial link between bioelectric states and cell behaviors they control. Recent work has shown this relationship to be true outside of the CNS as well [33, 75, 76].
Neurotransmitter signaling in cancer biology has attracted attention to molecules such as glutamate, glycine, acetylcholine, GABA, and dopamine [77-81]. Some of the most interesting data implicate serotonin [82-88], although the epidemiological picture is complicated by the fact that SSRIs are most often used on depressed patients who are undergoing stresses that may alter the
The familiar components of electrical signaling in neurons include resting potential, generated by the activity of ion channels in the cell membrane, and the electrical synapse which allows current to flow into neighboring cells (A). These electrical synapses occur in the CNS alongside the much more familiar chemical synapses [136-138], but are especially crucial outside of excitable tissues for cell regulation and tumor suppression [139-142]. These same components are present in most somatic cells (B), which likewise generate resting potentials and share them with their neighbors using the exact same ion channel and gap junction proteins. Even non-excitable tissues are composed of cell networks (C) in which cells coordinate activity and execute group decision-making during pattern generation and maintenance. These networks signal via the voltage-dependent movement of small molecules such as serotonin and other neurotransmitters. All of these components have been implicated in various stages of the cancer process. The result of this signaling is growth control, differentiation, pattern regulation – processes that go awry in cancer. Molecular tools now exist for manipulating bioelectric state of cells (intrinsic plasticity), connectivity of the bioelectric network (synaptic plasticity), or the movement of neurotransmitters within the tissue (network activity). Images courtesy of Jeremy Guay of Peregrine Creative and Alexis Pietak.
Each cell in the organism uses ion channels and pumps to maintain a resting potential (Vmem) across its plasma membrane (in addition to a number of subcellular gradients, such as nuclear envelope potential, and tissue-level gradients, such as trans-epithelial electric fields). Spatial patterns of resting potential can be detected in vivo using voltage-sensitive fluorescent dyes. Here, a fluorescent voltage reporter (Rhodamine 6G dye [143, 144], courtesy of Douglas J. Blackiston) applied to a stage 44 frog embryo (Xenopus laevis) illustrates an anterior-posterior physiological gradient across the middle flank of a frog tadpole. The signal has been pseudocolored according to a scale where red is depolarized and purple is hyper-polarized. Such gradients, and their time-dependent changes, reflect the activity of the bioelectric circuits that set up prepatterns driving downstream gene expression and morphogenesis. Schematic of frog embryo was used via Xenbase, originally sourced from [145]. Dye map image courtesy of Douglas Blackison. (The color version of the figure is available in the electronic copy of the article).
Cells are regulated by at least two layers of activity: the molecular genetic and the bioelectric (A). The molecular-genetic layer of pattern control is implemented by gene regulatory networks in which transcription factors control each other’s expression. The bioelectric layer of control is formed by the reciprocal interactions between ion channels and gap junctions, and cell resting potential: Vmem is determined by channels and electrical synapses, and at the same time, Vmem determines the gating properties of voltage sensitive channels and gap junctions. Thus, every cell has the opportunity to drive positive feedback loops that can amplify small differences (useful for spontaneous symmetry breaking) or negative feedback loops that confer stability and robustness to environmental stimuli. The genetic and bioelectric layers are functionally coupled, since Vmem changes can alter transcription of downstream target genes [146, 147], while changes in the transcription of ion channel genes alter electric circuit dynamics. Recent data have shown that manipulation of the complex dynamics of the bioelectrical layer reveal its endogenous patterning functions, resulting in the production of frogs with ectopic limbs (B), flatworms with 4 heads (C), or tadpoles in which gut tissue has been reprogrammed into complete eyes (D) [32, 46]. Panel A was drawn by Alexis Pietak. The ectopic-limb frog in panel B was obtained from an optogenetic transgenic EnPAC [148] line produced by Gufa Lin. Panels C and D come from references [46] and [32] respectively.
Human oncogenes, when introduced into tadpoles, cause the formation of tumor-like structures (A, closeup in A’) which exhibit all of the key characteristics of cancer (over-proliferation, tissue disorganization, invasiveness, cancer marker gene expression, etc.). Because aberrant bioelectric signaling is an early component of the carcinogenic process, this transformation is observed via voltage-sensitive fluorescent dye signaling (B, closeup in B) in vivo, revealing the tumor sites and margins before they become anatomically apparent. Consistent with a reversion of cancer into a unicellular phenotype, a wide range of data [149, 150] reveal that tumor cells, like stem and early embryonic cells, tend to be depolarized while mature, quiescent somatic cells tend to be hyperpolarized (C). Importantly, it is now known that this relationship is not merely a marker, but is actually functionally determinative of cell behavior [51, 52, 63, 151]. Panels A-B’ are taken from [63]. The schematic of panel C was drawn by Jeremy Guay of Peregrine Creative, while the voltage diagram of panel C is modified after [149].
background of carcinogenic induction and progression. Our strategy, based on the finding that blockade of serotonin transport efficiently rescued voltage-induced melanoma conversion [61, 62], was to explore an SSRI drug that did not cross the blood-brain barrier (an unusual requirement, since all known SSRIs were designed specifically for access to the brain). Such a compound could be expected to exert its protective effects throughout the body without causing the unwanted cognitive effects of SSRIs [89, 90]. Thus, we tested Prozac and an analog in vitro, to begin to characterize the function of molecules suggested by the above strategy. An additional component that must be considered is the cytoskeleton, as it is known to regulate the distribution of ion channels in a variety of cell types [91, 92]. It is also known that ion channels regulate NMDA receptors through microtubules [93]. Moreover, voltage-dependent anion channels are controlled by the c-termini of tubulin [94]. This taken together with previous observations indicates that the microtubule cytoskeleton and ion channels are involved in significant interactions such that regulation of one of these subsystems affects the other. Hence, ion channel regulators may cause downstream effects on microtubules and consequently the use of SSRIs could lead to hitherto unknown effects on cancer cells with potential therapeutic applications.
The cytoskeleton is a target for numerous chemotherapy drugs, many of which bind preferentially to tubulin (so-called tubulin-binding agents or TBAs), such as paclitaxel, vinca alkaloids, laulimalide, peloruside and others [95]. Some of these compounds stabilize microtubules (e.g. taxanes) while others destabilize them (e.g. vinca alkaloids). Interestingly, it has been also discovered that other classes of compounds, e.g. opioids such as noscapine [96] interact with microtubules as well as anesthetics [97]. It is, therefore, not unexpected that compounds which alter mood (psychoactives), for example the antidepressants such as Prozac and its analogs should also interact with microtubules and the cytoskeleton. It is known that they take several weeks for these compounds to achieve their clinical effects in human subjects, apparently because of the need
The black cells in these histological sections of a frog tadpole are melanocytes, pigment cells that normally have a round morphology. Here are shown sections of frog larvae in which a specific cell subpopulation has been artificially depolarized, but no other carcinogenic or mutagenic treatment was applied. In the context of a wild-type genome, the normally small number of round melanocytes seen in sections through the anterior torso (A) and the tail (B) become highly arborized and over-proliferative (C, D). These cells become highly migratory, invading the brain and neural tube (E, red arrows) and extend long processes as they reach throughout lateral tissues (F, red arrow). They also invade blood vessels, which are normally free of melanocytes (G) but become choked with these cells after depolarization by pharmacological agents [61] or injection of dominant negative potassium channel subunits (H). These animals’ normal vasculature (I) also begins to overgrow (J). Together, these data reveal that depolarization can trigger the key aspects of metastatic melanoma: rapid over-growth, cell shape change, and invasiveness. Panels A-F are taken from [61], panels G,H are taken from [152], and panels I,J are taken from [60]. (The color version of the figure is available in the electronic copy of the article).
for cytoskeletal reconfiguration. Anesthetics also appear to act in microtubules to prevent consciousness, not exclusively on membrane proteins as has been previously assumed [97]. However the effects of anesthesia cannot be readily explained via a simple mechanistic mode of action yet. Conversely, it is highly probable that psychoactive compounds should exert cytotoxic effects on cancer cells since microtubules are not only fundamentally important to neuronal cells but are the essential part of the mitotic apparatus (spindles) in dividing cells. Recently, a study was published that reports computational docking of three specific psychotropic compounds Lysergic Acid Diethylamide (LSD-25), heroin (morphine diacetate) and cocaine (benzoylmethylecgonine) to tubulin [89]. It demonstrated significant binding affinity of these compounds to tubulin with unique binding modes and locations compared to the standard control, chemotherapy drug paclitaxel, which has no known involvement in the cognitive process. It was also predicted that the binding affinity of these psychotropic drugs strongly depends on the conformational state of the tubulin dimer. Most importantly, this points to the possibility of off-target interactions of psychoactive drugs, not only binding to their standard receptor sites in neurons but also affecting the cytoskeleton and hence affecting other cellular functions including cell division of dividing cells including cancer cells.
Remarkably, the conversion of normal melanocytes to melanoma is not cell-autonomous (A): the depolarized ventral cells in this section through the tadpole trunk are marked with blue via b-galactosidase staining, which is at a distance from the cells that respond and convert (the dorsal melanocytes, red arrow). Mechanistic dissection of this process [60] revealed that the long-range communication is mediated by the voltage-dependent release of serotonin from instructor cells (a widely-distributed population that, when depolarized, releases serotonin that activates genes such as SLUG and other aspects of epithelial-to-mesenchymal transition in normal melanocytes). The whole process is shown in panel C, which reveals how a bioelectric signal is transduced and results in altered system-wide cell behavior. Panels A-B are taken from [152]. (The color version of the figure is available in the electronic copy of the article).
4. Effects of Psychoactive Compounds on Cancer and Normal Breast Cells
A biophysical perspective suggests serotonergic signaling to be an important control point in the cancer problem, both because of its role as a transducer of bioelectric state and because of its interaction with the cytoskeleton. To examine some of these predictions in a proof-of-concept investigation, we have exposed normal and cancer breast cells to Prozac (Fluoxetine) and its close analog. Fluoxetine, also known by trade names Prozac is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. Its chemical formula is C17H18F3NO and its molecular weight is 309.33. It is used for the treatment of major depressive disorder, OCD, bulimia nervosa, panic disorder, and premenstrual dysphoric disorder. The analog chosen was: ZINC ID = ZINC06811610 (http://zinc.docking.org/) with a chemical formula C15H23NO4 and a popular name = 2-[3-hydroxy-3-(4-propoxyphenyl)-propyl] aminopropanoic. It has a polar surface area = 86 in the units of Angstrom squared. Its molecular weight is 281.352. This is one of several compounds similar to Prozac that can be found in the ZINC database, which may have high DMSO solubility because of smaller radii of gyration and lower lipophilicity and hence are also less likely to cross the blood brain barrier.
Optogenetics [153-155] is the use of light-gated ion channels to gain spatio-temporal control of bioelectrical state of cells in vivo (A). Recent data have shown that when light-gated channels such as channelrhodopsin are co-injected with oncogenes (B), the incidence of resulting tumors can be reduced by light exposure which forces hyperpolarization and thus antagonizes the steps by which oncoproteins transform cells. This occurs by prevention of tumor formation and tumor normalization. Such therapies, especially when combined with novel chemical strategies to render ion cannels light-gated without the use of gene therapy [156, 157], are a promising novel path forward for cancer treatments that focus on tumor reprogramming instead of targeted toxicity. Panel A was drawn by Jeremy Guay of Peregrine Creative, while panels B, C are taken from [66].
The binding affinities of Fluoxetine for its receptor targets is in the range of 1 nM (for SERT) to 72.6 nM (5-HT2C, 200nM for 5-HT2A and 4 uM for 5-HT2B [98, 99]. A number of other receptors are characterized by Fluoxetine’s affinity in the low micromolar range (e.g. M1, M2, M3, M4, M5 and H1). To the best of our knowledge, it’s affinity for tubulin has not been reported so far. Based on previous studies of binding of psychoactive compounds to tubulin mentioned above we expect Fluoxetine to have an off-target interaction with tubulin and microtubules and hence cytotoxic properties we examined and report here for the first time.
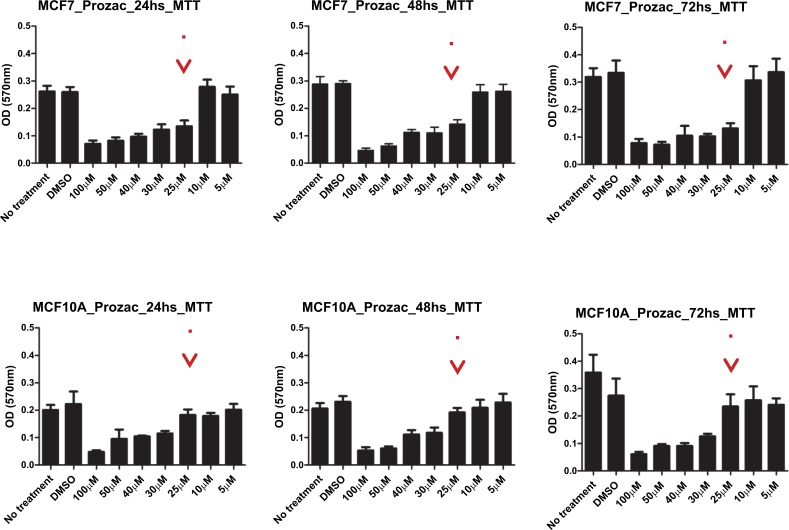
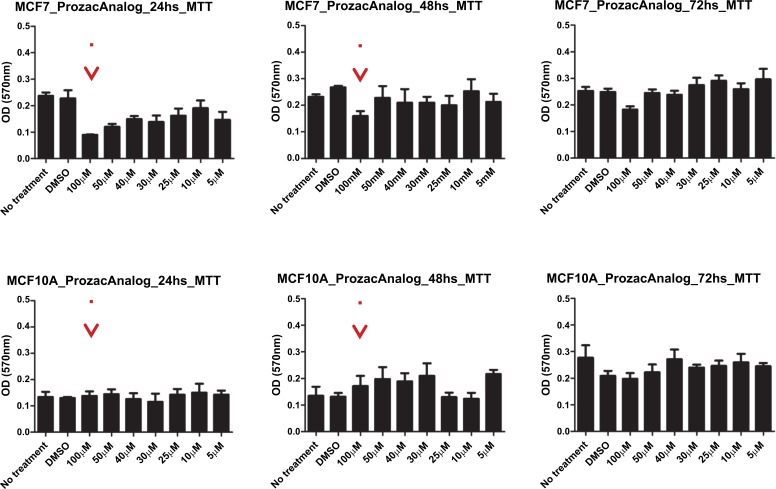
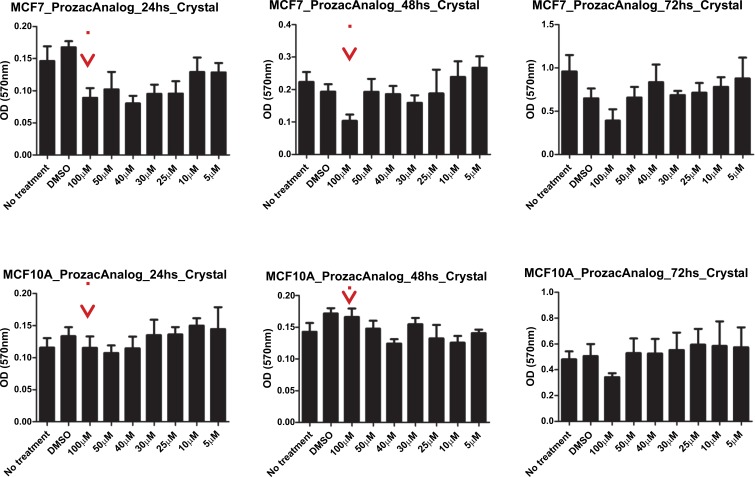
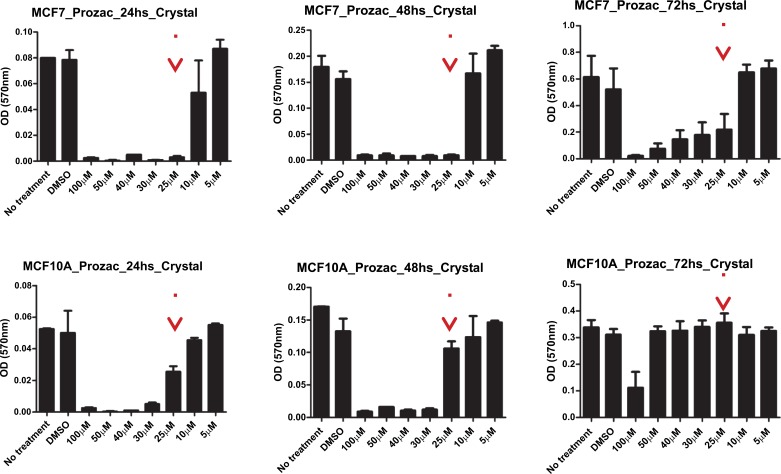
The survival of two cell lines: breast cancer cell line and a corresponding normal breast cell line (MCF7 and MCF10A) after exposure to the ligands was evaluated by an MTT and a proliferation assay. Here, MCF-10A was used as a reference since its immortal transformation allows its in vitro culture and it is not malignant. We performed an MTT assay to determine the survival of these cell lines and a Crystal violet staining assay for proliferation analysis for both compounds. In Figs. (10) and (11) we show the data for the MTT and proliferation assays for Prozac while in Figs. (12) and (13) similar data are shown for the Prozac analog. We found an IC50 value for the concentration of these compounds (25 μM for Prozac and 100 μM for the Prozac analog) at which these compounds inhibited tumor cell survival and proliferation. Additionally, at these concentrations we did not observe alterations in a non-tumoral cell (see the panels in Figs. (10-13) corresponding to MCF-10A). While these IC50 concentrations are higher than the concentration values for the kinetically determined affinities for the primary receptor targets of Prozac mentioned above, they are not negligible especially in view of pharmacokinetic considerations that may be brought to bear on the delivery of these compounds to the tumor site. Importantly, the bioavailability of fluoxetine is very high (72%), and peak plasma concentrations are reached in 6–8 hours. It is highly bound to plasma proteins, mostly albumin and α1-glycoprotein [z]. The extremely slow elimination of fluoxetine and its active metabolite norfluoxetine from the body distinguishes it from other antidepressants. Fluoxetine elimination half-life changes from 1 to 3 days, after a single dose, to 4 to 6 days, after long-term use [100]. Its therapeutic dose for depression depends ranges between 10 and 80 mg a day and a maximum dose is 90 mg. The latter translates into an approximate concentration in the blood at 300 mM, which is much higher than the IC50 value determined in this study.
Fig. (10).
MMT survival assay results for Prozac-exposed MCF7 breast cancer cells (top panel) and MCF10A non-malignant breast cells (bottom panel) at 24h, 48h and 72 h, respectively, from left to right.
Fig. (12).
MMT survival assay results for the Prozac analog-exposed MCF7 breast cancer cells (top panel) and MCF10A non-malignant breast cells (bottom panel) at 24h, 48h and 72 h, respectively, from left to right.
Fig. (13).
Crystal violet staining proliferation assay results for the Prozac analog-exposed MCF7 breast cancer cells (top panel) and MCF10A non-malignant breast cells (bottom panel) at 24h, 48h and 72 h, respectively, from left to right.
5. Materials and Methods
5.1. Reagents, Cell Lines and Culture Conditions
Unless stated otherwise, all chemical and tissue culture reagents were purchased from Sigma-Aldrich (Oakville, Canada) and tissue culture reagents from Invitrogen (Burlington, Canada), respectively. MCF-7 (Luminal A) and the non-tumoral breast epithelial cell line MCF-10A were maintained in MEM media supplemented with 10% FBS and 1% PSK.
5.2. Cell Proliferation and Survival Assays
For cell growth, replicate cultures were established 48 h after transfection in 24-well plates (Sarstedt, Canada) at 5 x 104 cells/well. At 24, 48, 72 and 96 h after plating, cultures were trypsinized, stained with trypan blue and counted using hemocytometer. The total number of cells/well for each cell line was calculated and plotted for each time point. For further verification, we also assessed cell proliferation by crystal violet staining. Cells were plated in 96-well microtiter plates at 5 x 103 cells/well. At specific time points, cells were washed with PBS, fixed with glutaraldehyde for 10 min, and stained with 0.1% (W/V) crystal violet in 0.2% (V/V) Triton X-100. Microtiter plates were read on a spectrophotometer at 570 nm.
For cell survival, cells were plated in 96-well plate at 5 x 103 cells/well. At 24, 48, 72 and 96 h, 20 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) was added to each well, and the plates were incubated at 37°C for another 4 h at which time the resulting formazan crystals were solubilized by the addition of 200 μl of MTT solubilization solution. The absorbance at 570 nm was recorded using a microplate reader (Bio Tek Instruments, Winooski, VT, USA). Each experiment for each cell lines was repeated 3-5 times.
Conclusion
Cancer is the uncontrolled cell growth in which the cells show invasive intrusion or destruction of adjacent tissues and metastasize to other locations in the body via lymph and /or blood. Cancer cells escape the normal control of cell division and programmed cell death. The conventional methods to treat metastatic cancer are chemotherapy and radiation therapy. But these have a major drawback of producing severe side effects as they cannot differentiate between the cancerous cells and the normal cells. The “normal” cells most commonly affected by chemotherapy are the blood cells, the cells in the mouth, stomach and bowel, and the hair follicles; resulting in low blood counts, mouth sores, nausea, diarrhea, and/or hair loss. Therefore, recently the focus has shifted to comparatively newer methods of cancer treatment, i.e. targeted cancer therapy, which uses drugs or other substances to identify and attack cancer cells thereby avoiding any damage to normal cells. In this paper, we have advanced a new hypothesis that reprogramming of tumor cells can be achieved by avoiding toxic chemotherapy and instead by using a completely different set of pharmacological agents, in particular psychoactive compounds. Their repurposing can be achieved by rational drug design [101] involving derivatization that should take into account the molecular structure of the target and such aspects as blood-brain-barrier permeation.
A view of cancer as a disease of pattern coordination is complementary and distinct from the prevailing paradigm that sees cancer as arising from genetically damaged cells, which are irrevocably broken [102, 103]. It has long been known that appropriate patterning environments, such as embryos and regenerating limbs [15, 104], can normalize aggressive transformed cells. Likewise, cancer can be induced by factors such as denervation, barriers made from non-carcinogenic substances, and even apposition of healthy but ectopic tissues [17]. It is clear that at least in some cases, the process is fundamentally physiological, not genetic, and thus could potentially be reversed [105, 106]. Strategies which seek to manipulate the environment to normalize or reprogram tumors [107, 108], and thus avoid toxic chemotherapy and the compensatory growth and tumor evolution that plagues conventional approaches, could be an important frontier in this field.
Future work in this emerging field will focus on the role of neurotransmitters in mediating long-range growth control signals, resulting in a better understanding of the limits of the “microenvironment” and strategies for manipulating endogenous morphogenetic cues for cancer prevention and normalization. The numerous drugs emerging from psychopharmacology form an exciting toolkit with which to address the subcellular and network-level mechanisms that go awry in cancer. In parallel with the modeling and in vivo studies of neurotransmitter pathways in computational psychiatry, a combined understanding of cytoskeletal, bioelectric, and neurotransmitter signaling in cellular networks is likely to have profound implications for novel approaches to the cancer problem.
Fig. (11).
Crystal violet staining proliferation assay results for Prozac-exposed MCF7 breast cancer cells (top panel) and MCF10A non-malignant breast cells (bottom panel) at 24h, 48h and 72 h, respectively, from left to right.
Acknowledgements
JT acknowledges support from the Canadian Breast Cancer Foundation, the Allard Foundation and the Alberta Cancer Foundation. M.L. gratefully acknowledges support by an Allen Discovery Center award from the Paul G. Allen Frontiers Group (No. 12171) and by the G. Harold and Leila Y. Mathers Foundation.
List of Abbreviations
- 5-HT
Serotonin
- SSRI
Selective Serotonin Reuptake Inhibitors
- Vmem
Resting Potential Across Plasma Membrane
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Knapp D., Tanaka E.M. Regeneration and reprogramming. Curr. Opin. Genet. Dev. 2012;22:485–493. doi: 10.1016/j.gde.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Stocum D.L., Cameron J.A. Looking proximally and distally: 100 years of limb regeneration and beyond. Dev. Dyn. 2011;240:943–968. doi: 10.1002/dvdy.22553. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum K.D., Alvarado A.S. Slicing across kingdoms: Regeneration in plants and animals. Cell. 2008;132:697–10. doi: 10.1016/j.cell.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin M. Morphogenetic fields in embryogenesis, regeneration, and cancer: Non-local control of complex patterning. Biosystems. 2012;109:243–261. doi: 10.1016/j.biosystems.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burr H.S. Biologic organization and the cancer problem. Yale J. Biol. Med. 1940;12:277–282. [PMC free article] [PubMed] [Google Scholar]
- 6.Needham J. Order and life. Cambridge: M.I.T. Press; 1968. [Google Scholar]
- 7.Rubin H. Cancer as a dynamic developmental disorder. Cancer Res. 1985;45:2935–2942. [PubMed] [Google Scholar]
- 8.Johnston R.N., Pai S.B., Pai R.B. The origin of the cancer cell: Oncogeny reverses phylogeny. Biochem. Cell Biol. 1992;70:831–834. doi: 10.1139/o92-130. [DOI] [PubMed] [Google Scholar]
- 9.Davies P.C., Demetrius L., Tuszynski J.A. Cancer as a dynamical phase transition. Theor. Biol. Med. Model. 2011;8:30. doi: 10.1186/1742-4682-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsonis P.A. Embryogenesis and carcinogenesis: Order and disorder. Anticancer Res. 1987;7:617–623. [PubMed] [Google Scholar]
- 11.Dean M. Cancer as a complex developmental disorder--nineteenth Cornelius P. Rhoads Memorial Award Lecture. Cancer Res. 1998;58:5633–5636. [PubMed] [Google Scholar]
- 12.Aranda-Anzaldo A. Towards a morphogenetic perspective on cancer. Riv. Biol. 2002;95:35–61. [PubMed] [Google Scholar]
- 13.Needham J. New advances in the chemistry and biology of organized growth: (Section of Pathology). Proc. R. Soc. Med. 1936;29:1577–1626. [PMC free article] [PubMed] [Google Scholar]
- 14.Kasemeier-Kulesa J.C., Teddy J.M., Postovit L.M., et al. Reprogramming multipotent tumor cells with the embryonic neural crest microenvironment. Dev. Dyn. 2008;237:2657–2666. doi: 10.1002/dvdy.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendrix M.J., Seftor E.A., Seftor R.E., Kasemeier-Kulesa J., Kulesa P.M., Postovit L.M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 16.Kulesa P.M., Kasemeier-Kulesa J.C., Teddy J.M., et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc. Natl. Acad. Sci. USA. 2006;103:3752–3757. doi: 10.1073/pnas.0506977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chernet B, Levin M. Endogenous voltage potentials and the microenvironment: bioelectric signals that reveal, induce and normalize cancer. J Clin Exp Oncol. 2013. [DOI] [PMC free article] [PubMed]
- 18.Levin M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell. 2014;25:3835–3850. doi: 10.1091/mbc.E13-12-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin M., Stevenson C.G. Regulation of cell behavior and tissue patterning by bioelectrical signals: Challenges and opportunities for biomedical engineering. Annu. Rev. Biomed. Eng. 2012;14:295–323. doi: 10.1146/annurev-bioeng-071811-150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin M. Molecular bioelectricity in developmental biology: New tools and recent discoveries: Control of cell behavior and pattern formation by transmembrane potential gradients. BioEssays. 2012;34:205–217. doi: 10.1002/bies.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates E. Ion channels in development and cancer. Annu. Rev. Cell Dev. Biol. 2015;31:231–247. doi: 10.1146/annurev-cellbio-100814-125338. [DOI] [PubMed] [Google Scholar]
- 22.Liebeskind B.J., Hillis D.M., Zakon H.H. Evolution of sodium channels predates the origin of nervous systems in animals. Proc. Natl. Acad. Sci. USA. 2011;108:9154–9159. doi: 10.1073/pnas.1106363108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keijzer F., van Duijn M., Lyon P. Adaptive Behavior. 2013. What nervous systems do: Early evolution, input-output, and the skin brain thesis. . [Google Scholar]
- 24.Sundelacruz S., Levin M., Kaplan D.L. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev Rep. 2009;5:231–246. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams D.S., Uzel S.G., Akagi J., et al. Bioelectric signalling via potassium channels: A mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J. Physiol. 2016 doi: 10.1113/JP271930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandenberg L.N., Morrie R.D., Adams D.S. V-ATPase-dependent ectodermal voltage and pH regionalization are required for craniofacial morphogenesis. Dev. Dyn. 2011;240:1889–1904. doi: 10.1002/dvdy.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emmons-Bell M., Durant F., Hammelman J., et al. Gap junctional blockade stochastically induces different species-specific head anatomies in genetically wild-type girardia dorotocephala flatworms. Int. J. Mol. Sci. 2015;16:27865–27896. doi: 10.3390/ijms161126065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beane W.S., Morokuma J., Adams D.S., Levin M. A Chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem. Biol. 2011;18:77–89. doi: 10.1016/j.chembiol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pai V.P., Lemire J.M., Pare J.F., Lin G., Chen Y., Levin M. Endogenous gradients of resting potential instructively pattern embryonic neural tissue via notch signaling and regulation of proliferation. J. Neurosci. 2015;35:4366–4385. doi: 10.1523/JNEUROSCI.1877-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perathoner S., Daane J.M., Henrion U., et al. Bioelectric signaling regulates size in zebrafish fins. PLoS Genet. 2014;10:e1004080. doi: 10.1371/journal.pgen.1004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams D.S., Tseng A.S., Levin M. Light-activation of the Archaerhodopsin H(+)-pump reverses age-dependent loss of vertebrate regeneration: sparking system-level controls in vivo. Biol. Open. 2013;2:306–313. doi: 10.1242/bio.20133665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pai V.P., Aw S., Shomrat T., Lemire J.M., Levin M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 2012;139:313–323. doi: 10.1242/dev.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin M., Buznikov G.A., Lauder J.M. Of minds and embryos: Left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev. Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- 34.Buznikov G.A., Lambert H.W., Lauder J.M. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- 35.Fukumoto T., Kema I.P., Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr. Biol. 2005;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 36.Fukumoto T., Blakely R., Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev. Neurosci. 2005;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- 37.Reisoli E., De Lucchini S., Nardi I., Ori M. Serotonin 2B receptor signaling is required for craniofacial morphogenesis and jaw joint formation in Xenopus. Development. 2010;137:2927–2937. doi: 10.1242/dev.041079. [DOI] [PubMed] [Google Scholar]
- 38.Moiseiwitsch J. The role of serotonin and neurotransmitters during craniofacial development. Crit. Rev. Oral Biol. Med. 2000;11:230–239. doi: 10.1177/10454411000110020601. [DOI] [PubMed] [Google Scholar]
- 39.Yavarone M.S., Shuey D.L., Tamir H., Sadler T.W., Lauder J.M. Serotonin and cardiac morphogenesis in the mouse embryo. Teratology. 1993;47:573–584. doi: 10.1002/tera.1420470609. [DOI] [PubMed] [Google Scholar]
- 40.Nebigil C.G., Hickel P., Messaddeq N., et al. Ablation of serotonin 5-HT(2B) receptors in mice leads to abnormal cardiac structure and function. Circulation. 2001;103:2973–2979. doi: 10.1161/01.cir.103.24.2973. [DOI] [PubMed] [Google Scholar]
- 41.Choi D.S., Ward S.J., Messaddeq N., Launay J.M., Maroteaux L. 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells. Development. 1997;124:1745–1755. doi: 10.1242/dev.124.9.1745. [DOI] [PubMed] [Google Scholar]
- 42.Colas J.F., Launay J.M., Vonesch J.L., Hickel P., Maroteaux L. Serotonin synchronises convergent extension of ectoderm with morphogenetic gastrulation movements in Drosophila. Mech. Dev. 1999;87:77–91. doi: 10.1016/s0925-4773(99)00141-0. [DOI] [PubMed] [Google Scholar]
- 43.Adams D.S., Masi A., Levin M.H. + pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- 44.Tseng A.S., Beane W.S., Lemire J.M., Masi A., Levin M. Induction of vertebrate regeneration by a transient sodium current. J. Neurosci. 2010;30:13192–13200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nogi T., Levin M. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev. Biol. 2005;287:314–335. doi: 10.1016/j.ydbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Oviedo N.J., Morokuma J., Walentek P., et al. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev. Biol. 2010;339:188–199. doi: 10.1016/j.ydbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blandino J.K., Viglione M.P., Bradley W.A., Oie H.K., Kim Y.I. Voltage-dependent sodium channels in human small-cell lung cancer cells: role in action potentials and inhibition by Lambert-Eaton syndrome IgG. J. Membr. Biol. 1995;143:153–163. doi: 10.1007/BF00234661. [DOI] [PubMed] [Google Scholar]
- 48.Grimes J.A., Fraser S.P., Stephens G.J., et al. Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: Contribution to invasiveness in vitro. FEBS Lett. 1995;369:290–294. doi: 10.1016/0014-5793(95)00772-2. [DOI] [PubMed] [Google Scholar]
- 49.Blackiston D.J., McLaughlin K.A., Levin M. Bioelectric controls of cell proliferation: Ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3519–3528. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binggeli R., Weinstein R.C., Stevenson D. Calcium ion and the membrane potential of tumor cells. Cancer Biochem. Biophys. 1994;14:201–210. [PubMed] [Google Scholar]
- 51.Cone C.D. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J. Theor. Biol. 1971;30:151–181. doi: 10.1016/0022-5193(71)90042-7. [DOI] [PubMed] [Google Scholar]
- 52.Cone C.D., Jr Variation of the transmembrane potential level as a basic mechanism of mitosis control. Oncology. 1970;24:438–470. doi: 10.1159/000224545. [DOI] [PubMed] [Google Scholar]
- 53.Stuhmer W., Alves F., Hartung F., Zientkowska M., Pardo L.A. Potassium channels as tumour markers. FEBS Lett. 2006;580:2850–2852. doi: 10.1016/j.febslet.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 54.Diss J.K., Stewart D., Pani F., et al. A potential novel marker for human prostate cancer: Voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8:266–273. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- 55.Arcangeli A., Becchetti A. New trends in cancer therapy: Targeting ion channels and transporters. Pharmaceuticals. 2010;3:1202. doi: 10.3390/ph3041202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Defamie N., Chepied A., Mesnil M. Connexins, gap junctions and tissue invasion. FEBS Lett. 2014;588:1331–1338. doi: 10.1016/j.febslet.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Yamasaki H., Mesnil M., Omori Y., Mironov N., Krutovskikh V. Intercellular communication and carcinogenesis. Mutat. Res. 1995;333:181–188. doi: 10.1016/0027-5107(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 58.Shishido S.N., Prasain K., Beck A., Nguyen T.D., Hua D.H., Nguyen T.A. Bioavailability and efficacy of a gap junction enhancer (PQ7) in a mouse mammary tumor model. PLoS One. 2013;8:e67174. doi: 10.1371/journal.pone.0067174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding Y., Nguyen T.A. Gap junction enhancer potentiates cytotoxicity of cisplatin in breast cancer cells. J. Cancer Sci. Ther. 2012;4:371–378. doi: 10.4172/1948-5956.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lobikin M., Chernet B., Lobo D., Levin M. Resting potential, oncogene-induced tumorigenesis, and metastasis: The bioelectric basis of cancer in vivo. Phys. Biol. 2012;9:065002. doi: 10.1088/1478-3975/9/6/065002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blackiston D., Adams D.S., Lemire J.M., Lobikin M., Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis. Model. Mech. 2011;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lobikin M., Lobo D., Blackiston D.J., Martyniuk C.J., Tkachenko E., Levin M. Serotonergic regulation of melanocyte conversion: A bioelectrically regulated network for stochastic all-or-none hyperpigmentation. Sci. Signal. 2015;8:ra99. doi: 10.1126/scisignal.aac6609. [DOI] [PubMed] [Google Scholar]
- 63.Chernet B.T., Levin M. Transmembrane voltage potential is an essential cellular parameter for the detection and control of tumor development in a Xenopus model. Dis. Model. Mech. 2013;6:595–607. doi: 10.1242/dmm.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chernet B.T., Fields C., Levin M. Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos. Front. Physiol. 2015;5:519. doi: 10.3389/fphys.2014.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chernet B.T., Levin M. Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range. Oncotarget. 2014;5:3287–3306. doi: 10.18632/oncotarget.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chernet B.T., Adams D.S., Lobikin M., Levin M. Use of genetically encoded, light-gated ion translocators to control tumorigenesis. Oncotarget. 2016 doi: 10.18632/oncotarget.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinha G. Charged by GSK investment, battery of electroceuticals advance. Nat. Med. 2013;19:654. doi: 10.1038/nm0613-654. [DOI] [PubMed] [Google Scholar]
- 68.Famm K., Litt B., Tracey K.J., Boyden E.S., Slaoui M. Drug discovery: A jump-start for electroceuticals. Nature. 2013;496:159–161. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li C., Levin M., Kaplan D.L. Bioelectric modulation of macrophage polarization. Sci. Rep. 2016;6:21044. doi: 10.1038/srep21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tseng A., Levin M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun. Integr. Biol. 2013;6:1–8. doi: 10.4161/cib.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sundelacruz S., Li C., Choi Y.J., Levin M., Kaplan D.L. Bioelectric modulation of wound healing in a 3D in vitro model of tissue-engineered bone. Biomaterials. 2013;34:6695–6705. doi: 10.1016/j.biomaterials.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sundelacruz S., Levin M., Kaplan D.L. Depolarization alters phenotype, maintains plasticity of predifferentiated mesenchymal stem cells. Tissue Eng. Part A. 2013;19:1889–1908. doi: 10.1089/ten.tea.2012.0425.rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lobikin M., Pare J.F., Kaplan D.L., Levin M. Selective depolarization of transmembrane potential alters muscle patterning and muscle cell localization in Xenopus laevis embryos. Int. J. Dev. Biol. 2015 doi: 10.1387/ijdb.150198ml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schloss P., Williams D.C. The serotonin transporter: A primary target for antidepressant drugs. J. Psychopharmacol. 1998;12:115–121. doi: 10.1177/026988119801200201. [DOI] [PubMed] [Google Scholar]
- 75.Buznikov G., Shmukler Y., Lauder J. From oocyte to neuron: do neurotransmitters function in the same way throughout development? Cell. Mol. Neurobiol. 1996;16:537–559. doi: 10.1007/BF02152056. [DOI] [PubMed] [Google Scholar]
- 76.Sullivan K.G., Levin M. Neurotransmitter signaling pathways required for normal development in Xenopus laevis embryos: A pharmacological survey screen. J. Anat. 2016;229:483–502. doi: 10.1111/joa.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stepulak A., Rola R., Polberg K., Ikonomidou C. Glutamate and its receptors in cancer. J. Neural Transm. (Vienna) 2014;121:933–944. doi: 10.1007/s00702-014-1182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sachlos E., Risueno R.M., Laronde S., et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149:1284–1297. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 79.Fitzgerald P.J. Beta blockers, norepinephrine, and cancer: An epidemiological viewpoint. Clin. Epidemiol. 2012;4:151–156. doi: 10.2147/CLEP.S33695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schuller H.M., Al-Wadei H.A. Neurotransmitter receptors as central regulators of pancreatic cancer. Future Oncol. 2010;6:221–228. doi: 10.2217/fon.09.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sontheimer H. Malignant gliomas: Perverting glutamate and ion homeostasis for selective advantage. Trends Neurosci. 2003;26:543–549. doi: 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Sarrouilhe D., Clarhaut J., Defamie N., Mesnil M. Serotonin and cancer: what is the link? Curr. Mol. Med. 2015;15:62–77. doi: 10.2174/1566524015666150114113411. [DOI] [PubMed] [Google Scholar]
- 83.Brandes L.J., Arron R.J., Bogdanovic R.P., et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res. 1992;52:3796–3800. [PubMed] [Google Scholar]
- 84.Kubera M., Grygier B., Wrona D., et al. Stimulatory effect of antidepressant drug pretreatment on progression of B16F10 melanoma in high-active male and female C57BL/6J mice. J. Neuroimmunol. 2011;240-241:34–44. doi: 10.1016/j.jneuroim.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Grygier B., Arteta B., Kubera M., et al. Inhibitory effect of antidepressants on B16F10 melanoma tumor growth. Pharmacol. Rep. 2013;65:672–681. doi: 10.1016/s1734-1140(13)71045-4. [DOI] [PubMed] [Google Scholar]
- 86.Kubera M., Grygier B., Arteta B., et al. Age-dependent stimulatory effect of desipramine and fluoxetine pretreatment on metastasis formation by B16F10 melanoma in male C57BL/6 mice. Pharmacol. Rep. 2009;61:1113–1126. doi: 10.1016/s1734-1140(09)70174-4. [DOI] [PubMed] [Google Scholar]
- 87.Ashbury J.E., Levesque L.E., Beck P.A., Aronson K.J. A population-based case-control study of selective serotonin reuptake inhibitors (SSRIs) and breast cancer: The impact of duration of use, cumulative dose and latency. BMC Med. 2010;8:90. doi: 10.1186/1741-7015-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu W., Tamim H., Shapiro S., Stang M.R., Collet J.P. Use of antidepressants and risk of colorectal cancer: A nested case-control study. Lancet Oncol. 2006;7:301–308. doi: 10.1016/S1470-2045(06)70622-2. [DOI] [PubMed] [Google Scholar]
- 89.Padala P.R., Padala K.P., Monga V., Ramirez D.A., Sullivan D.H. Reversal of SSRI-associated apathy syndrome by discontinuation of therapy. Ann. Pharmacother. 2012;46:e8. doi: 10.1345/aph.1Q656. [DOI] [PubMed] [Google Scholar]
- 90.Barnhart W.J., Makela E.H., Latocha M.J. SSRI-induced apathy syndrome: A clinical review. J. Psychiatr. Pract. 2004;10:196–199. doi: 10.1097/00131746-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 91.Cantiello H.F. Role of the actin cytoskeleton on epithelial Na+ channel regulation. Kidney Int. 1995;48:970–984. doi: 10.1038/ki.1995.379. [DOI] [PubMed] [Google Scholar]
- 92.Hamm-Alvarez S.F., Sheetz M.P. Microtubule-dependent vesicle transport: modulation of channel and transporter activity in liver and kidney. Physiol. Rev. 1998;78:1109–1129. doi: 10.1152/physrev.1998.78.4.1109. [DOI] [PubMed] [Google Scholar]
- 93.Yuen E.Y., Jiang Q., Chen P., Gu Z., Feng J., Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J. Neurosci. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rostovtseva T.K., Bezrukov S.M. VDAC inhibition by tubulin and its physiological implications. Biochim. Biophys. Acta. 2012;1818:1526–1535. doi: 10.1016/j.bbamem.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huzil J.T., Richard F.L., Jack T. Comparative modelling of human β tubulin isotypes and implications for drug binding. Nanotechnology. 2006;17:S90. doi: 10.1088/0957-4484/17/4/014. [DOI] [PubMed] [Google Scholar]
- 96.Gajewski M.M., Alisaraie L., Tuszynski J.A. Peloruside, laulimalide, and noscapine interactions with beta-tubulin. Pharm. Res. 2012;29:2985–2993. doi: 10.1007/s11095-012-0809-2. [DOI] [PubMed] [Google Scholar]
- 97.Craddock T.J., Hameroff S.R., Ayoub A.T., Klobukowski M., Tuszynski J.A. Anesthetics act in quantum channels in brain microtubules to prevent consciousness. Curr. Top. Med. Chem. 2015;15:523–533. doi: 10.2174/1568026615666150225104543. [DOI] [PubMed] [Google Scholar]
- 98.Roth B., Driscol J. 2013. PDSP Ki Database. [Google Scholar]
- 99.Owens M.J., Knight D.L., Nemeroff C.B. Second-generation SSRIs: Human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol. Psychiatry. 2001;50:345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- 100.Altamura A.C., Moro A.R., Percudani M. Clinical pharmacokinetics of fluoxetine. Clin. Pharmacokinet. 1994;26:201–214. doi: 10.2165/00003088-199426030-00004. [DOI] [PubMed] [Google Scholar]
- 101.Lorber D.M. Computational drug design. Chem. Biol. 1999;6:R227–R228. [Google Scholar]
- 102.Soto A.M., Sonnenschein C. One hundred years of somatic mutation theory of carcinogenesis: Is it time to switch? BioEssays. 2014;36:118–120. doi: 10.1002/bies.201300160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sonnenschein C., Soto A.M., Rangarajan A., Kulkarni P. Competing views on cancer. J. Biosci. 2014;39:281–302. doi: 10.1007/s12038-013-9403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tarin D. Clinical and Biological Implications of the Tumor Microenvironment. Cancer Microenviron. 2012 doi: 10.1007/s12307-012-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tarin D. Cell and tissue interactions in carcinogenesis and metastasis and their clinical significance. Semin. Cancer Biol. 2011;21:72–82. doi: 10.1016/j.semcancer.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 107.Bizzarri M., Cucina A. Tumor and the microenvironment: A chance to reframe the paradigm of carcinogenesis? BioMed Res. Int. 2014;2014:934038. doi: 10.1155/2014/934038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.D’Anselmi F., Masiello M.G., Cucina A., et al. Microenvironment promotes tumor cell reprogramming in human breast cancer cell lines. PLoS One. 2013;8:e83770. doi: 10.1371/journal.pone.0083770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Onkal R., Djamgoz M.B. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: Clinical potential of neonatal Nav1.5 in breast cancer. Eur. J. Pharmacol. 2009;625:206–219. doi: 10.1016/j.ejphar.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 110.House C.D., Vaske C.J., Schwartz A.M., et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957–6967. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perez-Neut M., Rao V.R., Gentile S. hERG1/Kv11.1 activation stimulates transcription of p21waf/cip in breast cancer cells via a calcineurin-dependent mechanism. Oncotarget. 2015 doi: 10.18632/oncotarget.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lansu K., Gentile S. Potassium channel activation inhibits proliferation of breast cancer cells by activating a senescence program. Cell Death Dis. 2013;4:E652. doi: 10.1038/cddis.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin H., Xiao J., Luo X., et al. Overexpression HERG K(+) channel gene mediates cell-growth signals on activation of oncoproteins SP1 and NF-kappaB and inactivation of tumor suppressor Nkx3.1. J. Cell. Physiol. 2007;212:137–147. doi: 10.1002/jcp.21015. [DOI] [PubMed] [Google Scholar]
- 114.Pei L., Wiser O., Slavin A., et al. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc. Natl. Acad. Sci. USA. 2003;100:7803–7807. doi: 10.1073/pnas.1232448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saito T., Schlegel R., Andresson T., Yuge L., Yamamoto M., Yamasaki H. Induction of cell transformation by mutated 16K vacuolar H+-atpase (ductin) is accompanied by down-regulation of gap junctional intercellular communication and translocation of connexin 43 in NIH3T3 cells. Oncogene. 1998;17:1673–1680. doi: 10.1038/sj.onc.1202092. [DOI] [PubMed] [Google Scholar]
- 116.Gupta N., Martin P.M., Prasad P.D., Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78:2419–2425. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 117.Roepke T.K., Purtell K., King E.C., et al. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5:e11451. doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weksberg R., Nishikawa J., Caluseriu O., et al. Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1. Hum. Mol. Genet. 2001;10:2989–3000. doi: 10.1093/hmg/10.26.2989. [DOI] [PubMed] [Google Scholar]
- 119.Lee M.P., Hu R.J., Johnson L.A., Feinberg A.P. Human KVLQT1 gene shows tissue-specific imprinting and encompasses Beckwith-Wiedemann syndrome chromosomal rearrangements. Nat. Genet. 1997;15:181–185. doi: 10.1038/ng0297-181. [DOI] [PubMed] [Google Scholar]
- 120.Than B.L., Goos J.A., Sarver A.L., et al. The role of KCNQ1 in mouse and human gastrointestinal cancers. Oncogene. 2013 doi: 10.1038/onc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.House C.D., Vaske C.J., Schwartz A., et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martino J.J., Wall B.A., Mastrantoni E., et al. Metabotropic glutamate receptor 1 (Grm1) is an oncogene in epithelial cells. Oncogene. 2012 doi: 10.1038/onc.2012.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Speyer C.L., Smith J.S., Banda M., DeVries J.A., Mekani T., Gorski D.H. Metabotropic glutamate receptor-1: A potential therapeutic target for the treatment of breast cancer. Breast Cancer Res. Treat. 2012;132:565–573. doi: 10.1007/s10549-011-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song Z., He C.D., Liu J., et al. Blocking glutamate-mediated signalling inhibits human melanoma growth and migration. Exp. Dermatol. 2012;21:926–931. doi: 10.1111/exd.12048. [DOI] [PubMed] [Google Scholar]
- 125.Zhang J.T., Jiang X.H., Xie C., et al. Downregulation of CFTR promotes epithelial-to-mesenchymal transition and is associated with poor prognosis of breast cancer. Biochim. Biophys. Acta. 2013;1833:2961–2969. doi: 10.1016/j.bbamcr.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 126.Xie C, Jiang XH, Zhang JT, et al. 2013.
- 127.Sirnes S., Bruun J., Kolberg M., et al. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int. J. Cancer. 2012;131:570–581. doi: 10.1002/ijc.26392. [DOI] [PubMed] [Google Scholar]
- 128.Schickling B.M., England S.K., Aykin-Burns N., Norian L.A., Leslie K.K., Frieden-Korovkina V.P. BKCa channel inhibitor modulates the tumorigenic ability of hormone-independent breast cancer cells via the Wnt pathway. Oncol. Rep. 2015;33:533–538. doi: 10.3892/or.2014.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Felder C.C., MacArthur L., Ma A.L., Gusovsky F., Kohn E.C. Tumor-suppressor function of muscarinic acetylcholine receptors is associated with activation of receptor-operated calcium influx. Proc. Natl. Acad. Sci. USA. 1993;90:1706–1710. doi: 10.1073/pnas.90.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Illmensee K., Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc. Natl. Acad. Sci. USA. 1976;73:549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seilern-Aspang F., Kratochwill L. 1965. Relation between regeneration and tumor growth. [Google Scholar]
- 132.Donaldson D.J., Mason J.M. Cancer-related aspects of regeneration research: A review. Growth. 1975;39:475–496. [PubMed] [Google Scholar]
- 133.Del Rio-Tsonis K., Tsonis P.A. Amphibian tissue regeneration - a model for cancer regulation. Int. J. Oncol. 1992;1:161–164. doi: 10.3892/ijo.1.2.161. [DOI] [PubMed] [Google Scholar]
- 134.Brockes J.P. Regeneration and cancer. Biochim. Biophys. Acta. 1998;1377:M1–M11. doi: 10.1016/s0304-419x(97)00029-2. [DOI] [PubMed] [Google Scholar]
- 135.Smithers D.W. An attack on cytologism. Lancet. 1962;1:493–499. doi: 10.1016/s0140-6736(62)91475-7. [DOI] [PubMed] [Google Scholar]
- 136.O’Brien J. The ever-changing electrical synapse. Curr. Opin. Neurobiol. 2014;29C:64–72. doi: 10.1016/j.conb.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Connors B.W., Long M.A. Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 138.Bennett M.V. Gap junctions as electrical synapses. J. Neurocytol. 1997;26:349–366. doi: 10.1023/a:1018560803261. [DOI] [PubMed] [Google Scholar]
- 139.Kandouz M., Batist G. Gap junctions and connexins as therapeutic targets in cancer. Expert Opin. Ther. Targets. 2010;14:681–692. doi: 10.1517/14728222.2010.487866. [DOI] [PubMed] [Google Scholar]
- 140.Trosko J.E. Gap junctional intercellular communication as a biological “Rosetta stone” in understanding, in a systems biological manner, stem cell behavior, mechanisms of epigenetic toxicology, chemoprevention and chemotherapy. J. Membr. Biol. 2007;218:93–100. doi: 10.1007/s00232-007-9072-6. [DOI] [PubMed] [Google Scholar]
- 141.Trosko J.E. The role of stem cells and gap junctions as targets for cancer chemoprevention and chemotherapy. Biomed. Pharmacother. 2005;59(Suppl. 2):S326–S331. doi: 10.1016/s0753-3322(05)80065-4. [DOI] [PubMed] [Google Scholar]
- 142.Mesnil M., Crespin S., Avanzo J.L., Zaidan-Dagli M.L. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta. 2005;1719:125–145. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 143.Mandala M., Serck-Hanssen G., Martino G., Helle K.B. The fluorescent cationic dye rhodamine 6G as a probe for membrane potential in bovine aortic endothelial cells. Anal. Biochem. 1999;274:1–6. doi: 10.1006/abio.1999.4253. [DOI] [PubMed] [Google Scholar]
- 144.Joshi P.G., Pant H.C. Depolarization of synaptosomal membranes: a study of mechanism by which rhodamine 6G measures membrane potential. Indian J. Biochem. Biophys. 1991;28:140–145. [PubMed] [Google Scholar]
- 145.Nieuwkoop P.D., Faber J. Normal Table of Xenopus laevis (Daudin). Amsterdam: North-Holland; 1967. [Google Scholar]
- 146.Dahal G.R., Rawson J., Gassaway B., et al. An inwardly rectifying K+ channel is required for patterning. Development. 2012;139:3653–3664. doi: 10.1242/dev.078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pai V.P., Martyniuk C.J., Echeverri K., Sundelacruz S., Kaplan D.L., Levin M. Genome-wide analysis reveals conserved transcriptional responses downstream of resting potential change in Xenopus embryos, axolotl regeneration, and human mesenchymal cell differentiation. Regeneration (Oxf.) 2015;3(1):3–25. doi: 10.1002/reg2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gradinaru V., Thompson K.R., Zhang F., et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Binggeli R., Weinstein R. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J. Theor. Biol. 1986;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- 150.Olivotto M., Arcangeli A., Carla M., Wanke E. Electric fields at the plasma membrane level: A neglected element in the mechanisms of cell signalling. BioEssays. 1996;18:495–504. doi: 10.1002/bies.950180612. [DOI] [PubMed] [Google Scholar]
- 151.Cone C.D., Tongier M. Control of somatic cell mitosis by simulated changes in the transmembrane potential level. Oncology. 1971;25:168–182. doi: 10.1159/000224567. [DOI] [PubMed] [Google Scholar]
- 152.Morokuma J., Blackiston D., Adams D.S., Seebohm G., Trimmer B., Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2008;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cohen A.E., Venkatachalam V. Bringing bioelectricity to light. Annu. Rev. Biophys. 2014;43:211–232. doi: 10.1146/annurev-biophys-051013-022717. [DOI] [PubMed] [Google Scholar]
- 154.Fenno L., Yizhar O., Deisseroth K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Adams D.S., Lemire J.M., Kramer R.H., Levin M. Optogenetics in Developmental Biology: Using light to control ion flux-dependent signals in Xenopus embryos. Int. J. Dev. Biol. 2014;58:851–861. doi: 10.1387/ijdb.140207ml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tochitsky I., Polosukhina A., Degtyar V.E., et al. Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells. Neuron. 2014;81:800–813. doi: 10.1016/j.neuron.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fortin D.L., Dunn T.W., Kramer R.H. Engineering light-regulated ion channels. Cold Spring Harb. Protoc. 2011;2011:579–585. doi: 10.1101/pdb.top112. [DOI] [PMC free article] [PubMed] [Google Scholar]