Abstract
Purpose
This study aimed to investigate the differential responses of trabecular meshwork stem cells (TMSCs) and trabecular meshwork (TM) cells to endoplasmic reticulum (ER) stress inducers.
Methods
Human TM cells and TMSCs were exposed to tunicamycin, brefeldin A, or thapsigargin. Cell apoptosis was evaluated by flow cytometry. ER stress markers were detected by quantitative PCR, Western blotting, and immunostaining. Morphologic changes were evaluated by transmission electron microscopy. Cells were treated with the PERK inhibitor GSK2606414 or the elF2α dephosphorylation inhibitor Salubrinal together with tunicamycin to evaluate their effects on ER stress.
Results
Both TMSCs and TM cells underwent apoptosis after 48- and 72-hour treatment with ER stress inducers. ER stress triggered the unfolded protein response (UPR) with increased expression of GRP78, sXBP1, and CHOP, which was significantly lower in TMSCs than TM cells. Swollen ER and mitochondria were detected in both TMSCs and TM cells. Neither GSK2606414 nor salubrinal alone activated UPR. GSK2606414 significantly reduced cell survival rates after tunicamycin treatment, and salubrinal increased cell survival rates. The increased expression of GRP78, sXBP1, CHOP, and GADD34 peaked at 6 or 12 hours and lasted longer in TM cells than TMSCs. Salubrinal treatment dramatically increased OCT4 and CHI3L1 expression in TMSCs.
Conclusions
In response to ER stress inducers, TMSCs activated a lower level of UPR and lasted shorter than TM cells. Inhibition of elF2α dephosphorylation had a protective mechanism against cell death. Stem cells combined with salubrinal may be a more effective way for TM regeneration in glaucoma.
Keywords: trabecular meshwork stem cells, trabecular meshwork cells, ER Stress, apoptosis, unfolded protein response, PERK inhibitors
Glaucoma, a chronic optic neuropathy, is the second leading cause of blindness worldwide.1 Primary open-angle glaucoma, the most common form of glaucoma, is usually accompanied by elevated IOP. The conventional outflow pathway, where aqueous humor circulates and drains through the trabecular meshwork (TM), accounts for up to 90% of aqueous humor outflow.2 Elevated IOP results from increased outflow resistance in TM, but the pathogenesis is still unclear. It has been suggested that reduced cellularity of the TM, aged TM cells, and abnormal extracellular matrix contribute to increased outflow resistance and cause elevated IOP in glaucoma patients.3–7
The endoplasmic reticulum (ER) is the organelle responsible for lipid manufacture, protein synthesis, folding, and the transport of secretory and transmembrane proteins. Various physiologic and pathophysiologic disturbances, including hypoxia, oxidative injury, glucose deprivation, viral infection, and mutant protein expression, can cause accumulation of misfolded and unfolded proteins in the ER,8 known as ER stress. ER stress triggers an adaptive cellular response termed the unfolded protein response (UPR), a signaling network that senses imbalances between protein synthesis, quality control, and degradation in the ER.9 Activated UPR regulates downstream effectors with the following three functions: adaptive response, feedback control, and apoptosis regulation.10 The UPR is initiated and regulated by three ER sensors: IRE1, PERK (protein kinase RNA-like endoplasmic reticulum kinase), and ATF6.11 These three sensors are inactive when binding with the molecular chaperone GRP78 (also called BiP) and are activated during GRP78 release. XBP1 mRNA spliced by activated IRE1α upregulates UPR target genes and increases protein-folding and degradation capacities.12,13 Activation of PERK phosphorylates eukaryotic translation initiation factor 2α (eIF2α), which leads to suppression of global mRNA translation and reduction of protein load.14,15 Protein disulfide isomerase (PDI) is a folding catalyst that facilitates misfolding proteins reaching their native arrangement of disulfides.16 These effectors and processes help cells restore ER homeostasis. However, when the UPR fails to restore ER homeostasis, persistent ER stress induces cell apoptosis via several mechanisms involving C/EBP homologous protein (CHOP), ER-specific caspase 12, BCl-2 family proteins, and other factors.8 CHOP is a significant mediator of apoptosis in ER stress.17 Researchers have found that CHOP deletion protects IOP elevation in glucocorticoid-induced ocular hypertension18 and promotes RGCs19 and β cells20 survival in an optic nerve crush mouse model and diabetic mouse model.
Recent studies demonstrated that ER stress plays an important role in myocilin-associated glaucoma21 and glucocorticoid-induced ocular hypertension,18 and reduction of ER stress can rescue glaucoma in mouse models. Peters et al.22 reported that chronic ER stress presents in human glaucomatous TM tissues and TM cells. The data support the hypothesis that sustained ER stress induces TM cell apoptosis and contributes to glaucoma development. Stem cell therapy for TM regeneration has been explored,23–26 which shows potential as a future glaucoma treatment. However, the characteristics of UPR in stem cells in response to ER stress are complicated. In the hematopoietic stem cell pool, ER stress selectively induces hematopoietic stem cell apoptosis, whereas progenitors are spared via the PERK pathway.27 GSK2606414 (GSK) inhibits PERK autophosphorylation.28 Salubrinal (Sal) is a selective inhibitor that blocks eIF2α dephosphorylation29; thus, the PERK pathway remains active. GADD34 is a negative feedback effector in the pathway that mediates dephosphorylation of elF2α and resumption of protein synthesis.30 Therefore, GADD34 mRNA indirectly reflects the p-elF2α level. Previously, we successfully isolated and characterized human TM stem cells (TMSCs).31 This study aims to discover whether responses of TMSCs and TM cells to ER stress are different and to understand the function of stem cells in glaucoma and the potential of stem cell–based therapy for glaucoma. We induced ER stress using three different inducers: tunicamycin (TUN, blocks glycoprotein biosynthesis in the ER), brefeldin A (BreA, inhibits transportation of proteins from the ER to Golgi), and thapsigargin (Thap, an inhibitor of the endoplasmic reticulum Ca2+-ATPase), and compared cell survival rates, UPR levels, and microstructural changes. We also detected the effects of GSK and Sal on the ER stress process in TMSCs and TM cells. To the best of our knowledge, this is the first study describing the differential responses of TMSCs and TM cells to ER stress inducers and underlying pathway mechanisms.
Methods
Primary Cell Culture
Human TMSCs and TM cells were isolated and cultured as previously described.31 In brief, de-identified human corneas were obtained from the Center for Organ Recovery and Education (Pittsburgh, PA, USA). TMSCs were isolated as clonal cultures and cultured in a medium requiring reduced serum (OptiMEM-1) supplemented with 5% FBS (ThermoFisher, Pittsburgh, PA, USA); 10 ng/mL epidermal growth factor (EGF), 0.08% chondroitin sulfate (Sigma-Aldrich Corp., St. Louis, MO, USA); 100 μg/mL bovine pituitary extract; 20 μg/mL ascorbic acid; 200 μg/mL calcium chloride; 100 IU/mL penicillin/100 μg/mL streptomycin; and 50 μg/mL gentamicin (ThermoFisher). TM cells were cultured from human TM tissue explants in Dulbecco's modified Eagle medium/F12 (DMEM/F12; Sigma-Aldrich Corp.) supplemented with 10% FBS and antibiotics.
ER Stress Induction and Inhibitor Treatment
Tunicamycin, brefeldin A, and thapsigargin were obtained from MP Biomedicals (Solon, OH, USA), and salubrinal and GSK2606414 were purchased from Calbiochem (EMD Millipore Corp., Billerica, MA, USA). These reagents were dissolved in dimethyl sulfoxide (DMSO; ThermoFisher) and stored at −20°C. Concentrations of ER stress inducers were decided by treating the cells with different concentrations with or without the presence of chaperon 4-phenylbutyric acid (PBA). Control groups were untreated cells in the presence of DMSO with the same concentration as the chemicals were dissolved in. Final DMSO concentration was always kept <1%. Cells were treated with the inhibitors at different concentrations for 1-hour, followed by adding ER stress inducer TUN at 5 μg/mL.
Cell Survival Rates Evaluated by Flow Cytometry
Cell apoptosis was measured using the Annexin V/7-Aminoactinomycin D (7-AAD) Apoptosis Detection kit (BD Biosciences, San Diego, CA, USA) according to the manufacturer's protocol. Cell suspensions were passed through 40-μm filter caps to remove possible cell clumps. After washing in PBS, cells were resuspended in 1× binding buffer. Annexin V and 7-AAD were added, and cells were incubated at room temperature for 15 minutes in dark. Stained cells were analyzed immediately using a flow cytometer (FACSAria; BD Biosciences).
Quantitative RT-PCR
Cells were lysed in RLT buffer (RNeasy mini kit; Qiagen, Hilden, Germany), and stored at −80°C. RNA was isolated following the manufacturer's instructions, including treatment with DNase I (New England Biolabs, Ipswich, MA, USA) and concentration by ethanol precipitation. RNA was reverse transcribed into cDNA using XLAScript cDNA MasterMix (WorldWide Medical Products, Bristol, PA, USA). Quantitative PCR (qPCR) was performed using SYBR Green RT-PCR Reagents (Applied Biosystems, Foster City, CA, USA) as previously described.31 The sequences of primers used in this study are shown in Supplementary Table S1. Relative mRNA abundance was calculated using the 2−ΔΔCt method. Gene expression was normalized to 18S rRNA. Individual gene expression values of two to four biological replicates (cell strains) were averaged to obtain a mean ± SEM.
Western Blotting
Cell lysates were collected using RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After heated at 75°C for 20 minutes, the samples were stored at −20°C. Before running, samples were sonicated and mixed with 2× Laemmli loading buffer (BIO-RAD, Hercules, CA, USA) and then heated at 95°C for 5 minutes. Each sample (30 μL) was loaded to 8% to 16% Tris-glycine gel (Invitrogen, Carlsbad, CA, USA), and electrophoresis was performed for 1 to 2 hours at 120 V. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane and incubated with Odyssey blocking buffer (LI-COR Biotechnology, Lincoln, NE, USA) for 1 hour at room temperature. The membrane was incubated with primary antibodies diluted in blocking buffer with 0.01% Tween-20 at 4°C overnight. Antibodies for GRP78 (BIP; 1:1000) and PDI (1:500) were purchased from Cell Signaling Technology. β-Actin antibody (1:500) was purchased from BioLegend, Inc. (San Diego, CA, USA). Then, membranes were incubated with goat anti-mouse and goat anti-rabbit secondary antibodies (IRDye 680LT, IRDye 800CW; LI-COR Biosciences). The fluorescent signals were captured on an infrared imager (Odyssey Infrared Imager; LI-COR Biosciences).
Immunocytochemistry
Cultured cells were washed with PBS, fixed in 4% paraformaldehyde at room temperature for 15 minutes, and washed with PBS twice. Then cells were permeabilized with 0.2% Triton X-100 for 10 minutes and blocked in 1% BSA for 30 minutes at room temperature. Cells were incubated overnight at 4°C with primary antibodies, including GRP78 (1:100; Santa Cruz Biotechnology), myocilin (1:50; Santa Cruz Biotechnology), and Alexa Fluor 633 Phalloidin (1:500; Life Technologies, Carlsbad, CA, USA). After three washes, fluorescent secondary antibodies (Invitrogen) and nuclear dye 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich Corp.) were added and incubated for 2 hours at room temperature. After additional washes, cells were mounted using fluorescence mounting medium (ThermoFisher). Samples were visualized and captured using a confocal microscope (Olympus, Center Valley, PA, USA) with a 60× oil objective. Microscopic analysis was carried out using the FluoView software (Olympus).
Transmission Electron Microscopy
Cultured cells were fixed with 2.5% glutaraldehyde (EM grade) in PBS for 1 hour at room temperature followed by postfixation in 1% osmium tetroxide (Electron Microscopy Sciences, Hatfield, PA, USA) with 1% potassium ferricyanide (ThermoFisher). Samples were dehydrated in a graded series of alcohol and changed three times in epon (1 hour each). After removal of epon, beam capsules full of resin were inverted over cells at 37°C overnight and then for 48 hours at 60°C. Cells were examined and photographed at 80 kV on a Jeol 1011 transmission electron microscope (Jeol, Peabody, MA, USA).
Statistical Evaluation
All the experiments were repeated with different cell strains from different donors. The strain numbers (n) for each experiment were marked in the Results and figure legends. All values are presented as mean ± SEM. The statistical differences were determined by ANOVA followed by Tukey or Dunnett's multiple comparison tests. Statistical significance was set at P < 0.05.
Results
Viability Changes of TMSCs and TM Cells in Response to ER Stress Inducers
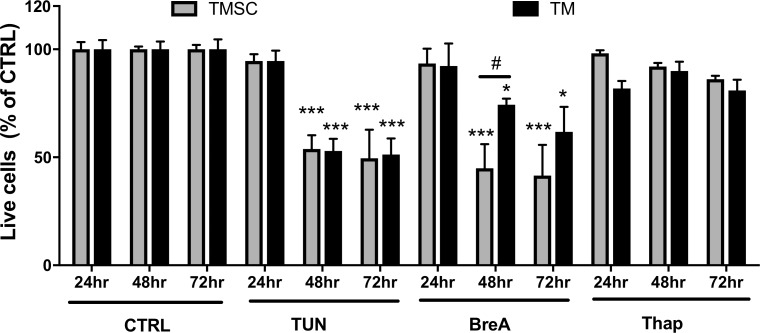
To determine the most suitable concentrations of selected ER stress inducers, TM cells were treated with TUN, BreA, and Thap at different concentrations with or without the presence of chaperon PBA at 10 mM for 72 hours. Western blotting results (Supplementary Fig. S1) show that TM cells treated with TUN at 5 μg/mL, BreA at 5 μg/mL, and Thap at 1 μg/mL had increased expression of GRP78 and PDI, whereas the increase was partially blocked by PBA. It indicated that those concentrations were able to induce ER stress in TM cells, and the ER stress could be partially rescued by a chaperon. The selected concentrations were used in the following experiments. Both TMSCs and TM cells were treated with 5 μg/mL TUN, 5 μg/mL BreA, or 1 μg/mL Thap for 24, 48, and 72 hours. Cell apoptosis and necrosis were detected by flow cytometry with Annexin V/7-AAD staining. Live cell counts (both Annexin V and 7-AAD negative) as a percentage of DMSO controls are shown in Figure 1. At 24 hours, ER stress inducers did not induce a significant reduction in viable cell numbers. However, significant reduced viability was observed in both TMSCs and TM cells after 48- and 72-hour treatment with TUN and BreA. The percentages of live cells after 48-hour TUN treatment were 53.8 ± 6.4% (n = 6) in TMSCs and 52.9 ± 5.6% (n = 6) in TM cells. After 72-hour TUN treatment, the percentages were 49.5 ± 13.3% (n = 4) in TMSCs and 51.2 ± 7.5% (n = 5) in TM cells. With BreA treatment, 44.9 ± 13.7% (n = 3) in TMSCs and 74.4 ± 3.4% (n = 3) in TM cells were alive after 48 hours; 41.6 ± 14.2% (n = 3) TMSCs and 61.7 ± 11.6% (n = 3) TM cells were alive after 72-hour treatment. More than 80% of both TMSCs and TM cells were alive in Thap treatment, and cell viability reduction was not statistically significant in both cell types. No statistically significant difference was found between TMSCs and TM cells at each time point with TUN and Thap treatments. With BreA treatment, TM cells survived more than TMSCs after 48-hour treatment (Fig. 1).
Figure 1.
ER stress inducers reduced cell viability in both TM cells and TMSCs. Cells were incubated with ER stress inducers TUN, BreA, or Thap for 24, 48, or 72 hours and stained with Annexin V and 7-AAD followed by flow cytometry analysis. Live cells are both Annexin V– and 7-AAD–negative stained. y-axis indicates percentage of live cells compared with no treatment controls at the same time points. TUN and BreA dramatically reduced cell viability at 48 and 72 hours in both TMSCs and TM cells. Data presented as means ± SEM (n ≥ 3). *Treated cells versus DMSO controls; #TMSCs versus TM cells. */#P < 0.05, ***P < 0.001. Two-way ANOVA followed by Tukey's multiple comparison test.
Expression of ER Stress Markers After 72-Hour Treatment
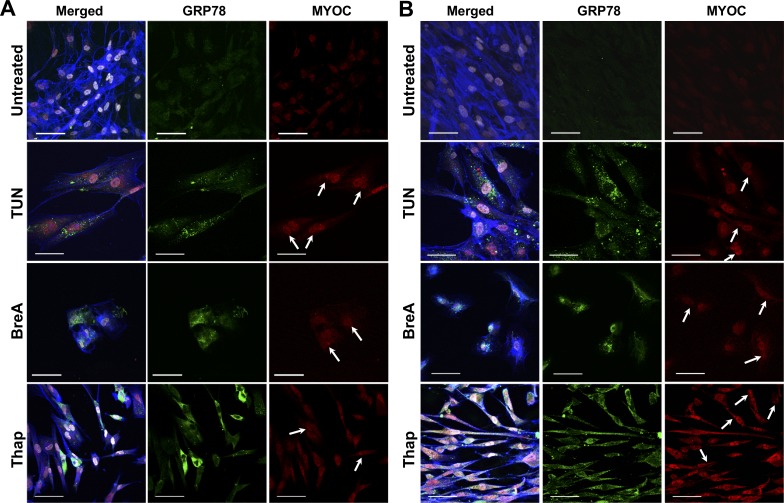
Both TMSCs and TM cells were treated with ER stress inducers for 72 hours, and the expression of ER stress markers was detected by immunofluorescent staining, Western blotting, and qPCR. Figure 2 shows representative images of immunostaining with GRP78 and myocilin antibodies. GRP78 and myocilin were detected at a very low or undetectable level in untreated TMSCs (Fig. 2A) and TM cells (Fig. 2B). In treated cells, GRP78 exhibited diffused distribution throughout the cytoplasm, and myocilin was mainly accumulated in the nuclei and ER regions. The distribution of GRP78 and myocilin partially overlapped. F-actin was stained with phalloidin (shown as blue). Although both TMSCs and TM cells increased GRP78 after Thap treatment, some TMSCs displayed higher expression of GRP78 than others (Fig. 2A).
Figure 2.
Expression of GRP78 and myocilin increased after 72-hour ER stress induction. Representative immunostaining images show GRP78 (green), myocilin (MYOC, red) merged with DAPI (white), and F-actin (blue) on TMSCs (A) and TM cells (B). Myocilin (red, arrows) accumulated in the perinuclear region where the ERs are and partially overlapped with GRP78 (green). Tun and BreA reduced attached live cell numbers. Thap treatment made both TMSCs and TM cells more elongated. Scale bars: 50 μm.
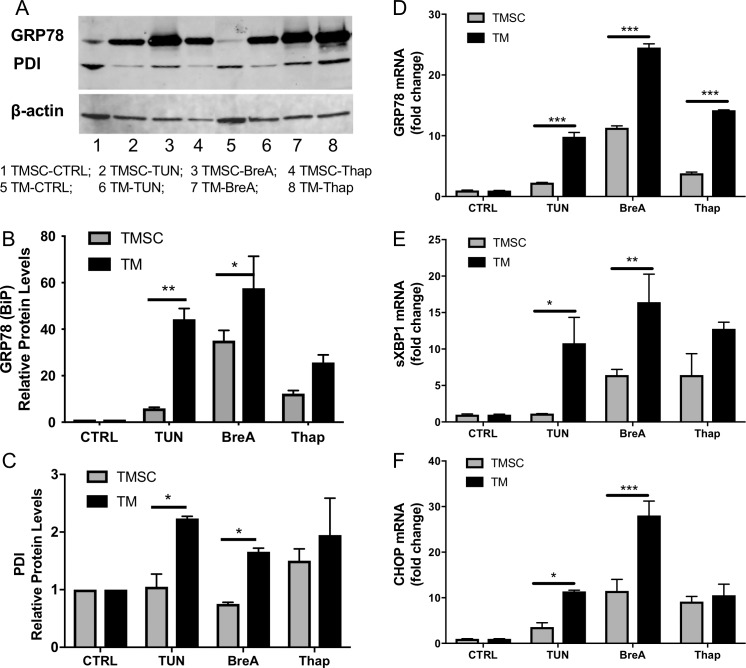
Figure 3A shows representative GRP78 and PDI expression bands in TMSCs and TM cells after 72-hour treatment by Western blotting. Figures 3B and 3C are the quantitative analysis of GRP78 and PDI expression averaged (mean ± SEM) from three independent experiment results with three different cell strains of TMSCs and TM cells. The protein levels were normalized with internal control β-actin and demonstrated as fold changes to the untreated controls. The expression of GRP78 and PDI in TMSCs were significantly lower than TM cells after 72-hour treatment with TUN and BreA. The protein level difference between TMSCs and TM cells after Thap treatment was not statistically significant (Figs. 3B, 3C). The qPCR results showed that the mRNA expression of GRP78, splicing XBP1 (sXBP1), and CHOP in TM cells was significantly higher than that in TMSCs after treatment with TUN and BreA (Figs. 3D–3F). After Thap treatment, TM cells also expressed significantly higher mRNA levels of GRP78 compared with TMSCs, but no statistically significant difference was observed for sXBP1 and CHOP mRNA expression (Figs. 3E, 3F).
Figure 3.
Expression of ER stress markers increased higher in TM cells than in TMSCs after ER stress induction. (A) Representative Western blotting bands that reveal protein expression levels. (B, C) Quantitative analysis of protein expression relative to β-actin and normalized to untreated TMSCs or TM cells, respectively, with three independent experiment results. (D–F) Relative gene expression of ER stress markers GRP78, sXBP1, and CHOP (normalized to untreated cells). At both protein (A–C) and mRNA (D–F) levels, the increase in TM cells was higher than TMSCs. All data represent mean ± SEM, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001. Two-way ANOVA followed by Dunnett's multiple comparison test.
Ultrastructural Changes in Response to ER Stress Inducers
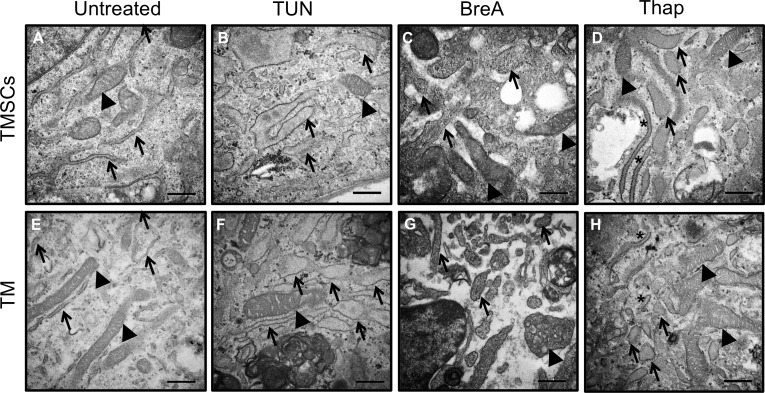
Ultrastructural analysis using electron microscopy was performed to gain deeper insights into cellular dynamics (Fig. 4). Abnormally large mitochondria with reduced cristae were observed in both cell types after 72-hour stress induction. These features suggested that mitochondria were swollen.32 Swollen ERs with remarkably enlarged lumen were detected in TUN-treated cells (Figs. 4B, 4F). BreA caused the most severe damages to cells, inducing fragmentation in many organelles, including ER (Figs. 4C, 4G). Thap caused the least damage compared with TUN and BreA, but swollen ER and mitochondria were detected in the cells as indicated by arrows (Figs. 4D, 4H).
Figure 4.
Swollen ER and mitochondria observed in TMSCs and TM cells after ER stress inducer treatment. Transmission electron microscopy shows the organelle changes of TMSCs (A–D) and TM cells (E–H) after TUN (B, F), BreA (C, G), or Thap (D, H) treatment for 72 hours. A and E show normal ER (arrows) and mitochondria (arrowheads). TUN and BreA induced obvious swollen ER and mitochondria. Thap treated cells had swollen ER (arrows) and normal sized ER (asterisks) (D, H). Scale bars: 500 nm.
PERK Inhibitor GSK Increased Cell Death
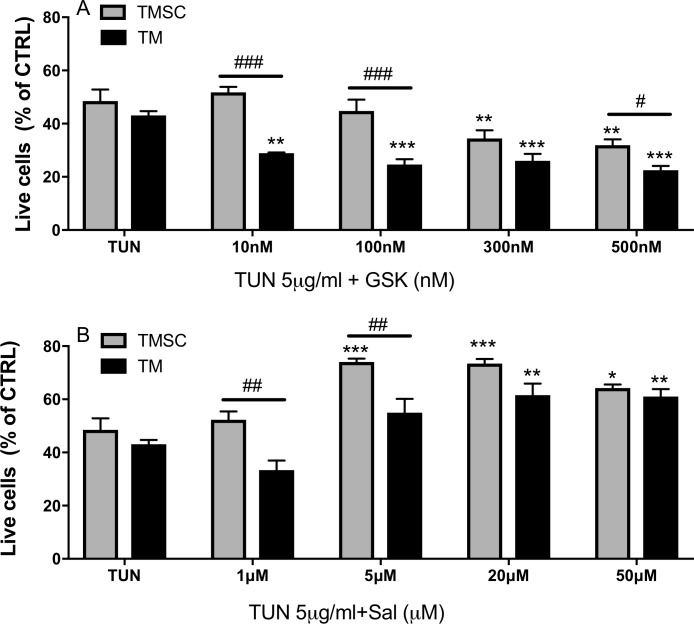
We were inquisitive about whether cell death induced by ER stress can be rescued by modulating the PERK pathway. To investigate this, TUN was used to induce cell ER stress because it has been confirmed with stable ER stress induction (Figs. 1–4). TMSCs and TM cells were incubated with GSK at concentrations of 0, 10, 100, 300, and 500 nM for 1 hour, followed by exposure to TUN at 5 μg/mL for 48 hours. A significant reduction of cell survival rates was observed in TM cells treated with GSK at all the concentrations tested (≥10 nM) and in TMSCs treated with GSK at 300 and 500 nM, compared with TUN treatment alone (Fig. 5A). The TMSC survival rates were significantly higher than TM cells after treatment with GSK at 10, 100, and 500 nM. With 300-nM GSK treatment, the difference between the two cell types was not significant. Cells treated with GSK at different concentrations without TUN did not present significant changes of cell viability compared with untreated controls (Supplementary Fig. S2A).
Figure 5.
GSK2606414 exaggerated TUN effect on cell viability and salubrinal attenuated that. Cells were treated with PERK pathway inhibitors GSK or Sal at different concentrations for 1 hour before TUN treatment for 48 hours. Flow cytometry results show GSK reduced TM cell survival rate at all concentrations and reduced TMSC survival at higher concentrations (A). Sal increased cell survival rate in TM cells starting at 20 μM, whereas as low as 5 μM Sal protected TMSC from apoptosis (B). Results were normalized to DMSO controls and present as means ± SEM (n = 4). *Versus TUN without inhibitor treatment; #TMSCs versus TM cells with the same treatment. */#P < 0.05, **/##P < 0.01, ***/###P < 0.001. Two-way ANOVA followed by Dunnett's multiple comparison test.
elF2α Dephosphorylation Inhibitor Sal Partially Rescued Cells After TUN Treatment
TMSCs and TM cells were treated with Sal at 0, 1, 5, 20, and 50 μM for 1 hour, followed by exposure to TUN at 5 μg/mL for 48 hours. There was a significant increase of cell survival rates in TMSCs treated with Sal at concentrations ≥5 μM and in TM cells treated with Sal at 20 and 50 μM (Fig. 5B). The TMSC survival rates were significantly higher than TM cells treated at 1 and 5 μM. With 20 and 50 μM Sal plus TUN, the difference between TMSCs and TM cells was not significant. Cells treated with Sal alone at different concentrations without TUN did not induce significant changes of cell viability compared with DMSO controls (Supplementary Fig. S2B).
Differential ER Stress Marker Expression in Cells Treated With TUN Together With GSK or Sal
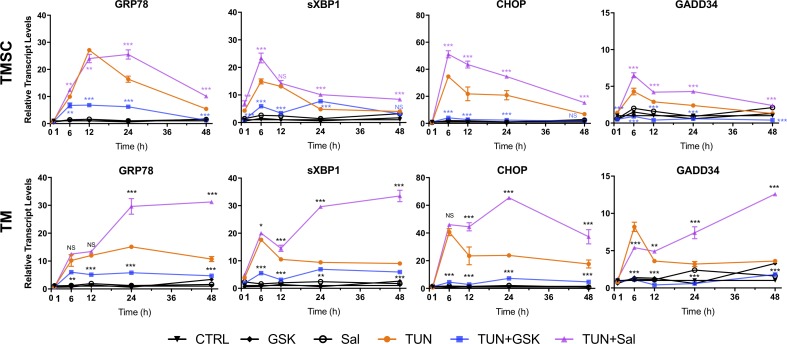
To explore the ER stress marker expression in response to TUN together with GSK or Sal, TMSCs and TM cells were treated with 300 nM GSK or 20 μM Sal for 1 hour, followed by 5 μg/mL TUN treatment for different durations (1, 6, 12, 24, and 48 hours); 300 nM GSK and 20 μM Sal were selected because both TMSCs and TM cells had dramatic reduced cell viability with 300 nM GSK (Fig. 5A) and increased cell viability with 20 μM Sal (Fig. 5B). At each time point, the expression levels of ER stress markers in cells treated with GSK or Sal without TUN were similar to DMSO controls, which indicated that the inhibitors themselves did not induce ER stress marker expression (Fig. 6).
Figure 6.
Sal increased the expression of ER stress markers, whereas GSK reduced that triggered by TUN. TMSCs and TM cells were incubated with 300 nM GSK or 20 μM Sal for 1 hour before exposed to TUN. mRNA levels by qPCR were normalized to untreated cells at each time point. Sal and GSK treatment without TUN did not change the expression of ER stress markers. Data are shown as mean ± SEM (n = 3). P value was calculated by comparing TUN treatment with TUN + inhibitor treatments at each time point. *P < 0.05, **P < 0.01, ***P < 0.001. Two-way ANOVA followed by Dunnett's multiple comparison test.
With TUN+GSK treatment, GRP78, sXBP1, CHOP, and GADD34 expression increased at lower levels compared with TUN treatment in both TMSCs and TM cells (Fig. 6). In contrast, the gene expression levels with TUN+Sal treatment in both TMSCs and TM cells increased dramatically compared with their DMSO controls, and the increased levels were comparable to TUN treatment (Fig. 6). The increases were higher and lasted longer in TM cells than in TMSCs.
The increase of sXBP1 expression in TMSCs treated with TUN and TUN+Sal started at 1 hour, whereas the increase in TM cells started at 6 hours. The increased expression of GRP78, sXBP1, CHOP, and GADD34 peaked at 6 or 12 hours after TUN treatment in both TMSCs and TM cells. With TUN+Sal treatment, the mRNA expression patterns in TMSCs were similar to TUN treatment alone, whereas the expression in TM cells lasted longer and were greater (Fig. 6).
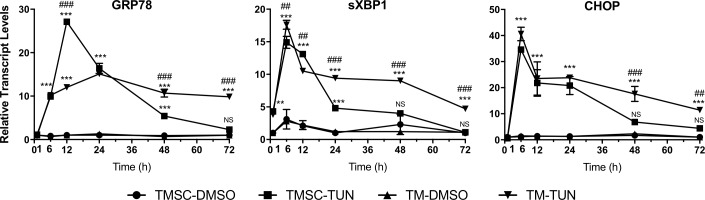
TMSCs treated with TUN significantly increased the expression of GRP78, sXBP1, and CHOP starting as early as 1 or 6 hours and lasted for 24 or 48 hours (Fig. 7). TM cells treated with TUN significantly increased the gene expression at the same time but lasted up to 72 hours (Fig. 7). GRP78 expression in TMSCs at 12 hours was significantly higher and at 48 and 72 hours was lower in TMSCs than TM cells (Fig. 7).
Figure 7.
Increased mRNA expression of ER stress markers started from 6 hours after TUN treatment. TMSCs and TM cells (TM) were incubated with 5 μg/mL TUN for different time points. mRNA levels were shown relative to DMSO controls at each time point. For DMSO controls, fold change was calculated based on the DMSO controls at 1 hour. Increase of GRP78 expression started at 6 hours and peaked at 12 hours in TMSCs and at 24 hours in TM cells. Increase of CHOP and sXBP1 expression peaked at 12 hours in TMSCs and TM cells, whereas the increase lasted longer in TM cells. Results are shown as mean ± SEM (n = 3). *TUN treatment groups versus DMSO controls; #TMSCs and TM cells with TUN treatment. */#P < 0.05, **/##P < 0.01, ***/###P < 0.001. Two-way ANOVA followed by Dunnett's multiple comparison test.
We were wondering whether TUN and Sal treatment could change the stemness and differentiation status of TMSCs. qPCR was performed to detect the expression of stem cell marker OCT4 and TM cell marker CHI3L1 in TMSCs after treatments. Supplementary Figure S3A shows as early as 1 hour after TUN and TUN+Sal treatment, OCT4 expression was dramatically reduced. At 6 hours after TUN treatment, OCT4 expression increased to a normal level up to 48 hours. With TUN+Sal treatment, OCT4 expression increased to the normal level at 6 hours and went to a greater level at 48 hours compared with DMSO controls and TUN treatment. With Sal treatment alone, OCT4 expression significant increased after 24 and 48 hours. CHI3L1 expression of TMSCs treated with TUN and TUN+Sal increased starting at 1 hour and went down to the normal level at 24 hours (Supplementary Fig. S3B). At 48 hours after treatment, CHI3L1 expression was reduced dramatically compared with the DMSO control. With Sal treatment, CHI3L1 expression significantly increased at 48 hours.
Discussion
In this study, we reported the responses of TMSCs and TM cells to ER stress inducers tunicamycin, brefeldin A, and thapsigargin at different time points and the effects of PERK pathway inhibitors GSK2606414 and salubrinal for ER stress. Both TMSCs and TM cells reduced cell viability after TUN and BreA treatment at 48 and 72 hours, whereas most of the cells were viable at 24 hours. Thap was found to be a milder ER stress inducer that did not cause severe cell apoptosis but increased GRP78 expression and swelling of ER and mitochondria, which might be a good candidate for in vivo animal model induction. In response to the ER stress inducers, both TMSCs and TM cells increased the expression of UPR markers GRP78, sXBP1, CHOP, and GADD34, and the glaucomatous-associated factor myocilin was increased in the ER and nuclei, which indicates the existence of ER stress. The increase was greather in TM cells than TMSCs, but the cell viability was similar at the same conditions. The ER and mitochondria in both cell types were swollen and abnormal in response to the inducers. The pPERK inhibitor GSK accelerated cell apoptosis and cell death induced by TUN treatment, whereas elf2α dephosphorylation inhibitor Sal increased cell viability after TUN treatment. More TMSC cells were viable at relatively low concentrations of GSK and Sal, which indicates TMSCs were less sensitive to inhibitor GSK and more sensitive to the protective inhibitor Sal. When treated TMSCs and TM cells with GSK and Sal at relatively high concentrations together with TUN treatment, GSK dramatically reduced the expression of UPR genes GRP78, sXBP1, CHOP, and GADD34 starting at 6 hours after TUN treatment. In contrast, Sal increased the UPR expression after TUN treatment starting as early as 1 hour and lasted about 24 hours in TMSCs and at least 72 hours in TM cells.
The reduced cellularity and impaired structure in the TM tissue were reported as clinical features in glaucoma patients.3,33 It is believed that chronic ER stress is involved in pathologic mechanisms of primary open angle glaucoma and may contribute to decreased TM cell numbers.18,21,22 Efforts have been made to explore the potential of stem cell therapy in regenerating TM cells and TM structure to recover normal outflow facility. However, it has not yet been elucidated whether TM stem cells in situ respond to ER stress and whether exogenous stem cells can better survive in ER stress environment. In this study, we observed that TMSCs and TM cells had similar survival rates in response to ER stress inducers in vitro, but TMSCs were less sensitive to negative PERK inhibitor GSK and more sensitive to protective inhibitor Sal, which indicates TMSCs might be able to be protected and be spared of ER stress in vivo.
In this study, GRP78, sXBP1, CHOP, and GADD34 were used to reflect ER stress response. GRP78 reflects an overall level of ER stress response. Spliced XBP1 mRNA acts as a transcriptional activator of UPR genes involved in protein folding and degradation; thus, sXBP1 serves as a protective effector to alleviate ER stress.12,34 CHOP is regulated under the PERK-eIF2α-ATF4 pathway and serves as a mediator of apoptosis.35 Our data revealed that TMSCs presented a lower level of activation of the UPR but experienced a similar death rate compared with TM cells. It has been reported that overexpression of CHOP decreases the expression levels of the antiapoptotic gene Bcl-2,36–38 but in our results, a higher expression of CHOP in TM cells did not cause greather cell death. Other researchers found that PERK−/− and eIF2α knock-in cells failed to induce CHOP but were hypersensitive to ER stress-induced apoptosis.39,40 Han et al.41 reported that coexpression of ATF4 and CHOP, but not CHOP alone, decreased cell survival by increasing protein synthesis. All these suggest that CHOP may require cooperation with other effectors to induce cell death. On the other hand, a higher expression of sXBP1 in TM cells demonstrated that these cells activated a more effective adaptive response to ER stress. Zode et al.21 and Peters et al.22 found that human glaucomatous TM tissues had chronic ER stress with significantly increased expression of GRP78 and CHOP. The starved glaucomatous TM cells also presented a higher level of ER stress response compared with normal TM cells. Contradictorily, Chai et al.42 reported a downregulation of GRP78 in stressed glaucomatous TM cells when using TUN as ER stress inducer. Considering TUN is a much stronger ER stress inducer than starvation, it is highly possible that glaucomatous TM cells cannot revoke the adaptive response or revoke an unbalanced response to ER stress. In the experiment of Peters et al.,22 glaucomatous TM cells lacked XBP1 splicing without eIF-2α increasing after dexamethasone treatment.22 Our data show that normal TMSCs and TM cells upregulated sXBP1 in response to TUN. Sal upregulated sXBP1 and CHOP expression and partially rescued cells from TUN treatment. All these support the idea that UPR is protective in response to ER stress, but the balance of UPR is critical for cell survival. Another interesting phenomenon is that Sal presented longer and stronger effects in TM cells than TMSCs, which needs further investigation.
Recently, accumulating evidence suggests that ER stress and the UPR play an important role in self-renewal and differentiation of stem cells. Hematopoietic stem cells have the remarkable ability of self-renewal. The study of van Galen et al. showed that the UPR selectively induced hematopoietic stem cell apoptosis to prevent propagation of damaged stem cells.27 In a similar study, intestinal epithelial stem cells quickly lost their stemness and were removed by differentiation under stress.43 Furthermore, the UPR signal network, especially PERK, was also involved in differentiation of muscle stem cells44 and esophageal epithelium.45 In our study, we observed that stem cell marker OCT4 expression was initially reduced and increased at 48 hours in TMSCs after TUN and TUN+Sal treatment, whereas the differentiated TM cell marker CHI3L1 expression decreased at 48 hours. Without TUN treatment, Sal alone increased OCT4 expression at 24 and 48 hours and increased CHI3L1 expression at 48 hours. This may indicate that some TMSCs are more prone to ER stress, whereas the “good” TMSCs are resistant to ER stress. At the meantime, Sal can promote stem cell proliferation and differentiation. Further studies are needed to unveil this.
Although our results show that TUN, BreA, and Thap can effectively induce ER stress in TMSCs and TM cells in vitro, the mechanism is different from the actual pathology of glaucoma. Zadoo et al.46 reported successful transfection of mutant myocilin into TM cells using plasmids. Jain et al.47 used CRISPR-Cas9 technology to transfect human TM cells with mutant myocilin and successfully induced ER stress in those cells. Further studies to transfect TMSCs and TM cells with mutant myocilin using the abovementioned techniques are needed to unveil the ER stress response and effects of activated PERK pathway.
In conclusion, our results revealed that TMSCs have a lower but effective UPR for cell survival compared with TM cells, which have higher UPR in response to ER stress. XBP splicing may play critical roles in the process of ER stress. Some TMSCs might be more resistant to ER stress than other TMSCs and TM cells. TMSCs are less sensitive to pPERK inhibitor GSK but more sensitive to protective inhibitor Sal. These findings provide a theoretical basis for protecting or regenerating TM in glaucoma patients by regulating the PERK signaling pathway in combination with stem cell therapy.
Supplementary Material
Acknowledgments
The authors thank Ming Sun (Center for Biologic Imaging at the University of Pittsburgh for Transmission Electron Microscopy) and Nancy Zurowski (Ophthalmology at the University of Pittsburgh) for flow cytometry.
Supported by BrightFocus Foundation Grant G2014086 (YD); National Institutes of Health Grants EY025643 (YD) and P30-EY008098; Eye and Ear Foundation, Pittsburgh, Pennsylvania, United States; Research to Prevent Blindness; and an anonymous philanthropic donation (YD).
Disclosure: Y. Wang, None; D. Osakue, None; E. Yang, None; Y. Zhou, None; H. Gong, None; X. Xia, None; Y. Du, None
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamer WD. The cell and molecular biology of glaucoma: mechanisms in the conventional outflow pathway. Invest Ophthalmol Vis Sci. 2012;53:2470–2472. doi: 10.1167/iovs.12-9483f. [DOI] [PubMed] [Google Scholar]
- 3.Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 4.Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond) 1987;1:204–210. doi: 10.1038/eye.1987.38. [DOI] [PubMed] [Google Scholar]
- 5.Lutjen-Drecoll E, Rittig M, Rauterberg J, Jander R, Mollenhauer J. Immunomicroscopical study of type VI collagen in the trabecular meshwork of normal and glaucomatous eyes. Exp Eye Res. 1989;48:139–147. doi: 10.1016/0014-4835(89)90027-4. [DOI] [PubMed] [Google Scholar]
- 6.Vranka JA, Kelley MJ, Acott TS, Keller KE. Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma. Exp Eye Res. 2015;133:112–125. doi: 10.1016/j.exer.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dan J, Belyea D, Gertner G, Leshem I, Lusky M, Miskin R. Plasminogen activator inhibitor-1 in the aqueous humor of patients with and without glaucoma. Arch Ophthalmol. 2005;123:220–224. doi: 10.1001/archopht.123.2.220. [DOI] [PubMed] [Google Scholar]
- 8.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 9.Athanasiou D, Aguila M, Bellingham J, Kanuga N, Adamson P, Cheetham ME. The role of the ER stress-response protein PERK in rhodopsin retinitis pigmentosa. Hum Mol Genet. 2017;26:4896–4905. doi: 10.1093/hmg/ddx370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oslowski CM, Urano F. The binary switch between life and death of endoplasmic reticulum-stressed beta cells. Curr Opin Endocrinol Diabetes Obes. 2010;17:107–112. doi: 10.1097/MED.0b013e3283372843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 13.Shen X, Ellis RE, Lee K, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 14.Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 15.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differentiation. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 18.Zode GS, Sharma AB, Lin X, et al. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest. 2014;124:1956–1965. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu YY, You YW, Ren XH, et al. Endoplasmic reticulum stress-mediated signaling pathway of gastric cancer apoptosis. Hepatogastroenterology. 2012;59:2377–2384. doi: 10.5754/hge12369. [DOI] [PubMed] [Google Scholar]
- 20.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zode GS, Kuehn MH, Nishimura DY, et al. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121:3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters JC, Bhattacharya S, Clark AF, Zode GS. Increased endoplasmic reticulum stress in human glaucomatous trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2015;56:3860–3868. doi: 10.1167/iovs.14-16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manuguerra-Gagne R, Boulos PR, Ammar A, et al. Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells. 2013;31:1136–1148. doi: 10.1002/stem.1364. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Hassan DW, Li X, Ryan EI, Acott TS, Kelley MJ. Induced pluripotent stem cells restore function in a human cell loss model of open-angle glaucoma. Stem Cells. 2015;33:751–761. doi: 10.1002/stem.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu W, Gramlich OW, Laboissonniere L, et al. Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc Natl Acad Sci U S A. 2016;113:E3492–E3500. doi: 10.1073/pnas.1604153113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu W, Jain A, Gramlich OW, Tucker BA, Sheffield VC, Kuehn MH. Restoration of aqueous humor outflow following transplantation of iPSC-derived trabecular meshwork cells in a transgenic mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2017;58:2054–2062. doi: 10.1167/iovs.16-20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Galen P, Kreso A, Mbong N, et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature. 2014;510:268–272. doi: 10.1038/nature13228. [DOI] [PubMed] [Google Scholar]
- 28.Axten JM, Medina JR, Feng Y, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2, 3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J Med Chem. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 29.Boyce M, Bryant KF, Jousse C, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 30.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Y, Roh DS, Mann MM, Funderburgh ML, Funderburgh JL, Schuman JS. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Invest Ophthalmol Vis Sci. 2012;53:1566–1575. doi: 10.1167/iovs.11-9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desjardin C, Chat S, Gilles M, et al. Involvement of mitochondrial dysfunction and ER-stress in the physiopathology of equine osteochondritis dissecans (OCD) Exp Mol Pathol. 2014;96:328–338. doi: 10.1016/j.yexmp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Gottanka J, Johnson DH, Grehn F, Lutjen-Drecoll E. Histologic findings in pigment dispersion syndrome and pigmentary glaucoma. J Glaucoma. 2006;15:142–151. doi: 10.1097/00061198-200604000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 35.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 36.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy KM, Ranganathan V, Farnsworth ML, Kavallaris M, Lock RB. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 2000;7:102–111. doi: 10.1038/sj.cdd.4400597. [DOI] [PubMed] [Google Scholar]
- 38.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 40.Fujita E, Kouroku Y, Isoai A, et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II) Hum Mol Genet. 2007;16:618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- 41.Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chai F, Luo R, Li Y, et al. Down-regulation of GRP78 in human glaucomatous trabecular meshwork cells. Mol Vis. 2010;16:1122–1131. [PMC free article] [PubMed] [Google Scholar]
- 43.Heijmans J, van Lidth de Jeude JF, Koo BK, et al. ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Rep. 2013;3:1128–1139. doi: 10.1016/j.celrep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 44.Bohnert KR, McMillan JD, Kumar A. Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J Cell Physiol. 2018;233:67–78. doi: 10.1002/jcp.25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosekrans SL, Heijmans J, Buller NV, et al. ER stress induces epithelial differentiation in the mouse oesophagus. Gut. 2015;64:195–202. doi: 10.1136/gutjnl-2013-306347. [DOI] [PubMed] [Google Scholar]
- 46.Zadoo S, Nguyen A, Zode G, Hulleman JD. A novel luciferase assay for sensitively monitoring myocilin variants in cell culture. Invest Ophthalmol Vis Sci. 2016;57:1939–1950. doi: 10.1167/iovs.15-18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain A, Zode G, Kasetti RB, et al. CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proc Natl Acad Sci U S A. 2017;114:11199–11204. doi: 10.1073/pnas.1706193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.