Abstract
Background:
Periodontitis is a public health concern since it is a major factor in tooth loss worldwide and has association with many systemic diseases. Sleep is a complex and essentially biological process and a critical factor for maintaining mental and physical health. Since inflammation is characteristic of both chronic periodontitis and sleep deprivation, few studies in recent years present the contradictory results regarding this potential association. The objective of the present study was to investigate the association between quality of sleep and chronic periodontitis.
Materials and Methods:
A total of 200 individuals participated in this study. All participants underwent a comprehensive clinical periodontal examination. Case–control were identified using the Centers for Disease Control and Prevention/American Academy of Periodontology case definitions for periodontal disease. The quality of sleep was assessed by Pittsburgh Sleep Quality Index. The univariate and multivariate logistic regression analysis was used to test the influence of variables (quality of sleep, age, sex, ethnicity, education, and socioeconomic status), in the occurrence of periodontitis. Odds ratio (OR) and respective confidence intervals (CIs) were calculated and reported. P =0.05 was considered statistically significant.
Results:
The prevalence of poor quality of sleep was 56.75% in cases (periodontitis group) and 43.24% in control group. There was positive association between quality of sleep and chronic periodontitis (OR = 3.04; 95% CI = 1.42–6.5; P = 0.004). In multivariate logistic regression analysis, only the age was significantly related to the periodontitis (OR = 1.11; 95% CI = 1.07–1.41; P < 0.001), other variables failed to reach the significant level.
Conclusion:
Poor quality of sleep was significantly associated with chronic periodontitis. Only the age was significantly related to periodontitis among the other covariable measured.
Keywords: Chronic periodontitis, periodontal medicine, quality of sleep, sleep deprivation
INTRODUCTION
Periodontal disease is the most common cause of tooth loss worldwide, referring to infection of the supporting structures of the tooth, principally the gingiva, periodontal ligament, cementum, and alveolar bone.[1] Periodontal diseases are inflammatory diseases in which microbial etiologic factors induce a series of host responses that mediate inflammatory events. Bacteria and inflammatory mediators not only confined to periodontal tissue but also may enter the blood and disseminate systemically having a measurable impact on systemic inflammation.[2] In susceptible individuals, dysregulation of inflammatory and immune pathways leads to chronic inflammation, tissue destruction, and disease.[3]
In recent years, there has been intense interest in potential associations between periodontal disease and various chronic systemic diseases and conditions, including cardiac diseases, diabetes, respiratory diseases, chronic kidney disease, rheumatoid arthritis, cognitive impairment, obesity, metabolic syndrome, and cancer.[4] A number of risk factors (smoking, diabetes, immunosuppression, genetic factors, stress, and age) contribute to the susceptibility of individuals to periodontal diseases and to the pathogenesis and severity of the disease. Studies of the influence of risk factors on disease progression have been focused on the inflammatory reaction. These studies concluded that a sound inflammatory host response is needed for successful periodontal defense.[5]
Sleep is a complex and essentially biological process that is required on a daily basis for all humans regardless of age, sex, or ethnic origin. Sleep has important roles in controlling the functions of many other body systems, and this becomes very evident in states of sleep deprivation.[6] Sleep deprivation is not limited to a special group of people, a nation, a gender, or a particular age group, rather, it is a new human behavior observed among millions of adults and children worldwide.[7,8,9]
Sleep deprivation has a profound effect on many aspects of physiologic function such as alertness, memory processing, the repair of cell damage, brain development, cognition, mood, hormonal regulation, risk of depression, increased cortisol, and ghrelin, impaired glucose metabolism, immune function, autonomic activities, increased inflammatory, and proinflammatory marker.[6,10] Both acute and chronic sleep deprivation may activate inflammatory processes, leading to increasing C-reactive protein (CRP) concentrations, increase peripheral circulation of leukocytes, increase levels of interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α).[11]
Recently, the investigators highlighted the association between short sleep duration with the risk of systemic infections, explained by the experimental evidence of host immunity.[12] Since inflammation is characteristic of both chronic periodontitis and sleep deprivation, few studies in recent years suggested the association of sleep duration and periodontitis with varying results in India, the USA, and South Korea.[5,13,14] Therefore, this study has been undertaken with the primary objective, to investigate the potential association between quality of sleep and chronic periodontitis. In addition to this, we also analyzed the influence of covariates such as age, gender, ethnicity, education, and household income associated with periodontitis.
MATERIALS AND METHODS
A case–control study was conducted from March to December 2016 after approval by the Institutional Research and Ethics Committee of Faculty of Dentistry (FOD), Melaka Manipal Medical College (MMMC), Manipal Academy of Higher Education, Melaka, Malaysia. Study individuals were recruited by purposive consecutive sampling from the outpatient department of polyclinic of FOD, reported for the dental treatment. Written informed consent was obtained from all participants.
The study included the individuals who were ≥21 years of age, and minimum 16 natural teeth were present in dentition.[15] Individuals who were smokers, pregnant, or lactating females, known systemic diseases (e.g., diabetes mellitus, HIV), had received any periodontal treatment in the past 6 months, and had a history of medication (antibiotics or anti-inflammatory drugs) in the past 3 months were excluded from the study.
Participants were also asked during an interview to report age, sex, race, education level, and economic status. The variables were categorized as follows: age (continuous variable), sex (male, female), race/ethnicity (Chinese, Malay, Indian, and others), education level (secondary or above; less than secondary level), and economic status (household monthly income <5000; ≥5000 Ringgit Malaysian {RM}). The periodontal status was assessed on all teeth excluding third molars using William's periodontal graduated probe by two calibrated examiners. The comprehensive clinical periodontal examination included pocket probing depth (PD) and clinical attachment loss (CAL) in millimeter (mm) at six points per tooth (mesiofacial, midfacial, distofacial, distolingual, midlingual, and mesiolingual). In addition to this, mean plaque index (PI)[16] and mean gingival index (GI)[17] were recorded for each patient. The intraexaminer reproducibility of clinical examination was provided before the study. The Cohen's kappa was 0.75 and 0.90 for PD and CAL, respectively.
Cases (group with periodontitis) were defined using the Centers for Disease Control and Prevention/American Academy of Periodontology (CDC/AAP) definitions of periodontal disease as described by Eke et al.:[18] (1) mild periodontitis, defined as two or more interproximal sites with CAL ≥3 mm and at least two interproximal sites with PD ≥4 mm (not on the same tooth) or one site with ≥5 mm; (2) moderate periodontitis, defined as two or more interproximal sites with CAL ≥4 mm (not on the same tooth) or two or more interproximal sites with PD ≥5 mm, also not on the same tooth; and (3) severe periodontitis, defined as two or more interproximal sites with CAL ≥6 mm (not on the same tooth) and one or more interproximal site(s) with PD ≥5 mm. Those participants who did not meet these criteria were considered control (no periodontitis) group. We used 1:1 case–control ratio, and included 100 cases with periodontitis and 100 controls.
All the recruited study individuals were administered validated Pittsburgh Sleep Quality Index (PSQI)[19] questionnaire, either in English, Malay, or Chinese language. The PSQI is an effective instrument used to measure the quality and pattern of sleep during the previous month, in the older[19] and young adults.[20] It provides a reliable, valid, and standardized measure of sleep quality.
PSQI consists of 19 self-rated item and these items were grouped into seven domains (subjective sleep quality, sleep latency, sleeps duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction) score, each weighted on 0–3 scale, whereby 3 reflected the negative extreme on the Likert scale. The seven component score was then summed to produce a global score (range 0–21); higher PSQI scores indicate the worse quality of sleep. A global sum of “5” or greater indicated a “poor” sleeper. It was stressed to the respondents that participation is voluntary and that they should skip any questions that they are not comfortable answering rather than providing false responses. The incomplete filled questionnaires were excluded from the study.
Statistical analysis
Descriptive analysis was undertaken and presented as mean, standard deviation, frequency, and percentage. We used independent t-test to find the PI, GI, PD, and CAL between cases and control. For relationship assessment, univariate and multivariate logistic regression analysis was performed. All variables except age were transformed into categorical variables in multivariate analysis. We calculated odds ratio (OR) and its 95% confidence interval (CI). P ≤ 0.05 was considered statistically significant. All data were analyzed using the statistical software (SPSS v. 24.0 INM, Chicago, IL, USA).
RESULTS
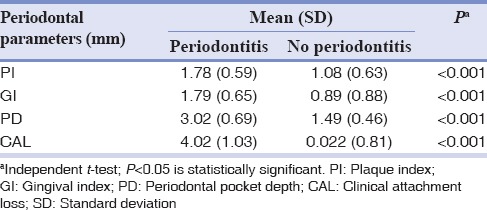
A total of 100 cases (periodontitis) and 100 controls (no periodontitis) were enrolled according to the CDC/AAP case definition for periodontitis. Table 1 summarizes the sociodemographic characteristic of the sampled population. The mean age of the cases was 45.74 ± 14.75 years, and in control group was 31.5 ± 9.77 years. Overall, 55.5% of participants having a poor quality of sleep in the study individuals, among them, 56.75% in periodontitis group. Table 2 summarizes the mean clinical measurements in accordance with the diagnosis of periodontitis. As expected the mean periodontal measurements (PI, GI, PD, and CAL) were higher in periodontitis group compared with no periodontitis group.
Table 1.
Social demographic characteristics of sampled population
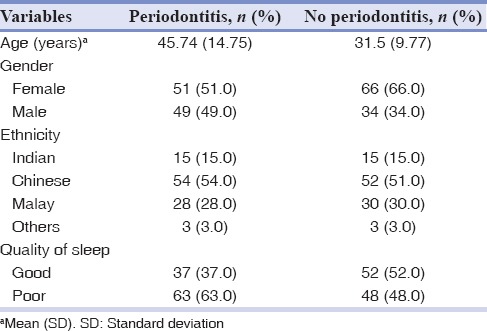
Table 2.
Mean clinical parameters in periodontitis and no periodontitis group
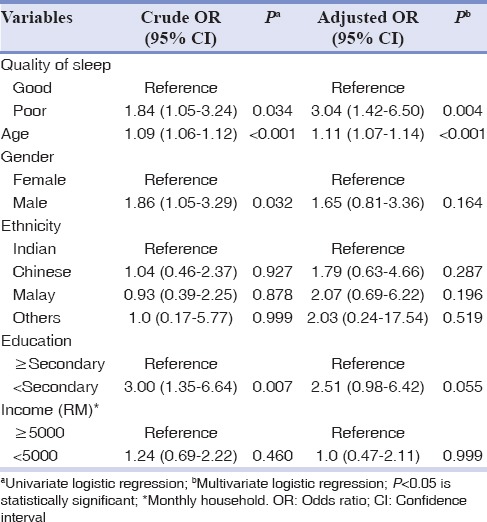
The results of the logistic regression model for selected variables are presented as crude OR (crude OR) for univariate and adjusted OR (adjusted OR) for multivariate analysis as shown in Table 3. The poor sleep quality was strongly associated to periodontitis in both univariate (crude OR = 1.85, 95% CI = 1.05–3.24; P = 0.034) and multivariate (adjusted OR = 3.04, 95% CI = 0.78–6.5; P = 0.004) analysis. Furthermore, age was positively associated with periodontitis (crude OR = 1.09, 95% CI = 1.06–1.12; P < 0.001 and adjusted OR = 1.11, 95% CI = 1.07–1.14; P < 0.001). The association of education with periodontitis was statistically significant, with P = 0.007 in univariate analysis but not in multivariate analysis. There was no significant association between sex, ethnicity, socioeconomic status, and periodontitis.
Table 3.
Logistic regression model for selected variables and periodontitis
DISCUSSION
The principal objective of this study was to analyze the association between quality of sleep and periodontitis. Few studies investigated this association as primary or secondary objective using various assessment methods with varying results. The present study found a significant association between periodontitis and quality of sleep.
Various sleep quantity and quality assessment method have been used in the past such as sleep diaries, clinical interviews, and questionnaire. In the present study, PSQI was used for assessing the quality of sleep. The PSQI has 86.5% specificity and 89.6% sensitivity for identifying “good” and “bad” sleep quality using a global cutoff score of 5. It also has internal consistency and a Cronbach's alpha (reliability coefficient) of 0.83 for its seven components. The validity and reliability of PSQI was supported by various studies in a variety of population internationally.[19,20]
Possible confounders such as smokers, pregnant or lactating females, known systemic diseases (e.g., diabetes mellitus and HIV), patients who had received any periodontal treatment in past 6 months, and had a history of medication (antibiotics or anti-inflammatory drugs) in the past 3 months were excluded using the strict exclusion criteria, thus providing a study sample with the least amount of patient-centered confounders. The periodontal examination on participants was performed before the completion of PSQI questionnaire, therefore, minimize the effect of examiner bias.
The results of the present study are in agreement with previously conducted studies,[5,14,21] which stated a possible association between periodontal disease and inadequate sleep. One of these studies[5] assessed the periodontal status based on measurement of PD at two proximal sites of each tooth, which may lead to overestimation/underestimation of the prevalence of periodontal disease. One of the other cross-sectional studies investigated the impact of lifestyle on periodontal health, and lack of sleep was identified as a significant lifestyle factor that played a role in the progression of periodontal disease (OR: 1.69; P = 0.008).[21] This study used the community periodontal index (CPI) and CAL with at least one site ≥4 mm was considered case-defining criteria for periodontitis compared to a six-point comprehensive periodontal examination and the CDC/AAP criteria used in our study.
Furthermore, one of the recent studies[14] found a novel, direct, and independent association between longer sleep duration and the prevalence of periodontitis in the Korean population. In particular, the author reported, for everyone more hour of sleep, the estimated odds of periodontitis increase of 17%. The authors of this study suggested the potential possibility of a “reverse causation” (effect of periodontitis on sleep duration) or a bidirectional relationship. This study used the CPI for the case definition of periodontitis and self-reported average number of hours of sleep per day for sleep assessment.
The findings of the present study are in disagreement with the recently conducted study, which investigated the association of sleep duration (<7 h/night), and periodontitis in individuals who were ages 30 years and above and failed to reach the association at a significant level in the adjusted analysis.[13] However, this study used data (2009–2012) from large, nationally representative survey, in which the multiple examiners performed periodontal examination, whereas, in our study, the periodontal data were collected by two calibrated examiners. The sleep quality was assessed by the dichotomized self-reported response (less or more than 7 h) in other study compared to PSQI score in our study.
Periodontitis is multifactorial plaque-induced chronic disease, in which environmental factors play a significant role over a period. In the present study, we analyzed the influence of age, sex, ethnicity, education, and socioeconomic status of participants. Age is considered an important risk factor for periodontal disease. Being the unmatched case–control design of the study, we did not attempt to match age or sex for selection of cases and control; moreover, both were selected from same pool of patients reported for dental treatment. We included participants of ≥21 years of age with no upper limit and as expected participants of younger age in control group compared to cases. The present study found a positive association of periodontitis with age (OR = 1.11), similar to finding reported in various other studies;[13,21,22,23] however, few authors[24] believed that severe periodontal disease in the elderly could be due to cumulative destruction over a lifetime, rather than the influence of age.
In the present study, sex was not significantly associated in adjusted analysis with periodontitis in accordance with other studies.[5,23] Although in contrast to this, few studies reported the prevalence of periodontitis is greater in males than in females.[13,25] Furthermore, no significant association was found in periodontitis and races in the present study are in agreement with Khan et al.;[26] however, few studies reported the racial variation (Hispanic vs. non-Hispanic) in contrast to the present study.[13]
The results of our study werein agreement with meta-analysis,[27] which concluded that low educational attainment is associated with increased risk for periodontitis in unadjusted analysis; however, a relatively weak association was demonstrated in the adjusted analysis.
Few studies[28] reported the progression of periodontal diseases influenced by the socioeconomic status. However, the results of the present study did not demonstrate an association between the severity of periodontitis and socioeconomic status. The possible recruitment of average income group individuals, from urban population might be the explanation of no association in this study.
The biological plausibility for such potential association exists that quality of sleep influences the modulation of host immune and inflammatory response. Researchers found increased lymphocyte activation with over productions of IL-1, IL-6, IL-7, and TNF-α in sleep-deprived individuals.[29,30] A study found that 4 h of sleep restriction in one night led to increasing in monocyte production of IL-6 and TNF-α messenger RNA.[31] However, researchers found a significant increase in IL-1β and IL-1ra and a significant decrease in CRP and IL-6 in 40 h sleep-deprived individuals.[30] In fact, some studies reported racial differences, particularly elevation in CRP level, only in long sleepers among Asians, compared to other races, which have exhibited elevated levels of CRP even in short sleepers.[32,33]
The case-–control design for higher level of evidence and full-mouth comprehensive clinical examination strengthening the finding of this study. However, due to the multifactorial etiology, some unknown confounding factors that might exist and influence both sleep pattern and periodontitis.
CONCLUSION
This is the first case–control study showing a significant association between poor quality of sleep and chronic periodontitis in the Malaysian population in both unadjusted and adjusted analysis. Furthermore, none of other measured variables except age was significantly associated with periodontitis. The future investigations are necessary to verify the role of sleep, including both subjective and objective measures for sleep pattern, to understand the underlying mechanism of this potential association.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declared that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
Acknowledgments
The authors would like to thank Prof. Dr Abdul Rashid Hj Ismail, Dean, FOD, MMMC, Manipal Academy of Higher Education, Melaka, for his constant support and encouragement. The authors thank Prof. Dr Adinegara Lutfi Abas, Dean, MMMC, Manipal Academy of Higher Education, Melaka, for his expertise in the statistical assistance.
REFERENCES
- 1.Zohrabian VM, Abrahams JJ. Inflammatory diseases of the teeth and jaws. Semin Ultrasound CT MR. 2015;36:434–43. doi: 10.1053/j.sult.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Van Dyke TE, van Winkelhoff AJ. Infection and inflammatory mechanisms. J Clin Periodontol. 2013;40(Suppl 14):S1–7. doi: 10.1111/jcpe.12088. [DOI] [PubMed] [Google Scholar]
- 3.Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57–80. doi: 10.1111/prd.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: Review of the evidence. J Clin Periodontol. 2013;40(Suppl 14):S8–19. doi: 10.1111/jcpe.12064. [DOI] [PubMed] [Google Scholar]
- 5.Grover V, Malhotra R, Kaur H. Exploring association between sleep deprivation and chronic periodontitis: A pilot study. J Indian Soc Periodontol. 2015;19:304–7. doi: 10.4103/0972-124X.154173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5:31–43. doi: 10.2174/1874306401105010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Basta M, et al. Insomnia with short sleep duration and mortality: The penn state cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro-Costa E, Dewey ME, Ferri CP, Uchôa E, Firmo JO, Rocha FL, et al. Association between sleep duration and all-cause mortality in old age: 9-year follow-up of the Bambuí cohort study, Brazil. J Sleep Res. 2011;20:303–10. doi: 10.1111/j.1365-2869.2010.00884.x. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, Takahashi K, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: A study with microneurographic technique. Sleep. 2003;26:986–9. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 11.Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Probl Cardiol. 2005;30:625–62. doi: 10.1016/j.cpcardiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW, et al. Aprospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35:97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiener RC. Relationship of routine inadequate sleep duration and periodontitis in a nationally representative sample. Sleep Disord 2016. 2016 doi: 10.1155/2016/9158195. 9158195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romandini M, Gioco G, Perfetti G, Deli G, Staderini E, Laforì A, et al. The association between periodontitis and sleep duration. J Clin Periodontol. 2017;44:490–501. doi: 10.1111/jcpe.12713. [DOI] [PubMed] [Google Scholar]
- 15.Loke W, Girvan T, Ingmundson P, Verrett R, Schoolfield J, Mealey BL, et al. Investigating the association between obstructive sleep apnea and periodontitis. J Periodontol. 2015;86:232–43. doi: 10.1902/jop.2014.140229. [DOI] [PubMed] [Google Scholar]
- 16.Silness J, Loe H. Periodontal disease in Pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 17.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 18.Eke PI, Thornton-Evans G, Dye B, Genco R. Advances in surveillance of periodontitis: The centers for disease control and prevention periodontal disease surveillance project. J Periodontol. 2012;83:1337–42. doi: 10.1902/jop.2012.110676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Manzar MD, Zannat W, Hussain ME, Pandi-Perumal SR, Bahammam AS, Barakat D, et al. Dimensionality of the pittsburgh sleep quality index in the collegiate young adults. Springerplus. 2016;5:1550. doi: 10.1186/s40064-016-3234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singla N, Acharya S, Prabhakar RV, Chakravarthy K, Singhal D, Singla R, et al. The impact of lifestyles on the periodontal health of adults in Udupi district: A cross sectional study. J Indian Soc Periodontol. 2016;20:330–5. doi: 10.4103/0972-124X.179405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad NE, Sanders AE, Sheats R, Brame JL, Essick GK. Obstructive sleep apnea in association with periodontitis: A case-control study. J Dent Hyg. 2013;87:188–99. [PubMed] [Google Scholar]
- 24.Albandar JM, Streckfus CF, Adesanya MR, Winn DM. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol. 2000;71:1874–81. doi: 10.1902/jop.2000.71.12.1874. [DOI] [PubMed] [Google Scholar]
- 25.Desvarieux M, Schwahn C, Völzke H, Demmer RT, Lüdemann J, Kessler C, et al. Gender differences in the relationship between periodontal disease, tooth loss, and atherosclerosis. Stroke. 2004;35:2029–35. doi: 10.1161/01.STR.0000136767.71518.36. [DOI] [PubMed] [Google Scholar]
- 26.Khan S, Saub R, Vaithilingam RD, Safii SH, Vethakkan SR, Baharuddin NA, et al. Prevalence of chronic periodontitis in an obese population: A preliminary study. BMC Oral Health. 2015;15:114. doi: 10.1186/s12903-015-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boillot A, El Halabi B, Batty GD, Rangé H, Czernichow S, Bouchard P, et al. Education as a predictor of chronic periodontitis: A systematic review with meta-analysis population-based studies. PLoS One. 2011;6:e21508. doi: 10.1371/journal.pone.0021508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchwald S, Kocher T, Biffar R, Harb A, Holtfreter B, Meisel P, et al. Tooth loss and periodontitis by socio-economic status and inflammation in a longitudinal population-based study. J Clin Periodontol. 2013;40:203–11. doi: 10.1111/jcpe.12056. [DOI] [PubMed] [Google Scholar]
- 29.Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van Beers P, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985) 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 30.Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 32.Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G, et al. Extreme sleep durations and increased C-reactive protein: Effects of sex and ethnoracial group. Sleep. 2013;36:769–779E. doi: 10.5665/sleep.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21:799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


