Abstract
Patient: Male, 59
Final Diagnosis: Chromophobe renal cell carcinoma, stage IV
Symptoms: Discomfort in the right hypochondrium
Medication: Oncolytic virus Rigvir
Clinical Procedure: Nephro-adrenalectomy
Specialty: Oncology
Objective:
Unusual or unexpected effect of treatment
Background:
Renal cell carcinoma is the most commonly diagnosed primary malignant tumor of the kidney in adults, and includes the variant of chromophobe renal cell carcinoma. Despite new targeted therapies that improve progression-free survival (PFS) and overall survival (OS) for early-stage renal cell carcinoma, the 5-year survival for patients with stage IV renal cell carcinoma remains below 10%, and the 50% OS is less than one year. Metastatic renal cell carcinoma can be resistant to cytotoxic chemotherapy. This report is of a case of stage IV chromophobe renal cell carcinoma that responded well to treatment with the oncolytic ECHO-7 virus, Rigvir®.
Case Report:
In December 2015, a 59-year-old man presented with a right-sided chromophobe renal cell carcinoma stage IV (pT1N0M1) with adrenal gland metastasis. He underwent right nephro-adrenalectomy followed by treatments with Rigvir® (≥106 TCID50/ml) by intramuscular (i.m.) injection on three consecutive days. Treatment with Rigvir® continued once per week for three months, and from March 2016, once per month, with continued treatment until computed tomography (CT) scans confirmed that the tumor metastases had stabilized.
Conclusions:
This case report has demonstrated that the oncolytic ECHO-7 virus, Rigvir® should be evaluated further as a potential treatment for advanced renal carcinoma.
MeSH Keywords: Carcinoma, Renal Cell; Oncolytic Virotherapy; Oncolytic Viruses
Background
Renal cell carcinoma is the most commonly diagnosed primary malignant tumor of the kidney in adults, accounting for 90% of all malignant tumors arising in the kidney, and includes the variant of chromophobe renal cell carcinoma [1]. Worldwide, each year there are more than 270,000 new cases of renal cancer, 116,000 of which about result in patient death [2]. In nearly 30% of all newly diagnosed patients, renal cell carcinoma is advanced with metastatic spread, with most metastases are found in the lungs, distant lymph nodes, liver, bone, or brain [3,4]. The primary treatment for localized renal cell carcinoma is surgery, but there is tumor recurrence in 30% of patients [5,6]. Current treatment approaches for advanced renal cell carcinoma rarely achieving a significant impact on the overall survival (OS) of the patient [7].
Metastatic renal cell carcinoma does not respond well to radiation therapy or cytotoxic chemotherapy, and the primary tumor or metastases might not be amenable to surgical re-section [7,8]. A review of the use of 72 cytotoxic chemotherapeutic agents in patients with renal cell carcinoma showed that in only 5.6% of patients was there is any improvement in OS when using a single chemotherapeutic agent [9].
Rigvir® is an oncolytic, non-pathogenic, genetically unmodified ECHO-7 virus, initially developed for the treatment of advanced melanoma, and has been approved and registered for the treatment of melanoma in Latvia since 2004 [10]. In a retrospective study of patients with melanoma, treatment with Rigvir® by intramuscular injection significantly reduced patient mortality by between 4.39–6.57-fold in patients with stage IB–IIC melanoma [11].
This report is of a case of stage IV chromophobe renal cell carcinoma that responded well to treatment with the oncolytic ECHO-7 virus, Rigvir®.
Case Report
On 12 December 2015, a 59-year-old man was diagnosed with chromophobe renal cell carcinoma, stage IV (pT1, N0, M1). He also had a history of duodenal diverticula, hypertension, dyscirculatory encephalopathy, hydrocephalus, cerebral atherosclerosis, osteochondrosis of the cervical and thoracic spine, deforming spondylosis, and coxarthrosis. The patient did not drink or smoke.
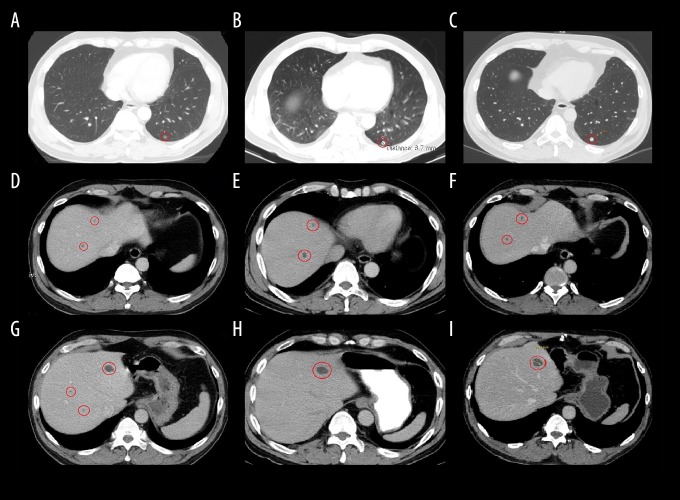
During a mandatory pre-operative assessment before right inguinal hernia repair surgery on 12 November 2015, abdominal ultrasound showed a hypoechoic focal right kidney lesion measuring 33×32 mm. Abdominal contrast – enhanced computed tomography (CT) confirmed the presence of a 31×34×40 mm well – defined, heterogeneous mass at the dorsal lower pole of the right kidney and bilateral involvement of the adrenal glands. There were multiple metastatic tumor foci measuring between 8×7×13 mm to 6×8×8 mm in the right adrenal gland, and 14×14×11 mm in the left adrenal gland. Also, multiple parenchymal cysts of the liver were seen on abdominal contrast-enhanced CT (Figure 1D, 1G), as well as a subpleural focus in the lower pole of the left lung (Figure 1A), which was most likely to have been a fibrotic nodule.
Figure 1.
Thoracic and abdominal contrast-enhanced computed tomography (CT) scans between November 2015 to September 2017 of a 61-year-old man with a chromophobe renal cell carcinoma treated with Rigvir®. (A) Thoracic contrast-enhanced computed tomography (CT) scan shows a subpleural nodule (13 November 2015). (B) Thoracic contrast-enhanced CT scan (20 June 2016). (C) Thoracic contrast-enhanced CT scan (21 September 2017). (D) Abdominal contrast-enhanced CT scan shows cysts in the liver parenchyma (13 November 2015). (E) Abdominal contrast-enhanced CT scan (20 June 2016). (F) Abdominal contrast-enhanced CT scan (21 September 2017). (G) Abdominal contrast-enhanced CT scan shows cysts in the liver parenchyma (13 November 2015). (H) Abdominal contrast-enhanced CT scan (20 June 2016). (I) Abdominal contrast-enhanced CT scan (21 September 2017).
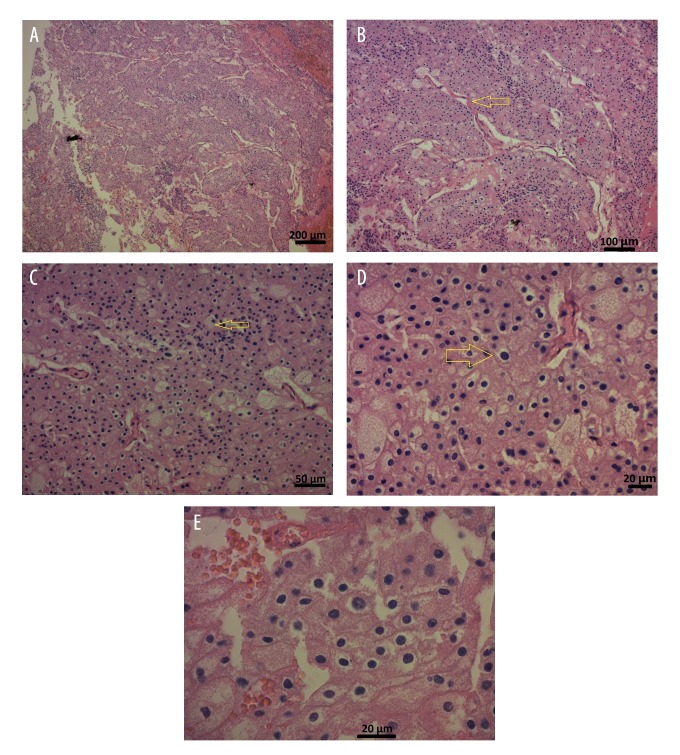
On 30th November 2015, a right nephro-adrenalectomy was performed. Biopsies from the left adrenal gland and the liver were taken. Histological examination showed a tumor with a solid growth pattern, with large pale cells with reticulated cytoplasm, and perinuclear halos, characteristic for a chromophobe renal cell carcinoma (Figure 2).
Figure 2.
Photomicrographs of the histopathology of a chromophobe renal cell carcinoma in a 59-year-old man. (A) The photomicrograph shows the characteristic solid growth pattern of a chromophobe renal cell carcinoma. Hematoxylin and eosin (H&E). Magnification ×50. Scale bar, 200 μm. (B) The photomicrograph shows the tumor cells growing in solid nests divided by vascular septae (arrow). H&E. Magnification ×100. Scale bar, 100 μm. (C) The photomicrograph shows that the tumor cells are large and pale with reticulated cytoplasm and perinuclear halos (arrow). H&E. Magnification ×200. Scale bar, 50 μm. (D) The photomicrograph shows large tumor cells with perinuclear halos or translucent zones (arrow). The cytoplasm is pale and flocculent but not clear. H&E. Magnification ×400. Scale bar, 20 μm. (E) The photomicrograph shows that the tumor cells have irregular, wrinkled, and angulated nuclei with perinuclear halos. H&E. Magnification ×630. Scale bar, 20 μm.
Chemotherapy with sunitinib was recommended, but the patient declined the treatment due to the possible long-term side-effects. Virotherapy with the oncolytic ECHO-7 virus, Rigvir® began on 20 December 2015. The TCID50 represents the viral titer that can result in a cytopathic effect (CPE). The first three treatments with Rigvir® (≥106 TCID50/ml) were by intramuscular (i.m.) injection given on three consecutive days. Then, treatment with Rigvir® was once per week for three months. From March 2016, treatment with Rigvir® was once per month, with Rigvir® therapy still ongoing. The patient did not receive any other cancer treatment pre-operatively or postoperatively.
Several ultrasound and CT imaging studies were performed between March 2016 and September 2017, including abdominal, thoracic, and prostate imaging. From this time, no cancer recurrence, metastases, or lymph node involvement were detected in the abdomen, retroperitoneum, pelvis, lungs, or bones. The thoracic CT scans showed that the subpleural focus remained stable between June 2016 and September 2017 (Figure 1B, 1C). Cysts within the parenchyma in the liver also had not changed in size (Figure 1E, 1F, 1H, 1I). There was no evidence of disease progression in the thorax, abdomen, or retroperitoneum.
Serum clinical chemistry parameters were graded according to the US National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), and values >Grade 1 (mild) were not present during Rigvir® therapy. At the time of writing this report, the patient’s condition remains stable, with an Eastern Cooperative Oncology Group (ECOG) performance status of Grade 0–1, and a Karnofsky Performance Status score of 90, indicating that he was active and able to perform routine tasks of daily living.
Discussion
Renal cell cancer has previously been reported to show sensitivity to immunotherapy, indicating the possible advantage of immunomodulating therapies over standard treatment. Treatment with oncolytic viruses has previously been tested in patients with urologic cancers including prostate cancer, bladder cancer, and renal cell cancer [12].
The overall survival (OS) for metastatic renal cell carcinoma is low, with a reported 5-year survival with current standard treatment in the USA of only 8.2%, an estimated 2-year survival of less than 20%, and with a 50% OS of less than one year [4]. More recently, the median survival for patients with renal cell carcinoma not receiving targeted therapy was 22.6 months and was 25.4 months for patients receiving tyrosine kinase inhibitors (TKIs) [13].
At the time of writing this report, this patient was diagnosed with metastatic renal cell carcinoma 33.7 months ago, and apart from surgery, he has only been treated with Rigvir® virotherapy. His condition has now stabilized, according to continued imaging using contrast-enhanced CT scans.
Conclusions
Currently, the median expected overall survival (OS) for a patient with stage IV renal cell carcinoma is less than 26 months. Considering the limited treatment options and poor prognosis for this disease, the findings in a 61-year-old man diagnosed with stage IV chromophobe renal cell carcinoma treated with the oncolytic ECHO-7 virus, Rigvir® are encouraging. Additional research in the field is required.
Footnotes
Department and Institution where work was done
Research and Development Department, International Virotherapy Center, Riga, Latvia.
Conflict of interest
Agnija Rasa, Katrīna Bandere, Andra Tilgase, and Pēteris Alberts are past and present employees of the International Virotherapy Center, and Linda Brokāne is an employee of the Global Virotherapy Cancer Clinic. Zaurbek Ismailov, Evija Olmane, and Jurijs Nazarovs state no conflict of interest.
References:
- 1.Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol. 2016;8(5):484–500. doi: 10.4329/wjr.v8.i5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah A, Jahan S, Najar L, et al. Metastatic clear cell variant of renal cell carcinoma of the mandible: Review and case report. Ann Maxillofac Surg. 2016;6(1):144–47. doi: 10.4103/2231-0746.186121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paule B, Brion N. Sunitinib re-challenge in metastatic renal cell carcinoma treated sequentially with tyrosine kinase inhibitors and everolimus. Anticancer Res. 2011;31(10):3507–10. [PubMed] [Google Scholar]
- 4.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC) cancer staging manual. 7th edition. New York, NY: Springer; 2010. pp. 1–648. [Google Scholar]
- 5.Athar U, Gentile T. Treatment options for metastatic renal cell carcinoma: A review. Can J Urol. 2008;15:3954–66. [PubMed] [Google Scholar]
- 6.Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349:g4797. doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson KA, Morris DG. Oncolytic virotherapy for renal cell carcinoma: A novel treatment paradigm? Expert Opin Biol Ther. 2012;12(7):891–903. doi: 10.1517/14712598.2012.685713. [DOI] [PubMed] [Google Scholar]
- 8.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373(9669):1119–32. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 9.Yagoda APD, Thompson S. Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol Clin North Am. 1993;20(2):303–21. [PubMed] [Google Scholar]
- 10.Alberts P, Tilgase A, Rasa A, et al. The advent of oncolytic virotherapy in oncology: The Rigvir® story. Eur J Pharmacol. 2018;837:117–26. doi: 10.1016/j.ejphar.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 11.Doniņa S, Strēle I, Proboka G, et al. Adapted ECHO-7 virus Rigvir immuno-therapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res. 2015;25(5):421–26. doi: 10.1097/CMR.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taguchi S, Fukuhara H, Homma Y, Todo T. Current status of clinical trials assessing oncolytic virus therapy for urological cancers. Int J Urol. 2017;24(5):342–51. doi: 10.1111/iju.13325. [DOI] [PubMed] [Google Scholar]
- 13.Achermann C, Stenner F, Rothschild SI. Treatment, outcome and prognostic factors in renal cell carcinoma – a single center study (2000–2010) J Cancer. 2016;7(8):921–27. doi: 10.7150/jca.15228. [DOI] [PMC free article] [PubMed] [Google Scholar]