Abstract
Context/Objective: Medically serious pressure injuries (MSPrIs), a common complication of spinal cord injury (SCI), have devastating consequences on health and well-being and are extremely expensive to treat. We aimed to test the efficacy of a lifestyle-based intervention designed to reduce incidence of MSPrIs in adults with SCI.
Design: A randomized controlled trial (RCT), and a separate study wing involving a nonrandomized standard care control group.
Setting: Rancho Los Amigos National Rehabilitation Center, a large facility serving ethnically diverse, low income residents of Los Angeles County.
Participants: Adults with SCI, with history of one or more MSPrIs over the past 5 years: N=166 for RCT component, N=66 in nonrandomized control group.
Interventions: The Pressure Ulcer Prevention Program, a 12-month lifestyle-based treatment administered by healthcare professionals, largely via in-home visits and phone contacts.
Outcome Measures: Blinded assessments of annualized MSPrI incidence rates at 12 and 24 months, based on: skin checks, quarterly phone interviews with participants, and review of medical charts and billing records. Secondary outcomes included number of surgeries and various quality-of-life measures.
Results: Annualized MSPrI rates did not differ significantly between study groups. At 12 months, rates were .56 for intervention recipients, .48 for randomized controls, and .65 for nonrandomized controls. At follow-up, rates were .44 and .39 respectively for randomized intervention and control participants.
Conclusions: Evidence for intervention efficacy was inconclusive. The intractable nature of MSPrI threat in high-risk SCI populations, and lack of statistical power, may have contributed to this inability to detect an effect.
Trial Registration: ClinicalTrials.gov NCT01999816
Keywords: Pressure ulcer, Pressure injury, Spinal cord injuries, Risk reduction behavior, Randomized controlled trial, Occupational therapy
Introduction
Individuals with spinal cord injury (SCI) remain at high risk of incurring medically serious (stage 3 or stage 4) pressure injuries (MSPrIs) even after receiving education and training in prevention techniques (e.g. pressure redistribution practices, skin checks, incontinence management) during rehabilitation.1,2 These same individuals face an uphill battle in preventing MSPrIs once they return home and in the years following discharge from post-acute rehabilitation.3 Consequently, MSPrIs remain one of the most frequent and costly reasons for unplanned hospitalizations among individuals with SCI.4 Not only can MSPrIs be life-threatening, they can also limit participation and compromise quality of life.
SCI-related pressure injuries: Prevalence and risk
Estimates of the prevalence of pressure injuries (PrIs) in community-dwelling adults with SCI vary in accordance with study characteristics such as the method of PrI detection, date of study, and patient variables (e.g. country of origin or time since spinal cord injury). In the majority of samples, prevalence rates for PrIs of all stages have ranged from 15–37%.5–9 The percentage of all PrIs that are medically serious approximates 25%,6,10,11 which suggests that in typical SCI populations the prevalence of MSPrIs is likely to average somewhat below 10%.5–14
A number of studies have identified correlates of PrI development in populations with SCI. Among these studies, risk factors have included such considerations as prior history of PrIs, spinal cord injury completeness, presence of co-morbidities, advancing age, lack of high school education, unemployment, smoking, poor diet, lack of fitness, and difficulty performing skin care procedures.15–17 An important additional risk consideration is membership in a socioeconomically disadvantaged or severely impoverished group. Individuals from impoverished subgroups must contend with the impact of social determinants that have been linked to inequality of health such as limited access to essential facilities and services, substandard material living and working conditions, and the unavailability of nutritious and affordable food.18 To a greater extent than other SCI populations, this group also has to negotiate a variety of other pervasive and often uncontrollable factors that potentially contribute to the development of MSPrIs such as inadequate transportation, life chaos, lack of knowledge surrounding prevention, emotional instability, and increased crisis situations such as homelessness.19 The results of a qualitative study brought this issue to light in that socioeconomically disadvantaged community-dwelling individuals with SCI were in perpetual danger of developing MSPrIs due to unpredictable events in daily life that ranged from seemingly innocuous (e.g. wearing ill-fitting hand-me-down shoes, sleeping on a dilapidated mattress, lacking funds for equipment purchase or repair) to highly disruptive (e.g. incarceration, extremely poor caregiving, or an untreated acute illness).20,21 These findings suggest that interventions that focus on distinct techniques (e.g. positioning, electrical stimulation) or isolated factors (e.g. insufficient PrI education) may be too narrowly focused and, therefore, ineffective in reducing the MSPrI threat for high-risk, socioeconomically disadvantaged individuals with SCI.22
Prior studies of complex preventive interventions
Only six prior studies have directly tested the efficacy of complex, behavioral interventions in preventing or managing MSPrIs in people with SCI.23–28 Each tested intervention included a combination of education on prevention behaviors and periodic follow-up with a healthcare provider, although the intensities varied widely. For example, one study had six planned contacts over 12 months23 and another used weekly contacts for 10 to 12 weeks.26
Results were null or inconclusive in all studies. However, each of the studies had one or more serious methodological problems that precluded the ability to provide a valid test of efficacy. Such problems included: (a) insufficient power or lack of power analysis;23–28 (b) poor intervention fidelity;23,24 and (c) low participant adherence.23–25 Therefore, it is arguable that behaviorally based interventions for preventing MSPrIs have yet to be adequately tested. Also of note, in all but two of the above six studies the primary mode of intervention delivery was via telephone or video phone, suggesting that the effectiveness of not only telehealth, but also of other approaches, needs to be rigorously assessed through well-designed studies in the future.
Rationale for current study
Due to the need to address limitations of previous studies and document effective MSPrI prevention interventions, particularly among socioeconomically disadvantaged individuals with SCI, we conducted a community-based RCT, known as the University of Southern California (USC) – Rancho Los Amigos National Rehabilitation Center (RLANRC) Pressure Ulcer Prevention Study (PUPS), from 2008 to 2013. The goal of this study was to identify an efficacious and cost-effective strategy for preventing MSPrIs in order to enhance health and quality of life among individuals with SCI, and consequently reduce the extremely high medical cost that results from treating advanced PrIs. Unlike the behaviorally based interventions previously examined, the intervention tested in this study: (1) targeted socioeconomically disadvantaged individuals with a past history of MSPrIs; (2) incorporated a broad spectrum of lifestyle/participation dimensions; (3) facilitated participants’ ability to implement sustainable, individualized preventive measures into their daily routines; (4) responded to the study population's risk-inducing life circumstances; and (5) included continuing face-to-face contact between therapists and clients over a 12-month period. The study's specific aims were as follows:
-
(1)
To assess the intervention's ability to reduce the incidence of MSPrIs during both the treatment year and the subsequent 12-month interval.
-
(2)
To examine the intervention's effects on the following secondary outcomes: incidence of PrI-related surgeries, participants’ health-related quality of life, satisfaction with life, and depression during the above two periods.
-
(3)
To model the intervening process mechanisms that mediate intervention effects.
Methods
A previous publication presents a detailed description of the methodological and logistical issues of PUPS.29 Methodology for the above aims is described below.
Study design
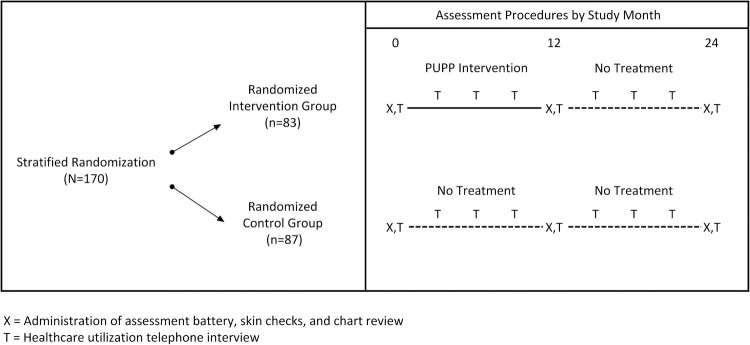
This study was a single-site, single-blind RCT that compared the lifestyle intervention entitled Pressure Ulcer Prevention Program (PUPP) to usual care. Figure 1 provides an overview of the study design. Participants were initially classified into one of two strata based on MSPrI history and current MSPrI status: (1) Risk Level I: ≤ 1 MSPrI in the past 2 years, and no current MSPrI; or (2) Risk Level II: ≥ 2 MSPrIs in the past 2 years and/or a current stable or healing stage 3 MSPrI. (Note: Individuals with stage 4 or unstable stage 3 PrIs were not eligible for this study. Stage 1 or Stage 2 PrIs at baseline that progressed to medically serious during the study were considered new MSPrI incidences. Stage 3 PrIs at baseline [N=5] were not counted as incident PrIs, since they were already present and serious at baseline.) Within each stratum, participants were randomized to the intervention group (Randomized Intervention group [RI]; N=83) or to the control group (Randomized Control group [RC]; N=87) using a block size of 8 (i.e. by forcing an equal split between intervention and control after each group of 8 participants was randomized in each stratum). During their first 12 months of study involvement, RI participants received the intervention, while RC participants received usual care. After the initial 12 months were completed, both the RI and RC participants were followed for an additional 12 months without treatment.
Figure 1.
Assessment procedures by study month.
Participants were assessed on demographic, primary and secondary outcomes, and moderating/mediating variables at baseline (pretest, prior to randomization), 12 months, and 24 months. The assessment was multi-faceted and included: (1) medical chart review; (2) skin examinations performed by specially trained nurses; and (3) questionnaires administered by trained university students. Additionally, during quarterly phone interviews we collected information about MSPrIs and health care utilization from the participants. All personnel involved in gathering these data were blinded to participant group assignment.
In the mediation model of facilitated intervention effects,29 we posited that increased self-efficacy,30 PrI-relevant knowledge, and social support31 would subsequently reduce MSPrI incidence.
Participants
Prospective participants for this study were adults (≥ 18 years of age) with SCI (paraplegia or tetraplegia) who had a history of at least one stage 3 or stage 4 PrI in the past five years, were currently utilizing RLANRC services, and had an existing medical chart at the facility. Additional inclusion criteria were: (1) English- or Spanish-speaking; (2) contactable by telephone or cell phone; (3) cognitively intact (based on unadjusted score ≥ 7 on the Short Portable Mental Status Questionnaire [SPMSQ]32); (4) willing to undertake recommended lifestyle changes for MSPrI prevention;29 and (5) residing within 100 miles of RLANRC. Participants were excluded if they: (1) were ambulatory; (2) were less than 6 months post-injury; (3) had an unstable or worsening stage 3, or any stage 4, PrI present; or (4) participated in any of our prior research studies.
Based on the findings of two preliminary studies, we determined that a sample size of 160 participants was necessary to adequately power this study for the primary outcome of annualized MSPrI incidence. We arrived at this estimate in a twofold manner. First, a review of RLANRC medical charts found an annualized mean MSPrI rate of 1.33±1.15. In the second preliminary study, two independent raters analyzed qualitative data to estimate the probability (0%-100%) that the PrIs would not have arisen or otherwise advanced to stage 3 or stage 4 had the individual with SCI received a multifaceted, intensive, lifestyle-based intervention. This resulted in mean decreases in MSPrI incidence estimates of 61.7% during the intervention and 56.4% in the following, post-intervention year. Based on these findings, we expected the annualized mean of 1.33 to decrease by half (d=0.578). Sample size calculations indicated that 112 participants (=160 reduced by 30% for attrition) would be necessary for 80% power to detect this posited (50%) reduction in MSPrI incidence using a 2-sided hypothesis test with an alpha level of 0.05.
Furthermore, when we began the study, we did not consider that the quarterly phone interviews conducted with RC participants could potentially function as an “intervention.” In other words, by merely asking RC participants PrI-relevant questions, we inadvertently may have made them aware of certain issues (e.g. the integrity/state of their skin). As a result, they might have altered their behavior. To assess the magnitude of this effect, we obtained university funding in November 2011 to recruit 70 additional participants to document the MSPrI rate among controls whose prior PrI histories were comparable to participants in the main study, but who received neither the intervention nor quarterly phone calls. This latter, nonrandomized group, which we term the No-Contact Control group (NCC), was followed for only one year, without quarterly contact. At baseline and after 12 months of study enrollment, we assessed NCC participants via skin checks and medical chart review, and administered a streamlined set of the same questionnaires that we used with the randomized groups. Inclusion of the NCC group enabled us to conduct a prospective observational comparative study to supplement and clarify findings of the main RCT.
Procedures
We recruited the experimental study sample from February 2009 to November 2011 and the NCC group from January 2012 through February 2013. All recruitment took place at RLANRC, which treats primarily low income, minority residents of Los Angeles County—one of the most medically underserved populations in the US. To successfully attract prospective participants, we adopted strategies to ensure cultural sensitivity, gain trust, and build rapport among the RLANRC patients accessing services in either the Spinal Cord Injury Primary Care or Pressure Ulcer Management clinics.19,29 To this end, we enlisted a bilingual, Hispanic member of our investigative team to oversee the recruitment process for both the main experimental and the observational control studies. We also assembled a culturally diverse recruitment team, including two bilingual Hispanic recruiters, an African American recruiter, and an individual with SCI. Staff and clinicians employed in the Spinal Cord Injury Primary Care or Pressure Ulcer Management clinics assisted in recruitment by endorsing the study and referring prospective participants.
Prior to study enrollment, each participant completed the RLANRC IRB informed consent process. All study protocols and modifications were approved by the RLANRC IRB. An external data safety monitoring board (DSMB) semi-annually monitored study progression. Patients were financially compensated for testing, skin checks, and quarterly phone interviews.
Intervention and control group protocols
The PUPP intervention consisted of six modules and was fully manualized. In general, this lifestyle-based intervention was designed to instill knowledge on prevention and application to a person's unique life circumstances. Its content was based on clinical guidelines for prevention,33,34 literature reviews concerning risk factors,15–17 and the results of our investigative team's qualitative studies on the real-world events that heighten PrI risk during participation in home and community life. The modules were: (1) Understanding Lifestyle and Pressure Injury Risk; (2) Advocacy; (3) Equipment and the Physical Environment; (4) Social Support; (5) Happiness and Well-Being; and (6) Planning the Future. Table 1 lists the fixed elements (addressed for all participants) and variable (optional) elements (tailored based on individual needs/challenges) specified within each module.29
Table 1. PUPP modular intervention content.
| Modules | Fixed elements | Variable (optional) elements |
|---|---|---|
| Module 1 - Understanding Lifestyle and Pressure Ulcer Risk | Importance of lifestyle Prevention practices in daily life Personal risk profile Development of personal prevention plan |
Activity versus health; life events; exercise; nutrition and weight; smoking; alcohol/substance abuse; prevention techniques; pressure reliefs; pressure and shearing; stages of pressure ulcers; response to emerging pressure ulcers |
| Module 2 - Advocacy | Attendant care Partnering with your health-care professional Self-advocacy Fine-tuning of personal prevention plan |
Access to health care; medical treatments; medical administration issues; medical complications; selecting/managing care attendants; emotions, attitudes, and self-efficacy; decision making |
| Module 3 - Equipment and the Physical Environment | Equipment Transportation and use of environmental options Further refinement of personal prevention plan |
Personally relevant transportation options; overcoming environmental barriers; detail on specific equipment options; living situations; safety in and outside the home |
| Module 4 - Social Support | Social support Family and intimate relationships Review of current use of personal prevention plan |
Developing friendships; social networking; e-mailing; dealing with family problems; job issues; social contact and pressure ulcer risk; overcoming loneliness |
| Module 5 - Happiness and Personal Well-Being | Accomplishing a sense of well-being Relation of mental health to pressure ulcer risk Further refinement of personal prevention plan |
Coping strategies; managing pain; depression; stress; risk taking; alcohol and drugs; healthy activity; maintaining a positive outlook |
| Module 6 - Planning the Future | Successfully anticipating change Making healthy habits permanent Review of personal prevention plan |
Aging skin; finances; strategizing for continued success; personal organization skills; aging and spinal cord injury |
Note. Table used with permission from SAGE publications (Clark F, Pyatak EA, Carlson M, Blanche EI, Vigen C, Hay J, et al. Implementing trials of complex interventions in community settings: the USC-Rancho Los Amigos Pressure Ulcer Prevention Study (PUPS). Clin Trials. 2014;11(2):218–29.).
Participants randomized to the intervention (RI) group received ongoing and intensive exposure to the PUPP modular content, which included information, activities, and exercises. Designed to increase prevention-facilitating behaviors, the program emphasized choice, skill mastery, and coaching, while also incorporating motivational interviewing techniques.35 The intervention was ultimately aimed at enabling participants not only to gain a more comprehensive awareness of the impact of lifestyle factors on PrI risk, but also to be highly motivated and able to ensconce sound, personally relevant MSPrI prevention strategies into their daily routines on an ongoing basis. Sessions were individually tailored based on professional judgement and participant need.36–39 During each session, the interveners implemented a carefully selected combination of the practices listed in Table 2.
Table 2. Practices implemented by interveners during PUPP sessions.
| 1. Soliciting the participant's active role in MSPrI management40–44 |
| 2. Fostering the participant's self-determination to alter behaviors because of increased knowledge, success experiences in implementing strategies (mastery), and intervener coaching45–47 |
| 3. Assisting the participant in resolving personal concerns related to self-advocacy, stress management, or the ability to create a balance between living a full life while simultaneously avoiding participation-related pressure injuries |
| 4. Enabling the participant to set personal goals pertaining to MSPrI prevention, taking one's strengths and challenges into account |
| 5. Facilitating problem solving with respect to daily dilemmas that heighten MSPrI risk |
| 6. Encouraging practice of relevant prevention techniques (e.g. pressure redistribution, regular skin checks, pressure reliefs, and prompt response to a potentially emerging pressure injury) |
| 7. Familiarizing the participant with information on MSPrI prevention and assuring they begin to apply it when relevant to their pressure injury risk |
| 8. Utilizing motivational interviewing techniques to enable participants to be receptive to behavioral change35 |
The intervention and assessment visits occurred in participants’ homes or a location of their choosing and were conducted in English or Spanish based on participant preference. Professional guidance was an essential element of PUPP. Therefore, licensed occupational therapists delivered the intervention and consulted with registered nurses when medical wound care issues arose. The intervention was divided into an intensive phase (months 1–6) followed by a tapered phase (months 7–12). The schedule of contacts is indicated in Supplementary Material 1. During the intensive phase, pre-planned weekly contact included nine face-to-face one on one home sessions and fifteen telephone calls. The fixed elements of each module (see Table 1) were delivered primarily in the intensive phase. During the tapered phase, contact was bi-weekly and included two in-person one on one home visits and nine telephone calls. Contacts in the tapered phase centered on review of prevention practices, reinforcement of personalized lifestyle applications, and responses to emergent risk situations. Throughout the intervention, face-to-face sessions lasted on average 1.5 hours, and phone call duration averaged 30 minutes. Additionally, intervention participants were encouraged to contact their occupational therapist if they detected a new PrI or experienced an event that elevated their MSPrI risk. In such cases, if necessary, the occupational therapist or nurse made an in-home incident visit. Finally, during the intervention, all RI group participants were provided with up to $400 to cover the costs of prevention-related equipment, and were given a consumer manual developed in connection with our preliminary qualitative study. This manual contains descriptive information, written in lay language, about a host of issues that pertain to pressure injury prevention.
Several aspects of the intervention reflected the intent to treat a socioeconomically disadvantaged population. Such adaptations included: (a) specialized safety training for interveners, who frequently needed to travel into dangerous neighborhoods when performing in-home visits; (b) during weekly therapist meetings, open discussion of participants’ emergent issues pertaining to extreme poverty or crime; (c) greater-than-usual attention devoted to issues such as homelessness or substance abuse; (d) use of therapist-client matching on language, sex (when possible), and ethnicity, so as to establish rapport with potentially distrustful participants; and (e) enactment of a relatively intensive treatment plan, as a means of heightening dosage in response to an anticipated extremely high PrI risk.
To ensure that the intervention protocol was properly implemented, we adhered to a multi-faceted treatment fidelity plan consistent with guidelines for monitoring complex interventions.48–50 According to this plan, interveners received 30 hours of standardized training, were assessed monthly in session delivery using a standardized rating scale, and attended weekly troubleshooting meetings to mitigate protocol drift.50–52 All interveners were blind to the study hypotheses and design.
RC group participants did not receive any study-based intervention. However, along with the RI group, they had continual access to the services provided to all SCI clients at RLANRC. Standard care included clinic visits to undergo skin checks and receive necessary medical treatment (e.g. cleansing, bandaging, surgery, hospitalization) and advice (e.g. recommendations for rest) when a PrI was present. Beyond this, following their study involvement RC participants were given a copy of the PUPP Consumer Manual, up to $400 to cover the cost of PrI prevention-related equipment, and one in-home visit by an occupational therapist and a nurse who presented educational information on MSPrI prevention.
Study outcomes and measures
The annualized incidence rate of MSPrIs (stages 3 and 4) was the primary study outcome. If an unstageable PrI or deep tissue injury was identified, it was also classified as an MSPrI. Because MSPrI stage is often difficult to determine (e.g. wounds may appear and heal within less than 12 months), we collected PrI-relevant data from several sources and used the following multi-tiered process to reconcile any discrepant findings.
In Tier 1, study-blinded (to group assignment and study hypotheses) nurses trained in PrI staging performed skin checks at baseline, 12, and 24 months and administered the Bates-Jensen Wound Assessment Tool (BWAT).53 The BWAT includes the following 13 wound characteristics, each rated on a scale from 1 (best) to 5 (worst): size, depth, edges, undermining/tunneling, necrotic tissue type, necrotic tissue amount, exudate type, exudate amount, surrounding skin color, peripheral tissue edema, peripheral tissue induration, granulation tissue, and epithelialization. The ratings are then summed to indicate the final score, or overall severity, of tissue damage. The reported inter-rater reliability of the BWAT is 0.92.53 Following the skin check and BWAT, the nurses classified each PrI into a stage. In addition, patients and study team members also identified and reported PrI occurrences and stages—the patients during quarterly phone interviews, and the study team members while reviewing participants’ medical charts and billing records, including those from non-RLANRC providers.
In Tier 2, two additional nurses trained in wound categorization (also unaware of study hypotheses and treatment assignments) independently reviewed the wound data of each PrI identified in Tier 1. These nurses carefully verified that all wounds reported were in fact PrIs (and not non-pressure wounds such as diabetic ulcers), confirmed whether the PrIs were medically serious, and ensured that PrIs were not double counted from multiple sources or over a long time span. Concurrently, the nurses examined medical and billing records to assess incidence of PrI-related surgeries (secondary outcome).
Finally, in Tier 3, a PrI reconciliation team comprised of four research team members who were blinded to treatment assignment reviewed the Tier 2 MSPrI occurrence data. All inconsistencies in PrI and/or surgery counts, or in PrI stage, were resolved through discussion. If necessary, the team consulted with a wound care specialist.
We measured the remaining secondary outcomes and mediating variables2,54–60 using the instruments presented in Table 3. We selected these scales for their brevity, sensitivity, language (English or Spanish), and/or previous validation for use with people with SCI. Assessments available only in English were translated into Spanish and reviewed by a committee for accuracy.61–63
Table 3. Measurement of secondary outcomes and mediating process variables.
| Construct | Instrument | Number of Items |
|---|---|---|
| Secondary outcomes | ||
| Health-related quality of life | Adapted RAND 36-Item Health Survey 1.0 (SF-36)58 | 36 |
| Life satisfaction | Satisfaction with Life Scale (SWLS)55 | 5 |
| Depression | Quasi-Adaptive Short Form for the Patient Reported Outcomes Measurement Information System (PROMIS) Version 1 Depression Item Bank59 | 5 |
| Mediating process variables | ||
| Social support | Interpersonal Support Evaluation List (ISEL)54 | 6 |
| Knowledge of pressure injury prevention | Pressure Ulcer Knowledge Test57 | 14 |
| Performance of preventive behaviors | Garber, et al., Procedure for Assessing Performance of Preventive Behaviors2 with the addition of caregiver experience | 10 |
| Self-efficacy | Adapted Moorong Self Efficacy Scale60 | 27 |
| Substance abuse | Adapted Cut Down, Annoyed, Guilty, and Eye Opener (CAGE) Questionnaire56 | 11 |
Descriptive measures included participant demographics, SCI factors (i.e. completeness of spinal cord injury, extent of paralysis, years since SCI), and self-reported comorbidities (e.g. diabetes, urinary tract infection). We attempted to gather information about substance abuse, but later found that the data were unreliable. For example, many participants who reported current or former substance use at baseline later indicated that they never used the same substance. Therefore, we excluded these variables from our analyses. We also collected data on adherence/process variables associated with intervention delivery, including number of in-home preplanned intervention sessions attended and phone contacts, client-initiated phone calls, and in-home incident visits.
Statistical analyses
All data entry, data management, and statistical analyses were performed through the project's data analysis center. We used recommended algorithms to calculate summary scores on each assessment. Missing responses were accommodated using each instrument's specifications.
We obtained baseline descriptive statistics for all study variables for the entire sample and for the RI, RC, and NCC groups separately. We calculated means, standard deviations, and ranges for continuous variables and frequency distributions for categorical variables. We performed Fisher's exact tests or analyses of variance to test for demographic and injury-related differences at baseline between the RI, RC, and NCC groups.
For the intent-to-treat (ITT) analysis, we ran Poisson regression analyses of between-group (RC vs. RI) differences in the number of MSPrIs over the 12-month intervention and 12-month follow-up phases to test intervention efficacy. To supplement the ITT findings, we also compared differences in the number of MSPrIs from baseline to 12 months between the NCC group and the RC and RI groups. A two-tailed test with alpha=0.05 was employed for each of the above comparisons.
Additionally, we employed logistic regression analyses to investigate whether baseline values or changes from baseline in secondary outcome or mediating variables were associated with the occurrence of one or more MSPrIs in year 1 (N=232) as well as in years 1 and 2 combined (N=158). We also adjusted for randomization strata as well as background variables associated with MSPrI in either year 1 or years 1 and 2 combined. In selecting covariates, we used stepwise logistic regression with p≤0.10 required for entry or retention in the model.
Changes in mediators, covariates, and secondary outcome variables from baseline to 12 months were assessed within treatment group using paired sample t-tests, and across treatment groups using independent sample t-tests. Although we originally planned to use structural equation modeling to examine the hypothesized intervention mediation model, the ITT findings (see Results section) rendered the rationale for this analysis unsuitable. Consequently, we conducted ANOVAs and ANCOVAs as described above, treating the mediating variables as potential secondary intervention outcomes.
Results
Recruitment and allocation of experimental study cohort
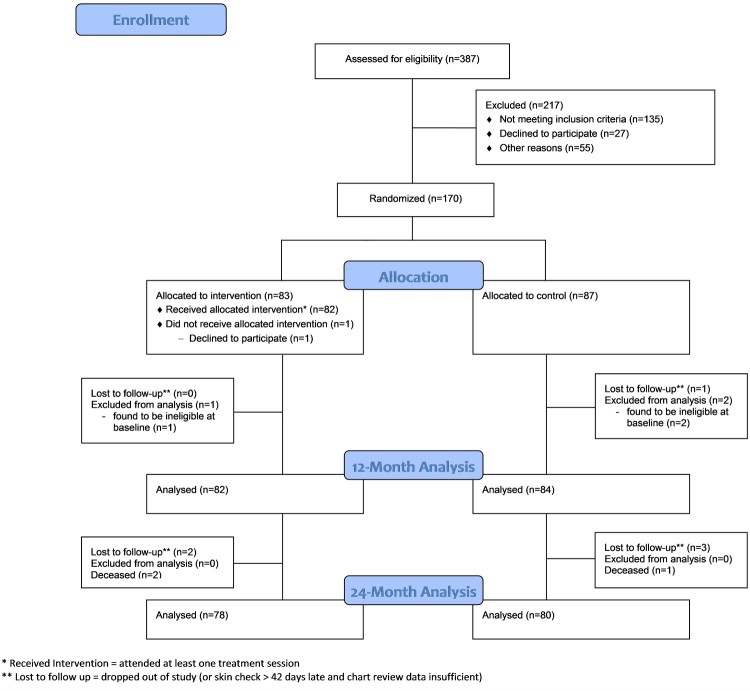
Approximately midway into the study, preliminary blinded analysis of MSPrI incidence indicated that the number of serious PrIs in the total sample was much lower than what we had anticipated at study inception. Therefore, to partially counteract any resulting loss of power in detecting an intervention effect, we recruited an additional 10 participants (final N=170). Figure 2 presents the CONSORT flow diagram for the study. Of 387 individuals assessed for eligibility, 170 enrolled in the study. Of the 170 enrollees, 83 were randomly assigned to the RI group and 87 to the RC group.
Figure 2.
CONSORT flow diagram.
Evaluability
A total of 166 out of 170 randomized participants were evaluable for the ITT analysis in that they: (a) received a 12-month skin check on or within 42 days after the 12-month due date; (b) had sufficient chart review data (i.e. regular chart entries and updates at RLANRC during the study period); or (c) participated in at least one data-collection phone interview. Eight of the 166 participants were only partially evaluable for the intent-to-treat analysis due to insufficient chart data (n=1), death (n=3), relocation out of the area (n=2), loss to follow-up (n=1), or withdrawal from the study (n=1). Of the 70 participants enrolled in the NCC group, 66 were evaluable at 12 months.
Baseline characteristics
Table 4 presents baseline characteristics of participants in the RI (n=82), RC (n=84), and non-randomized NCC (n=66) groups. Results of Fisher's exact tests or ANOVA analyses for between-group differences (RI, RC, NCC comparisons as well as RI, RC comparisons) were non-significant (all P values ≥ 0.13) on all baseline characteristics, indicating that the treatment groups were balanced with respect to age, sex, race/ethnicity, SCI level and completeness, time since SCI injury, education, income, diabetes and other comorbidities, body mass index (BMI), and risk level based on past PrI incidence.
Table 4. Baseline characteristics of participants by group.
| Variable | RC (n=84) n (%) or M ± SD |
RI (n=82) n (%) or M ± SD |
NCC (n=66) n (%) or M ± SD |
|---|---|---|---|
| Sex | |||
| Male | 72 (85.7%) | 69 (84.1%) | 60 (90.9%) |
| Female | 12 (14.3%) | 13 (15.9%) | 6 (9.1%) |
| Race/ethnicity | |||
| Caucasian | 11 (13.1%) | 10 (12.2%) | 9 (13.6%) |
| African American | 28 (33.3%) | 23 (28.0%) | 22 (33.3%) |
| Hispanic | 42 (50.0%) | 41 (50.0%) | 27 (40.9%) |
| Other | 3 (3.6%) | 8 (9.8%) | 8 (12.1%) |
| Paralysis level | |||
| Paraplegia | 61 (72.6%) | 58 (70.7%) | 46 (69.7%) |
| Tetraplegia | 22 (26.2%) | 21 (25.6%) | 12 (18.2%) |
| Undetermined | 1 (1.2%) | 3 (3.7%) | 8 (12.1%) |
| SCI completeness | |||
| Complete | 55 (65.5%) | 61 (74.4%) | 49 (74.2%) |
| Incomplete | 28 (33.3%) | 21 (25.6%) | 17 (25.8%) |
| Undetermined | 1 (1.2%) | 0 | 0 |
| Risk group | |||
| Risk level I | 51 (60.7%) | 46 (56.1%) | 41 (62.1%) |
| Risk level II | 33 (39.3%) | 36 (43.9%) | 25 (37.9%) |
| Education level | |||
| Less than high school | 34 (40.5%) | 30 (36.6%) | 22 (33.3%) |
| High school graduate | 26 (31.0%) | 19 (23.2%) | 20 (30.3%) |
| Some college | 16 (19.0%) | 25 (30.5%) | 18 (27.3%) |
| College graduate | 8 (9.5%) | 8 (9.8%) | 6 (9.1%) |
| Household Income | |||
| $0-$999/month | 46 (54.8%) | 43 (52.4%) | 41 (62.1%) |
| $1000-$1999/month | 16 (19.0%) | 19 (23.2%) | 11 (16.7%) |
| ≥ $2000/month | 20 (23.8%) | 18 (22.0%) | 13 (19.7%) |
| Undetermined | 2 (2.4%) | 2 (2.4%) | 1 (1.5%) |
| Diabetes | |||
| Yes | 9 (11.1%) | 16 (19.5%) | 14 (21.2%) |
| No | 72 (88.9%) | 64 (78.0%) | 47 (71.2%) |
| Undetermined | 3 (3.6%) | 2 (2.4%) | 5 (7.6%) |
| Other comorbidities (excluding diabetes) | |||
| ≤ 6 | 49 (58.3%) | 57 (69.5%) | 45 (68.2%) |
| ≥ 7 | 35 (41.7%) | 25 (30.5%) | 21 (31.8%) |
| Age | 42.5 ± 12.2 | 41.7 ± 12.9 | 44.0 ± 14.0 |
| BMI | 25.1 ± 5.4 | 26.2 ± 7.0 | 25.0 ± 5.3 |
| Years since SCI | 16.3 ± 12.3 | 18.1 ± 12.1 | 16.5 ± 13.5 |
BMI, body mass index.
The total sample (RI, RC, and NCC) was comprised primarily of males (87%) and racial/ethnic minorities (47% Hispanic, 31% African American). Thirty-seven percent of the sample had less than a high school education, and 57% had a household income of less than $1000 per month. Most participants were paraplegic (75%), as opposed to tetraplegic (25%), and had complete spinal cord injuries (71%). Ninety-eight percent of participants reported at least 1 comorbidity, and 39% reported 7 or more comorbidities. The majority of participants (59%) were classified as Risk Level I (≤ 1 MSPrI in the past 2 years and no current MSPrI) for MSPrIs. The cause of injury for intervention participants was recorded in treatment notes and indicated the following percentages: gunshot wounds (52%), motor vehicle accidents (35%), other/unknown (13%).
Intent-to-treat analysis
As shown in Table 5, 41 MSPrIs occurred in the RC group and 45 occurred in the RI group during year 1, resulting in annual incidence rates of 0.48 and 0.56, respectively. Using the randomized control group as the reference, the rate ratio for serious MSPrIs in the RI group was 1.15 (95% confidence interval [CI] = [0.76, 1.76]). This ratio is not statistically significant. During year 2, the rate ratio remained non-significant, with 32 and 35 PrIs in RC and RI groups, respectively (rate ratio = 1.14, CI=0.72, 1.82).
Table 5. Numbers and rates of MSPrIs by treatment group.
| Year 1 | Year 2 | ||||
|---|---|---|---|---|---|
| Statistic | RC | RI | NCC | RC | RI |
| Number of evaluable participants | 84 | 82 | 66 | 80 | 78 |
| Number of participants with MSPrIs | 27 | 30 | 24 | 17 | 22 |
| Number of MSPrIs | 41 | 45 | 41 | 32 | 35 |
| MSPrI rate per year | 0.48 | 0.56 | 0.65 | 0.39 | 0.44 |
| Rate ratio compared to randomized control group (95% CI) | — | 1.15 (0.76–1.76) P=0.86* |
1.34 (0.87–2.07) P=0.33** |
— | 1.14 (0.72–1.82) P=0.80* |
*Adjusted for risk group, diabetes, other comorbidities (≤6 vs. ≥7), and baseline preventive behaviors.
**Adjusted for risk group, diabetes, and other comorbidities (≤6 vs. ≥7).
Prospective comparison
Results of the prospective cohort comparison analysis revealed that 41 MSPrIs occurred in the NCC group for an annual incidence rate of 0.65 (Table 5). Although the rate for this group exceeded the rates for each of the two randomized groups, the differences failed to achieve statistical significance. The distribution of number of serious MSPrIs by individual was substantially the same in all three groups (data not shown).
Secondary analyses
We also investigated if baseline values of selected variables were associated with MSPrI incidence (Table 6). Many of these analyses only included the RI and RC groups, as we did not test the NCC group on most of the hypothesized mediators and secondary outcome measures. Risk Level II (≥ 2 MSPrIs in the past 2 years and/or a current stable or healing stage 3 MSPrI) had the strongest association with MSPrI incidence for year 1 (odds ratio [OR]=6.1, 95% confidence interval [CI]=3.4,11.0) as well as for year 1 and 2 combined (OR=4.2, 95% CI=2.2,8.3). Other baseline variables associated with MSPrI risk appeared in year 1: diabetes compared to no diabetes (OR=2.4, 95% CI=1.2,4.8), ≥7 compared to <7 other comorbidities (OR=2.0, 95% CI=1.2,3.6), and increased practice of preventive behaviors (OR=1.6, 95% CI=1.1,2.3 per 1 standard deviation in baseline score). In years 1 and 2 combined, the following variables were associated with MSPrI incidence: male sex (OR=2.7, 95% CI=1.0,7.4), household income <$1000 per month compared to $1000–1999 per month (OR=2.5, 95% CI=1.1,5.8), ≥7 compared to <7 other comorbidities (OR=2.1, 95% CI=1.1,4.1), and practice of preventive behaviors (OR=1.5, 95% CI=1.1,2.2 per 1 standard deviation in baseline score). There was no evidence that adjustment for any of these variables changed the treatment effect on MSPrI incidence.
Table 6. Odds ratio (OR) for incidence of one or more MSPrIs in year 1 and in years 1 and 2 combined by baseline risk factors – using logistic regression.
| Year 1 | Years 1 and 2 combined | ||||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||||
| Variable | OR (95% CI) | P value | P value* | OR (95% CI) | P value | P value* | |
| Participant Characteristics | |||||||
| Female | 0.733 (0.320–1.677) | 0.46 | — | 0.365 (0.136–0.975) | 0.04 | — | |
| Race/ethnicity | |||||||
| Caucasian | Ref | Ref | |||||
| African American | 0.671 (0.284–1.586) | 0.36 | 0.28 | 1.017 (0.352–2.940) | 0.97 | 0.46 | |
| Hispanic | 0.533 (0.234–1.213) | 0.13 | 0.14 | 0.551 (0.201–1.511) | 0.25 | 0.13 | |
| Other | 0.408 (0.117–1.422) | 0.16 | 0.14 | 0.514 (0.112–2.359) | 0.39 | 0.23 | |
| Paralysis Level | |||||||
| Paraplegia | Ref | Ref | |||||
| Tetraplegia | 1.399 (0.749–2.614) | 0.29 | 0.85 | 1.047 (0.508–2.161) | 0.90 | 0.52 | |
| Complete SCI | 1.224 (0.667–2.248) | 0.51 | 0.66 | 1.208 (0.602–2.422) | 0.59 | 0.67 | |
| Risk Level II | 6.075 (3.361–10.980) | <0.001 | — | 4.239 (2.160–8.321) | <0.001 | — | |
| Education | |||||||
| < HS, GED | Ref | Ref | |||||
| High School | 1.310 (0.672–2.555) | 0.43 | 0.25 | 1.019 (0.466–2.225) | 0.96 | 0.37 | |
| Some College | 1.008 (0.500–2.030) | 0.98 | 0.80 | 1.048 (0.466–2.353) | 0.91 | 0.59 | |
| College Graduate | 0.737 (0.261–2.084) | 0.57 | 0.84 | 0.611 (0.186–2.005) | 0.42 | 0.91 | |
| Household Income | |||||||
| $0–999/month | Ref | Ref | |||||
| $1000–1999/month | 0.667 (0.315–1.413) | 0.29 | 0.35 | 0.40 (0.171–0.935) | 0.03 | 0.04 | |
| ≥ $2000/month | 1.433 (0.739–2.777) | 0.29 | 0.25 | 0.75 (0.339–1.660) | 0.48 | 0.65 | |
| Diabetes | 2.391 (1.186–4.821) | 0.01 | — | 2.056 (0.830–5.089) | 0.12 | — | |
| ≥ 7 Other comorbidities | 2.045 (1.167–3.582) | 0.01 | — | 2.122 (1.097–4.106) | 0.03 | — | |
| Age (per year) | 0.99 (0.97–1.01) | 0.35 | 0.09 | 0.99 (0.96–1.01) | 0.40 | 0.40 | |
| BMI (per 5 kg/m2) | 0.95 (0.76–1.20) | 0.69 | 0.17 | 0.96 (0.74–1.23) | 0.73 | 0.51 | |
| Duration of SCI (per yr.) | 0.99 (0.96–1.01) | 0.24 | 0.17 | 0.99 (0.96–1.02) | 0.49 | 0.97 | |
| Baseline Mediators** | |||||||
| Interpersonal support | 0.92 (0.70–1.20) | 0.52 | 0.94 | 0.78 (0.56–1.07) | 0.12 | 0.35 | |
| Self-efficacy | 1.04 (0.74–1.45) | 0.84 | 0.89 | 1.20 (0.86–1.67) | 0.28 | 0.18 | |
| General | 1.03 (0.74–1.44) | 0.85 | 0.88 | 1.18 (0.85–1.64) | 0.33 | 0.22 | |
| Pressure injury | 1.05 (0.76–1.47) | 0.76 | 0.90 | 1.19 (0.86–1.64) | 0.29 | 0.24 | |
| Preventive behaviors | 1.57 (1.08–2.27) | 0.02 | 0.09 | 1.52 (1.07–2.15) | 0.02 | 0.06 | |
| Baseline Covariates** | |||||||
| SF-36 | |||||||
| Physical function | 0.80 (0.57–1.11) | 0.18 | 0.40 | 0.88 (0.64–1.21) | 0.45 | 0.60 | |
| Role limitations - physical | 0.76 (0.54–1.06) | 0.11 | 0.16 | 0.95 (0.69–1.31) | 0.76 | 0.81 | |
| Role limitations - emotional | 0.94 (0.68–1.29) | 0.69 | 0.62 | 1.11 (0.81–1.52) | 0.52 | 0.63 | |
| Energy/fatigue | 1.00 (0.72–1.38) | 0.99 | 0.67 | 1.11 (0.81–1.52) | 0.52 | 0.76 | |
| Emotional well-being | 0.98 (0.71–1.35) | 0.91 | 0.62 | 1.24 (0.90–1.71) | 0.19 | 0.26 | |
| Social functioning | 0.89 (0.65–1.23) | 0.48 | 0.63 | 1.10 (0.80–1.51) | 0.57 | 0.56 | |
| Pain | 0.78 (0.56–1.07) | 0.13 | 0.05 | 0.76 (0.55–1.04) | 0.09 | 0.04 | |
| General health | 0.99 (0.72–1.37) | 0.97 | 0.60 | 1.05 (0.76–1.44) | 0.77 | 0.45 | |
| Life satisfaction | 0.80 (0.58–1.11) | 0.19 | 0.61 | 0.80 (0.59–1.10) | 0.17 | 0.56 | |
| PrI knowledge | 1.13 (0.82–1.57) | 0.45 | 0.30 | 0.87 (0.63–1.19) | 0.37 | 0.30 | |
| Depression | 1.04 (0.79–1.37) | 0.77 | 0.83 | 0.93 (0.68–1.26) | 0.63 | 0.72 | |
*Adjusted for sex, risk group, diabetes, and other comorbidities.
**Odds ratios and confidence intervals are per one standard deviation in baseline values for the specified variable.
Eight PrI-related surgeries occurred during the treatment phase in each of the RI and RC groups, and 7 in the NCC group within the same time span. This incidence rate was too small to draw conclusions about an intervention effect. As shown in Table 7, analyses of other secondary outcomes revealed that both the RI and RC groups improved significantly from baseline on many measures of health and well-being, including physical functioning (effect size=0.40 for RI and 0.50 for RC), physical role limitations (effect size=0.72 for RI and 0.32 for RC), emotional role limitations (effect size=0.31 for RI and 0.38 for RC), social functioning (effect size=0.28 for RI and 0.38 for RC), pain (effect size=0.41 for RI and 0.33 for RC), and depression (effect size=-0.36 for RI and -0.33 for RC). Further, a significant beneficial intervention vs. control group effect was detected for SF-36: Role Limitations-Physical (P=0.0472). However, due to the number of hypotheses tests, this latter finding potentially reflects a Type 1 error.
Table 7. Changes in quality of life measures from baseline to 12 months by treatment group.
| RC (n=70) | RI (n=73) | |||||
|---|---|---|---|---|---|---|
| Variables | Baseline mean (SD)1 |
Signed 12-month change (SD) |
P value2 | Signed 12-month change (SD) |
P value2 | P value (change: RI vs. RC)3 |
| Baseline Mediators | ||||||
| Interpersonal support | 12.35 (2.95) | 0.48 (3.88) | 0.31 | –0.19 (2.79) | 0.56 | 0.24 |
| Self-efficacy | 23.84 (3.04) | 0.45 (2.20) | 0.12 | 0.82 (3.44) | 0.06 | 0.47 |
| General | 13.92 (2.22) | 0.28 (1.67) | 0.19 | 0.60 (2.55) | 0.06 | 0.41 |
| Pressure injury | 9.92 (1.30) | 0.12 (1.08) | 0.37 | 0.27 (1.33) | 0.09 | 0.48 |
| Preventive behaviors | 8.58 (1.43) | -0.11 (1.20) | 0.43 | 0.52 (1.55) | 0.005 | 0.01 |
| Baseline Covariates | ||||||
| SF36 | ||||||
| Physical functioning | 50.56 (25.22) | 12.66 (24.96) | <0.0001 | 10.18 (26.35) | 0.002 | 0.56 |
| Role limitations - physical | 38.86 (39.36) | 12.50 (45.39) | 0.02 | 28.42 (49.53) | <0.0001 | 0.05 |
| Role limitations - emotional | 65.46 (40.02) | 15.24 (50.34) | 0.01 | 12.33 (45.31) | 0.02 | 0.72 |
| Energy/fatigue | 62.65 (20.31) | 7.24 (20.11) | 0.004 | 2.33 (19.06) | 0.30 | 0.14 |
| Emotional well-being | 76.37 (18.84) | 5.86 (17.30) | 0.006 | 2.30 (19.40) | .31 | 0.25 |
| Social functioning | 69.43 (27.85) | 10.71 (22.95) | 0.0002 | 7.88 (33.69) | 0.05 | 0.56 |
| Pain | 55.62 (27.92) | 9.11 (29.34) | 0.01 | 11.51 (26.70) | 0.0004 | 0.61 |
| General health | 60.78 (23.19) | 2.43 (16.70) | 0.23 | 2.12 (23.69) | 0.45 | 0.93 |
| Satisfaction with life | 21.51 (7.06) | 1.26 (6.76) | 0.12 | 0.72 (7.76) | 0.43 | 0.66 |
| PrI knowledge | 0.54 (0.15) | 0.01 (0.16) | 0.70 | 0.01 (0.15) | 0.68 | 1.00 |
| Depression | -0.26 (0.85) | -0.28 (0.89) | 0.01 | -0.31 (0.80) | 0.002 | 0.86 |
1RC and RI groups combined. These groups did not differ significantly at baseline on any of these variables.
2One-sample t-test.
3Independent-sample t-test comparing changes in RI group to changes in RC group.
Mediation variable analysis
Although our mediation model cannot be validated due to the null relationship between the intervention and the primary outcome, it is informative to investigate the effects of the intervention on the hypothesized mediators and of the hypothesized mediators on the primary outcome. Our model had predicted that increased practice of preventive behaviors (due directly to intervention effects on self-efficacy, PrI knowledge, and social support) would reduce MSPrI incidence. We found that the intervention compared to the RC group increased self-reported preventive behaviors significantly (P = 0.007; decrease of 0.11 in the RC group and increase of 0.52 in the RI group). Preventive behavior items included skin inspection, special mattresses, weight shifts, hygiene, diet, limiting sitting, safety, early notification of health care providers, use of wheelchair cushions,2 and having an experienced caregiver. No single item appeared to dominate the overall improvement. However, increases in preventive behaviors were not significantly associated with decreases in MSPrI incidence (P = 0.08; data not shown). Neither group evidenced significant changes in interpersonal support, PrI knowledge, or self-efficacy for PrI prevention (Table 7).
Discussion
Intervention efficacy
Counter to the main study hypothesis, the annualized MSPrI incidence rate did not differ significantly between the RI and RC groups during the intervention or follow-up phases. This non-significant outcome was puzzling because the intervention was very thorough and participants strongly adhered to the program requirements. Below, we note several considerations to account for the lack of ability to detect an intervention effect.
First, it is likely that the extreme pervasiveness of the MSPrI threat militated against the intervention's ability to effect prevention. Consistent with what we observed among socioeconomically disadvantaged individuals with SCI in our earlier publications,20,21 the participants’ lives were frequently characterized by chaotic circumstances such as homelessness, incarceration, or extremely limited finances.19 In this regard, we observed in this study's intervener treatment notes that many MSPrIs occurred when participants did not have the necessary resources to respond to unforeseeable events: an air mattress suddenly broke, a participant's skin was exposed to prolonged pressure during an emergency plane flight, or an individual was incarcerated for two days and lacked appropriate medical care. Many of these occurrences demonstrated social determinants of health that extended beyond the intervention's reach.
From a statistical vantage point, the highly haphazard development of PrIs likely created error variability, thereby impeding the ability to document the intervention's effect. Thus, despite the non-significant hypothesis test result, the intervention could have in fact been effective. What the study lacked, however, was sufficient power. In this regard, we chose the sample size based on an optimistic expected effect magnitude (50% MSPrI reduction) and overestimated the per-participant annualized MSPrI incidence (1.33). Given the obtained annualized incidence rate in the chief comparator group (0.48 in the RC group) and our final sample size (84 RC, 82 RI), power was only 57%, even after assuming a 50% PrI reduction effect. Further, although the intervention would still be meritorious if it reduced PrIs by far less than 50%, power for detecting such an effect would be minimal. For example, the average intervention cost was approximately $5,200 per participant, and treatment costs fall between $20,000 and $152,000 per MSPrI.64 Therefore, an intervention that reduces MSPrIs by 25% is likely to remain cost-effective in similar high-risk populations. Yet, the probability of achieving a significant result for such an intervention, given the sample size and actual PrI rate, would have only been 17%. Moreover, the 95% confidence intervals for intervention-based PrI rates in relation to the control groups are as follows: 24% decrease to 76% increase vs. RC; 44% decrease to 31% increase vs. NCC; and 30% decrease to 45% increase vs. combination of RC and NCC. Because these confidence intervals include regions of acceptable intervention effect, it remains statistically plausible that the intervention does, in fact, meaningfully reduce PrIs and that the experimentally obtained negative result reflects a type 2 error.
The intent-to-treat result is also consistent with the possibility that the intervention has no effect, or that it increases PrI incidence. In this regard, the above confidence intervals for intervention-related MSPrI outcomes include high as well as near-zero rates relative to controls. With respect to the possibility of a near-zero effect, even if interveners can anticipate many PrI-inducing events, there is no guarantee that: (a) all risk events will be detected, (b) intervention recipients will consistently adhere to preventive recommendations, or (c) the recommendations will be effective, even if followed. The lack of correlation, both in the present study as well as in other investigations, between knowledge or practice of prescribed preventive procedures (e.g. pressure reliefs) and the development of MSPrIs corresponds with this interpretation.25,27
It is also possible that some aspects of the treatment supported prevention, but that in other ways the treatment inadvertently induced PrIs, resulting in a cancellation effect. A review of therapist treatment notes indicates that the interveners commonly expressed that a potential PrI was avoided because the participant followed prescribed recommendations. However, in other instances the intervention could have indirectly caused PrIs by increasing engagement in personally chosen, but nonetheless risk-inducing, activity. Although one intervention component entailed teaching participants to consider carefully any unintended consequences of increasing activity levels on PrI risk, the actual effect of such admonitions is unclear. In this regard, in our previous qualitative study we found that participants frequently incurred PrIs from engagement in personally valued activities such as work, school, or social outings, despite their knowledge that such activities would increase PrI risk.20,21,65
Our study team made a considerable effort to reconcile diverse sources of information when determining whether an MSPrI was present. Therefore, it is unlikely that the non-significant experimental result was due to measurement unreliability. We realized during the course of the study that at least two commonly used methods for documenting PrIs—namely self-report and chart review—frequently produced surprisingly inaccurate and inconsistent results. For example, patient medical files often included contradictory PrI-relevant information. In light of such discrepancies, we undertook a cautious and extremely thorough assessment of medical charts, we relied on direct observation via skin checks and the BWAT, and we incorporated triangulation of all sources of information to assess PrI status. We have concluded that relying on any single source as the sole means of identifying or determining PrI stage in SCI populations is fraught with potential difficulties.
As previously noted, the NCC condition was included to gauge the MSPrI rate among similarly at-risk RLANRC consumers with SCI who did not receive quarterly research-related phone calls and other aspects of the RCT that might have affected study outcomes. Although the annualized MSPrI rate was higher in the NCC group than in both the RI and RC during the intervention year, these differences did not attain statistical significance. During Year 2 (follow-up) MSPrI rates for the RI and RC groups were 21% and 26% lower than the rate for the NCC group. If genuine, this degree of advantage for the two experimentally randomized groups would likely constitute effective prevention (due to elimination of one-fifth to one-fourth of MSPrIs, with probable intervention-based cost-savings). However, these Year 2 differences from the NCC in annualized rates were not statistically reliable. Further, between-year differences in PrI outcomes, both within and between comparison groups, could in principle reflect time-based trends in MSPrI incidence. For example, changes in RLANRC Pressure Ulcer Management Clinic practices or shifts in the national economy could affect the ability to enact preventive options such as equipment repair.
Pre-to-post changes in secondary outcomes
At 12 months (and at 24 months -- data not shown), both the RI and RC groups evidenced statistically significant gains relative to baseline on the majority of health-related quality of life variables, as measured by the SF-36. We offer two explanations for this finding. First, it is possible that both the RI and RC groups improved simultaneously due to regular contact via quarterly phone calls (RI and RC) and/or the PUPP intervention (RI). Because quality of life was not measured in the NCC group, it is difficult to evaluate this hypothesis. However, we did assess participants in the NCC group on depression, a construct reflecting quality of life. A comparison of mean change at 12 months from baseline revealed a trend toward greater reduction in depression among the two experimental groups relative to the NCC group (for combination of RI and RC groups, P=0.07, two-tailed). This partially supports the notion of genuine positive change in the RI and RC groups. A second possibility for this finding is that the health-related quality of life gains are artificial and stem from concerns such as social desirability, adaptations to repeated measurement, or anticipation of remuneration for study participation shortly following testing. Therefore, further research is needed to sort out potential intervention effects on quality of life.
As we anticipated, self-reported enactment of preventive behaviors increased more in the RI condition than in the RC condition. If these self-reports are accurate, then this effect reveals that the intervention was beneficial in facilitating important intermediate steps that are theorized to prevent MSPrIs. Nevertheless, the RI group's mean degree of change in prevention behaviors was only 0.52 on a 10-point scale. It is also possible that RI participants over-reported more often than RC participants due to the intervention's prolonged focus on practicing prevention techniques. In any event, it is noteworthy that, within the RI condition, self-reported increases in preventive behaviors were unrelated to MSPrI incidence over the 12-month treatment interval—an observation consistent with the high occurrence of MSPrIs in the study population.
Prediction of MSPrIs
As expected, when we combined the RI and RC groups, risk level determined by previous history of MSPrI or a current MSPrI was the strongest predictor at baseline of new MSPrIs over the total 24-month observation period; those in the Risk Level II stratum were 4.2 times more likely to incur PrIs than those in the Risk Level I stratum. Based on non-experimental observations, individuals in the Level II stratum frequently face two types of threats: (a) susceptibility to MSPrIs repeatedly occurring in the same bodily location because of extensive damage due to frequent surgical repair, and (b) multiple additional factors such as substance abuse, depression, homelessness, or lack of caution (evidenced by a failure to adhere to recommended bed rest). Keeping these considerations in mind, it may be possible to identify a subgroup of individuals within the SCI population who are in dire need of effective preventive services.
The other persistent predictor of MSPrIs consisted of the overall number of co-morbidities and, specifically, the presence of diabetes (Year 1). Prior studies also support this claim by indicating that various co-morbidities, such as UTI, obesity, or CVD problems, compromise skin integrity.33 Additional potential predictors, including tetraplegia (versus paraplegia) and spinal cord injury completeness, were related to MSPrIs, but failed to achieve statistical significance.
Self-reported enactment of preventive behaviors at baseline was associated with more MSPrIs at both 12 and 24 months. The association was non-significant, however, after we adjusted for sex, risk group, diabetes, and other comorbidities. This lack of association is counter-intuitive and may potentially reflect the complex interplay of considerations such as the following: (a) individuals who know that they are at higher risk due to other reasons may be more likely to practice prevention techniques; (b) self-reports of preventive behaviors may frequently be inaccurate in this study population; or (c) the techniques in question may not be effective or may often be performed incorrectly. Nevertheless, the lack of observed association does not rule out the possibility that many participants performed the techniques correctly and, hence, reduced MSPrIs.
Limitations
Apart from the previously discussed lack of statistical power, a key limitation of this study pertains to the issue of generalizability. Compared to most SCI populations, the current study's participants have a much higher MSPrI rate, require a more intensive intervention, and sustain greater PrI risk even with intervention services. Consequently, the results may not be directly applicable to more typical SCI populations, which are likely to experience far fewer MSPrIs and are arguably better able to respond to PrI risk events that do occur. Likewise, it is important to note that results cannot be generalized to other geographical areas or types of healthcare settings. In this regard, different patterns of care, such as decisions about surgical intervention or longer appointment wait times, could affect the MSPrI incidence as well as intervention effectiveness. Despite our best efforts to tailor the intervention to our disadvantaged population, it is possible that it was overwhelming or otherwise a poor fit for some members of our study sample. An additional limitation is that the lack of a randomized no contact control group obscured the results insofar as it is difficult to estimate the effects of the regular phone interviews administered in the RC condition. This fact poses a final limitation: the rate of MSPrIs in the two control groups may have been deflated to some degree if a higher percentage of PrIs were reported in the RI condition due to increased vigilance or therapy-based prompts for treatment. This latter limitation reflects the possibility that some MSPrIs in one or both of the control groups may have emerged, and then improved, prior to the 12- or 24-month skin checks. However, the quarterly interviews as well as the frequency of medical visits in the control groups suggest that any between-group differences in detection are likely to have been minimal.
Future directions
Although the present study's intervention effect remains unclear, there is no denying that the severe human suffering and exorbitant treatment costs associated with MSPrIs make it a priority to identify strategies to enhance prevention among individuals at high risk. To this end, our study team is currently investigating the causes of MSPrIs during the course of the PUPP intervention, as reported in the therapist notes. Our hope is that such investigation may provide clues to enhance future preventive efforts. At present, one potential cause of MSPrIs that we are considering is equipment issues; ostensibly preventable PrIs were noted to appear when items such as wheelchair cushions or mattresses broke down, or when vitally needed equipment was not available in a timely manner. Second, clients’ personalized medical issues may have provoked the development of MSPrIs. Two such areas of concern include: (1) anatomical sites of previous skin breakdown which, given that this population had typically sustained multiple prior MSPrIs, proved to be the same location for a substantial percentage of newly emergent lesions;66 and (2) medical co-morbidities, especially diabetes, which can indirectly weaken skin integrity and impede wound healing.42,66
In this study we confirmed that previous PrIs, as well as diabetes and other comorbidities, predict the development of MSPrIs. These and other hypothesized risk factors should be studied further to better understand the course and critical events that lead from the risk factor to an incident low-stage PrI to a subsequent MSPrI. In 2014, the Pressure Ulcer Programme of Research published a pressure ulcer conceptual framework67 that has implications for prevention-relevant theory development and research guidance. Broadening this framework from its biomechanical focus to additionally incorporate increased attention to social determinants may provide new ways to model PrI development and, thereby, enact prevention.
Recently, hospitals have been particularly interested in reducing MSPrIs, partly because the 2008 Centers for Medicare and Medicaid Services policies denied Medicare payment for certain hospital-acquired conditions such as MSPrIs. Although community-dwelling individuals are outside of the direct influence of the healthcare system, researchers can benefit from the successes of hospitals that have implemented prevention programs by modifying their procedures to fit the SCI community-dwelling population.
When testing preventive interventions, given the low base rate of MSPrIs in SCI study populations, researchers should take particular care to ensure an adequate sample size. In conducting RCTs on populations with relatively low PrI rates, it is possible that only a very large multi-site study would suffice. On the other hand, restricting study participants to only those at the very highest levels of risk could increase RCT power. Even within our relatively high-risk sample, variation in risk was apparent at baseline. To test a promising intervention, researchers can use Salzberg's Pressure Ulcer Risk Assessment Scale for Individuals with Spinal Cord Injury68 or a similar assessment tool to identify a very high-risk group. If a given intervention should prove to be effective for such individuals, then further research could investigate how to adapt it to the wider SCI population. Because multiple sites might be necessary to recruit the restricted population, it would also be very important to account for geographic and other site-related differences, which could affect PrI rates or intervention success.
Conclusions
-
1.
Study results had wide confidence intervals, indicating that the true effect of the PUPP intervention remains uncertain.
-
2.
Our results are consistent with the notion that regularly administered phone calls may facilitate prevention of MSPrIs; such an approach should continue to be pursued in future research because, if successful, it would likely be highly cost-effective.
-
3.
The extreme nature of the current sample warrants caution in extrapolating the results to more typical SCI populations.
-
4.
In the effort to identify successful interventions, there is a need to conduct research on the efficacy of various standard prevention practices, which currently have a weak evidence base and in the present study failed to correlate with PrI-related outcomes.
-
5.
In future studies, measurement of PrIs should be undertaken cautiously, considering as much information as possible, as a simplistic reliance on participant reports or medical chart information could in many cases prove misleading.
-
6.
Future researchers should be aware that there may be a tendency for participants who receive a behavioral intervention to report PrI incidence more frequently than those in a control condition.
-
7.
The methodological and substantive findings of our study can inform future research in this area. They provide greater clarity with respect to such critical concerns as effect size estimation, constitution of control groups, PrI occurrence measurement methods, and the impact of social determinants on intervention outcomes. Despite the challenges inherent in conducting RCTs to evaluate the effectiveness of complex PrI prevention programs, such research is vitally important in attempting to improve the lives of people with SCI.
Supplementary Material
Acknowledgements
This work was supported by grant R01HD056267 from the National Center for Medical Rehabilitation Research (NCMRR) within the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health to the University of Southern California.
Conflict of interest: No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
The Pressure Ulcer Prevention Study is registered at ClinicalTrials.gov (NCT01999816).
The authors thank Michael Weinrich, MD and Louis Quatrano, PhD from the NICHD National Center for Medical Rehabilitation Research (NCMRR) for their programmatic assistance for this project. In addition, the investigators thank those who served on the Data Safety and Monitoring Board for their ongoing guidance and support: Richard (Sal) Salcido, MD, EdD (Chair); Tom Belin, PhD; Joy Hammel, PhD, OTR; Forrest Pendleton; Denise G. Tate, PhD; and Louis Quatrano, PhD. Finally, the PUPS investigators acknowledge all trial participants, without whom the study would not be possible.
Consultants: Marcus J. Fuhrer, PhD; Nancy Gibbs, MD. Interveners: Arameh Anvarizadeh, OTD, OTR/L; Mark Armstrong, RN; Jane Baumgarten, BS, OTR/L; Delphine O. Mangan, BSN, RN, CWCN; Annee Deering-Fitzgerald, MS, RN; Celso Delgado, Jr, OTD, OTR/L; Yvette Ngann, BSN, RN, WOCN, CRRN; Kelly Peck, RN, MSN; Clarissa Saunders-Newton, PhD, OTR/L; Jenna Trammel, MS, RN; Lisbeth Vega, OTD, OTR/L; Ana Verran, MA, OTR/L, CDRS. Project staff: Daniella Florindez, MPH; Samruddhi Ghaisas, OTD, OTR/L; Kiley Hanish, OTD, OTR/L; Cynthia L. Kushi, OTR/L; Jeremy Seip, OTD, OTR/L; Michael Tien, MS, MPH; Ashwini Vaishampayan, OTD, OTR. Ulcer data reconciliation team: Jingwen Claire Li, BS, OTD, OTR; Brooke Bianco, MSN, RN; Robert Maxwell, MSN, RN; Tina Wayne, RN, MPH. Assessment team: Jardine Cordero-Pagunsan, NP-C; Hilda Diaz, RN, MSN, ACNP; Donald Fogelberg, PhD, OTR/L; Jinpei Hong, RN; German Sanchez, RN; Alison J. Stoneham, LVN. Rancho Los Amigos National Rehabilitation Center (RLANRC) site support: Deandra Pedroza, BA; Angie Rivera. Fiscal administration: Patricia H. Gutierrez, Janis Wise. Manuscript preparation: Erin Rice, BA; Sarah Gleason, BA; Stephanie Mielke, OTD, OTR/L.
ORCID
Cheryl LP Vigenhttp://orcid.org/0000-0002-9789-1502
Erna Imperatore Blanchehttp://orcid.org/0000-0003-0177-6073
Barbara Bates-Jensenhttp://orcid.org/0000-0001-8490-4012
Elizabeth A Pyatakhttp://orcid.org/0000-0003-3280-8912
Trudy Mallinsonhttp://orcid.org/0000-0002-4888-5579
Jennifer B Ungerhttp://orcid.org/0000-0001-9064-6603
Alison Coganhttp://orcid.org/0000-0002-6800-1988
Florence Clarkhttp://orcid.org/0000-0001-7401-0824
References
- 1.Dorsett P, Geraghty T.. Health-related outcomes of people with spinal cord injury—a 10 year longitudinal study. Spinal Cord 2008;46(5):386–91. doi: 10.1038/sj.sc.3102159 [DOI] [PubMed] [Google Scholar]
- 2.Garber SL, Rintala DH, Rossi CD, Hart KA, Fuhrer MJ.. Reported pressure ulcer prevention and management techniques by persons with spinal cord injury. Arch Phys Med Rehabil 1996;77(8):744–9. doi: 10.1016/S0003-9993(96)90251-8 [DOI] [PubMed] [Google Scholar]
- 3.Guilcher SJ, Craven BC, Lemieux-Charles L, Casciaro T, McColl MA, Jaglal SB.. Secondary health conditions and spinal cord injury: an uphill battle in the journey of care. Disabil Rehabil 2013;35(11):894–906. doi: 10.3109/09638288.2012.721048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeVivo M, Farris V.. Causes and costs of unplanned hospitalizations among persons with spinal cord injury. Topics in Spinal Cord Injury Rehabilitation 2011;16(4):53–61. doi: 10.1310/sci1604-53 [DOI] [Google Scholar]
- 5.Eslami V, Saadat S, Arejan RH, Vaccaro A, Ghodsi S, Rahimi-Movaghar V.. Factors associated with the development of pressure ulcers after spinal cord injury. Spinal Cord 2012;50(12):899–903. doi: 10.1038/sc.2012.75 [DOI] [PubMed] [Google Scholar]
- 6.Fuhrer MJ, Garber SL, Rintala DH, Clearman R, Hart KA.. Pressure ulcers in community-resident persons with spinal cord injury: prevalence and risk factors. Arch Phys Med Rehabil 1993;74(11):1172–7. [PubMed] [Google Scholar]
- 7.McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ.. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil 1999;80(11):1402–10. doi: 10.1016/S0003-9993(99)90251-4 [DOI] [PubMed] [Google Scholar]
- 8.Saladin LK, Krause JS.. Pressure ulcer prevalence and barriers to treatment after spinal cord injury: comparisons of four groups based on race-ethnicity. NeuroRehabilitation 2009;24(1):57–66. [DOI] [PubMed] [Google Scholar]
- 9.Saunders LL, Krause JS, Acuna J.. Association of race, socioeconomic status, and health care access with pressure ulcers after spinal cord injury. Arch Phys Med Rehabil 2012;93(6):972–7. doi: 10.1016/j.apmr.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarkony G, Heinemann AW.. Pressure Ulcers. In: Stover SL, DeLisa JA, Whiteneck GG, editors. Spinal cord injury: clinical outcomes from the model systems. Gaithersburg, MD: Aspen Publishers; 1995. p. 100–19. [Google Scholar]
- 11.Young J, Burns P.. Pressure sores and the spinal cord injured. Sci Digest 1981;3(3):9–17. [Google Scholar]
- 12.Smith BM, Guihan M, LaVela SL, Garber SL.. Factors predicting pressure ulcers in veterans with spinal cord injuries. Am J Phys Med Rehabil 2008;87(9):750–7. doi: 10.1097/PHM.0b013e3181837a50 [DOI] [PubMed] [Google Scholar]
- 13.Whiteneck GG, Charlifue SW, Frankel HL, Fraser MH, Gardner BP, Gerhart KA, et al. . Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia 1992;30(9):617–30. [DOI] [PubMed] [Google Scholar]
- 14.Carlson C, King R, Kirk P, Temple R, Heinemann A.. Incidence and correlates of pressure ulcer development after spinal cord injury. J Rehabil Nurs Res 1992;1:34–40. [Google Scholar]
- 15.Krause JS, Broderick L.. Patterns of recurrent pressure ulcers after spinal cord injury: identification of risk and protective factors 5 or more years after onset. Arch Phys Med Rehabil 2004;85(8):1257–64. doi: 10.1016/j.apmr.2003.08.108 [DOI] [PubMed] [Google Scholar]
- 16.Krause JS, Vines CL, Farley TL, Sniezek J, Coker J.. An exploratory study of pressure ulcers after spinal cord injury: relationship to protective behaviors and risk factors. Arch Phys Med Rehabil 2001;82(1):107–13. doi: 10.1053/apmr.2001.18050 [DOI] [PubMed] [Google Scholar]
- 17.Garber SL, Rintala DH, Hart KA, Fuhrer MJ.. Pressure ulcer risk in spinal cord injury: predictors of ulcer status over 3 years. Arch Phys Med Rehabil 2000;81(4):465–71. doi: 10.1053/mr.2000.3889 [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson RG, Marmot MG.. Social determinants of health: the solid facts: World Health Organization; 2003.
- 19.Pyatak EA, Blanche EI, Garber SL, Diaz J, Blanchard J, Florindez L, et al. . Conducting intervention research among underserved populations: lessons learned and recommendations for researchers. Arch Phys Med Rehabil 2013;94(6):1190–8. doi: 10.1016/j.apmr.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark FA, Jackson JM, Scott MD, Carlson ME, Atkins MS, Uhles-Tanaka D, et al. . Data-based models of how pressure ulcers develop in daily-living contexts of adults with spinal cord injury. Arch Phys Med Rehabil 2006;87(11):1516–25. doi: 10.1016/j.apmr.2006.08.329 [DOI] [PubMed] [Google Scholar]
- 21.Jackson J, Carlson M, Rubayi S, Scott MD, Atkins MS, Blanche EI, et al. . Qualitative study of principles pertaining to lifestyle and pressure ulcer risk in adults with spinal cord injury. Disabil Rehabil 2010;32(7):567–78. doi: 10.3109/09638280903183829 [DOI] [PubMed] [Google Scholar]
- 22.Dunn CA, Carlson M, Jackson JM, Clark FA.. Response factors surrounding progression of pressure ulcers in community-residing adults with spinal cord injury. Am J Occup Ther 2009;63(3):301–9. doi: 10.5014/ajot.63.3.301 [DOI] [PubMed] [Google Scholar]
- 23.Bloemen-Vrencken JH, de Witte LP, Post MW, Pons C, van Asbeck FW, van der Woude LH, et al. . Comparison of two Dutch follow-up care models for spinal cord-injured patients and their impact on health problems, re-admissions and quality of care. Clin Rehabil 2007;21(11):997–1006. doi: 10.1177/0269215507079835 [DOI] [PubMed] [Google Scholar]
- 24.Guihan M, Bombardier CH, Ehde DM, Rapacki LM, Rogers TJ, Bates-Jensen B, et al. . Comparing multicomponent interventions to improve skin care behaviors and prevent recurrence in veterans hospitalized for severe pressure ulcers. Arch Phys Med Rehabil 2014;95(7):1246–53.e3. doi: 10.1016/j.apmr.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 25.Houlihan BV, Jette A, Friedman RH, Paasche-Orlow M, Ni P, Wierbicky J, et al. . A pilot study of a telehealth intervention for persons with spinal cord dysfunction. Spinal Cord. 2013;51(9):715–20. doi: 10.1038/sc.2013.45 [DOI] [PubMed] [Google Scholar]
- 26.Phillips VL, Temkin A, Vesmarovich S, Burns R, Idleman L.. Using telehealth interventions to prevent pressure ulcers in newly injured spinal cord injury patients post-discharge. Results from a pilot study. Int J Technol Assess Health Care 1999;15(4):749–55. [PubMed] [Google Scholar]
- 27.Rintala DH, Garber SL, Friedman JD, Holmes SA.. Preventing recurrent pressure ulcers in veterans with spinal cord injury: impact of a structured education and follow-up intervention. Arch Phys Med Rehabil 2008;89(8):1429–41. doi: 10.1016/j.apmr.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 28.Jones ML, Mathewson CS, Adkins VK, Ayllon T.. Use of behavioral contingencies to promote prevention of recurrent pressure ulcers. Arch Phys Med Rehabil 2003;84(6):796–802. doi: 10.1016/S0003-9993(02)04943-2 [DOI] [PubMed] [Google Scholar]
- 29.Clark F, Pyatak EA, Carlson M, Blanche EI, Vigen C, Hay J, et al. . Implementing trials of complex interventions in community settings: the USC-Rancho Los Amigos Pressure Ulcer Prevention Study (PUPS). Clin Trials 2014;11(2):218–29. doi: 10.1177/1740774514521904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandura A.Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84(2):191–215. doi: 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- 31.DiMatteo MR.Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol 2004;23(2):207–18. doi: 10.1037/0278-6133.23.2.207 [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer E.A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975;23(10):433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 33.Consortium for Spinal Cord Medicine Clinical Practice Guidelines Pressure ulcer prevention and treatment following spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med 2001;24Suppl 1:S40–101. [DOI] [PubMed] [Google Scholar]
- 34.Medicine CfSC Pressure ulcer prevention and treatment following injury: a clinical practice guideline for health-care providers. 2nd ed Washington, DC: Paralyzed Veterans of America; 2014. [Google Scholar]
- 35.Miller WR, Rollnick S.. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press; 1991. [Google Scholar]
- 36.Richards KC, Enderlin CA, Beck C, McSweeney JC, Jones TC, Roberson PK.. Tailored biobehavioral interventions: a literature review and synthesis. Res Theory Nurs Pract 2007;21(4):271–85. doi: 10.1891/088971807782428029 [DOI] [PubMed] [Google Scholar]
- 37.Crepeau EB, Cohn ES, Schell BAB, editors. Willard & Spackman's occupational therapy. 11th ed Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 38.Pendleton HM, Schultz-Krohn W, editors. Pedretti's occupational therapy: practice skills for physical dysfunction. 7th ed St Louis, MO: Elsevier; 2013. [Google Scholar]
- 39.Radomski MV, Latham CAT.. Occupational therapy for physical dysfunction. 6th ed Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 40.Burton KD, Lydon JE, D'Alessandro DU, Koestner R.. The differential effects of intrinsic and identified motivation on well-being and performance: prospective, experimental, and implicit approaches to self-determination theory. J Pers Soc Psychol 2006;91(4):750–62. doi: 10.1037/0022-3514.91.4.750 [DOI] [PubMed] [Google Scholar]
- 41.Clark F, Carlson M, Zemke R, Frank G, Patterson K, Ennevor BL, et al. . Life domains and adaptive strategies of a group of low-income, well older adults. Am J Occup Ther 1996;50(2):99–108. doi: 10.5014/ajot.50.2.99 [DOI] [PubMed] [Google Scholar]
- 42.Guihan M, Bombardier CH.. Potentially modifiable risk factors among veterans with spinal cord injury hospitalized for severe pressure ulcers: a descriptive study. J Spinal Cord Med 2012;35(4):240–50. doi: 10.1179/2045772312Y.0000000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guihan M, Garber SL, Bombardier CH, Durazo-Arizu R, Goldstein B, Holmes SA.. Lessons learned while conducting research on prevention of pressure ulcers in veterans with spinal cord injury. Arch Phys Med Rehabil 2007;88(7):858–61. doi: 10.1016/j.apmr.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 44.Ward MM.Sense of control and self-reported health in a population-based sample of older Americans: assessment of potential confounding by affect, personality, and social support. Int J Behav Med 2013;20(1):140–7. doi: 10.1007/s12529-011-9218-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aujoulat I, d'Hoore W, Deccache A.. Patient empowerment in theory and practice: polysemy or cacophony? Patient Educ Couns 2007;66(1):13–20. doi: 10.1016/j.pec.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 46.Lauver DR, Ward SE, Heidrich SM, Keller ML, Bowers BJ, Brennan PF, et al. . Patient-centered interventions. Res Nurs Health 2002;25(4):246–55. doi: 10.1002/nur.10044 [DOI] [PubMed] [Google Scholar]
- 47.Whalley Hammell K.Experience of rehabilitation following spinal cord injury: a meta-synthesis of qualitative findings. Spinal Cord 2007;45(4):260–74. doi: 10.1038/sj.sc.3102034 [DOI] [PubMed] [Google Scholar]
- 48.Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. . Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23(5):443–51. doi: 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- 49.Breitenstein SM, Gross D, Garvey CA, Hill C, Fogg L, Resnick B.. Implementation fidelity in community-based interventions. Res Nurs Health 2010;33(2):164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parham LD, Cohn ES, Spitzer S, Koomar JA, Miller LJ, Burke JP, et al. . Fidelity in sensory integration intervention research. Am J Occup Ther 2007;61(2):216–27. doi: 10.5014/ajot.61.2.216 [DOI] [PubMed] [Google Scholar]
- 51.Horner SD.Best practices for improving intervention fidelity that every nurse should know. J Spec Pediatr Nurs. 2012;17(2):171–4. doi: 10.1111/j.1744-6155.2012.00327.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oakley A, Strange V, Bonell C, Allen E, Stephenson J, Team RS.. Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332(7538):413–6. doi: 10.1136/bmj.332.7538.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bates-Jensen BM, Vredevoe DL, Brecht ML.. Validity and reliability of the Pressure Sore Status Tool. Decubitus 1992;5(6):20–8. [PubMed] [Google Scholar]
- 54.Cohen S, Mermelstein R, Kamarck T, Hoberman HM.. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social support: theory, research, and applications. The Hague, Holland: Martinus Nijhoff; 1985. p. 73–94. [Google Scholar]
- 55.Diener E, Emmons RA, Larsen RJ, Griffin S.. The Satisfaction With Life Scale. J Pers Assess 1985;49(1):71–5. doi: 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- 56.Ewing JA.Detecting alcoholism. The CAGE questionnaire. JAMA 1984;252(14):1905–7. doi: 10.1001/jama.1984.03350140051025 [DOI] [PubMed] [Google Scholar]
- 57.Garber SL, Rintala DH, Holmes SA, Rodriguez GP, Friedman J.. A structured educational model to improve pressure ulcer prevention knowledge in veterans with spinal cord dysfunction. J Rehabil Res Dev 2002;39(5):575–88. [PubMed] [Google Scholar]
- 58.Hays RD, Sherbourne CD, Mazel RM.. The RAND 36-Item Health Survey 1.0. Health Econ 1993;2(3):217–27. doi: 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 59.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, et al. . Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment 2011;18(3):263–83. doi: 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Middleton JW, Tate RL, Geraghty TJ.. Self-efficacy and spinal cord injury: psychometric properties of a new scale. Rehabil Psych 2003;48(4):281. doi: 10.1037/0090-5550.48.4.281 [DOI] [Google Scholar]
- 61.Azen SP, Palmer JM, Carlson M, Mandel D, Cherry BJ, Fanchiang SP, et al. . Psychometric properties of a Chinese translation of the SF-36 health survey questionnaire in the Well Elderly Study. J Aging Health 1999;11(2):240–51. doi: 10.1177/089826439901100206 [DOI] [PubMed] [Google Scholar]
- 62.Jackson J, Blanche EI, Mandel D.. A cultural adaptation of the well elderly intervention for spanish-speaking older adults. Paper presented at: 91st Annual American Occupational Therapy Association Conference; 2011 Apr 14; Philadelphia, PA.
- 63.Jackson J, Kennedy BL, Mandel D, Carlson M, Cherry BJ, Fanchiang SP, et al. . Derivation and pilot assessment of a health promotion program for Mandarin-speaking Chinese older adults. Int J Aging Hum Dev 2000;50(2):127–49. doi: 10.2190/9V9H-E4L7-BTJP-9WMJ [DOI] [PubMed] [Google Scholar]
- 64.Agency for Healthcare Research and Quality Are we ready for this change? [document on the Internet].2014 [updated 2014 October; cited 2015 September 24]. Available from: http://www.ahrq.gov/professionals/systems/hospital/pressureulcertoolkit/putool1.html.
- 65.Fogelberg D, Atkins M, Blanche EI, Carlson M, Clark F.. Decisions and dilemmas in everyday life: daily use of wheelchairs by individuals with spinal cord injury and the impact on pressure ulcer risk. Top Spinal Cord Inj Rehabil 2009;15(2):16–32. doi: 10.1310/sci1502-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guihan M, Garber SL, Bombardier CH, Goldstein B, Holmes SA, Cao L.. Predictors of pressure ulcer recurrence in veterans with spinal cord injury. J Spinal Cord Med 2008;31(5):551–9. doi: 10.1080/10790268.2008.11754570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coleman S, Nixon J, Keen J, Wilson L, McGinnis E, Dealey C, et al. . A new pressure ulcer conceptual framework. J Adv Nurs 2014;70(10):2222–34. doi: 10.1111/jan.12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salzberg CA, Byrne DW, Cayten CG, van Niewerburgh P, Murphy JG, Viehbeck M.. A new pressure ulcer risk assessment scale for individuals with spinal cord injury. Am J Phys Med Rehabil 1996;75(2):96–104. doi: 10.1097/00002060-199603000-00004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.