Abstract
Objective
To examine the effects of a six-week body weight-support treadmill training (BWSTT) program on center-of-mass control and gait independence in chronic, incomplete spinal cord injury (iSCI) patients.
Design
Descriptive.
Setting
Clinica Los Coihues. Neurorehabilitation center in Santiago, Chile.
Participants
17 chronic iSCI patients and 17 healthy subjects.
Outcome Measures
An instrumented sway (ISway) test was performed before and after the implementation of a six-week BWSTT program. The standing balance of participants was measured by Normalized jerk (NJ) and root mean square (RMS). These values were used to assess the standing balance of participants, and were correlated with the scores obtained on the Walking Index Spinal Cord Injury (WISCI) II test.
Results
Significant differences were found in standing balance (i.e., through NJ) after the BWSTT program (P = 0.016), but no significant differences were found in RMS values for postural sway (P = 0.693). None of the patients obtained improved WISCI II scores pre- vs. post-intervention.
Conclusion
While a BWSTT program can improve center-of-mass control in iSCI patients, no effects were recorded for gait independence.
Trial Registration
National Clinical Trials, registry number NCT02703883.
Keywords: Spinal cord injury, Body weight-support treadmill, Locomotor training, Jerk, Center of mass
Introduction
Spinal cord injury (SCI) is a devastating event, the degree and severity of which determines impacts to sensorimotor and autonomous functions.1 The global yearly incidence of SCI is an estimated 83 cases per million persons, with 12,000 new cases reported in the United States each year.2 Epidemiological studies report that approximately 50% of traumatic SCI patients suffer incomplete lesions.3,4 The International Standards for Neurological Classification of SCI, as developed by the American Spinal Injury Association (ASIA), further defines approximately 40% of these patients as either motor non-functional (ASIA Impairment Scale (AIS) C) or motor functional (AIS D).3 This population can usually execute many functional activities, such as standing balance and gait.5 In fact, 80–100% of AIS D patients at least partially recover walking functions within a year after injury.6
Recovering locomotor function is a high priority for SCI patients, independent of injury severity and the time elapsed.7 Specifically for incomplete SCI (iSCI) patients, the ability to walk can be limited by lower limb paresis, increased spasticity, poor coordination, and impaired postural control, all clinical factors that contribute to low walking speed and biomechanical gait inefficiencies.8,9 Locomotor training promotes the plasticity of neural spinal circuits, which can induce functional recovery in iSCI patients.1,10 Body weight-support treadmill training (BWSTT), in particular, is positively associated with increased muscle strength, kinematics, and spatiotemporal gait parameters, thereby promoting locomotor function recovery of the central nervous system in iSCI patients.11–13 However, the contribution of BWSTT towards the recovery of walking independence in chronic iSCI patients is unclear.13–15
Reduced balance during walking is associated with a high risk of falls.16 This risk is up 75% after iSCI,17 which directly impacts on autonomy and functional independence. Currently, clinical rating scales such as the Berg Balance Scale (BBS) are regularly used to assess balance in SCI patients, and several studies using this scale have shown that BWSTT improves balance.4,5,18 However, while clinical rating scales assess general functional aspects of balance and transitions,19 analytical components of postural control are not evaluated, including center-of-mass (COM) displacement and associated derivatives. These postural-control components are directly related to proper body stability and reflect how the nervous system controls the complex sensorimotor task of maintaining bipedal equilibrium.20 No clinical rating scale has, as yet, proven to accurately measure postural stability in iSCI patients.
Force plates that analyze center-of-pressure displacement in a quiet stance are the gold standard for balance assessments in patients with musculoskeletal and neurological injuries.21 However, force plates are expensive, not easy to move, and infrequently available in rehabilitation centers.22 Inertial sensors are a possible valid and reliable alternative for assessing postural control in people with disabilities.23,24 More specifically, body-worn accelerometers show promise as a practical and low-cost option for measuring instrumented postural sway (ISway) and, consequently, replacing force-plate posturography.25
The ISway test establishes sway through time-domain, frequency-domain, and JERK measures. These data respectively characterize the amplitude, frequency, and smoothness of body sway.23 JERK, a time derivative of acceleration, is used as an empirical measure of sway smoothness,26 and, together with time-domain parameters, is more reliable than frequency-domain parameters.23 Evaluating the basic accelerometric parameters associated with JERK analysis might permit discriminating between postural control strategies in different neurological diseases.26,27 These data might also be applicable in obtaining objective parameters of sensory-motor abilities and establishing the efficacy of therapeutic tools.27
The implementation of BWSTT during early rehabilitation seems to help restore walking functions in iSCI patients, as widely associated with improvements in balance and muscle strength.11,13 However, the effectiveness of BWSTT in improving analytical measures of postural control and functional independence during locomotion has not been determined for long-term rehabilitation of chronic injuries.19,27 Therefore, the aim of this study was to assess if a six-week BWSTT program improved COM control in chronic iSCI patients during a standing balance task. This was measured using the ISway test, which included an inertial sensor positioned at L5. Additionally, evaluations were conducted to determine if changes in COM control were correlated with the achievement of independent walking, where the degree of gait independence was measured by the Walking Index Spinal Cord Injury (WISCI) II scale.4 The WISCI II scale is more sensitive than other functional scales for assessing balance during gait in SCI patients.4,9 Overall, the assessed six-week BWSTT program positively impacted COM control, as measured by the normalized JERK (NJ) parameter. However, this improvement in standing balance did not correlate with improvements in gait independence.
Methods
Design
Patients were recruited from Clínica Los Coihues (Santiago, Chile), which provides outpatient and hospital care. Consolidated Standards of Reporting Trials (CONSORT) recommendations for nonpharmacological studies were followed. This study was approved by the Ethics Committee of the Universidad de Chile (Santiago, Chile), and is registered in the National Clinical Trials database under registry number NCT02703883. Written informed consent was obtained from all subjects prior to participation.
Participants
The study included 17 iSCI patients and 17 healthy subjects. The degree of SCI was determined through a physical examination by a rehabilitation physician. The physician evaluated the presence of dermatomes and myotomes, as established by ASIA, for each patient (Table 1).28 The healthy control group was statistically similar in age and sex to the patient group (Table 2).
Table 1. Demographic traits of the assessed spinal cord injury patients.
| Patient | Sex | Age (years) | Height (cm) | Level of injury | Time elapsed since injury (months) | AIS classification |
|---|---|---|---|---|---|---|
| 1 | M | 65 | 167 | T10 | 33 | C |
| 2 | F | 51 | 163 | C7 | 18 | D |
| 3 | M | 64 | 167 | L2 | 29 | C |
| 4 | M | 58 | 170 | C6 | 31 | C |
| 5 | F | 28 | 157 | C5 | 14 | C |
| 6 | M | 45 | 178 | C5 | 14 | C |
| 7 | M | 33 | 170 | T12 | 17 | D |
| 8 | M | 25 | 168 | C6 | 15 | D |
| 9 | M | 19 | 167 | T9 | 18 | D |
| 10 | M | 26 | 177 | T6 | 27 | D |
| 11 | M | 30 | 175 | C6 | 23 | C |
| 12 | M | 33 | 170 | C7 | 31 | C |
| 13 | M | 45 | 173 | T10 | 28 | C |
| 14 | M | 48 | 165 | C7 | 13 | C |
| 15 | F | 59 | 161 | C6 | 17 | D |
| 16 | M | 47 | 170 | C7 | 19 | D |
| 17 | M | 63 | 173 | C6 | 48 | C |
M, male; F, female; AIS, American Spinal Cord Injury Association impairment scale.
Table 2. Statistical comparisons between the control and iSCI groups.
| Control Group | iSCI Group | P | |
|---|---|---|---|
| (n = 17) | (n = 17) | value | |
| Age (years) | 37.5 ± 8.9 | 43.5 ± 3.7 | 0.181 (ns) |
| Sex | |||
| Male, n (%) | 13(76.5%) | 14 (82.4%) | |
| Female, n (%) | 4 (23.5%) | 3 (17.6%) | |
| Height (cm) | 171.6± 6.6 | 168 ± 1.3 | 0.208 (ns) |
| NJ | 4.055± 0.8 | 5.803± 1.7 | 0.009 |
| RMS | 0.041±0.008 | 0.115±0.05 | 0.0001 |
| WISCI-II | - | 18 (13 – 20) | |
| AIS Classification | |||
| AIS C, n (%) | - | 10 (58.8%) | |
| AIS D, n (%) | - | 7 (41.2%) | |
| Level of injury | |||
| Cervical, n (%) | - | 11 (64.7%) | |
| Thoracic, n (%) | - | 5 (29.4%) | |
| Lumbar, n (%) | - | 1 (5.9%) | |
| Time elapsed since injury, months | - | 19 (16 – 30) |
iSCI, incomplete spinal cord injury; NJ, normalized jerk; RMS, root mean square, ns = non-significant. Values are expressed as the mean ± SD if data is normally distributed or as the median (P25-P75) if data distribution is skewed.
Inclusion criteria were as follows: individuals ≥ 18 years-old; neurological level of C5 or below; AIS C or D classifications; traumatic and non-traumatic injury; non-progressive lesions; onset > 12 months; ability to ambulate with or without assistive devices; ability to maintain a standing position for 30 s without assistance; and ability to follow verbal or visual commands.29
Exclusion criteria were as follows: unstable orthopedic injuries; osteoporosis with a high risk for pathological fracturing; cutaneous lesions and/or pressure ulcers; pregnancy; body weight >150 kg.29
Training protocol
The BWSTT program was distributed across 18 sessions over a six-week period (i.e., three sessions per week). Each session consisted of three 6-minute series of locomotor treadmill training, with 2 minute breaks between each series. In the first training session, a trained therapist selected the appropriate amount of body weight support for each participant. This weight was determined according to motor commitment and exercise tolerance. For safety, initial treadmill speed was 0.5 km/h. Furthermore, two physical therapist sat on either side of the treadmill and assisted participants in the gait cycle when it was necessary.29
Training sessions were conducted on an h/p/cosmos® treadmill system, which included a specialized built-in weight support device, namely, the h/p/cosmos® airwalk ap (HP Cosmos sports & medical GmbH, Nussdorf-Traunstein, Germany). A pulley system was used to hoist participants into a standing position over the treadmill. Once upright, a second set of cables was used to connect participants to weight stacks located at the front of the treadmill. Weight stacks were set at a predetermined percentage of each participant’s body weight. The speed of the h/p/cosmos® treadmill system ranges between 0.1 and 22 km/h, and speed can be adjusted in 0.1 km/h increments.29
Prior to and after the six-week BWSTT program, participants were assessed i) by a physical therapist; ii) through a standing balance test (i.e., ISway); and iii) using WISCI II to determine walking independence. Standing balance was measured via an ISway test that employed the APDM Mobility Lab™ (APDM Inc., Portland, OR, USA).23 Briefly, a wireless Opal™ inertial sensor with a docking station was attached with Velcro straps to the waist of each participant at level L5. The sensor recorded 2D linear accelerations and angular velocity, which were transmitted to a wireless receiver that streamed data to a laptop. Outcome measures were recorded and automatically generated using the Mobility Lab™ software (APDM Inc.).23 For the ISway test, participants were instructed to maintain an upright standing position, with the arms crossed over the chest, and a fixed heel-to-heel distance of 10 cm.23 Data were recorded for three quiet-standing trials (30 s each).
The obtained ISway measures were validated against postural sway values previously determined through center-of-pressure displacement tests with a force plate.23 The recorded ISway parameters included the root mean square (RMS, m/s2) of sway trajectory and JERK normalized to the excursion and duration of the sway trajectory. JERK was defined as the third derivative of displacement, and the subsequently NJ was used as the index of trajectory smoothness.26,28 The NJ was calculated as shown in Eq. (1):30,31
| (1) |
where Pi is the COM position at the ith sample; t1 is the onset of movement; t2 is the offset of movement; d3p/dt3 is the third derivative of displacement (i.e., JERK); t is movement time; and Pt2−Pt1 is movement range.
Statistical analysis
Statistical analyses were performed using the SPSS v.22 software (IBM Corp., Armonk, NY, USA). All data were analyzed for normal distribution with the Shapiro-Wilk test. Continuous variables were presented, where appropriate, as means ± standard deviation (S.D.) or as medians and 25th-75th percentiles. Data were analyzed using one-way ANOVA with repeated measures in each group. Differences were considered statistically significant at P < 0.05. Since the WISCI II test data had a non-parametric distribution, Spearman’s rank correlation coefficient was used to analyze relationships between basal NJ and basal performance in the WISCI II test. A post-hoc power analysis was conducted using the G*Power v.3.1.8.2 statistical software (Dusseldorf, Germany).
Results
The 17 included iSCI patients presented C7 to T10 injury levels classified as AIS C or D. Participants were allowed to wear their regular ankle-foot orthosis during all training sessions and to use their usual walking devices during assessments. All participants successfully completed the six-week BWSTT program and the ISway test. Traits of the study groups (i.e., patients and healthy subjects) are given in Tables 1 and 2.
Standing balance
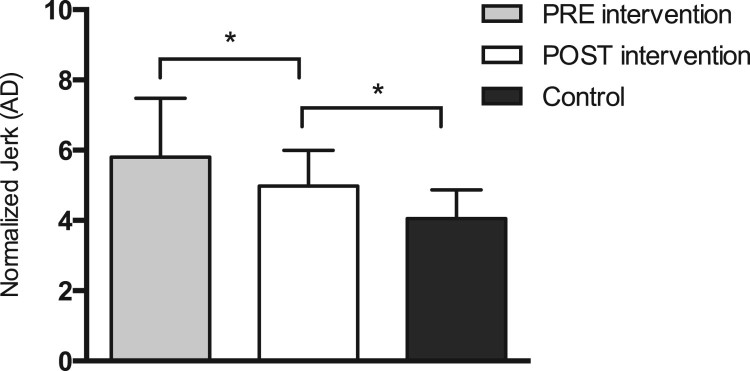
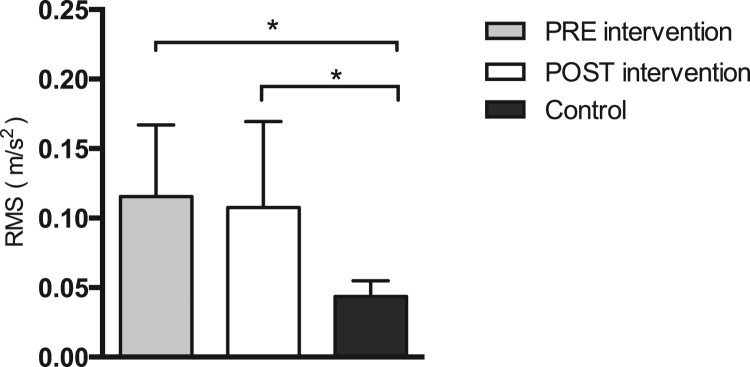
Differences in standing balance between iSCI patients and healthy participants were established with the NJ and RMS, both computed during the ISway test. Significant differences in initial NJ measurements existed between groups (P < 0.001) (Fig. 1). Furthermore, iSCI patients had an increased initial RMS value as compared to the control group (P < 0.001) (Fig. 2).
Figure 1.
Differences in normalized JERK (NJ) for iSCI patients pre- and post-intervention (P = 0.016), between pre- intervention values for iSCI and control subjects (P < 0.001), and between post-intervention values for iSCI patients and control subjects (P = 0.004). Error bars represent one standard error (SE) of the mean. Significant differences between groups are denoted by an asterisk (*). AD, amplitude duration.
Figure 2.
Differences in root mean square (RMS) values for iSCI patients pre- and post-intervention (P = 0.693), between pre-intervention values for iSCI patients and control subjects (P < 0.001), and between post-intervention values for iSCI patients and control subjects (P < 0.001). Error bars represent one standard error (SE) of the mean. Significant differences between groups are denoted by an asterisk (*).
After the six-week BWSTT program, no significant differences in ISway RMS values were found between groups (P = 0.693) (Fig. 2). A noteworthy post-intervention change was found for the NJ of iSCI patients (P = 0.016) (Fig. 1), but this improvement remained poorer than values obtained in control participants.
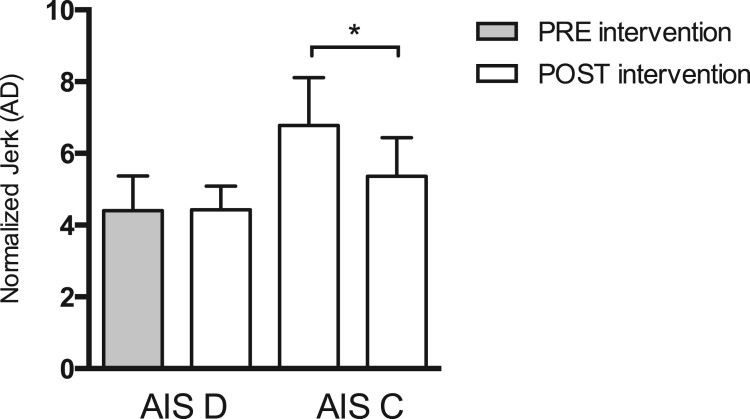
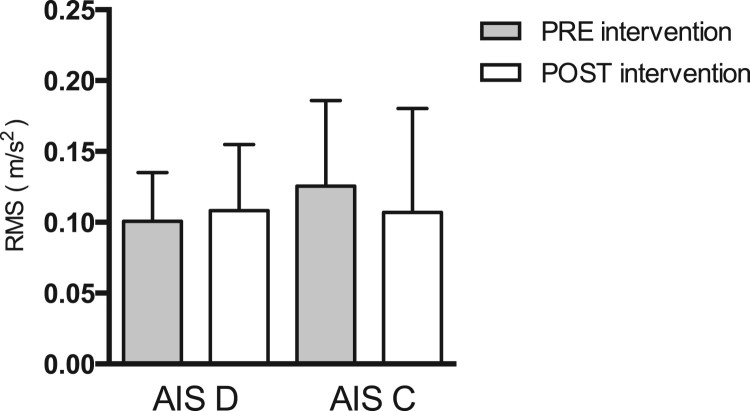
Initial and post-training NJ (Fig. 3) and RMS (Fig. 4) values were also compared within the AIS C and AIS D patient sub-groups (Table 3). AIS C patients showed a significant decrease in the NJ after training (P = 0.008). In contrast, AIS D patients evidenced no significant changes in this indicator (P = 0.931). No significant differences in RMS were found in either iSCI patient group.
Figure 3.
Differences in normalized JERK (NJ) values pre- and post-intervention in AIS D (P = 0.931) and AIS C (P = 0.008) patients. Error bars represent one standard error (SE) of the mean. Significant differences between groups are denoted by an asterisk (*). AD, amplitude duration.
Figure 4.
Differences in root mean square (RMS) values pre- and post-intervention in AIS D (P = 0.630) and AIS C (P = 0.244) patients. Error bars represent one standard error (SE) of the mean.
Table 3. Normalized JERK and RMS values pre- and post-BWSTT.
| iSCI Group AIS C | iSCI Group AIS D | |||||
|---|---|---|---|---|---|---|
| (n = 10) | (n = 7) | |||||
| Initial | Post-BWSTT | P-value | Initial | Post-BWSTT | P-value | |
| RMS | 0.107 ± 0.07 | 0.126 ± 0.06 | 0.244 (ns) | 0.101 ± 0,03 | 0.108 ± 0.05 | 0.630 (ns) |
| NJ | 6.80 ± 1.33 | 5.37 ± 1.07 | 0.008 | 4.41 ± 0.97 | 4.43 ± 0.67 | 0.931 (ns) |
| Abbreviations: iSCI, incomplete spinal cord injury; NJ, normalized jerk; RMS, root mean square, ns = non-significant. Values are expressed as the mean ± SD. | ||||||
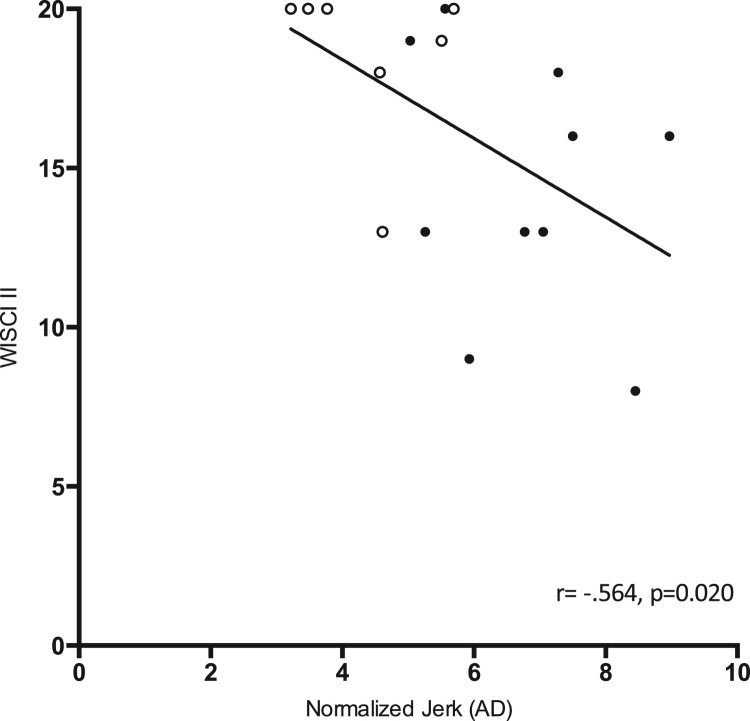
Despite achieving decreased NJ values post-training, no changes in gait independence were found. To determine this, initial and post-training NJ values and WISCI II scores were correlated for all iSCI patients (Fig. 5). An inverse correlation was found between the initial measures (P = 0.020, r = 0.564). However, no statistically significant correlation was observed between post-training NJ values and WISCI II scores (P = 0.526, r = 0.151).
Figure 5.
Inverse correlation between initial WISCI II scores and normalized JERK (NJ) values in iSCI patients. Black dots = AIS C patient values; White dots = AIS D patient values. Spearman’s correlation coefficients and P values are provided for each association. The solid line indicates the computed linear correlation. r = 0.564; P = 0.020. AD, amplitude duration.
Discussion
To our knowledge, this is the first study to evaluate the impact of a six-week BWSTT program in chronic iSCI patients (AIS C and D) using an ISway test with inertial sensors (i.e., an accelerometer, a magnetometer, and a gyroscope).The main findings were as follows: (1) the NJ of chronic iSCI patients significantly decreased after completion of the BWSTT protocol; (2) no significant pre- vs. post-BWSTT differences in RMS values were recorded; and (3) postural control improvements in the standing balance of iSCI patients, measured by the NJ, had no impact on gait independence.
Since the ISway test provides sensitive measures of the complex sensorimotor control loop responsible for regulating standing balance, it is an excellent method for measuring postural instability.23 Indeed, the ISway test could be used to initially assess standing balance before implementing interventions. Some research shows that postural sway measurements are more affected by a physical therapy postural training program than clinical rating measurements.23,32 The ISway test establishes JERK, which is an indicator of sway smoothness for the assessed movement.20,26 Furthermore, this test is the most sensitive measurement for discriminating postural-control differences in Parkinson’s disease.23,26 Nevertheless, the potential applications of the ISway test are not limited to Parkinson’s disease. This method can be used to obtain the amplitude, smoothness, and frequency measures needed to characterize body sway,33 all parameters that would be useful for testing any individual with postural and motor control deficits.
Effects of the BWSTT program on COM oscillatory dynamics
The assessed iSCI patients presented altered initial values of NJ (Fig. 1) and RMS (Fig. 2). In other words, despite being able to generate postural adaptations to environmental challenges and being able to complete the standing balance task, these patients could not fully compensate for the postural control changes caused by their sensory and motor impairments.
Standing balance requires appropriate sensory inputs, brain integration, and an intact neuromuscular system. iSCI patients may present increased postural sway as a result of deficient motor responses related to timing muscle contractions. This, in turn, would be the consequence of the residual motor pathways left after SCI being insufficient to react and generate appropriate postural adjustments. Increased postural sway can also result from damaged somatosensory pathways.6 These pathways are often compromised after SCI and subsequently reflect noisy somatosensory feedback from foot pressure, muscle proprioceptors, and joint receptors.34 Damaged somatosensory pathways can ultimately provide inaccurate information about body position in space and result in an abnormal internal map of stability limits. Altogether, these possible consequences of SCI can generate frequent, abrupt corrections of postural sway direction and may be responsible for higher JERK values as compared to healthy individuals (Fig. 1).
The presently obtained results also show that NJ significantly decreased in iSCI patients after the BWSTT program (Fig. 1). Although the NJ was measured in only 17 iSCI patients, the respective post hoc power was 98.8%. In contrast, RMS values did not change significantly (Fig. 2). These results indicate that the BWSTT program helped iSCI patients improve their ability to spontaneously compensate smoothness, the amount of sway trajectory was unaffected. In other words, iSCI patients did not decrease COM oscillation levels after the BWSTT program (Fig. 2). However, their ability to control COM acceleration changes during the ISway test was improved after program completion (Fig. 1).
A more detailed analysis revealed that 8 of the 17 iSCI patients underwent a significant decrease in sway NJ. All eight were classified as AIS C patients and evidenced the worst initial postural control measures (Fig. 3). These results indicate that the primary benefactors of a BWSTT program would be individuals with poor motor performance and static postural control. Regarding RMS measures, no significant differences were found among the AIS C patients following completion of the BWSTT program (Fig. 4).
Correlation between ISway measures and WISCI II scores
An inverse correlation was found between initial JERK values and WISCI II scores for iSCI patients (Fig. 5), i.e., individuals with worse indicators of postural control (increased JERK) presented poorer gait independence. This finding supports the sensitivity of accelerometer-based measures of sway in differentiating degrees of gait functionality, specifically as related to the NJ indicator.
Despite the inverse correlation between accelerometer-based measures of sway and WISCI II scale scores, significant changes in the NJ after the six-week BWSTT program did not correlate with improvements in gait independence. These results indicate that changes in static postural control (measured by the NJ) did not generate functional improvements (measured by the WISCI II scale).
Effects of the BWSTT program on gait independence in chronic iSCI patients
No changes were found in the gait independence of iSCI patients after the six-week BWSTT program. Individuals with neurological disorders that participate in motor learning processes display significant, correlative changes in sensorimotor performance and functional independence. Recent research suggests that after neurological damage there is a short plasticity window (i.e., 3–6 months) during which motor learning can be achieved through physical and pharmacological therapies.35,36 In turn, improvements achieved in the chronic stages of injury are related to optimization processes of compensatory behaviors, which may be less effective in achieving functional goals. In the present study, all of the participating iSCI patients were in the chronic stage of injury (i.e. > 12 months post-injury). The lack of improvements in gait independence as a result of improvements in postural control indicators (NJ) could be due to an absence of experiencing-learning processes, which likely would have allowed the iSCI patients to significantly improve their motor performance.
Another possible explanation for the obtained results is that various approaches exist for gait training in iSCI patients, and while many show improvement potential, no approach has been decidedly established as superior.37 The recommended methodology is to use different therapeutic strategies for recovering gait and to combine conventional therapies with conventional over-ground training and BWSTT. In the present study, only one intervention was tested. While this intervention led to significant changes in static postural control, these improvements did not correlate with improvements in gait independence. These results support the notion that the neural controls of balance and gait are relatively independent. That is, measures of postural sway while standing on a firm surface with the eyes open appear insufficient for predicting clinical improvements in gait for individuals with SCI. Although many measures of postural sway and gait were abnormal in the present study, balance control while standing was not related to gait control, suggesting that postural sway in these static conditions could not predict dynamic postural instability while walking. Postural control models further demonstrate that instability of the postural control loop is also reflected by an increased postural sway frequency, as resulting from the nervous system increasing the stiffness and frequency of postural corrections.38,39
In summary, no single measure of balance or gait can fully characterize mobility impairments in individuals with SCI, but a small set of relatively independent measures is useful for strategic assessments and targeted rehabilitation. Finally, a significant limitation of this study in correlating NJ improvements and changes in gait independence was that five of the iSCI patients achieved a maximum WISCI II score before beginning the BWSTT program.
Conclusion
Balance is an important ambulation component for those with spinal cord injury. This study evidenced that a six-week body weight-support treadmill training program modified center-of-mass control, as measured by the normalized JERK parameter. Nevertheless, this improvement in static postural control did not correlate with improvements in gait independence. Further research is needed to assess the impact of longer training protocols or of a similar program in acute iSCI patients. Future studies should also to better establish the relationship between postural control and functional independence.
Disclaimer statement
Conflicts of interest The authors declare no conflict of interest.
ORCID
Felipe Covarrubias-Escuderohttp://orcid.org/0000-0001-5228-5763
Gonzalo Rivera-Lillohttp://orcid.org/0000-0002-8157-4086
Rodrigo Torres-Castrohttp://orcid.org/0000-0001-7974-4333
Gonzalo Varas-Díazhttp://orcid.org/0000-0002-4621-056X
References
- 1.Hubli M, Dietz V.. The physiological basis of neurorehabilitation locomotor training after spinal cord injury. J Neuroeng Rehabil 2013;10(5):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astorino TA, Harness ET, White AC.. Efficacy of acute intermittent hypoxia on physical function and health status in humans with spinal cord injury: A brief review. Neural Plast 2015;2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datta S, Lorenz DJ, Morrison S, Ardolino E, Harkema SJ.. A Multivariate Examination of Temporal Changes in Berg Balance Scale Items for Patients With ASIA Impairment Scale C and D Spinal Cord Injuries. Arch Phys Med Rehabil 2009;90(7):1208–17. [DOI] [PubMed] [Google Scholar]
- 4.Tamburella F, Scivoletto G, Molinai M.. Balance training improves static stability and gait in chronic incomplete spinal cord injury subjects: a pilot study. Eur J Phys Rehabil 2013;49(3):353–64. [PubMed] [Google Scholar]
- 5.Lemay JF, Nadeau S.. Standing balance assessment in ASIA D paraplegic and tetraplegic participants: concurrent validity of the Berg Balance Scale. Spinal Cord 2010;48(3):245–50. [DOI] [PubMed] [Google Scholar]
- 6.Scivoletto G, Di Donna V, Scivoletto G, Di Donna V.. Prediction of walking recovery after spinal cord injury. Brain Res Bull 2009;78(1):43–51. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KD.Targeting Recovery: Priorities of the Spinal Cord-Injured Population. J Neurotrauma 2004;21(10):1371–83. [DOI] [PubMed] [Google Scholar]
- 8.Barbeau H, Nadeau S, Garneau C.. Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. J Neurotrauma 2006;23(3):571–85. [DOI] [PubMed] [Google Scholar]
- 9.Scivoletto G, Romanelli A, Mariotti A, Marinucci D, Tamburella F, Mammone A, et al Clinical factors that affect walking level and performance in chronic spinal cord lesion patients. Spine 2008;33(3):259–64. [DOI] [PubMed] [Google Scholar]
- 10.Tansey KE.Neural plasticity and locomotor recovery after spinal cord injury. PM&R 2010;2(12):220–6. [DOI] [PubMed] [Google Scholar]
- 11.Wernig A, Muller S.. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Spinal Cord 1992;30(4):229–38. [DOI] [PubMed] [Google Scholar]
- 12.Dietz V, Colombo G, Jensen L.. Locomotor activity in spinal man. The Lancet 1994;344(8932):1260–3. [DOI] [PubMed] [Google Scholar]
- 13.Alexeeva N, Sames C, Jacobs PL, Hobday L, Distasio MM, Mitchell SA, et al. Comparison of training methods to improve walking in persons with chronic spinal cord injury: a randomized clinical trial. J Spinal Cord Med 2011;34(4):362–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiPiro ND, Embry AE, Fritz SL, Middleton A, Krause JS, Gregory CM.. Effects of aerobic exercise training on fitness and walking-related outcomes in ambulatory individuals with chronic incomplete spinal cord injury. Spinal Cord 2016;54(9):675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, et al. Effectiveness of Automated Locomotor Training in Patients With Chronic Incomplete Spinal Cord Injury: A Multicenter Trial. Arch Phys Med Rehabil 2005;86(4):672–80. [DOI] [PubMed] [Google Scholar]
- 16.Phonthee S, Saengsuwan J, Amatachaya S.. Falls in independent ambulatory patients with spinal cord injury: incidence, associated factors and levels of ability. Spinal Cord 2013;51(5):365–8. [DOI] [PubMed] [Google Scholar]
- 17.Brotherton SS, Krause JS, Nietert PJ.. Falls in individuals with incomplete spinal cord injury. Spinal Cord 2007;45(1):37–40. [DOI] [PubMed] [Google Scholar]
- 18.Forrest GF, Lorenz DJ, Hutchinson K, Vanhiel LR, Basso DM, Datta S, et al. Ambulation and balance outcomes measure different aspects of recovery in individuals with chronic, incomplete spinal cord injury. Arch Phys Med Rehabil 2012;93(9):1553–64. [DOI] [PubMed] [Google Scholar]
- 19.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL.. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training based rehabilitation. Arch Phys Med Rehabil 2012;93(9):1508–17. [DOI] [PubMed] [Google Scholar]
- 20.Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB.. Postural Sway as a Marker of Progression in Parkinson's disease: a Pilot Longitudinal Study. Gait Posture 2012;36(3):471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harel NY, Asselin PK, Fineberg DB, Pisano TJ, Bauman WA, Spungen AM.. Adaptation of computerized posturography to assess seated balance in persons with spinal cord injury. J Spinal Cord Med 2013;36(2):127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leach JM, Mancini M, Peterka RJ, Hayes TL, Horak FB.. Validating and calibrating the Nintendo Wii balance board to derive reliable center of pressure measures. Sensors 2014;14(10):18244–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mancini M, Salarian A, Carlson-kuhta P, Zampieri C, King L, Chiari L, et al. ISway: a sensitive, valid and reliable measure of postural control. J. Neuroeng Rehabil 2012;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitney SL, Roche JL, Marchetti GF, Steed DP, Furman GR, Redfern MS.. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: A measure of balance. Gait Posture 2016;33(4):594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moe-Nilssen R, Helbostad JL.. Trunk accelerometry as a measure of balance control during quiet standing. Gait Posture 2002;16(1):60–8. [DOI] [PubMed] [Google Scholar]
- 26.Mancini M, Horak FB, Zampieri C, Carlson-kuhta P, Nutt JG, Chiari L.. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Park Relat Disord 2011;17(7):557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fazio P, Granieri G, Casetta I, Cesnik E, Mazzacane S, Calandrio P, et al. Gait measures with a triaxial accelerometer among patients with neurological impairment. Neurol Sci 2013;34(4):435–40. [DOI] [PubMed] [Google Scholar]
- 28.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (Revised 2011). J Spinal Cord Med 2011;34(6):535–46. 10.1179/107902611X13186000420242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ditor DS, MacDonald MJ, Kamath MV, Bugaresti J, Adams M, McCartney N, et al. The effects of body-weight supported treadmill training on cardiovascular regulation in individuals with motor-complete SCI. Spinal Cord 2005;43(11):664–73. [DOI] [PubMed] [Google Scholar]
- 30.Tsao CC, Mirbagheri MM.. Upper limb impairments associated with spasticity in neurological disorders. J Neuroeng Rehabil 2007;4(45):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Hinrichs R, Payne G, Thomas J, Normalized Jerk: A Measure to Capture Developmental Characteristics of Young Girls’ Overarm Throwing. J Appl Biomech 2000;16(2):196–203. [Google Scholar]
- 32.King LA, Salarian A, Mancini M, Priest KC, Nutt J, Serdar A, et al. Exploring Outcome Measures for Exercise Intervention in People with Parkinson’s Disease. Parkinson’s Disease 2013;2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menant JC, Latt MD, Menz HB, Fung VS, Lord SR.. Postural Sway Approaches Center of Mass Stability Limits in Parkinson’s Disease. Mov Disord 2011;26(4):637–43. [DOI] [PubMed] [Google Scholar]
- 34.Dietz V, Fouad K.. Restoration of sensorimotor functions after spinal cord injury. Brain 2014;137(3):654–67. [DOI] [PubMed] [Google Scholar]
- 35.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF.. Getting neurorehabilitation right what can be learned from animal models?. Neurorehabil Neural Repair 2012;26(8):923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeiler SR, Hubbard R, Gibson EM, Zheng T, Ng K, O’Brien R, et al. Paradoxical motor recovery from a first stroke after induction of a second stroke: reopening a postischemic sensitive period. Neurorehabil Neural Repair 2016;30(8):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morawietz C, Moffat F.. Effects of locomotor training after incomplete spinal cord injury: A systematic review. Arch Phys Med Rehabil 2013;94(11):2297–308. [DOI] [PubMed] [Google Scholar]
- 38.Peterka RJ.Postural control model interpretation of stabilogram diffusion analysis. Biol Cybern 2000;82(4):335–43. [DOI] [PubMed] [Google Scholar]
- 39.Maurer C, Mergner T, Peterka RJ.. Multisensory control of human upright stance. Exp Brain Res 2006;171(2):231–50. [DOI] [PubMed] [Google Scholar]