Abstract
Context/objective
Acute care readmission has been identified as an important marker of healthcare quality. Most previous models assessing risk prediction of readmission incorporate variables for medical comorbidity. We hypothesized that functional status is a more robust predictor of readmission in the spinal cord injury population than medical comorbidities.
Design
Retrospective cross-sectional analysis.
Setting
Inpatient rehabilitation facilities, Uniform Data System for Medical Rehabilitation data from 2002 to 2012
Participants
traumatic spinal cord injury patients.
Outcome measures
A logistic regression model for predicting acute care readmission based on demographic variables and functional status (Functional Model) was compared with models incorporating demographics, functional status, and medical comorbidities (Functional-Plus) or models including demographics and medical comorbidities (Demographic-Comorbidity). The primary outcomes were 3- and 30-day readmission, and the primary measure of model performance was the c-statistic.
Results
There were a total of 68,395 patients with 1,469 (2.15%) readmitted at 3 days and 7,081 (10.35%) readmitted at 30 days. The c-statistics for the Functional Model were 0.703 and 0.654 for 3 and 30 days. The Functional Model outperformed Demographic-Comorbidity models at 3 days (c-statistic difference: 0.066-0.096) and outperformed two of the three Demographic-Comorbidity models at 30 days (c-statistic difference: 0.029-0.056). The Functional-Plus models exhibited negligible improvements (0.002-0.010) in model performance compared to the Functional models.
Conclusion
Readmissions are used as a marker of hospital performance. Function-based readmission models in the spinal cord injury population outperform models incorporating medical comorbidities. Readmission risk models for this population would benefit from the inclusion of functional status.
Keywords: Spinal cord injury, Readmission, Functional status, Rehabilitation, Patient outcomes
Introduction
Every year, one-third of traumatic spinal cord injury (SCI) patients will experience unplanned readmission, often for preventable conditions (e.g. urinary tract infections, respiratory infections, and pressure ulcers).1–8 Patients are particularly vulnerable in the early phase after injury. One of the goals of inpatient rehabilitation is imparting the knowledge and skills needed for effective management of self-care needs to avoid secondary complications that result in unplanned readmissions. However, patients and families have often had mere weeks to adjust to the injury and its consequences, which may diminish their ability to retain this new knowledge.9 As a result, patients and families often leave the hospital feeling overwhelmed, socially isolated, and unprepared to assume responsibility for their health and care needs.9 This has resulted in a rise in the prevalence of medical complications after discharge and a concomitant increase in hospitalizations.10 Once rehospitalized, these patients may require additional rehabilitation to regain strength and function that were lost during rehospitalization, disrupting and undermining rehabilitation gains. Furthermore, rehospitalization can impact an individual’s ability to sustain employment and to otherwise participate in the community, thereby impacting overall quality of life.11,12 In addition to diminishing an individual’s ability to live actively and independently, there is a significant long-term financial burden associated with readmission in SCI, with costs due to hospitalizations ranging from $600 to 6300 in the first year after injury and $3500 to 15,800 per person in the subsequent five years.13 Ultimately, the personal toll of readmissions cannot be overestimated, as rehospitalization has been found to be a primary risk factor for early mortality.14
In addition to the quality-of-life and cost implications for SCI individuals, acute care readmission is an increasingly important marker of healthcare quality in the broader national regulatory environment. In 2010, the Hospital Readmission Reduction Program (HRRP) was enacted as part of the Patient Protection and Affordable Care Act with the goal of reducing readmissions within 30 days of hospital discharge, imposing financial penalties against hospitals with higher-than-expected readmission rates beginning in 2012.15 While 30-day readmission rates initially declined when the penalties were announced, they later plateaued,16 suggesting that the reasons for readmission are complex and remain incompletely understood.17
To date, the majority of attention to readmissions has focused on acute care hospitalization. However, post-acute care is a major source of healthcare costs with a trend toward increasing utilization of post-acute care services.18 As such, the focus of quality improvement efforts is also shifting. To document unplanned 30-day readmissions from inpatient rehabilitation facilities (IRF), the Center for Medicare and Medicaid Services (CMS) developed the All-Cause Unplanned Readmission Measure for 30 Days Post Discharge from Inpatient Rehabilitation Facilities. Public reporting began in 2016, but as of yet, there are no associated financial penalties.19 Extrapolating from acute care, it can be expected that the post-acute setting will be an increasingly scrutinized area of cost reduction and quality optimization, presenting an opportunity and incentive to shift the focus of examining readmission from acute care to the IRF setting.
These recent trends in healthcare policy and the imposed financial penalties have raised awareness about the importance of reducing hospital readmissions not only to avoid fines but also to improve patient care. As such, a growing number of studies have sought to identify predictors of readmission with the aim of developing preventative strategies and interventions.20 Previous readmission risk prediction models have yielded limited discriminative ability (c-statistics 0.55-0.65).20 In a review of such models in general medical populations, most models focused on medical comorbidities, demographics (i.e. age, sex, and race/ethnicity), and use of prior medical services, whereas few considered mental health, functional status, or social determinant variables (i.e. socioeconomic status, insurance status, marital status, caregiver availability, access to care, and discharge location).20 One model that included functional status demonstrated that functional status improved model performance compared to use of medical services/comorbidities (c = 0.83 vs. 0.77).20,21 There is growing evidence that functional status is an important predictor of patient outcomes and mortality21–25 and that interventions to improve functional status improve outcomes.26,27 While specific, quantifiable measures of functional status may be difficult to obtain from retrospective acute care data sets, standardized data regarding functional status and medical comorbidities are routinely collected in the inpatient rehabilitation population. In studies of patients admitted to IRFs, there is growing evidence that functional status outperforms medical comorbidities in predicting acute care readmissions.28–31 Notably, the addition of medical comorbidities to risk prediction models incorporating functional status did not enhance predictive ability.28,30,31
Previous studies of readmission in the SCI population have found significant relationships between functional status and acute care readmission. Cardenas et al. found that lower motor Functional Independence MeasureTM (FIM) score is a significant predictor of rehospitalization at one and five years post injury.1 Similarly, Eastwood et al. found that lower discharge FIMTM predicts rehospitalization.32 Other functional and psychosocial factors that have been associated with increased likelihood of rehospitalization include education level, bladder management method, motor complete injuries, dependence in self-care, and dependence in ambulation.7 Others have found that sex, race, payor source, and more severe case mix are associated with increased odds of rehospitalization.3,33,34 These studies were limited by the use of self-report of occurrence and reason for rehospitalization, low follow-up rate, samples of exclusively SCI Model Systems populations or single-institution populations, and high proportions of missing data.1,3,7,32–34 The variability in functional status measures makes it difficult to compare and generalize the results of prior work. Though the 30-day readmission rate has been chosen as a benchmark of quality by CMS, few of these previous studies in SCI have examined 30-day readmission specifically, and those that have did not include standardized measures of functional status in their risk prediction models.33,34 None of these studies have directly compared the predictive power of functional status to that of medical comorbidities in the SCI population using a large, national sample.
In light of the considerable morbidity, mortality, and cost burden associated with acute care readmission in SCI patients, as well as the regulatory trends incentivizing reduction of readmissions, this study seeks to examine the role of functional status, as compared to medical comorbidities, in predicting readmission in the SCI population. We hypothesize that a risk prediction model that incorporates functional status for the inpatient rehabilitation traumatic SCI population would be more predictive than a model using medical comorbidities alone. Furthermore, the addition of comorbidities to a model that includes functional status would not meaningfully improve predictive power.
Methods
Study design and population
This study uses a retrospective cross-sectional design. We analyzed data from the Uniform Data System for Medical Rehabilitation (UDSMR), a repository for IRF functional outcome data. CMS requires IRFs to complete the Inpatient Rehabilitation Facility Patient Assessment Instrument (IRF-PAI), which contains demographic, social, medical, and functional data. UDSMR services approximately 70% of IRFs in the United States. Data was obtained from the UDSMR from 2002–2012. Inclusion criteria were Medicare-established Impairment Group codes associated with traumatic SCI (04.210-04.230, 14.1, 14.3).35 Exclusion criteria were age <18 or >108 years, time from SCI onset to IRF admission >90 days, and admission to IRF from a setting other than acute care. Patients with >90 days from SCI onset were excluded in an effort to maximize homogeneity of the study population. Patients with greater time from SCI onset likely represent patients with prolonged acute care hospital courses, possibly due to complications not directly related to SCI, or chronic SCI patients who were admitted to acute care for medical complications. This study received exemption from the Institutional Review Board at Spaulding Rehabilitation Hospital given the de-identified nature of the data set.
Primary outcome and study variables
The primary outcomes were readmission to acute care at 3 and 30 days after admission to inpatient rehabilitation. Predictor variables included age, race, sex, functional status on admission, and medical comorbidities. The race categories available in the UDSMR were grouped into white, black, and other categories. Functional status was measured using the FIMTM instrument. The FIMTM, which has been widely used in the assessment of disability in the SCI population,36 is a standardized tool that assesses function consisting of eighteen items in either a motor (13 items) or cognitive domain (5 items), each rated on a seven-level ordinal scale from completely dependent (1) to independent (7).35 The IRF-PAI includes up to ten comorbidities per patient, coded according to the International Classification of Diseases 9th edition Clinical Modification (ICD-9-CM). Transfer to acute care is a designated disposition category in the IRF-PAI. Comorbidities were assessed using three classification systems: Deyo-Charlson comorbidity index,37–39 Elixhauser comorbidity measure,40 and Medicare comorbidity tier system (Table 1).41
Table 1. Comorbidity classification systems.
| Index | Description |
|---|---|
| Deyo-Charlson | The Deyo-Charlson index places ICD-9 codes into one of seventeen comorbidity categories and assigns weights to each comorbidity category. This index was developed to identify comorbid illnesses that increase the risk of acute hospital mortality. |
| Elixhauser | The Elixhauser measure was developed for use with ICD-9 data to address some of the perceived shortcomings of the Charlson index. Elixhauser incorporates twenty-nine disease categories to assess the impact of each category on outcomes. |
| CMS Comorbidity Tiers | The CMS Comorbidity Tiers rely on a four-tiered system for grading medical complexity as part of the prospective payment system of inpatient rehabilitation facilities developed by RAND for Medicare. The most complex tier receives the highest payment for a given diagnosis and age. Comorbidity data are obtained for up to ten ICD-9-CM codes. |
The Deyo-Charlson index, originally developed by Charlson et al. in 1987 and adapted for use with ICD-9-CM codes in administrative data sets by Deyo et al., predicts one-year mortality based on the presence of 17 potential comorbid conditions, including heart disease, malignancy, or AIDS.37–39 As this index was developed for predicting mortality in breast cancer patients being considered for clinical trials, concerns have been raised regarding the generalizability of this index in other populations. Further considerations when using indices such as the Deyo-Charlson index to control for comorbidities include taking into account the complexity of ICD-9-CM coding as well as separately estimating weights of particular comorbidities for different populations and different outcomes, as their predictive values differ by patient groups.42,43 The Elixhauser comorbidity measure is comprised of 29 comorbidities which were associated with increased length of stay, hospital charges, and mortality; this measure sought to mitigate the shortcomings of previous comorbidity measures by taking a comprehensive approach to identifying the included comorbidities through a survey of the literature and the ICD-9-CM manual and by distinguishing comorbidities from the primary reason for hospitalization.40 The CMS Comorbidity Tiers use a four-tiered grading level for medical complexity as part of the CMS prospective payment system for IRF Medicare payments, with the most complex tier receiving the highest payment for a given diagnosis and age. Comorbidity data are obtained from ICD-9-CM codes reported to UDSMR, with a maximum of ten reported comorbidities.41
Statistical analyses
We hypothesized that (i) readmission models based on demographics and functional status would outperform models based on demographics and comorbidities and (ii) the addition of comorbidity data to function-based models would not improve predictive ability. To test the hypotheses, we developed a series of logistic regression models: the “Functional” model that included demographic variables (age, sex, and race) and functional status (admission FIMTM motor score and FIMTM cognitive score), the “Functional-Plus” models that added comorbidity data to the Functional Model according to each comorbidity scoring system, and the “Demographic-Comorbidity” models that included only demographic variables and comorbidities from each scoring system. Predictive ability for readmission of each model was examined at 3 and 30 days after admission to IRF. Model performance was assessed using the area under the receiver operator curve (c-statistic). The c-statistic captures the absolute ability of a model to discriminate between those readmitted and those not readmitted and has been used in prior readmission studies and in a systematic review of readmission risk prediction.20,28,30,31 A c-statistic of 0.50 signifies that a model performs no better than chance. A c-statistic of 0.70-0.80 signifies modest to acceptable discriminative ability, and a c-statistic greater than 0.80 signifies good discriminative ability.44,45 We used the difference between c-statistics for two models at the same time point as a comparison method. A c-statistic difference of 0.05 was considered meaningful based on prior literature.28,31 Any Functional-Plus model meeting this threshold and any failure of the Functional model to outperform a Demographic-Comorbidity model by at least 0.05 was considered evidence against our hypotheses. Tests of significance were not performed on the differences between c-statistics calculated from our models given that even negligible differences would likely be statistically significant given the large size of our sample.46 The potential effect of non-normal distributions of age in our population was examined using linear, linear spline, and restricted cubic spline transformations. Model calibration at 3 and 30 days was assessed using lowess calibration plots. Internal validation was performed using boot-strapping.
Results
Between 2002 and 2012, there were 5,630,451 adult IRF admissions in the UDSMR database. Of those, 91,810 had Impairment Group codes associated with traumatic SCI. We excluded 5861 patients who had >90 days between SCI onset and IRF admission, as well as 17,554 patients who were not admitted to inpatient rehabilitation directly from an acute hospital. No patients were excluded based on age <18 or >108 years. The final sample was 68,395 patients from 1097 IRFs. The mean age was 49.58 years, 71.0% (48,559/68,395) were male, and 69.8% (47,748/68,395) were white. Of the study population, 1469 (2.15%) were readmitted to an acute hospital within three days, and 7081 (10.35%) were readmitted within 30 days after IRF admission. Table 2 shows demographic, medical, and facility data for the study population.
Table 2. Patient characteristics.
| Number of patients, n | 68395 |
| Number of facilities, n | 1097 |
| Age, mean (SD) | 49.58 (19.87) |
| Male, n (%) | 48559 (71.00) |
| Race, n (%) | |
| White | 47748 (69.81) |
| Black | 10169 (14.87) |
| Hispanic | 5830 (8.52) |
| Asian | 1558 (2.28) |
| Multi-racial | 413 (0.60) |
| Other | 1077 (1.57) |
| Admission motor FIM, mean (SD) | 28.81 (13.57) |
| Admission cognitive FIM, mean (SD) | 27.67 (6.94) |
| Level of impairment, n (%) | |
| Incomplete paraplegia | 8391 (12.27) |
| Complete paraplegia | 7829 (11.45) |
| Unspecified paraplegia | 3779 (5.52) |
| Incomplete quadriplegia, C1-4 | 6746 (9.86) |
| Incomplete quadriplegia, C5-8 | 8331 (12.18) |
| Complete quadriplegia, C1-4 | 1937 (2.83) |
| Complete quadriplegia, C5-8 | 3215 (4.70) |
| Unspecified quadriplegia | 2560 (3.74) |
| Other | 25607 (37.44) |
| Married, n (%) | 29070 (42.50) |
| Living alone pre-injury, n (%) | 14215 (20.78) |
| Employed pre-injury, n (%) | 32427 (47.41) |
| Primary payor source, n (%) | |
| Medicare | 20845 (30.48) |
| Medicaid | 9653 (14.11) |
| Commercial | 22094 (32.30) |
| Unreimbursed | 6202 (9.07) |
| Workers’ compensation | 4254 (6.22) |
| Other | 5342 (7.81) |
| Number of comorbidities, mean (SD) | 7.64 (2.92) |
| Length of IRF stay, mean days (SD) | 25.84 (23.31) |
| Discharge disposition, n (%) | |
| Community | 48417 (70.79) |
| Acute facility | 8239 (12.05) |
| Skilled nursing/subacute | 5087 (7.44) |
| Other | 4628 (6.77) |
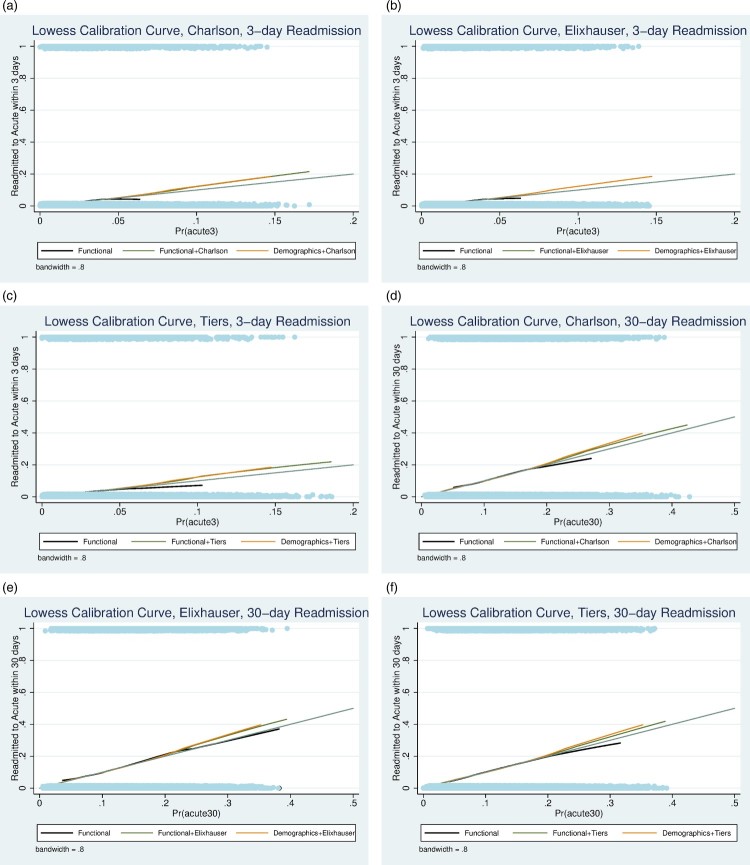
Logistic regression coefficients for each of the models are summarized in Tables 3 and 4. The c-statistics for each model at 3 and 30 days are shown in Table 5. The c-statistics for the Functional Model are 0.703 and 0.654 for 3 and 30 days respectively. The Functional-Plus models performed marginally better than the Functional Model at each time point, but changes in c-statistics did not exceed the threshold of 0.05 at either time point. At three days, the Functional model outperformed the Demographic-Comorbidity models with c-statistic improvements of 0.096, 0.098, and 0.066 compared to the Demographic-Deyo-Charlson, Demographic-Elixhauser, and Demographic-CMS Tiers models respectively. At 30 days, the Functional model outperformed the Demographic-Deyo-Charlson and Demographic-Elixhauser models with c-statistic differences of 0.056 and 0.053 respectively; however, the Functional model did not meet the c-statistic threshold of 0.05 compared to the Demographic-CMS Tiers model, with a c-statistic difference of 0.029. The best-performing Functional-Plus model was the Functional-Plus CMS Tiers Model (3-day c-statistic 0.707, 30-day c-statistic 0.664). Though it demonstrated limited to modest discriminative ability, the Functional-Plus CMS Tiers model failed to outperform the Functional Model at 3 or 30 days, with c-statistic differences of 0.004 and 0.010 respectively. Transformations of age did not qualitatively affect the results. The Functional model at 3 and 30 days had good calibration based on calibration plots (Fig. 1). All models were internally valid based on boot-strapping.
Table 3. Logistic regression results, 3-day readmission.
| Odds ratio | P-value | 95% CI | AIC | BIC | |
|---|---|---|---|---|---|
| Functional | |||||
| Age | 1.013 | 0.000 | 1.009 -1.017 | 12996.54 | 13060.31 |
| Sex | |||||
| Female | 0.827 | 0.004 | 0.726 - 0.941 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 0.911 | 0.300 | 0.764 - 1.087 | ||
| Other | 0.868 | 0.127 | 0.725 - 1.041 | ||
| Motor FIM | 0.961 | 0.000 | 0.955 - 0.967 | ||
| Cognitive FIM | 0.952 | 0.000 | 0.945 - 0.959 | ||
| Constant | 0.113 | 0.000 | 0.075 - 0.171 | ||
| Functional + Deyo | |||||
| Age | 1.012 | 0.000 | 1.008 - 1.016 | 12989.70 | 13071.68 |
| Sex | |||||
| Female | 0.828 | 0.004 | 0.727 - 0.942 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 0.902 | 0.251 | 0.756 - 1.076 | ||
| Other | 0.872 | 0.137 | 0.727 - 1.045 | ||
| Motor FIM | 0.960 | 0.000 | 0.954 - 0.967 | ||
| Cognitive FIM | 0.953 | 0.000 | 0.946 - 0.960 | ||
| Charlson index | 0.990 | 0.883 | 0.870 - 1.127 | ||
| Constant | 0.115 | 0.000 | 0.076 - 0.172 | ||
| Functional + Elixhauser | |||||
| Age | 1.013 | 0.000 | 1.009 - 1.017 | 12998.51 | 13071.38 |
| Sex | |||||
| Female | 0.826 | 0.004 | 0.726 - 0.941 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 0.911 | 0.300 | 0.765 - 1.086 | ||
| Other | 0.869 | 0.129 | 0.724 - 1.042 | ||
| Motor FIM | 0.961 | 0.000 | 0.955 - 0.967 | ||
| Cognitive FIM | 0.952 | 0.000 | 0.945 - 0.959 | ||
| Elixhauser weighted sum | 0.999 | 0.891 | 0.985 - 1.013 | ||
| Constant | 0.113 | 0.000 | 0.075 - 0.172 | ||
| Functional + CMS Tiers | |||||
| Age | 1.015 | 0.000 | 1.010 - 1.019 | 12960.51 | 13051.60 |
| Sex | |||||
| Female | 0.818 | 0.003 | 0.718 - 0.932 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 0.903 | 0.257 | 0.756 - 1.077 | ||
| Other | 0.874 | 0.144 | 0.729 - 1.047 | ||
| Motor FIM | 0.961 | 0.000 | 0.955 - 0.968 | ||
| Cognitive FIM | 0.953 | 0.000 | 0.945 - 0.960 | ||
| CMS Tiers | |||||
| No cost | 1.000 | ||||
| Low cost | 1.083 | 0.433 | 0.887 - 1.323 | ||
| Med cost | 0.667 | 0.000 | 0.538 - 0.827 | ||
| High cost | 1.289 | 0.009 | 1.066 - 1.558 | ||
| Constant | 0.104 | 0.000 | 0.065 - 0.165 | ||
| Demo + Deyo | |||||
| Age | 1.017 | 0.000 | 1.013 - 1.021 | 13631.53 | 13695.30 |
| Sex | |||||
| Female | 0.726 | 0.000 | 0.636 - 0.830 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 0.933 | 0.441 | 0.782 - 1.113 | ||
| Other | 0.919 | 0.353 | 0.768 - 1.099 | ||
| Charlson index | 0.958 | 0.509 | 0.843 - 1.089 | ||
| Constant | 0.010 | 0.000 | 0.007 - 0.013 | ||
| Demo + Elixhauser | |||||
| Age | 1.018 | 0.000 | 1.013 - 1.022 | 13633.48 | 13688.14 |
| Sex | |||||
| Female | 0.732 | 0.000 | 0.640 - 0.837 | ||
| Race | |||||
| Black | 0.937 | 0.466 | 0.786 - 1.116 | ||
| Other | 0.911 | 0.310 | 0.761 - 1.091 | ||
| Elixhauser weighted sum | 1.014 | 0.036 | 1.001 - 1.028 | ||
| Constant | 0.009 | 0.000 | 0.007 - 0.012 | ||
| Demo + CMS Tiers | |||||
| Age | 1.019 | 0.000 | 1.015 - 1.024 | 13509.31 | 13582.19 |
| Sex | |||||
| Female | 0.746 | 0.000 | 0.653 - 0.853 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 0.938 | 0.473 | 0.787 - 1.118 | ||
| Other | 0.920 | 0.364 | 0.769 - 1.101 | ||
| CMS Tiers | |||||
| No cost | 1.000 | ||||
| Low cost | 1.265 | 0.013 | 1.051 - 1.522 | ||
| Med cost | 1.008 | 0.080 | 0.828 - 1.228 | ||
| High cost | 2.596 | 0.000 | 2.202 - 3.061 | ||
| Constant | 0.007 | 0.000 | 0.006 - 0.010 |
Table 4. Logistic regression results, 30-day readmission.
| Odds Ratio | P-value | 95% CI | AIC | BIC | |
|---|---|---|---|---|---|
| Functional | |||||
| Age | 1.015 | 0.000 | 1.012 - 1.018 | 42500.14 | 42563.91 |
| Sex | |||||
| Female | 0.877 | 0.000 | 0.823 - 0.935 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 1.093 | 0.103 | 0.982 - 1.216 | ||
| Other | 1.010 | 0.861 | 0.900 - 1.134 | ||
| Motor FIM | 0.970 | 0.000 | 0.966 - 0.974 | ||
| Cognitive FIM | 0.976 | 0.000 | 0.972 - 0.980 | ||
| Constant | 0.235 | 0.000 | 0.171 - 0.321 | ||
| Functional + Deyo | |||||
| Age | 1.013 | 0.000 | 1.011 - 1.016 | 42405.77 | 42487.75 |
| Sex | |||||
| Female | 0.878 | 0.000 | 0.824 - 0.936 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 1.077 | 0.171 | 0.969 - 1.197 | ||
| Other | 1.015 | 0.796 | 0.905 - 1.140 | ||
| Motor FIM | 0.969 | 0.000 | 0.966 - 0.973 | ||
| Cognitive FIM | 0.977 | 0.000 | 0.973 - 0.981 | ||
| Charlson index | 1.048 | 0.130 | 0.986 - 1.115 | ||
| Constant | 0.240 | 0.000 | 0.176 - 0.327 | ||
| Functional + Elixhauser | |||||
| Age | 1.014 | 0.000 | 1.011 - 1.017 | 42436.39 | 42509.26 |
| Sex | |||||
| Female | 0.884 | 0.000 | 0.829 - 0.941 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 1.085 | 0.117 | 0.980 - 1.201 | ||
| Other | 1.003 | 0.952 | 0.897 - 1.122 | ||
| Motor FIM | 0.971 | 0.000 | 0.967 - 0.974 | ||
| Cognitive FIM | 0.977 | 0.000 | 0.972 - 0.981 | ||
| Elixhauser weighted sum | 1.021 | 0.000 | 1.011 - 1.031 | ||
| Constant | 0.216 | 0.000 | 0.158 - 0.297 | ||
| Functional + CMS Tiers | |||||
| Age | 1.015 | 0.000 | 1.012 - 1.018 | 42289.78 | 42389.98 |
| Sex | |||||
| Female | 0.879 | 0.000 | 0.824 - 0.937 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 1.093 | 0.097 | 0.984 - 1.213 | ||
| Other | 1.021 | 0.732 | 0.907 - 1.149 | ||
| Motor FIM | 0.990 | 0.161 | 0.975 - 1.004 | ||
| Cognitive FIM | 0.978 | 0.000 | 0.974 - 0.982 | ||
| CMS Tiers | |||||
| No cost | 1.000 | ||||
| Low cost | 1.558 | 0.000 | 1.392 - 1.744 | ||
| Med cost | 1.153 | 0.012 | 1.032 - 1.289 | ||
| High cost | 1.511 | 0.000 | 1.337 - 1.709 | ||
| Constant | 0.142 | 0.000 | 0.093 - 0.215 | ||
| Odds Ratio | P-value | 95% CI | AIC | BIC | |
| Demo + Deyo-Charlson | |||||
| Age | 1.015 | 0.000 | 1.013 - 1.018 | 43699.56 | 43763.32 |
| Sex | |||||
| Female | 0.803 | 0.000 | 0.752 - 0.857 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 1.108 | 0.047 | 1.001 - 1.226 | ||
| Other | 1.049 | 0.402 | 0.938 - 1.174 | ||
| Charlson index | 1.030 | 0.335 | 0.970 - 1.093 | ||
| Constant | 0.052 | 0.000 | 0.042 - 0.064 | ||
| Demo + Elixhauser | |||||
| Age | 1.016 | 0.000 | 1.013 - 1.019 | 43636.28 | 43690.94 |
| Sex | |||||
| Female | 0.816 | 0.000 | 0.764 - 0.871 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 1.110 | 0.033 | 1.008 - 1.223 | ||
| Other | 1.034 | 0.545 | 0.929 - 1.151 | ||
| Elixhauser weighted sum | 1.031 | 0.000 | 1.023 - 1.040 | ||
| Constant | 0.047 | 0.000 | 0.038 - 0.058 | ||
| Demo + CMS Tiers | |||||
| Age | 1.017 | 0.000 | 1.014 - 1.020 | 43245.04 | 43317.91 |
| Sex | |||||
| Female | 0.829 | 0.000 | 0.776 - 0.886 | ||
| Race | |||||
| White | 1.000 | ||||
| Black | 1.119 | 0.025 | 1.014 - 1.236 | ||
| Other | 1.052 | 0.392 | 0.937 - 1.181 | ||
| CMS Tiers | |||||
| No cost | 1.000 | ||||
| Low cost | 1.717 | 0.000 | 1.558 - 1.892 | ||
| Med cost | 1.469 | 0.000 | 1.341 - 1.610 | ||
| High cost | 2.299 | 0.000 | 2.067 - 2.557 | ||
| Constant | 0.039 | 0.000 | 0.031 - 0.049 |
Table 5. C-statistics.
| Functional Model | Functional-Plus models | Demographic-comorbidity models | |||||
|---|---|---|---|---|---|---|---|
| Demo + FIM | Func + Deyo | Func + Elixhauser | Func + CMS Tiers | Demo + Deyo | Demo + Elixhauser | Demo + CMS Tiers |
|
| 3 days | 0.703 | 0.705 | 0.703 | 0.708 | 0.607 | 0.606 | 0.637 |
| 30 days | 0.654 | 0.658 | 0.656 | 0.664 | 0.598 | 0.601 | 0.625 |
Figure 1.
(a) Lowess calibration curve, Deyo-Charlson Index, 3-day readmission; (b) Lowess calibration curve, Elixhauser Index, 3-day readmission; (c) Lowess calibration curve, CMS Tiers, 3-day readmission; (d) Lowess calibration curve, Deyo-Charlson Index, 30-day readmission; (e) Lowess calibration curve, Elixhauser Index, 30-day readmission; (f) Lowess calibration curve, CMS Tiers, 30-day readmission.
Discussion
This study has clinical and policy implications. Clinically, unnecessary hospital readmissions are psychologically and physically detrimental to patients. Thus, preventing avoidable hospital readmissions is of critical importance in improving both the patient experience and clinical outcomes. Regarding policy, with the current regulatory environment of penalties for readmissions, development of accurate risk prediction models becomes increasingly relevant. There is an increasing emphasis on improving prediction of rehospitalization to better allocate limited resources. Our results support a growing body of evidence that functional status outperforms demographics and medical comorbidities in predicting readmissions while negligible improvements in model discrimination are realized with the addition of age and comorbidities to functional status-based models. As functional status is routinely documented in the IRF setting and is predictive of readmission risk, its inclusion in future readmission penalty frameworks targeted towards IRFs would be a feasible and important consideration.
Our study is one of the first to examine functional status as a predictor of acute care readmission in traumatic SCI patients undergoing inpatient rehabilitation in a national array of centers and to directly compare the predictive ability of functional status to that of demographics and medical comorbidities. The results showed that demographic factors and functional status on admission to inpatient rehabilitation predict the risk of acute care readmission, with good model calibration, at 3 and 30 days from IRF admission. Models incorporating demographics and functional status alone (Functional model) consistently demonstrated better discriminative ability than models based on demographics and comorbidities (Demographic-comorbidity models). The addition of comorbidities to the Functional model (Functional-Plus models) did not meaningfully enhance predictive ability at 3 and 30 days, while leading to increased model complexity. At three days, the Functional model performed comparably to other rehabilitation populations28–31 and demonstrated better predictive ability than large, non-SCI-population-based models incorporating medical comorbidities.20 The predictive ability of the Functional model was slightly less at 30 days, though still superior to corresponding Demographic-Comorbidity models.
There are several potential reasons for the enhanced predictive ability of models incorporating functional status compared to models focusing on medical comorbidities for readmission in the inpatient rehabilitation traumatic SCI population. While ICD-9-CM codes are readily available in acute care administrative data sets and indicate the presence of medical comorbidities, there is less information on disease severity and clinical instability, which have been proposed as important to improving risk prediction model performance.47 Furthermore, the Deyo-Charlson, Elixhauser, and CMS Tiers may be limited methods of capturing prevalent comorbidities in inpatient rehabilitation populations, including the SCI population. Despite existing administrative data and a growing interest in functional outcomes, no comorbidity indices have been developed specifically to examine outcomes in inpatient rehabilitation populations, and most comorbidity indices have been validated in acute care hospital populations.48 In a study of burn patients in inpatient rehabilitation, the Deyo-Charlson Comorbidity Index, Elixhauser Comorbidity Index, and the CMS Comorbidity tiers did not capture 67%, 27%, and 58% of the subjects’ reported comorbidities.49 Moreover, there were 107 unique comorbidities that occurred with frequency of greater than one percent, of which 67% were not captured in all three comorbidity indices.49 Several of the most frequently observed comorbidities not captured by the comorbidity indices denoted comorbid factors that influence rehabilitation and impact functional outcomes, including dysphagia, joint contractures, and gait impairment,49 comorbidities that are also prevalent in the SCI population. The observation that commonly used comorbidity indices did not accurately capture the burden of comorbid illness in the burn population in inpatient rehabilitation suggests that the development of rehabilitation-specific comorbidity indices for this and other rehabilitation populations may be valuable.
In contrast to medical comorbidities, functional status is a dynamic indicator of disease burden and is tied to patient outcomes, as evidenced by higher functional status being associated with transition to home and community reintegration as well as with lower likelihood of medical complications and readmission among SCI patients.32 Furthermore, functional status likely represents a surrogate measure of increased risk of immobility-related complications, such as UTI, pneumonia, and pressure ulcers, which are common reasons for readmissions among SCI patients.1,3,5,6
Though FIMTM score was robustly predictive of readmission in our population at 3 days, it was less so at 30 days. Despite an overall trend of evidence in previous studies to support functional status as an important factor in readmission risk, the predictive ability of the FIMTM score for readmission in SCI has not been consistently established. Cardenas et al. found that lower discharge motor FIMTM was a significant predictor of readmission at one and five years post-injury.3 Dejong et al. found an association between increased odds of rehospitalization at one year and admission cognitive FIMTM, hours per day of physical therapy, and the Comprehensive Severity Index, which combines physiological, functional, and psychosocial complexity into a single continuous score.3 Alternately, Ivie et al. found that while discharge FIMTM was a significant predictor of readmission at one year on univariate analysis, it did not improve model accuracy on multivariate analysis. Notably, their multivariate analysis included functional independence measured by self-reported ability to perform self-care ADLs unassisted and ambulatory status as significant predictors of readmission.7 This finding suggests that functional status is indeed an important marker of readmission risk. However, it stands to reason that measures of function taken closer to the time of readmission more accurately represent the patient’s functional status at that time point and would more strongly predict readmission.
These contrasting findings may reflect the limitations of the FIMTM as a measurement of disability in the SCI population. The reliability, internal consistency, and construct validity of FIMTM have been found to be variably adequate in the SCI population; moreover, a negative ceiling effect, whereby the instrument only detects changes up to a certain threshold, has been consistently documented.50 These effects could explain the decreased discriminative ability of admission FIMTM we observed at 30 days compared to 3 days. The optimal method of measuring disability in SCI patients is an area of active investigation and debate. Alternative proposed measures include the Spinal Cord Injury Measure (SCIM), which seeks to ameliorate the shortcomings of the FIMTM, such as the negative ceiling effect,50,51 and the Spinal Cord Injury-Functional Index (SCI-FI), which seeks to mitigate the limitations of previous measures of function in SCI by measuring a broadened range of functional domains and increasing generalizability.52
The results of this study must be interpreted within the context of their limitations. Due to the retrospective, observational method, we are unable to draw a causal relationship between functional status and readmission, and our findings require prospective validation. Comorbidity data was limited to a maximum of ten ICD-9-CM codes per patient rather than all potential comorbidities. Moreover, the documented presence of medical comorbidities is not a reliable indicator of illness severity or clinical stability. We addressed this by using three validated comorbidity indices in our risk prediction models. We were unable to distinguish between planned and unplanned readmissions using our database. However, we included 3-day readmissions, as these likely represent unplanned readmissions.53 Our hypotheses were supported at 3 days and demonstrated similar trends at 30 days. Socioeconomic data and broad categories of neurologic level of impairment are documented within the IRF-PAI but were not incorporated in our models. Previous studies have not consistently shown socioeconomic factors such as marital status, living environment, or payor source to significantly affect rates of rehospitalization.7,54,55 Furthermore, the goal of this study was not to create the most comprehensive readmission prediction model but to identify a parsimonious set of variables to perform a focused comparison of function-based and comorbidity-based readmission models. Evaluation of the effect of neurologic level of injury on readmission risk using our data set would be difficult to interpret given the broad categories of neurologic level in the IRF-PAI as well as the high percentage of patients with either no level of injury documented or categorized as traumatic SCI with concurrent brain injury or polytrauma, from which no level of injury could be obtained. Prior studies have not demonstrated a consistent association between neurologic level and readmission risk.4,7, 32,55 It remains unclear whether FIMTM is a proxy marker for measures of physiologic impairment such as the American Spinal Injury Association (ASIA) impairment scale (AIS), which has previously been shown to be a significant predictor of readmission in traumatic SCI.56
Future research is needed to determine whether measures of function tailored to the SCI population such as the SCIM and the SCI-FI might have better predictive power for readmission in SCI patients as well as to examine how these functional measures relate to measures of physiologic/neurologic dysfunction (e.g. ASIA classification). Our results support the hypothesis that functional status outperforms medical comorbidities as a predictor of readmission, and the FIMTM retains the strength of being a widely-used and standardized method of measuring functional status. Despite its limitations, our study has the advantages of a large, national sample, systematic documentation of functional status and readmission, and examines 30-day readmission, an outcome that has become increasingly important and scrutinized given current regulatory and fiscal trends.
Conclusions
Functional status is an effective predictor of readmission after traumatic SCI in the inpatient rehabilitation population. Models using admission FIMTM, despite its potential limitations, and demographics showed better predictive ability compared to models using medical comorbidities and demographics alone when applied to a large, administrative data set. Furthermore, the addition of medical comorbidities to models with functional status did not enhance model performance. Our findings contribute to increasing evidence that functional status is an important and modifiable metric of health and a predictor of adverse outcomes after traumatic SCI.1,3,4,7,32 The identification of key determinants of readmission risk, as well as investigating optimal timing and methods of capturing disability specific to SCI, are areas of future inquiry that are critical to the creation of high-quality, cost-effective strategies to predict and prevent acute care readmissions.
Acknowledgements
FIMTM and UDSMR are trademarks of UDSMR, a division of UB Foundation Activities, Inc.
Disclaimer statements
Contributors None.
Funding The authors received no specific funding for this work.
Conflicts of interest None.
Ethics approval None.
ORCID
Julie K. Silver http://orcid.org/0000-0001-9711-0713
References
- 1.Cardenas DD, Hoffman JM, Kirshblum S, McKinley W.. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil 2004;85(11):1757-63. doi: 10.1016/j.apmr.2004.03.016 [DOI] [PubMed] [Google Scholar]
- 2.Jaglal SB, Munce SE, Guilcher SJ, Couris CM, Fung K, Craven BC, et al. Health system factors associated with rehospitalizations after traumatic spinal cord injury: a population-based study. Spinal Cord 2009;47(8):604-9. doi: 10.1038/sc.2009.9 [DOI] [PubMed] [Google Scholar]
- 3.DeJong G, Tian W, Hsieh CH, Junn C, Karam C, Ballard PH, et al. Rehospitalization in the first year of traumatic spinal cord injury after discharge from medical rehabilitation. Arch Phys Med Rehabil 2013;94(4 Suppl):S87-97. doi: 10.1016/j.apmr.2012.10.037 [DOI] [PubMed] [Google Scholar]
- 4.Hammond FM, Horn SD, Smout RJ, Chen D, DeJong G, Scelza W, et al. Acute rehospitalizations during inpatient rehabilitation for spinal cord injury. Arch Phys Med Rehabil 2013;94(4 Suppl):S98-105. doi: 10.1016/j.apmr.2012.11.051 [DOI] [PubMed] [Google Scholar]
- 5.Dryden DM, Saunders LD, Rowe BH, May LA, Yiannakoulias N, Svenson LW, et al. Utilization of health services following spinal cord injury: a 6-year follow-up study. Spinal Cord 2004;42(9):513-25. doi: 10.1038/sj.sc.3101629 [DOI] [PubMed] [Google Scholar]
- 6.Dorsett P, Geraghty T.. Health-related outcomes of people with spinal cord injury--a 10 year longitudinal study. Spinal Cord 2008;46(5):386-91. doi: 10.1038/sj.sc.3102159 [DOI] [PubMed] [Google Scholar]
- 7.Ivie CS, DeVivo MJ.. Predicting unplanned hospitalizations in persons with spinal cord injury. Arch Phys Med Rehabil 1994;75(11):1182-8. doi: 10.1016/0003-9993(94)90002-7 [DOI] [PubMed] [Google Scholar]
- 8.McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ.. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil 1999;80(11):1402-10. doi: 10.1016/S0003-9993(99)90251-4 [DOI] [PubMed] [Google Scholar]
- 9.Barclay-Goddard R, King J, Dubouloz CJ, Schwartz CE, Group RSTTW.. Building on transformative learning and response shift theory to investigate health-related quality of life changes over time in individuals with chronic health conditions and disability. Arch Phys Med Rehabil 2012;93(2):214-20. doi: 10.1016/j.apmr.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Apple DF, Hudson LM, Bode R.. Medical complications during acute rehabilitation following spinal cord injury--current experience of the Model Systems. Arch Phys Med Rehabil 1999;80(11):1397-401. doi: 10.1016/S0003-9993(99)90250-2 [DOI] [PubMed] [Google Scholar]
- 11.Harvey C, Wilson SE, Greene CG, Berkowitz M, Stripling TE.. New estimates of the direct costs of traumatic spinal cord injuries: results of a nationwide survey. Paraplegia 1992;30(12):834-50. [DOI] [PubMed] [Google Scholar]
- 12.Trenaman L, Miller WC, Querée M, Escorpizo R, Team SR.. Modifiable and non-modifiable factors associated with employment outcomes following spinal cord injury: A systematic review. J Spinal Cord Med 2015;38(4):422-31. doi: 10.1179/2045772315Y.0000000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dryden DM, Saunders LD, Jacobs P, Schopflocher DP, Rowe BH, May LA, et al. Direct health care costs after traumatic spinal cord injury. J Trauma 2005;59(2):464-7. doi: 10.1097/01.ta.0000174732.90517.df [DOI] [PubMed] [Google Scholar]
- 14.Krause JS, Carter RE, Pickelsimer EE, Wilson D.. A prospective study of health and risk of mortality after spinal cord injury. Arch Phys Med Rehabil 2008;89(8):1482-91. doi: 10.1016/j.apmr.2007.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hospital Readmission Reduction Program, Patient Protection and Affordable Care Act, S. 3025(2010).
- 16.Desai NR, Ross JS, Kwon JY, Herrin J, Dharmarajan K, Bernheim SM, et al. Association Between Hospital Penalty Status Under the Hospital Readmission Reduction Program and Readmission Rates for Target and Nontarget Conditions. JAMA 2016;316(24):2647-56. doi: 10.1001/jama.2016.18533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks E.Complexity science and the readmission dilemma. JAMA Intern Med 2013;173(8):629-31. doi: 10.1001/jamainternmed.2013.4065 [DOI] [PubMed] [Google Scholar]
- 18.Table: Medicare Spending by State and Category: Kaiser Health News; 2013 [Available from: http://khn.org/news/post-acute-care-table-by-state/.
- 19.CMS National Dry Run summary: all-cause unplanned readmission measure for 30 days post discharge from inpatient rehabilitation facilities. Special open door forum.: RTI International; 2015 [Available from: https://www.cms.gov/Available from: 12_10_15.pdf.
- 20.Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, et al. Risk prediction models for hospital readmission: a systematic review. JAMA 2011;306(15):1688-98. doi: 10.1001/jama.2011.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman EA, Min SJ, Chomiak A, Kramer AM.. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res 2004;39(5):1449-65. doi: 10.1111/j.1475-6773.2004.00298.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider JC, Gerrard P, Goldstein R, Divita MA, Niewczyk P, Ryan CM, et al. Predictors of transfer from rehabilitation to acute care in burn injuries. J Trauma Acute Care Surg 2012;73(6):1596-601. doi: 10.1097/TA.0b013e318270d73d [DOI] [PubMed] [Google Scholar]
- 23.Hoyer EH, Needham DM, Miller J, Deutschendorf A, Friedman M, Brotman DJ.. Functional status impairment is associated with unplanned readmissions. Arch Phys Med Rehabil 2013;94(10):1951-8. doi: 10.1016/j.apmr.2013.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Peng X, Zhu B, Zhang Y, Xi X.. Active mobilization for mechanically ventilated patients: a systematic review. Arch Phys Med Rehabil 2013;94(3):551-61. doi: 10.1016/j.apmr.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 25.Ntoumenopoulos G.Rehabilitation during mechanical ventilation: Review of the recent literature. Intensive Crit Care Nurs 2015;31(3):125-32. doi: 10.1016/j.iccn.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 26.Needham DM.Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA 2008;300(14):1685-90. doi: 10.1001/jama.300.14.1685 [DOI] [PubMed] [Google Scholar]
- 27.Serra-Prat M, Sist X, Domenich R, Jurado L, Saiz A, Roces A, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing 2017. [DOI] [PubMed] [Google Scholar]
- 28.Gerrard P, Goldstein R, DiVita MA, Slocum C, Ryan CM, Mix J, et al. Functional status and readmissions in unilateral hip fractures. Am J Manag Care 2015;21(4):e282-7. [PubMed] [Google Scholar]
- 29.Shih SL, Gerrard P, Goldstein R, Mix J, Ryan CM, Niewczyk P, et al. Functional Status Outperforms Comorbidities in Predicting Acute Care Readmissions in Medically Complex Patients. J Gen Intern Med 2015;30(11):1688-95. doi: 10.1007/s11606-015-3350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih SL, Zafonte R, Bates DW, Gerrard P, Goldstein R, Mix J, et al. Functional Status Outperforms Comorbidities as a Predictor of 30-Day Acute Care Readmissions in the Inpatient Rehabilitation Population. J Am Med Dir Assoc 2016;17(10):921-6. doi: 10.1016/j.jamda.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 31.Slocum C, Gerrard P, Black-Schaffer R, Goldstein R, Singhal A, DiVita MA, et al. Functional Status Predicts Acute Care Readmissions from Inpatient Rehabilitation in the Stroke Population. PLoS One 2015;10(11):e0142180. doi: 10.1371/journal.pone.0142180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eastwood EA, Hagglund KJ, Ragnarsson KT, Gordon WA, Marino RJ.. Medical rehabilitation length of stay and outcomes for persons with traumatic spinal cord injury--1990-1997. Arch Phys Med Rehabil 1999;80(11):1457-63. doi: 10.1016/S0003-9993(99)90258-7 [DOI] [PubMed] [Google Scholar]
- 33.Yarbrough CK, Gamble PG, Janjua MB, Tang M, Ghenbot R, Zhang AJ, et al. Readmission after spinal cord injury: analysis of an institutional cohort of 795 patients. J Neurosurg Sci 2016. [DOI] [PMC free article] [PubMed]
- 34.Yarbrough CK, Bommarito KM, Gamble PG, Hawasli AH, Dorward IG, Olsen MA, et al. Population-based approaches to treatment and readmission after spinal cord injury. J Neurosurg Sci 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The inpatient rehabilitation facility-patient assessment instrument (IRF-PAI) training manual.: UB Foundation Activities; 2004 [Available from: http://www.cms.gov/Availablefrom:irfpaimanual040104.pdf.
- 36.Kirshblum S, Millis S, McKinley W, Tulsky D.. Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil 2004;85(11):1811-7. doi: 10.1016/j.apmr.2004.03.015 [DOI] [PubMed] [Google Scholar]
- 37.Charlson M, Szatrowski TP, Peterson J, Gold J.. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47(11):1245-51. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 38.Deyo RA, Cherkin DC, Ciol MA.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45(6):613-9. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 39.Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373-83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 40.Elixhauser A, Steiner C, Harris DR, Coffey RM.. Comorbidity measures for use with administrative data. Med Care 1998;36(1):8-27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 41.Centers for Medicare & Medicaid Services (CMS) HHS Medicare Program; Inpatient Rehabilitation Facility Prospective Payment System for Federal Fiscal Year 2017. Final rule. Fed Regist 2016;81(151):52055-141. [PubMed] [Google Scholar]
- 42.Romano PS, Roos LL, Jollis JG.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46(10):1075-9; discussion 81-90. doi: 10.1016/0895-4356(93)90103-8 [DOI] [PubMed] [Google Scholar]
- 43.Romano PS, Roos LL, Jollis JG.. Further evidence concerning the use of a clinical comorbidity index with ICD-9-CM administrative data. Journal of Clinical Epidemiology 1993;46(10):1085-90. doi: 10.1016/0895-4356(93)90106-B [DOI] [PubMed] [Google Scholar]
- 44.Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ.. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001;154(9):854-64. doi: 10.1093/aje/154.9.854 [DOI] [PubMed] [Google Scholar]
- 45.Ohman EM, Granger CB, Harrington RA, Lee KL.. Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA 2000;284(7):876-8. doi: 10.1001/jama.284.7.876 [DOI] [PubMed] [Google Scholar]
- 46.Lin M, Lucas H, Shmueli G.. Too big to fail: large samples and the p-value problem. Information Systems Research 2013;24:906-17. doi: 10.1287/isre.2013.0480 [DOI] [Google Scholar]
- 47.Donzé J, Aujesky D, Williams D, Schnipper JL.. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med 2013;173(8):632-8. doi: 10.1001/jamainternmed.2013.3023 [DOI] [PubMed] [Google Scholar]
- 48.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM.. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol 2003;56(3):221-9. [DOI] [PubMed] [Google Scholar]
- 49.Slocum CS, Goldstein R, DiVita MA, Mix J, Niewczyk P, Gerrard P, et al. Assessing the ability of comorbidity indexes to capture comorbid disease in the inpatient rehabilitation burn injury population. Am J Phys Med Rehabil 2015;94(5):373-84. doi: 10.1097/PHM.0000000000000180 [DOI] [PubMed] [Google Scholar]
- 50.Furlan JC, Noonan V, Singh A, Fehlings MG.. Assessment of disability in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma 2011;28(8):1413-30. doi: 10.1089/neu.2009.1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catz A, Itzkovich M, Agranov E, Ring H, Tamir A.. SCIM--spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord 1997;35(12):850-6. doi: 10.1038/sj.sc.3100504 [DOI] [PubMed] [Google Scholar]
- 52.Jette AM, Tulsky DS, Ni P, Kisala PA, Slavin MD, Dijkers MP, et al. Development and initial evaluation of the spinal cord injury-functional index. Arch Phys Med Rehabil 2012;93(10):1733-50. doi: 10.1016/j.apmr.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 53.Carney ML, Ullrich P, Esselman P.. Early unplanned transfers from inpatient rehabilitation. Am J Phys Med Rehabil 2006;85(5):453-60; quiz 61-3. doi: 10.1097/01.phm.0000214279.04759.45 [DOI] [PubMed] [Google Scholar]
- 54.Davidoff G, Schultz JS, Lieb T, Andrews K, Wardner J, Hayes C, et al. Rehospitalization after initial rehabilitation for acute spinal cord injury: incidence and risk factors. Arch Phys Med Rehabil 1990;71(2):121-4. [PubMed] [Google Scholar]
- 55.Gabbe BJ, Nunn A.. Profile and costs of secondary conditions resulting in emergency department presentations and readmission to hospital following traumatic spinal cord injury. Injury 2016;47(8):1847-55. doi: 10.1016/j.injury.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 56.Middleton JW, Lim K, Taylor L, Soden R, Rutkowski S.. Patterns of morbidity and rehospitalisation following spinal cord injury. Spinal Cord 2004;42(6):359-67. doi: 10.1038/sj.sc.3101601 [DOI] [PubMed] [Google Scholar]