Abstract
Context: Ventral longitudinal intraspinal fluid collection (VLISFC) presenting as hand amyotrophy has been described only in a few cases and there are no reports on associated intracranial CSF hypovolemia (ICH). We describe the clinical and imaging findings of a case with combined brachial amyotrophy and ICH secondary to VLISFC.
Findings: A 31 year old man presented with severe positional neck discomfort, radiating pain, progressive asymmetrical wasting and weakness of distal upper limbs. Contrast Magnetic Resonance Imaging (MRI) of the spine demonstrated a ventral extradural intraspinal fluid collection extending from upper border of C6 to lower border of T3 vertebra with pockets of dorsal collection. Three-dimensional constructive interference in steady state (CISS 3D) used in spinal imaging for identification of CSF leak corroborated with the extent seen on T2 sagittal sections; however, the site of the leak was not identified. After a year he developed disturbing postural headache which was relieved in recumbent position. Follow up MRI of brain was normal while spine demonstrated significant cervical cord atrophy and bilateral cord white matter hyperintensities.
Conclusion / Clinical Relevance: We report this unusual case where local compression by VLISFC located at the cervical and upper thoracic level not only caused distal bi-brachial amyotrophy mimicking Hirayama disease but also led to secondary intracranial hypotension. An early identification and intervention could possibly have prevented the onset of ICH.
Keywords: Ventral longitudinal intraspinal fluid collection, VLISFC, Brachial amyotrophy, Cerebrospinal fluid hypovolemia, Spontaneous intracranial hypotension, MRI
Introduction
Cerebrospinal fluid (CSF) hypovolemia due to CSF leak at different levels has several modes of presentation. Intracranial hypovolemia most commonly presents with orthostatic headaches. Other manifestations include neck pain, ocular and vestibulo-cochlear disturbances, upper limb pains or paraesthesias and dysgeusia.1,2 CSF lumbar puncture showing low opening pressures and diffuse pachymeningeal enhancement on contrast magnetic resonance imaging (MRI) with or without subdural fluid / descent of the brain are additional clues towards the diagnosis.1 Extracranial CSF leak leading to intra spinal fluid collection causing hand amyotrophy has been rarely reported. The precise pathophysiology of upper limb amyotrophy associated with ventral longitudinal intraspinal fluid collection (VLISFC) is yet to be elucidated. However, there are a few studies in the English literature describing the clinical pattern and possible pathomechanism of brachial amyotrophy associated with VLISFC.3–8 De Luca et al. coined the term VLISFC and enumerated the typical MRI findings of an elevated dura and extended extradural fluid collection and attributed it to CSF leak.5 In the present report, we describe the rare clinical evolution and MR imaging characteristics of a man presenting initially as progressive bilateral distal amyotrophy of upper limbs who later developed classical features of spontaneous intracranial hypotension.
Case report
A 31-year-old man, software professional presented during July 2013 with one year history of severe neck discomfort and radiating pain along the medial aspect of arms and forearms on flexing the neck or on attempting to raise objects high above the shoulder level. He reported inability to extend the left forearm due to weakness of triceps muscle. Interestingly this weakness appeared particularly on flexing the neck. Simultaneously he developed progressive distal wasting and weakness of the left upper limb (UL) followed soon by right UL distal muscles. He experienced infrequent and brief lasting cramps of the small muscles of hands at rest. There was no history of injury to the neck or UL's. There were no other neurological symptoms.
Patient had also been diagnosed as having non-insulin dependent diabetes mellitus since age 26 and hypertension since age 30. Examination revealed mild to moderate asymmetrical wasting (left > right) of hypothenar, medial forearms, triceps and shoulder girdle muscles. Prominent minipolymyoclonus was observed (left > right). Tone was reduced in the ULs. Power in the shoulder girdle muscles was normal although there was very minimal wasting. According to Medical Research Council (MRC) grading, power of biceps was grade 4+, left triceps was 1 and right triceps was grade 4. The flexors of the wrists and fingers were grade 4 and the extensors were grade 3. The intrinsic muscles of the left hand was grade 3 and on the right side, the power was 4+ (Fig. 1). Tone and power in lower limbs were normal. The biceps, supinator and triceps jerks were absent in the upper limbs whereas the knee and ankle jerks were normal. There was no Babinski's sign.
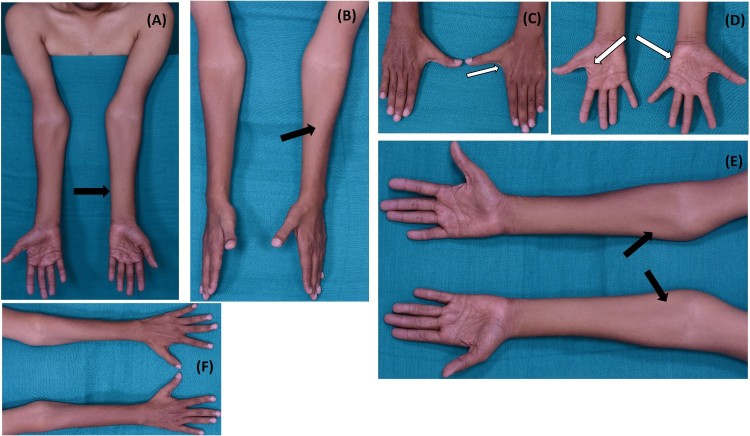
Figure 1.
(A-F). Wasting of bilateral medial forearm (left > right) with relative sparing of brachioradialis (oblique atrophy) (thick black arrows). Severe wasting of small muscles of hands (thick white arrows). Note: First dorsal interossei and hypothenar muscles are more wasted than thenar muscles.
Patient was initially evaluated by the neurosurgeons for cervical compressive myelopathy and he underwent X-rays and contrast MRI of the spine. Subsequently he was evaluated by the neurologist for anterior horn cell disease. Routine biochemical tests were normal. Nerve conduction studies included motor and sensory conductions of median, ulnar, common peroneal and sural nerves. A comprehensive concentric needle electromyography was performed from bilateral Abductor Digiti Minimi (ADM), biceps, triceps, right vastus lateralis and right tibialis anterior. The electrophysiological findings are summarized in Table 1.
Table 1. Electrophysiological findings of the patient with ventral longitudinal intraspinal fluid collection (VLISFC).
| Nerve conduction study | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nerve | Distal / proximal latency (ms) | Distal Amplitude (mV) | Distance (mm) | Velocity (m/s) | F-wave latency (ms) | ||||
| MOTOR | Rt | Lt | Rt | Lt | Rt | Lt | Rt | Lt | |
| Median | 3.4 / 8.5 | 3.2 / 8.6 | 7.3 | 6.9 | 75/230 | 45.1 | 42.5 | 33.3 | 34.0 |
| Ulnar | 3.0 / 8.8 | 2.8 / 8.9 | 7.8 | 6.0 | 70/260 | 44.8 | 42.6 | absent | absent |
| Common peroneal | 5.2 / 13.2 | 5.3 / 12.9 | 5.9 | 6.0 | 110/320 | 40.0 | 42.1 | 59.0 | 58.2 |
| SENSORY | Latency (ms) | Amplitude (µV) | Distance (mm) | Velocity (m/s) | - | ||||
| Median | 2.4 | 2.3 | 13.0 | 14.0 | 140 | 58.3 | 60.9 | - | |
| Ulnar | 2.5 | 2.6 | 10.0 | 10.5 | 130 | 52.0 | 50.0 | - | |
| Sural | 2.5 | 2.4 | 18.5 | 19.0 | 135 | 54.0 | 56.3 | - | |
| Electromyography | |||||||||
| Muscle | Innervation | Insertional activity | Spontaneous activity | MUPs | Recruitment | ||||
| Rt | Lt | Rt | Lt | Rt | Lt | Rt | Lt | ||
| Abductor digiti minimi | Ulnar C8,T1 | No | No | No | Fas | N | N | IC | IC |
| Biceps | Musculocutaneous C5,C6 | No | No | No | N | N | N | C | C |
| Triceps | Radial C6,C7 | No | No | No | Fas, Fib | N | N | C | IC |
| Vastus lateralis | Femoral L2-L4 | No | ND | No | ND | N | ND | C | ND |
| Tibialis anterior | Common peroneal L5,S1 | No | ND | No | ND | N | ND | C | ND |
Rt, right; Lt, left; MUPs, motor unit potentials; Fas, fasciculations; Fib, fibrillations; IC, incomplete; C, complete; N, normal; ND, not done.
Review of the MRI demonstrated a ventral extradural intraspinal fluid collection extending from upper border of C6 to lower border of T3 vertebra (Fig. 2). Constructive interference in steady state (CISS) 3D images of the whole spine corroborated with the extent seen on T2 sagittal sections, however, the exact site of leak was not detected. Axial sections did not reveal any signal changes / cord atrophy. Whole spine X-rays were normal (Fig. 2). CT myelography / intrathecal gadolinium scan MRI was advised to identify the site of leak but was declined by the patient. He was referred for physiotherapy without any specific intervention.
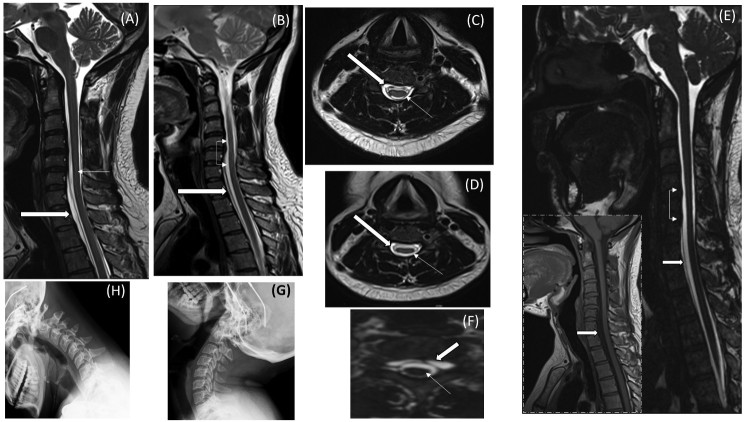
Figure 2.
(A-H). Clockwise from top. (A) Sagittal T2 image of the cervical and upper thoracic spine during first visit demonstrates a hyperintense extradural collection from upper border of C6 vertebra to lower border of T3 vertebra displacing the cord posteriorly. (B) Follow up sagittal T2 image demonstrates the stable collection extending from C6-T3 (block arrow) with cord atrophy (thin arrows). (C, D) Axial T2 images of spine at lower border of C5 vertebrae during initial visit and follow up respectively, shows pockets of CSF collection (block arrows) and cord atrophy appearing on follow up scan (thin arrows). (E, F) CISS 3D sagittal and axial images (inset with dash borders- T1W sagittal) reveals cord atrophy (thin arrows) and unidentifiable site of CSF leak with collection (block arrows). (G, H) Normal X-Rays of cervical spine in extension and flexion.
Patient reported again during September 2014 with progressive severe postural headache appearing after 2 to 3 hours of erect posture. Symptoms began as a pulling sensation in the lower back followed by stiffness of the neck and at the peak of headache he would experience tinnitus, nausea and vomiting which dramatically reduced by 10–15 minutes of recumbent posture. Notably patient reported rapid alleviation of headache and tinnitus on sneezing and this prompted him to induce a sneeze at the height of headache. He also reported worsening of upper limb tremulousness, weakness and wasting for 6 months. Repeat spine MRI had interesting new findings that included asymmetrical cord atrophy with central T2 hyperintensities in the white matter of the cord extending from C6 to T3 vertebral levels (Fig. 2). Flexion studies did not show any evidence of posterior epidural detachment / enhancing soft tissue to suggest Hirayama disease. The extradural CSF collection was stable which was predominantly present on the ventral side with pockets of dorsal collection also. There was no evidence of siderosis, root avulsion or spine fractures. CISS 3D image revealed the largest fluid retention at C7 mid vertebral level with a maximum width of 5 mm. He was again advised to undergo intrathecal gadolinium enhanced MR myelography to identify the site of leak, simultaneous CSF fluid collection for analysis and pressure monitoring followed by epidural blood patch. However, the patient declined any form of invasive investigation or intervention.
At last follow-up in January 2016, he reported mild subjective improvement of the muscle bulk in the proximal medial forearms. The symptom of postural headache was less frequent as patient avoided prolonged upright position. A non-contrast MRI of brain was normal. The mammilo-pontine distance was 5.7 mm (normal >5.5 mm). There was no evidence of tonsillar herniation. A repeat spine MRI showed no new findings.
Discussion
In the present report we describe the clinical and serial radiological findings of VLISFC in an adult man presenting initially as distal bi-brachial amyotrophy9 and later with symptoms of cerebrospinal fluid hypovolemia. This sequence of disease evolution with two pathologies is being reported for the first time. Presently there are 6 reports with 12 cases associating brachial amyotrophy with VLISFC.3–8 In 2000, Hader and Fairholm described three cases of progressive upper limb weakness due to pseudo-meningocoeles. The collections extended from cervical to lumbar level. The theory of venous congestive myelopathy and/or direct compression of cord leading to anterior horn cell loss was proposed.3 Schmalbach et al. reported three patients suspected to have possible amyotrophic lateral sclerosis who several years later developed nuchal pain and paraesthesias of upper limbs and they had anterior spinal cysts on MR Imaging.4 De Luca et al. reported on three patients with symptoms of segmental atrophy of upper limbs, related it to intraspinal ventral extra-arachnoid fluid collection and termed this condition as VLISFC.5 Authors hypothesized that similar to Hirayama disease, the amyotrophy could be a consequence of dynamic pressure by the anterior cysts on the ventral spinal cord / conduction block over a tethered motor root by dorsal cord displacement.5 Our patient initially presented with progressive asymmetric amyotrophy mimicking distal bimelic amyotrophy (DBMA).9 However, presence of nuchal and radicular pains were unusual features. Mihaylova et al. reported a case of proximal amyotrophy preceded many years earlier by symptoms of CSF leak. Brain MRI had revealed Chiari malformation and the CSF collection extended from C1 to L2. Authors postulated that cervical anterior cord involvement was secondary to the mechanical compression by VLISFC.6 In contrast to this pattern; our patient had distal brachial amyotrophy and subsequently developed intracranial hypovolemia. In a report in 2014, one among the three patients with upper limb amyotrophy had VLISFC, while another report with a case having asymmetric bilateral intrinsic hand and finger extensor muscle weakness had VLISFC secondary to spinal trauma. Both authors propose the pathophysiology to be venous congestive myelopathy.7,8 In these cases MR imaging performed after several years demonstrated ventral cord signal changes which was again attributed to congestive myelopathy.7 Our patient had focal cord atrophy with signal changes in the second MRI similar to that described by Foster et al.7 and Löscher et al.8 Thus, the possibility of dynamic anterior horn cell loss is most likely. All the studies have reported CSF collection extending variably over a long stretch of the ventral extra-arachnoid space.3–8 In comparison, our patient had relatively shorter collection extending from C6 to T3 level.
Further, in all the reports the illness remained restricted to the spinal cord and none developed intracranial symptoms.7,8 Interestingly, in our case, the onset and evolution of illness was unique. The rarer manifestations of CSF hypovolemia includes chorea, radicular pains in upper limbs, encephalopathy, bulbar palsy, facial numbness with weakness and gait abnormalities.1,10,11 Our patient had none of these unusual features reported to occur in intracranial hypotension.
The loss of anterior horn cells in VLISFC is attributed to chronic compression.5–7 Taylor and Byrnes showed in monkeys that anterior compression of upper cervical segments causes ischemic damage to the lower cervical anterior horns.12,13 This pathophysiology could explain the cord signal changes in long term follow-up of VLISFC.4,7,8 In our patient the site of fluid collection corresponded with the affected segments and also showed changes secondary to ischemia.
In patients with minimal symptoms of VLISFC, the clinical course could be closely monitored for any progression, while in individuals with disturbing symptoms and identified site of leak a targeted epidural blood patch is recommended and if unsuccessful surgical correction of the dural defect / excision of cysts is advisable.15,16 Our patient preferred to have medical management despite having disabling symptoms. The outcome following surgical intervention is generally satisfactory but depends on factors like age, duration of deficits and the degree of existing medullary damage.16 Schmalbach et al.4 and De Luca et al.5 have reported significant improvement following surgical therapy. In conclusion, hitherto unreported our patient with VLISFC presented with brachial amyotrophy which unusually evolved to manifest also as intracranial hypovolemia. It is important to identify these cases at the earliest and appropriate intervention might prevent the cranio-caudal / caudo-cranial progression.
Disclaimer statement
Conflicts of interest No potential conflict of interest.
ORCID
Veeramani Preethish-Kumarhttp://orcid.org/0000-0003-1158-0971
References
- 1.Mokri B, Piepgras DG, Miller GM.. Syndrome of orthostatic headaches and diffuse pachymeningeal gadolinium enhancement. Mayo Clin Proc 1997;72(5):400–13. doi: 10.4065/72.5.400 [DOI] [PubMed] [Google Scholar]
- 2.Mokri B.Spontaneous cerebrospinal fluid leaks: from intracranial hypotension to cerebrospinal fluid hypovolemia—evolution of a concept. Mayo Clin Proc 1999;74(11):1113–23. doi: 10.4065/74.11.1113 [DOI] [PubMed] [Google Scholar]
- 3.Hader WJ, Fairholm D.. Giant intraspinal pseudomeningoceles cause delayed neurological dysfunction after brachial plexus injury: report of three cases. Neurosurgery 2000;46(5):1245–9. doi: 10.1097/00006123-200005000-00044 [DOI] [PubMed] [Google Scholar]
- 4.Schmalbach S, Petri S, Götz F, Dengler R, Krampfl K.. Anterior cysts of the spine: a difficult differential diagnosis to amyotrophic lateral sclerosis. J Neurol 2008;255(11):1662–9. doi: 10.1007/s00415-008-0951-2 [DOI] [PubMed] [Google Scholar]
- 5.Deluca GC, Boes CJ, Krueger BR, Mokri B, Kumar N.. Ventral intraspinal fluid-filled collection secondary to CSF leak presenting as bibrachial amyotrophy. Neurology 2011;76(16):1439–40. doi: 10.1212/WNL.0b013e3182166e6f [DOI] [PubMed] [Google Scholar]
- 6.Mihaylova T, Biondo A, Zak I, Lewis RA.. Anterior horn cell loss from subdural hygroma: a consequence of spontaneous spinal fluid leak. J Neurol Sci 2011;305(1–2):156–9. doi: 10.1016/j.jns.2011.02.029 [DOI] [PubMed] [Google Scholar]
- 7.Foster E, Tsang BK, Kam A, Stark RJ.. Mechanisms of upper limb amyotrophy in spinal disorders. J Clin Neurosci 2014;21(7):1209–14. doi: 10.1016/j.jocn.2013.10.035 [DOI] [PubMed] [Google Scholar]
- 8.Löscher WN, Tschugg A, Wanschitz JV, Stark RJ, Grams AE.. Hand amyotrophy and ventral intraspinal fluid collection. Amyotroph Lateral Scler Frontotemporal Degener 2015;16(5–6):412–3. doi: 10.3109/21678421.2015.1025795 [DOI] [PubMed] [Google Scholar]
- 9.Preethish-Kumar V, Nalini A, Singh R-JJ, Saini J, Prasad C, Polavarapu K et al. Distal bimelic amyotrophy (DBMA): Phenotypically distinct but identical on cervical spine MR imaging with brachial monomelic amyotrophy/Hirayama disease. Amyotroph Lateral Scler Frontotemporal Degener 2015:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Chung SJ, Kim JS, Lee MC.. Syndrome of cerebral spinal fluid hypovolemia: clinical and imaging features and outcome. Neurology 2000;55(9):1321–7. doi: 10.1212/WNL.55.9.1321 [DOI] [PubMed] [Google Scholar]
- 11.Balgera R, Rigamonti A, Sozzi G, Agostoni E.. An atypical case of spontaneous intracranial hypotension. Neurol Sci 2009;30(1):71–3. doi: 10.1007/s10072-009-0011-4 [DOI] [PubMed] [Google Scholar]
- 12.Taylor AR, Byrnes DP.. Foramen magnum and high cervical cord compression. Brain 1974;97(3):473–80. doi: 10.1093/brain/97.1.473 [DOI] [PubMed] [Google Scholar]
- 13.Becske T, Nelson PK.. The vascular anatomy of the vertebro-spinal axis. Neurosurg Clin N Am 2009;20(3):259–64. doi: 10.1016/j.nec.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 14.Singh RJ, Preethish-Kumar V, Polavarapu K, Vengalil S, Prasad C, Nalini A.. Reverse split hand syndrome: Dissociated intrinsic hand muscle atrophy pattern in Hirayama disease/brachial monomelic amyotrophy. Amyotroph Lateral Scler Frontotemporal Degener 2017;18(1–2):10–16. doi: 10.1080/21678421.2016.1223140 [DOI] [PubMed] [Google Scholar]
- 15.Takagaki T, Nomura T, Toh E, Watanabe M, Mochida J.. Multiple extradural arachnoid cysts at the spinal cord and cauda equina levels in the young. Spinal Cord 2006;44(1):59–62. doi: 10.1038/sj.sc.3101799 [DOI] [PubMed] [Google Scholar]
- 16.Choi JY, Kim SH, Lee WS, Sung KH.. Spinal extradural arachnoidal cyst. Acta Neurochir (Wien) 2006;148(5):579–85. doi: 10.1007/s00701-006-0744-2 [DOI] [PubMed] [Google Scholar]