Abstract
Primary liver carcinomas with both hepatocytic and cholangiocytic differentiation have been referred to as “combined (or mixed) hepatocellular-cholangiocarcinoma.” These tumors, although described over 100 years ago, have attracted greater attention recently because of interest in possible stem cell origin and perhaps because of greater frequency and clinical recognition. Currently, because of a lack of common terminology in the literature, effective treatment and predictable outcome data have been challenging to accrue. This article represents a consensus document from an international community of pathologists, radiologists, and clinicians who have studied and reported on these tumors and recommends a working terminology for diagnostic and research approaches for further study and evaluation.
Conclusion:
It is recommended that diagnosis is based on routine histopathology with hematoxylin and eosin (H&E); immunostains are supportive, but not essential for diagnosis. (HEPATOLOGY 2018; 00:000-000).
A dichotomous classification of primary liver carcinoma (PLC) into either hepatocellular carcinoma (HCC) or peripheral, intrahepatic cholangiocarcinoma CCA (iCCA) is challenged by increasing recognition of malignant epithelial hepatic tumors that share features of both cell types.
Classical HCC and iCCA demonstrate hepatocytic or cholangiocytic differentiation, respectively, and can be considered to represent two ends in a spectrum of PLCs. The distinction is important clinically because treatment considerations differ for classic HCC and iCCA, particularly with regard to transplant options, localized ablative treatments and systemic chemotherapy, and for prognostication.
It is currently recognized that PLCs exist that do not neatly fit into either category of HCC or iCCA cytologically or architecturally. These carcinomas are, in fact, not a homogenous tumor type, but have been broadly categorized as “mixed” or “combined” HCC-cholangiocarcinoma (CCA) (cHCC-CCA), or “biphenotypic” PLC. PLCs with mixed phenotypes have been reported for more than a century,(1) albeit with inconsistent nomenclature (Supporting Table S1). This lack of uniform terminology is likely attributed to the complex morphological and immunohistological diversity of these tumors, rather than their rarity, given that several large series have reported that mixed tumors represent 2%-5% of PLC.(2-6) Lack of uniform terminology has impeded systematic study of this important category of PLC. The most recent edition of the World Health Organization’s (WHO) Classification of Tumors of the Digestive System(7) specified a classic form of cHCC-CCA (i.e., a single tumor with both differentiations, not a collision between separate HCC and iCCA) and three variants with stem cell features: “typical,” “intermediate cell,” and “cholangiolocellular” subtypes. Significant recent work has shown, however, that “stem cell” phenotypes can be demonstrated in many forms of PLC, and the WHO categories are not as clearly separable as once thought.(8,9) Furthermore, improvements in laboratory techniques to demonstrate hepatocytic and cholangiocytic differentiation have resulted in increasingly common recognition of a spectrum of PLCs with mixed differentiation, the biological significance—and impact on tumor classification, staging, prognosis, and treatment—of which remains to be determined.
For progress to be made in unraveling the biological behavior and natural history of these tumors, we must necessarily begin by agreeing on terminology to classify them. With the promises of advances in the molecular biology of PLCs, and the development of more specialized therapeutics on the horizon, standardized nomenclature is critical for appropriate clinical-radiological-molecular-pathological correlations. Thus, an international group of hepatic pathologists, radiologists, surgeons, and clinicians previously published in this area have worked to formulate proposed nomenclature for these heterogeneous carcinomas with the goals of (1) creating uniformity of histological approach for diagnostic and research purposes and (2) facilitating scientific studies.
Primary Liver Carcinoma, Not Classic HCC or iCCA: Diagnostic Categories
Included in this heterogeneous group of carcinomas are three types:
Those in which there are varying degrees of hepatocytic and cholangiocytic cytologies and architectures, either admixed or as separate areas within the same tumor, referred to as cHCC-CCA.
PLC purely comprised of “intermediate cells,” referred to as intermediate cell carcinoma. These rare tumors contain relatively monomorphic populations of malignant epithelial cells that are phenotypically neither classic HCC nor classic iCCA. Immunophenotypically, they display variably mixed hepatocytic and cholangiocytic markers on a cellular basis.
PLC comprised of cholangiolocarcinoma (CLC).
Examples of each are illustrated in Supporting Fig. S1. Whether intermediate cell carcinoma and CLC are best categorized within cHCC-CCA, or as unique and separate entities,(10-12) has yet to be fully determined. However, it is now recognized that stem/progenitor cell features and desmoplastic stromal alterations may be detected in many PLCs; thus, these features are no longer considered characteristic of unique, specific diagnostic subtypes of cHCC-CCA.
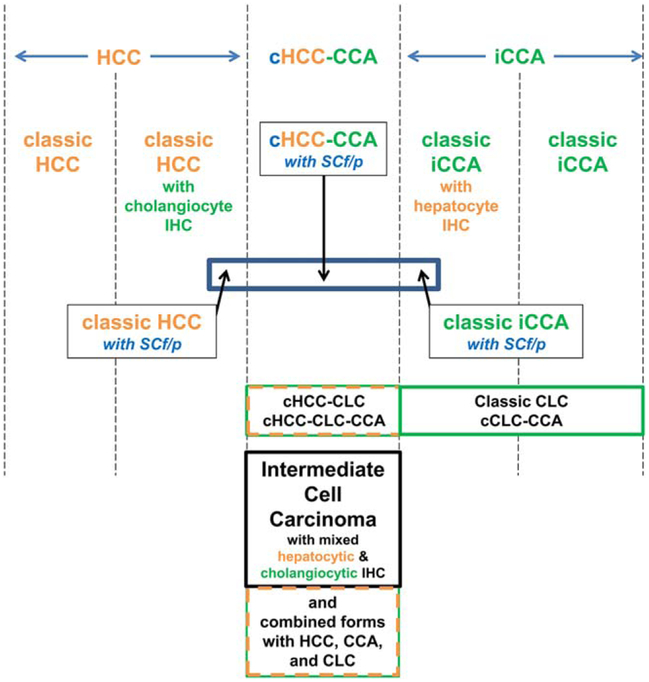
Finally, all PLCs, including classic HCC and iCCA, cHCC-CCA, intermediate cell carcinoma, and CLC, have been reported to occur alone or in combination with one another(8,9) (Fig. 1).
FIG. 1.
Differentiative and histological relationships between PLCs. PLCs show an array of differentiative states from hepatocytic (left) through combined types (middle) to cholangiocytic (right). Thus, there are classic HCCs that are morphologically only hepatocytic, some of which, however, display immunophenotypes, including cholangiocytic marker antigens (e.g., K19). There are also classic iCCAs that are morphologically pure adenocarcinomas, some of which may display immunophenotypes of hepatocytic marker antigens or mRNA. In the middle are the cHCC-CCAs—these are histologically partly morphological HCC and partly morphologically iCCA; immunophenotyping of such lesions can be helpful in confirming the histological impression, but morphology remains the primary criterion. CLC is a separate form of generally lower-grade biliary malignancy. CLC, as indicated, may be found in combination with any of the other forms of PLC in the diagram. Intermediate cell carcinoma is also distinctive: For this tumor, the morphology is neither that of HCC nor that of iCCA, but the mixture of cholangiocytic and hepatocytic features is observed on a cell-by-cell basis on the basis of immunophenotyping. Thus, for this tumor type alone, morphology requires confirmatory immunophenotyping to demonstrate the mixture of differentiation markers.
Fibrolamellar HCC is a distinct, unique PLC that has yet to be reported as a component in “combined” tumors; thus, it will not be further discussed in this consensus document. Further caveats for this nomenclature apply; cHCC-CCA is not considered appropriate terminology for:
distinct (multifocal) HCC and iCCA;
collision tumors of HCC and iCCA arising separately in the same liver;
any form of hepatoblastoma or variants, such as those with cholangiocytic or ductal plate components;
the pediatric “transitional liver cell tumor” or variants (13);
morphologically typical HCCs with only immunohistochemical expression of keratin (K)19 or other cholangiocytic or stem/progenitor cell markers;*
morphologically typical iCCAs with only immunohistochemical expression of hepatocytic or stem/progenitor cell markers, or iCCA with in situ hybridization markers for hepatocytic differentiation (i.e., albumin)†; and
sclerosing/scirrhous HCC, a rare variant of HCC with some areas that may be suggestive of iCCA (adenocarcinoma in sclerotic stroma).
Diagnostic Terminology of cHCC-CCA
Important considerations for diagnostic categorization of mass lesions in the liver include knowledge of sex and background liver disease, as well as adequacy of tissue sample. cHCC-CCA have been reported in both cirrhotic and noncirrhotic livers,(6,16,17) in contrast to HCC which is significantly more common in cirrhosis, and iCCA, which is more common in patients without cirrhosis. No sex distinction has been found in cHCC-CCA.
In an appropriately sectioned and sampled resection specimen, there is a high likelihood of visualization and correct characterization of all components. Biopsy-based tissue samples are common for diagnosis and tumor characterization, such as molecular profiling, but may not contain salient tumor components, and intratumoral heterogeneity of most cHCC-CCA can lead to incorrect diagnosis if a biopsy does not contain adequate tissue. Exactly what an “adequate” biopsy should be is an area that will require multi-institutional collaborative discussion. But the importance of recognizing the risk of “sampling error” cannot be overstated particularly when radiographical features are not uniformly typical for classic HCC.
It remains the consensus of this international group that histopathological diagnoses of PLCs are based primarily on routine stains (i.e., H&E ± histochemical stains for matrix proteins or mucins); immunohistochemical stains are secondary, providing supplemental evidence. It is also now recommended there should no longer be formal diagnostic subtypes based on the identification of stem/progenitor cells, but rather if stem/progenitor cell features are observed, they are noted in a comment as “stem/progenitor cell features present.” Furthermore, if combinations of PLC are present, it is recommended the diagnostic terminology include which forms of PLC are “combined” (e.g., cHCC-CCA; cHCC-CLC; ciCCA-CLC, cHCC-CCA-CLC, cHCC-CCA-intermediate cell ca, etc.). Some investigators recommend reporting percent of each component present,(18) even in biopsy samples, although the challenges of this are recognized. Supporting Table S2 compares descriptive, WHO 2010, and this proposed terminology.
Microscopic Pathology
As the nomenclature indicates, cHCC-CCA contains areas of both typical HCC and typical iCCA, the former having any/all of the possible cytological and architectural features of HCCs and the latter distinctly being an adenocarcinoma with malignant glands, usually lying within a dense stromal background (Fig. 2). The two components may be intermixed, or lie in separate regions of a tumor, though focal areas of merging can often be discerned. At the current time, there are no published consensus guidelines for minimum amounts of HCC or iCCA to qualify for the diagnosis, either in biopsy material or in resected/explant samples. Examples have been illustrated.(7-9,18-22) In regions in which the tumors seem to merge, the cellular morphology may be difficult to identify as either hepatocellular or cholangiocytic by pure H&E evaluation alone. Tumor cells of either hepatocytic or cholangiocytic morphologic type may contain intracytoplasmic inclusions common to hepatocytes such as Mallory-Denk bodies, steatosis, α1AT globules (even in an α1AT genotypically normal individual), fibrinogen, etc. Mucin stains, if utilized, are most often negative.
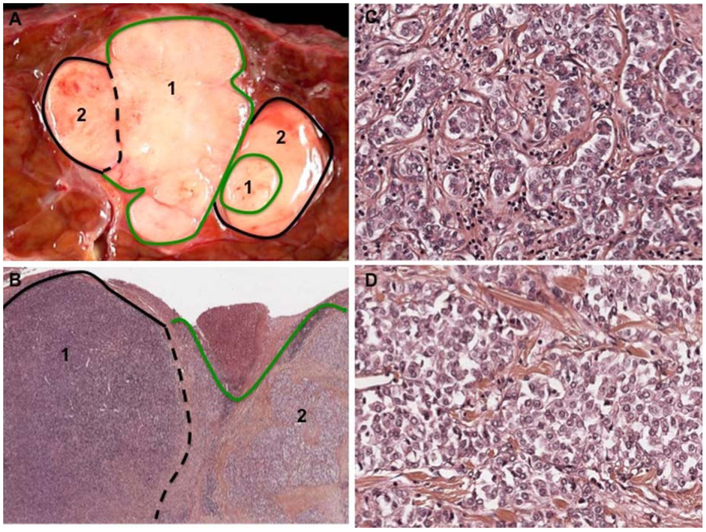
FIG. 2.
Combined HCC-CCA, defined by histochemical staining. A 67-year-old man with chronic hepatitis B and cirrhosis. Imaging diagnosis of a 3-cm hypervascular nodule in segment VII resulted in the segmentectomy specimen illustrated. (A) Gross evaluation shows a well-demarcated, lobulated tumor with two grossly distinct areas, labeled {1} and {2}. {1} is whiter and firmer while {2} is smoother and more yellow. (B) At low magnification, H&E evaluation shows that {1} is densely packed basophilic cells and {2} has more apparent nests of tumor cells within a fibrous stroma. (H&E, ×10). (C) Higher magnification of component {1} shows small tumoral cells arranged in glandular structures consistent with cholangiocytic differentiation. (D) is a higher magnification of component {2}, which is composed of nests of larger, eosinophilic cells with round nuclei and focally prominent nucleoli consistent with hepatocellular differentiation (C,D; H&E, ×20). IHC was positive for K7 and EpCAM in both tumor components. Positive K19, Glypican 3, and AFP staining were primarily in component {2}. Around 10% of cells were CD56 positive, preferentially in component{1}.
“STEM/PROGENITOR CELL” FEATURES/PHENOTYPES
Stem/progenitor cell features or phenotypes can be observed by both light microscopy and immunohistochemistry (IHC), however, in and of themselves, are not taken as proof of origin of carcinoma. These features/ phenotype consist of small cells with scant cytoplasm, a high nuclear/cytoplasmic ratio, and hyperchromatic nuclei (Fig. 3A). These cells are most often found at the interface between a nest of carcinoma and the adjoining tumoral fibrous or desmoplastic stroma.(23) Mitotic activity is uncommon. Lineage-like progressions may be noted by routine stains from the small cells at the periphery (those with stem/progenitor features) to more differentiated neoplastic hepatocytes or glands within the nests. Tumor cells may be variably highlighted by IHC for stem/progenitor markers, such as, but not limited to, keratin (K)19, cluster of differentiation (CD)56, epithelial cell adhesion molecule (EpCAM), ckit (aka, cluster of differentiation, CD117), and others.(22,24)
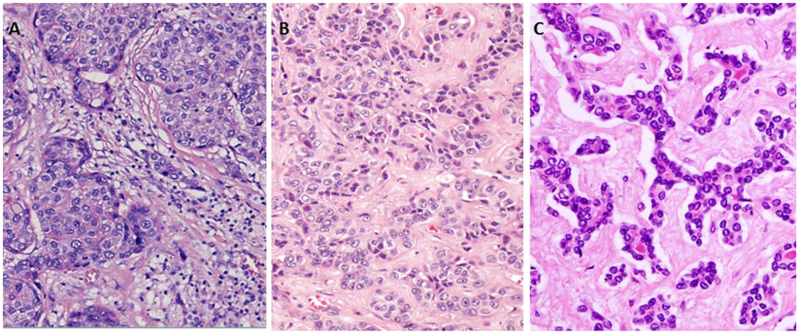
FIG. 3.
Morphological variants of stem/progenitor cell features. (A) HCC with stem cell/progenitor cell features. Nests of obvious hepatocytic tumor (often with little atypia) surrounded by stroma at the interface within which there are small cells with dark nuclei and high nuclear: cytoplasmic ratio (H&E, ×20). (B) Intermediate cell carcinoma with cords and trabeculae of cells with “intermediate” size between hepatocytes and cholangiocytes, without clear gland formation, often in a desmoplastic stroma. This tumor may be mistaken for iCCA. (H&E, ×20). (C) CLC in which slender, malignant ductules are present in a tubular, cord-like, anastomosing (“antler-like”) pattern within a dense stroma (H&E, ×20).
INTERMEDIATE CELL CARCINOMA
Intermediate cell carcinoma has been inconsistently described.(9,19,20) However, with growing evidence of the presence of such a lesion,(7-9) the term is applied to carcinomas in which a monomorphic tumor is comprised of tumor cells smaller than normal hepatocytes, but larger than the above-described stem/progenitor cell phenotype, and have features intermediate between hepatocytes and cholangiocytes (Fig. 3B). The tumor cells may be cuboidal to oval-shaped, with pale or pink cytoplasm. Tumor cells may be arranged in trabeculae, or cords, solid nests, or strands, and set within a background of marked desmoplastic or acellular hyalinized stroma. Elongated, ill-defined gland-like structures may be present, suggestive of tubules, but well-defined glands are not observed. Tumor cell atypia and mitoses are uncommon. Mucin production is absent. Supportive IHC displays some features of both hepatocytic and cholangiocytic lineage within individual cells.(22)
The entire tumor is commonly comprised of intermediate cells, and when present in a desmoplastic stroma, this tumor may be misdiagnosed as iCCA. Tumor nests, on the other hand, may appear as HCC. Intermediate cell carcinomas have been reported to have typical invasive patterns of spread of both HCC (intravascular and intrabiliary) and iCCA (lymphatic and perineural).
CLC
CLC (aka, cholangiolocellular carcinoma), first described in 11 African cases by Steiner and Higginson in 1959,(25) was conjectured to arise from cells within the canal of Hering. It remains as an incompletely understood form of PLC. Although both CLC and iCCA have bililary features, studies to date have shown clinicopathological, radiological, and molecular characteristics distinct from both iCCA and HCC(10,11,26-30) (Fig. 3C). CLC may comprise the entire PLC, or variably as a component of an HCC, an iCCA,(31) or as a component in a cHCC-CCA. The conventionally accepted criteria for CLC being the “sole component” of PLC is that >80% of tumor consists of CLC. This implies, however, that the entire lesion has been removed and histologically sampled. By microscopic locale and by histology, CLC closely resembles the epithelial components of a “ductular reaction,” because it appears to arise near and/or surround residual portal tracts. CLC consists of thin, malignant ductular-like structures that may appear to radiate from or surround a portal tract in a tubular, cord-like, anastomosing pattern (“antler-like” pattern) within a dense, hyalinized stroma. The tumor may show trabecular and replacing growth at its interface with the surrounding nontumorous liver. Although these latter architectural features may be suggestive of HCC, they are not by themselves sufficient evidence of a hepatocellular phenotype and may also occur in typical iCCA. CLC is mucin negative. Immunohistochemically, CLC demonstrates characteristic luminal reactivity with antibodies to epithelial membrane antigen(30) and polyclonal CEA (pCEA), which contrasts with the cytoplasmic positivity of iCCA with these antibodies.(27,29) There may be a range of differentiation patterns within CLC, and a rare variant with spindle cell pattern has been reported.(30)
Whether CLC is the sole diagnosis, or only a component of another PLC, its presence and percent amount may be useful information to document in the pathology report. The recent study of Rhee et al.(31) indicates that iCCA containing CLC in a portion of tumor with a CLC differentiation trait, confirmed by molecular and IHC investigations, carries a better prognosis than iCCA without it.
IHC of PLC
IHC is best considered a supplement for PLC, inclusive of cHCC-CCA, intermediate cell carcinoma, and CLC, but IHC should not define the diagnosis, in and of itself, and requires expertise in interpretation. The uses of IHC in PLC have been summarized.(18,22) The markers can be divided into those characteristic of hepatocytes (hepatocyte in paraffin 1), alpha fetoprotein (AFP; pCEA [canalicular], CD10 [canalicular], arginase-1, and glypican-3), and biliary cells (pCEA [cytoplasmic and/or membranous], CD10 [cytoplasmic], and K7 and K19). Malignant transformation may alter expression of these markers, and familiarity with them in settings of malignancy is recommended.
The use of markers for “stem/progenitor cell” features/phenotypes in cHCC-CCA can be challenging. Because at least some stem/progenitor cell niches in the liver are located within the biliary tree, their immunophenotypes overlap with cholangiocytic markers. Therefore, some “stem/progenitor cell markers” such as EpCAM and K19 are markers of cholangiocytes in various stages of development. Interpretation of these markers should thus rely primarily on morphological characteristics of the positive cells. If these cells have the morphological characteristics of cholangiocytes and form ductules or glands, they should be regarded as cholangiocytes. If, on the other hand, the cellular morphology of the reactive cells is that of stem/progenitor cells, they can be considered to represent stem/progenitor cells. At the other end of the spectrum, CD117 (c-kit), CD133, and others(24) are not diffusely expressed throughout the biliary tree and therefore can be considered markers that represent “stemness.” CD56 (aka neural cell adhesion marker) lies somewhere in between this spectrum because, while not staining the entire biliary tree, it often stains the full extent of ductular reactions. As with EpCAM and K19, interpretation of CD56 is therefore best based on the cellular morphology of the positive cells.
Molecular Pathology OF cHCC-CCA
Although limited in number, molecular studies have highlighted significant heterogeneity in cHCC-CCA at the molecular level.(10,32) The most recent studies have identified a group of cHCC-CCA displaying expression of stemness features. Indeed, using a genome-wide transcriptional analysis, Coulouarn et al.’s study(32) exhibited stem/progenitor features associated with down-regulation of the hepatocyte differentiation program and a commitment to the biliary lineage in a series of 20 cHCC-CCA histologically belonging to the type with stem cell features. This result was confirmed in a recent integrative genomic analysis performed in formalin-fixed paraffin-embedded samples from 18 cHCC-CCA representative of the different tumor subtypes of cHCC-CCA, revealing stem cell subtype characterized by spaltlike transcription factor 4 expression, enrichment of progenitor-like signatures, and activation of MYC and insulin-like growth factor pathways.(10)
In addition, CLC is suggested to be unique, showing low chromosomal instability, enrichment of transforming growth factor beta (TGF-β) signaling, and biliary cell lineage markers, as compared to other sub-types.(10,32) Such results are in accord with previous data comparing loss of heterozygosity using 400 microsatellite markers, p53, and β-catenin mutations in a series of 15 cHCC-CCAs with 9 iCCAs, and 137 HCCs, suggesting that cHCC-CCA were genetically closer to iCCA than to HCC.(33) TGF-β and Wnt/β-catenin were identified as the two major signaling pathways involved in cHCC-CCA.(32) Interestingly, increase in TGF-β-signaling pathway has also been reported in a subset of HCC characterized by the presence of prominent fibrous stroma, namely “scirrhous HCC,”(34) as well as CLC,(10) and could be attributed to the presence of the tumoral fibrous stroma.
Whereas the identification in cHCC-CCA of common molecular traits with more aggressive HCC and iCCA is now well established, significant attention should be paid in respect to the background nontumoral liver, which could have a strong impact on the liver’s mutational landscape.(35) Indeed, in Fujimoto et al.’s study,(35) a whole-genome sequencing analysis performed on 30 PLCs displaying biliary phenotype, including 7 cHCC-CCAs, 2 CLCs, and 21 iCCAs, showed that the genome-wide substitution patterns regardless of tumor type arising in a background of chronic hepatitis overlapped with those of 60 HCCs, whereas those of arising within hepatitis-negative livers diverged from HCC. In addition, mutations of KRAS and IDH genes were more frequent in the hepatitisnegative tumors, whereas the TERT promoter mutation was more frequent in cHCC-CCA and HCCs, which mainly developed in a background of chronic hepatitis.
The molecular signature of stemness in cHCC-CCA has supported the concept of a stem/progenitor cell origin of cHCC-CCA.(14,23,36) Although this concept is still debated, it implies the notion of clonality, for which no consensus has been reached to date.(37) Nevertheless, a clonal origin has been successfully demonstrated in a subset of cHCC-CCA (8 of 11 cases) by studying allelic status of a number of selected chromosomes’ arms following laser microdissection.(38) Clonality is further supported by the demonstration of significant correlation in the copy number variation between iCCA and HCC components of the classical type of cHCC-CCA.(10) The presence of a stemness molecular signature is usually viewed, and confirmed in a recent small series as an adverse prognostic factor in hepatic malignancies.(10) Whereas the worse prognosis for HCC with K19 positivity has been well demonstrated,(14,36,39-41) there is good prognostic evidence from two recent Asian clinicopathological studies for CLC: One compared CLC to iCCA(11) and another compared ciCCA-CLC to iCCA without CLC.(31) Nevertheless, a worse postoperative survival rate has been reported in the group of cHCC-CCA exhibiting >5% stem cells.(42) Moreover, cHCC-CCA has been recently shown to have a larger “side population” of tumor cells, which implies more chemoresistance of these tumors compared to classical HCC and HCC expressing K19.(43)
Radiology
In contrast to classical HCC and iCCA, there are few publications that describe the radiological appearances of cHCC-CCA. Additionally, the relative infrequency of these lesions, the evolving pathological definition, and inconsistent use of radiology terminology, provide formidable challenges to accurate analysis of the existing literature. In single-center, retrospective case reports and series, cHCC-CCA are described as demonstrating some form of arterial hyperenhancement (APHE), most commonly peripheral or rim-like, on dynamic contrast-enhanced magnetic resonance imaging (MRI) and computed tomography (CT; Table 1).(6,16,17,27,44-51) Washout appearance is common and is often peripheral in location. Delayed central enhancement is frequently observed. These imaging features most commonly overlap with those of iCCA(17,48) (Fig. 4). Rarely, cHCC-CCA may resemble classical HCC (Fig. 4), by displaying diffuse APHE and diffuse or patchy washout appearance, or have features of both classical HCC and iCCA (Fig. 5).(16,44,50) Imaging features that are preponderantly HCC or iCCA appear to correspond to the predominant histopathology component of HCC and iCCA respectively(44,50) Discordance of tumor markers with imaging appearances (i.e., elevated CA19-9 in a lesion resembling an HCC or elevated AFP in a lesion more resembling iCCA) provide another clue to accurately identify cHCC-CCA(6,16,17) (Fig. 6). Imaging may also be used to target the biopsy needle to specific HCC-like and iCCA-like areas in the cHCC-CCA tumor.
TABLE 1.
Summary of Published Imaging Features of Presumed cHCC-CCA
| Citation | No. | Modality | Major Features | Ancillary Features |
|---|---|---|---|---|
| Fowler K, AJR 2013(17) | 29 cases | Dynamic phase CT and MRI | 100% APHE (14%-16% peripheral) 33%-41% washout 22%-26% capsule |
17%-35% biliary duct dilation 0% intralesional lipid 48%-74% delayed enhancement 42%-45% capsular retraction 10%-11% peripheral APHE/rim washout/delayed enhancement central |
| De Campos, JMRI 2012(46) | 11 cases | Dynamic phase MRI | 100% APHE (6/11 ring) 45% washout 18% capsule |
72% delayed enhancement (or a component of it mixed with washout) 9% biliary duct dilation 27% vascular invasion |
| Hwang, JMRI 2012(47) | 20 cases | Gadoxetate disodium MRI | 85% APHE (rim) | 50% target on HBP/10 minutes (complete or partial) 85% T2 hypointense area 60% irregular shape |
| Willekens, World J Gastroenterol 2009(51) | 1 case | Dynamic phase MRI | APHE | Mild T2 hyperintensity |
| Aoki, Hepatology 1993(44) | 20 cases | Dynamic CT | 64% (9/14) APHE (rim) 29% (4/14) confluent APHE 29% (4/14) washout |
64% (9/14) central progressive |
| Sanada, Hepatology Res 2005(50) | 11 cases | Dynamic CT | 100% APHE (20% rim) 40% washout |
60% delayed enhancement |
| Wells, Abdom Imaging 2015(16) | 39 cases | Dynamic CT, dynamic MRI, PET/CT, US | 100% APHE (rim 86%) 7% washout 10% capsule |
11% necrosis 3% intralesional fat 10% vascular invasion 26% capsular retraction |
| Komuta, Hepatology 2012(27) | 24 cases | Dynamic MRI | APHE (rim or diffuse 54.2%) 75% washout (rim or multinodular) |
79% heterogenous signal intensity at T2 weighted imaging (central hypointensity) |
Abbreviations: PET, positron emission tomography; US, ultrasound; HBP, hepatobiliary phase
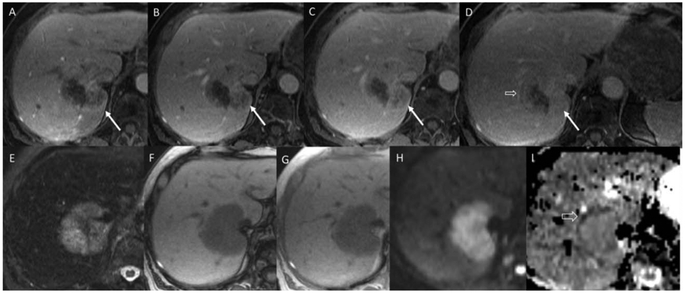
FIG. 4.
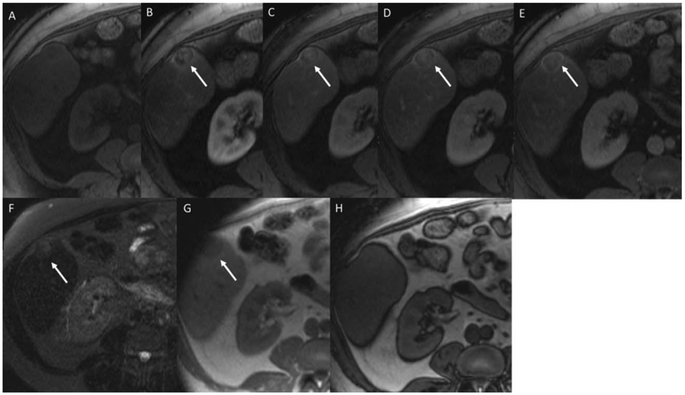
A 73-year-old woman with abdominal pain and lesion detected on imaging. Arterial phase, portal venous, and early and late delayed phase postcontrast magnetic resonance images (A-D) show a large mass in the central liver encasing the inferior vena cava (IVC; arrows). Note the progressive enhancement on the dynamic postcontrast images using an extracellular contrast agent. There are some regions of peripheral washout on the second delayed image (open arrow). The lesion shows T2 hyperintensity (E), no intralesional lipid or blood (F,G), and target appearance on diffusion weighted imaging/apparent diffusion coefficient (H,I, open arrow). The overall pattern is most compatible with a non-HCC malignancy, such as iCCA. The patient’s tumor markers were elevated (AFP-37,197 ng/mL and CA19-9-2168). Resection of the mass showed fairly uniform population of large tumor cells with abundant cytoplasm and large nuclei and nucleoli and brisk mitotic activity. The cells were arranged in solid nests and within lymphovascular spaces with no glandular formation. The IHC showed heterogeneous cell populations. AFP highlighted a majority of tumor cells whereas the hepatocyte-specific antigen showed only scattered positivity. Further IHC showed canalicular pCEA, diffuse K7 and K19 positivity, thyroid transcription factor-1, and K20 negativity.
FIG. 5.
A 66-year-old woman at risk for HCC, with lesion detected on surveillance imaging. Precontrast, arterial phase, portal venous phase, and early and late delayed phase magnetic resonance images show a segment 5 hepatic lesion with mild central nodular arterial phase hyperenhancement followed by washout and capsule appearance on the delayed images using an extracellular contrast agent (arrows). This is a pattern most compatible with HCC. The lesion is mildly T2 hyperintense (F) and shows no intralesional fat or blood products (G,H). Tumor markers were negative. Biopsy of the lesion demonstrated moderately differentiated HCC with central hemorrhage and necrosis. Several tumor nodules were embedded fibrous stroma and IHC showed diffuse K7 and K19, weak nuclear p53, and canalicular pCEA.
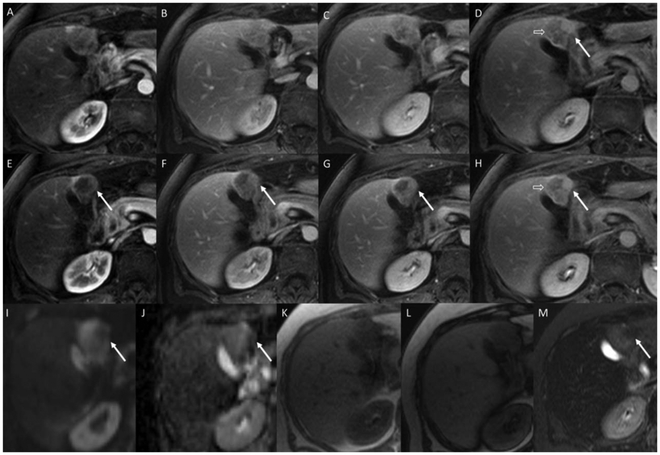
FIG. 6.
A 77-year-old woman with incidentally detected liver lesion. Arterial, portal venous, and early and late delayed magnetic resonance images obtained with an extracellular contrast agent at two slice positions (A-D and E-H) demonstrate a mixed enhancement pattern with features of both HCC and other malignancy. Arrows indicate a region of progressive enhancement along the medial border, which would be more compatible with iCCA. Open arrows indicate a region demonstrating washout and capsule appearance as observed in HCC. Diffusion weighted, apparent diffusion coefficient, in-phase, opposed phase, and fat suppressed T2 weighted images (I-M) also show a mixed picture. The arrows indicate a region of T2 hypointensity and different diffusion pattern. The in- and opposed-phase images demonstrate no intralesional blood products or fat. The overall picture would be indeterminate by imaging, not meeting algorithmic criteria for HCC. Tumor markers were all negative. Biopsy of the mass demonstrated a moderately differentiated primary liver carcinoma with morphological features of HCC (cells with abundant eosinophilic cytoplasm) and gland forming iCCA. IHC stains showed positivity for K7 and K19 and canalicular pCEA.
In a single-center retrospective study,(45) most (93.5%) cHCC-CCAs were categorized as an unknown liver malignancy (LR-M; probably malignant, not specific for HCC) using the American College of Radiology’s liver imaging reporting and data system (LI-RADS).(52) However, unlike the majority of HCCs, cHCC-CCA may arise in patients without cirrhosis or other known risk factors for HCC, as noted above, thus placing them outside of the context of LI-RADS and other similar diagnostic systems, which were formulated for the diagnosis of HCC in at-risk populations.(53) No studies have compared the biological behavior or imaging appearance of cHCC-CCA arising in patients with versus without cirrhosis or other HCC risk factors.
Translation of cHCC-CCA into the Clinic: Next Steps
This consensus document hopes to represent substantial progress in standardizing the pathological definition and diagnosis of cHCC-CCA and to be of use to clinical and research pathologists, hepatologists, surgeons, radiologists, and oncologists. The document provides a platform for future clinical and translational investigations regarding cHCC-CCA, an increasingly recognized malignancy. Although little is known clinically about these cancers, the data available suggest they are aggressive and likely signify a unique subset of primary liver cancers, which merit clinical distinction.
cHCC-CCA is important to characterize because a major source of clinical uncertainty are mass lesions in advanced stage (cirrhotic) liver that do not demonstrate radiological features typical for HCC. The LI-RADS criteria for diagnosing HCC are predicated on a high specificity of the radiographical findings, rather than sensitivity. In theory, the combination of specific imaging features and an appropriate pretest probability (i.e., applied in the at-risk population) allow sufficiently high specificity to obviate the need for biopsy. Thus, in the appropriate setting and with typical radiological features of HCC, a confident diagnosis can be made without biopsy.
However, for lesions that do not meet these stringent criteria (no established clinical risk factors, atypical imaging features), LI-RADS suggests biopsy as a possible appropriate diagnostic pathway. Many of these may turn out to be HCC upon biopsy, but some may not. These are lesions best described by the LI-RADS category LR-M, probably or definitely malignant but not specific for HCC.
The challenges associated with imaging cHCC-CCA raise two immediate areas that require investigation. The first regards the means of making the diagnosis of cHCC-CCA: If tumors are radiologically heterogeneous, how many areas must be biopsied to have confidence of a complete assessment of the nature of the malignancy and what are the appropriate methods and criteria to target those biopsies? Correlation between preresection imaging and resected tumors, mapping the pathology to the radiology in detail, will play an important role. The second challenge regards the management of cHCC-CCA in cirrhosis, once diagnosed: (1) What is the outcome with liver transplantation?; (2) How do these cancers respond to locoregional therapies, particularly when bridging patients to liver transplantation?; and (3) How do these tumors respond to systemic therapies traditionally given for HCC or iCCA, in particular, the newer targeted agents? These are urgent questions that need to be addressed with more abundant data than published in small series to date.(54,55)
A recent study, the first to analyze recurrences and metastases after identification of a primary cHCC-CCA, indicates that just as the primary tumors are undoubtedly heterogeneous, the recurrent and metastatic lesions are as well.(56) Mixed tumors reflective of the primary may be observed in recurrence or metastases, or an individual component of the original may metastasize. Different single components even have unique organ tropisms. This study, the first of its kind and only 4 cases, is sufficient, however, to reaffirm the need for multi-institutional collaborative work to study the natural history of recurrence and spread of combined PLC.
What are other next steps to establish the clinical-pathological relevance of cHCC-CCA as a distinct entity even in the absence of cirrhosis? First, the interobserver variability for this pathological definition needs to be rigorously and robustly examined. It will be impossible to make progress if the diagnosis of cHCC-CCA cannot be standardized across institutions. Second, continued molecular profiling of well-defined cHCC-CCA needs to be performed; we need to know whether these tumors are associated with unique genetic signatures, genetic aberrations, or epigenetic markers. Given their phenotypic diversity by pathological evaluation, it is possible that the genetic profiles of cHCC-CCA are even more heterogeneous than classic HCC or iCCA. In fact, it is possible that, if cHCC-CCA have a high mutational burden, they may be more responsive to immunotherapy than other PLC. Third, outcomes data for surgical resection, liver transplantation, locoregional therapy, and systemic agents need to be examined and compared to classic HCC and iCCA for clinical, pathological, and radio-graphical factors for potential prognostic and predictive values. We need to know whether cHCC-CCA should be treated as HCC or iCCA or as a completely different tumor entity. Fourth, the question of whether locoregional therapy can drive a classic HCC to exhibit the phenotypic morphology of cHCC-CCA, as reported(57) needs clarification. For example, is the presence of angular glands on the periphery of presumed HCC lesions treated by transarterial chemoembolization (TACE) a treatment effect representing change in the cancer’s phenotype or were these cancers cHCC-CCA prior to TACE, or does TACE simply unmask the complexity of these tumors?(57) Of course, these questions are difficult to assess in the absence of pretreatment biopsies. This issue returns us to the questions of biopsies: how many, and how they should be targeted if an untreated lesion shows atypical imaging. It also raises the issue of whether radiology is truly sufficient, without confirmatory biopsy, for excluding iCCA, or other components of cHCC-CCA in the first place. Fifth, as exemplified by recent changes in the American Joint Committee on Cancer Tumor Node Metastasis staging criteria for intra- and extrahepatic biliary tract cancers,(58) we need to better understand how cHCC-CCA tumors should be staged. Finally, none of these questions can be answered by a single institution given that these cHCC-CCA likely comprise ~2% of all primary liver cancers. Consortia of investigators will need to be formulated to efficiently and correctly address these and other questions. This consensus document therefore represents the first of many steps toward an understanding of these forms of PLC. Table 2 provides current Practice Points based on this consensus document.
TABLE 2.
Practice Points Summary
| • The proposed terminology of primary liver carcinomas with both hepatocellular and cholangiocytic differentiation within the same tumor is combined hepatocellular-cholangiocarcinoma, cHCC-CCA. |
| • The diagnosis of cHCC-CCA relies on routine histochemical stains; IHC is used as a supplemental diagnostic tool. |
| • Stem cell phenotypes/features may exist within cHCC-CCA, and can be noted in a descriptive report. The finding does not warrant a separate subclassification. |
| • Two other types of PLC discussed include CLC and intermedate cell carcinoma. Both may coexist with HCC, iCCA, or cHCC-CCA. |
| • As molecular advances in all PLC, including cHCC-CCA are progressing, the importance of pathological confirmation of tumor type under study cannot be overemphasized. |
| • Radiological findings ofcHCC-CCA to date indicate features between those of typical HCC and iCCA, but often are not specific for either. Biopsy confirmation may be indicated. |
| • Radiological evidence of different components with different imaging features within a single tumor raises the as yet unanswered question of whether to biopsy each distinct region for accurate assessment. |
| • Clinical advances in patient management are occurring as the recognition of these tumors, both in cirrhosis and in noncirrhotic livers, grows. The exact incidence and prevalence in different diseases and in different clinical settings remain in question, partially attributed to terminology differences. |
| • It may be beneficial to establish an international registry with centralized pathology and radiology for further clinical study of these tumors. |
Supplementary Material
Abbreviations:
- AFP
alpha fetoprotein
- APHE
arterial phase hyperenhancement
- CCA
cholangiocarcinoma
- cHCC-CCA
combined (or mixed) hepatocellular-cholangiocarcinoma
- CLC
cholangiolocarcinoma
- CT
computed tomography
- EpCAM
epithelial cell adhesion molecule
- HCC
hepatocellular carcinoma
- H&E
hematoxylin and eosin
- iCCA
intrahepatic cholangiocarcinoma
- IHC
immunohistochemistry
- K
keratin
- MRI
magnetic resonance imaging
- pCEA
polyclonal CEA
- PLC
primary liver carcinoma
- TACE
transarterial chemoembolization
- TGF-β
transforming growth factor beta
- WHO
World Health Organization
Footnotes
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.29789/suppinfo.
The authors of this article met in November 2015 and 2016 at the initiative of Drs. Theise and Brunt. The term combined HCC-CCA (cHCC-CCA) was agreed upon.
Disclaimer: The manuscript was neither submitted nor endorsed by the AASLD Governing Board.
Potential conflict of interest: Dr. Sirlin is on the speakers’ bureau for and received grants from GE. He is on the speakers’ bureau for Bayer. He received grants from Siemens. Dr. Kagen consults for and is on the speakers’ bureau for Bayer.
HCC with expression of K19 in >5% of tumor cells have a worse prognosis than HCC without this immunophenotype staining, however, is usually not within an area with stem/progenitor cell morphology, but in tumor cells that are clearly hepatocytic. Published data regarding other stem cell markers and similar thresholds for “positive” are still less robust.(14)
mRNA in an iCCA is not necessarily reflective of protein production.(15)
REFERENCES
- 1).Wells HG. Primary carcinoma of the liver. Am J M Sc 1903; 126:403–417. [Google Scholar]
- 2).European Association for the Study of the Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–943. [DOI] [PubMed] [Google Scholar]
- 3).Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- 4).Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 1985;55:124–135. [DOI] [PubMed] [Google Scholar]
- 5).Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol 1998;13:34–40. [DOI] [PubMed] [Google Scholar]
- 6).Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 2002;94:2040–2046. [DOI] [PubMed] [Google Scholar]
- 7).Theise ND, Nakashima O, Park YN, Nakanuma Y. Combined hepatocellular-cholangiocarcinoma In: Bosman FT, Carneiro F, Hruban R, Theise ND, eds. WHO Classification of Tumours of the Digestive System Volume 3 4th ed. Lyon, France: International Agency for Research on Cancer; 2010:225–227. [Google Scholar]
- 8).Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol 2013;37:496–505. [DOI] [PubMed] [Google Scholar]
- 9).Sasaki M, Sato H, Kakuda Y, Sato Y, Choi JH, Nakanuma Y. Clinicopathological significance of ‘subtypes with stem-cell feature’ in combined hepatocellular-cholangiocarcinoma. Liver Int 2015;35:1024–1035. [DOI] [PubMed] [Google Scholar]
- 10).Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, et al. Mixed hepatocellular cholangiocarcinoma tumors: cholan-giolocellular carcinoma is a distinct molecular entity. J Hepatol 2017;66:952–961. [DOI] [PubMed] [Google Scholar]
- 11).Chen J, He J, Deng M, Wu HY, Shi J, Mao L, et al. Clinico-pathological, radiologic, and molecular study of 23 combined hepatocellular-cholangiocarcinomas with stem cell features, cholangiolocellular type. Hum Pathol 2017;64:118–127. [DOI] [PubMed] [Google Scholar]
- 12).Sasaki M, Sato Y, Nakanuma Y. Mutational landscape of combined hepatocellular carcinoma and cholangiocarcinoma, and its clinicopathological significance. Histopathology 2017;70:423–434. [DOI] [PubMed] [Google Scholar]
- 13).Zhou S, Venkatramani R, Gupta S, Wang K, Wang L, Mascarenhas L. Hepatocellular malignant neoplasm-NOS: a clinicopathologic study of 11 cases from a single institution. Histopathology 2017;71:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006;49:138–151. [DOI] [PubMed] [Google Scholar]
- 15).Ferrone CR, Ting DT, Shahid M, Konstantinidis IT, Sabbatino F, Goyal L, et al. The ability to diagnose intrahepatic cholangio-carcinoma definitively using novel branched DNA-enhanced albumin RNA in situ hybridization technology. Ann Surg Oncol 2014;23:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Wells ML, Venkatesh SK, Chandan VS, Fidler JL, Fletcher JG, Johnson GB, et al. Biphenotypic hepatic tumors: imaging findings and review of literature. Abdom Imaging 2015;40:2293–2305. [DOI] [PubMed] [Google Scholar]
- 17).Fowler KJ, Sheybani A, Parker RA, Doherty S, Brunt EM, Chapman WC, Menias CO. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. Am J Roentgenol 2013;201:332–339. [DOI] [PubMed] [Google Scholar]
- 18).Sempoux C, Paradis V, Saxena R. Variant differentiation patterns in primary liver carcinoma. Semin Diagn Pathol 2017;34: 176–182. [DOI] [PubMed] [Google Scholar]
- 19).Akiba J, Nakashima O, Hattori S, Naito Y, Kusano H, Kondo R, et al. The expression of arginase-1, keratin (K) 8 and K18 in combined hepatocellular-cholangiocarcinoma, subtypes with stem-cell features, intermediate-cell type. J Clin Pathol 2016;69: 846–851. [DOI] [PubMed] [Google Scholar]
- 20).Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol 2004;40:298–304. [DOI] [PubMed] [Google Scholar]
- 21).Park HS, Bae JS, Jang KY, Lee JH, Yu HC, Jung JH, et al. Clinicopathologic study on combined hepatocellular carcinoma and cholangiocarcinoma: with emphasis on the intermediate cell morphology. J Korean Med Sci 2011;26:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Brunt EM, Paradis V, Sempoux C, Theise ND. Biphenotypic (hepatobiliary) primary liver carcinomas: the work in progress. Hepat Oncol 2015;2:255–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, et al. Hepatic ‘stem cell’ malignancies in adults: four cases. Histopathology 2003;43:263–271. [DOI] [PubMed] [Google Scholar]
- 24).Leung CO, Mak WN, Kai AK, Chan KS, Lee TK, Ng IO, Lo RC. Sox9 confers stemness properties in hepatocellular carcinoma through Frizzled-7 mediated Wnt/β-catenin signaling. Oncotar-get 2016;7:29371–29386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Steiner PE, Higginson J. Cholangiolocellular carcinoma of the liver. Cancer 1959;12:753–759. [DOI] [PubMed] [Google Scholar]
- 26).Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. HEPATOLOGY 2008;47:1544–1556. [DOI] [PubMed] [Google Scholar]
- 27).Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology 2012;55:1876–1888. [DOI] [PubMed] [Google Scholar]
- 28).Ariizumi S, Kotera Y, Katagiri S, Nakano M, Yamamoto M. Combined hepatocellular-cholangiocarcinoma had poor outcomes after hepatectomy regardless of Allen and Lisa class or the predominance of intrahepatic cholangiocarcinoma cells within the tumor. Ann Surg Oncol 2012;19:1628–1636. [DOI] [PubMed] [Google Scholar]
- 29).Kondo F, Fukusato T. Pathogenesis of cholangiolocellular carcinoma: possibility of an interlobular duct origin. Intern Med 2015;54:1685–1694. [DOI] [PubMed] [Google Scholar]
- 30).Nakano M, Ariizumi SI, Yamamoto M. Intrahepatic cholangiocarcinoma. Semin Diagn Pathol 2017;34:160–166. [DOI] [PubMed] [Google Scholar]
- 31).Rhee H, Ko JE, Chung T, Jee BA, Kwon SM, Nahm JH, et al. Transcriptomic and histopathological analysis of cholangiolocellular differentiation trait in intrahepatic cholangiocarcinoma. Liver Int 2018;38:113–124. [DOI] [PubMed] [Google Scholar]
- 32).Coulouarn C, Cavard C, Rubbia-Brandt L, Audebourg A, Dumont F, Jacques S, et al. Combined hepatocellular-cholangiocarcinomas exhibit progenitor features and activation of Wnt and TGFβ signaling pathways. Carcinogenesis 2012;33: 1791–1796. [DOI] [PubMed] [Google Scholar]
- 33).Cazals-Hatem D, Rebouissou S, Bioulac-Sage P, Bluteau O, Blanché H, Franco D, et al. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol 2004; 41:292–298. [DOI] [PubMed] [Google Scholar]
- 34).Seok JY, Na DC, Woo HG, Roncalli M, Kwon SM, Yoo JE, et al. A fibrous stromal component in hepatocellular carcinoma reveals a cholangiocarcinoma-like gene expression trait and epithelial-mesenchymal transition. Hepatology 2012;55:1776–1786. [DOI] [PubMed] [Google Scholar]
- 35).Fujimoto A, Furuta M, Shiraishi Y, Gotoh K, Kawakami Y, Arihiro K, et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun 2015;6:1–8. [DOI] [PubMed] [Google Scholar]
- 36).Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, et al. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology 2011;54:1707–1717. [DOI] [PubMed] [Google Scholar]
- 37).Alizadeh AA, Aranda V, Bardelli A, Blanpain C, Bock C, Borowski C, et al. Toward understanding and exploiting tumor heterogeneity. Nat Med 2015;21:846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Fujii H, Zhu XG, Matsumoto T, Inagaki M, Tokusashi Y, Miyokawa N, et al. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol 2000;31:1011–1017. [DOI] [PubMed] [Google Scholar]
- 39).Uenishi T, Kubo S, Yamamoto T, Shuto T, Ogawa M, Tanaka H, et al. Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence. Cancer Sci 2003;94:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Lee JI, Lee JW, Kim JM, Kim JK, Chung HJ, Kim YS. Prognosis of hepatocellular carcinoma expressing cytokeratin 19: comparison with other liver cancers. World J Gastroenterol 2012;18:4751–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut 2014;63:674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Ikeda H, Harada K, Sato Y, Sasaki M, Yoneda N, Kitamura S, et al. Clinicopathologic significance of combined hepatocellular-cholangiocarcinoma with stem cell subtype components with reference to the expression of putative stem cell markers. Am J Clin Pathol 2013;140:329–340. [DOI] [PubMed] [Google Scholar]
- 43).Govaere O, Wouters J, Petz M, Vandewynckel YP, Van den Eynde K, Van den Broeck A, et al. Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. J Hepatol 2016;64:609–617. [DOI] [PubMed] [Google Scholar]
- 44).Aoki K, Takayasu K, Kawano T, Muramatsu Y, Moriyama N, Wakao F, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features and computed tomographic findings. Hepatology 1993;18:1090–1095. [PubMed] [Google Scholar]
- 45).Potretzke TA, Tan BR, Doyle MB, Brunt EM, Heiken JP, Fowler KJ. Imaging features of biphenotypic primary liver carcinoma (hepatocholangiocarcinoma) and the potential to mimic hepatocellular carcinoma: LI-RADS analysis of CT and MRI features in 61 cases. Am J Roentgenol 2016;207:25–31. [DOI] [PubMed] [Google Scholar]
- 46).de Campos RO, Semelka RC, Azevedo RM, Ramalho M, Heredia V, Armao DM, Woosley JT. Combined hepatocellular carcinoma-cholangiocarcinoma: Report of MR appearance in eleven patients. J Magn Reson Imaging 2012;36:1139–1147. [DOI] [PubMed] [Google Scholar]
- 47).Hwang J, Kim YK, Park MJ, Lee MH, Kim SH, Lee WJ, Rhim HC. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imaging 2012;36:881–889. [DOI] [PubMed] [Google Scholar]
- 48).Nishie A, Yoshimitsu K, Asayama Y, Irie H, Aibe H, Tajima T, et al. Detection of combined hepatocellular and cholangiocarcinomas on enhanced CT: comparison with histologic findings. Am J Roentgenol 2005;184:1157–1162. [DOI] [PubMed] [Google Scholar]
- 49).Ebied O, Federle MP, Blachar A, Brancatelli G, Grazioli L, Cazals-Hatem D, et al. Hepatocellular-cholangiocarcinoma: helical computed tomography findings in 30 patients. J Comput Assist Tomogr 2003;27:117–124. [DOI] [PubMed] [Google Scholar]
- 50).Sanada Y, Shiozaki S, Aoki H, Takakura N, Yoshida K, Yamaguchi Y. A clinical study of 11 cases of combined hepatocellular-cholangiocarcinoma. Assessment of enhancement patterns on dynamics computed tomography before resection. Hepatol Res 2005;32:185–195. [DOI] [PubMed] [Google Scholar]
- 51).Willekens I, Hoorens A, Geers C, Op de Beeck B, Vandenbroucke F, de Mey J. Combined hepatocellular and cholangiocellular carcinoma presenting with radiological characteristics of focal nodular hyperplasia. World J Gastroenterol 2009;15: 3940–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).American College of Radiology. Liver Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2014. [Google Scholar]
- 53).Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61:1056–1065. [DOI] [PubMed] [Google Scholar]
- 54).Zhou A, Amin M, Fowler KJ, Brunt EM, Keller J, Tan B. Complete response to erlotinib and bevacizumab in a patient with biphenotypic (hepatobiliary) primary liver carcinoma. J Natl Compr Canc Netw 2015;13:1468–1473. [DOI] [PubMed] [Google Scholar]
- 55).Kohler BC,Waldburger N,Schlamp K,Jager D,Weiss KH,Schulze-Bergkamen H Schirmacher P, et al. Liver cancers with stem/progenitor-cell features—a rare chemotherapy-sensitive malignancy. Oncotarget 2017;8:59991–59998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).De Vito C, Sarker D, Ross P, Heaton N, Quaglia A. Histological heterogeneity in primary and metastatic classic combined hepatocellular-cholangiocarcinoma: a case series. Virchows Arch 2017;471:619–629. [DOI] [PubMed] [Google Scholar]
- 57).Zen C, Zen Y, Mitry RR, Corbeil D, Karbanova J, O’Grady J, et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transpl 2011;17:943–954. [DOI] [PubMed] [Google Scholar]
- 58).Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A, eds. Intrahepatic Bile Ducts AJCC Cancer Staging Manual. New York, NY: Springer; 2009:201–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.