Abstract
Vasopressin (VP) and oxytocin (OT) are involved in modulating basic physiology and numerous social behaviors. Although the anatomical distributions of nonapeptide neurons throughout development have been described, the functional roles of VP and OT neurons during development are surprisingly understudied, and it is unknown whether they exhibit functional changes throughout early development. We utilized an acute social isolation paradigm to determine if VP and OT neural responses in eight nonapeptide cell groups differ at three different stages of early development in prairie voles. We tested pups at ages that are representative of the three rapid growth stages of the developing brain: postnatal day (PND)2 (closed eyes; poor locomotion), PND9 (eye opening; locomotion; peak brain growth spurt), and PND21 (weaning). Neural responses were examined in pups that (1) were under normal family conditions with their parents and siblings, (2) were isolated from their parents and siblings and then reunited, and (3) were isolated from their parents and siblings. We found that VP and OT neural activity (as assessed via Fos co-localization) did not differ in response to social condition across development. However, remarkably rapid VP and OT synthesis in response to social isolation was observed only in the paraventricular nucleus of the hypothalamus (PVN) and only in PND9 pups. These results suggest that PVN nonapeptide neurons exhibit distinct cellular properties during a critical period of development, allowing nonapeptide neurons to rapidly upregulate peptide production in response to stressors on a much shorter timescale than has been observed in adult animals.
Keywords: Development, Vasopressin, Oxytocin, Critical period, Prairie vole, PVN
Introduction
The nonapeptides (vasopressin, VP, and oxytocin, OT) are key modulators of basic physiological processes and social behavior (Landgraf and Neumann 2004). Understanding the development of this system can provide insight into how individual differences in social behavior arise, and elucidate sources of behavioral dysfunction. The ontogeny of VP and OT neuronal populations has been examined in rodents (DiBenedictis et al. 2017; Grinevich et al. 2014; Swaab and Ter Borg 1981; Boer et al. 1980; Yamamoto et al. 2004), demonstrating that these cell groups are present at parturition, although dendritic and axonal growth, and neuron number, continue to grow in the first weeks after birth (Hammock 2015; Grinevich et al. 2014; Tamborski et al. 2016). Although the anatomical distribution of nonapeptide neurons throughout development has been described, their functional roles during the early development are surprisingly understudied.
The nonapeptide cell groups of the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus are particularly versatile cell groups that can have both central and peripheral effects. They represent the largest populations of nonapeptide cell groups (Landgraf and Neumann 2004), and also contain the largest number of magnocellular neurons (Brown et al. 2013). Magnocellular neurons in the PVN and SON send direct axonal projections to the posterior pituitary whereby they release peptide into the periphery. In this way, VP and OT exert direct and indirect effects on the hypothalamic–pituitary–adrenal (HPA) axis in response to stress (Landgraf and Neumann 2004; Sivukhina and Jirikowski 2016). These direct connections to the periphery distinguish the PVN and SON nonapeptide cell groups from other accessory VP and OT cell groups [e.g., bed nucleus of the stria terminalis (BST), medial preoptic nucleus (MPO), and anterior hypothalamus (AH)], which send axonal projections throughout the basal forebrain and midbrain (Brown et al. 2013). Thus, unlike accessory nonapeptide cell groups, the PVN and SON nonapeptide cell groups can have a direct impact on peripheral physiology. These cell groups are involved in cardiovascular function, hydromineral balance, water retention, energy metabolism, and smooth muscle function (Engelmann et al. 2004; Goodson and Thompson 2010; Smith et al. 2015). The PVN also contains parvocellular nonapeptide neurons, which send axonal projections throughout the brain. Importantly, both magnocellular and parvocellular nonapeptide neurons exhibit somatic, axonal, and dendritic release (Ludwig and Leng 2006), so both VP and OT producing neurons in the PVN and SON can impact the brain, and subsequently behavior. Because of their multidimensional nature (i.e., having both central and peripheral effects), PVN and SON nonapeptide cell groups may alter their functions across early development as the physiological needs of a developing animal change during maturation. In addition, although accessory nonapeptide cell groups do not directly impact the periphery as the PVN and SON nonapeptide cell groups do, accessory cell groups may also alter functional properties across development in response to changes in how a maturing animal processes and interacts with the social environment.
Neurodevelopmental milestones have been described for rodents and humans (Clancy et al. 2007; Semple et al. 2013), and studies in rats and mice have identified three rapid growth stages of brain development that occur between postnatal days (PND) 0–6, PND8–12, and PND17–23 (Gottlieb et al. 1977). Key benchmarks of neural maturation also occur during these rapid growth stages. For example, between PND1–3, the blood–brain barrier is established. A peak brain growth spurt, peak in gliogenesis and neurogenesis, and increases in axonal and dendritic density are seen at PND7–10 (Bockhorst et al. 2008; Dean et al. 2011; Semple et al. 2013). At PND20–21, the brain reaches 90–95% of its adult weight, and there are peaks in myelination rate and synaptic density (Dekaban 1987; Qiu et al. 2007; Semple et al. 2013). In addition, cellular functions change dramatically during development (Sperelakis 2001).
The ontogeny of rodent nonapeptide neuron and receptor anatomical distributions has been described in rats (Boer et al. 1980; DiBenedictis et al. 2017; Krisch 1980; Smith et al. 2017; Swaab and Ter Borg 1981), mice (Godefroy et al. 2017), jirds (Rabhi et al. 1996, 1999), and voles (lateral septum nonapeptide receptors only, Wang and Young 1997; PVN VP and OT neurons only; Yamamoto et al. 2004). However, to our knowledge, it is largely unknown whether nonapeptide neurons exhibit distinct functional properties or play different functional roles in behavior at different stages of development, particularly during rapid brain growth stages. Notably, we recently showed that BST VP neurons exhibit changes in functional response to novel conspecifics in male prairie voles as they mature (Kelly et al. 2018), but this study examined developmental stages from PND15–60 and did not examine very early development. Nonetheless, this study demonstrates the potential for nonapeptide neuronal functional plasticity during development.
Here, we sought to examine whether nonapeptide neurons exhibit changes in functional responses across early development in prairie voles (Microtus ochrogaster) at different developmental stages representing the main neural growth stages above (PND2, 9, 21). We used prairie voles, because this species has emerged as a compelling model for understanding the impact of nonapeptides on social behavior and development (Hammock 2015). Importantly, developmental milestones in the brain occur alongside and in conjunction with developmental milestones in physiology, anatomy, and behavior (Fox 1965; Lagerspetz 1966; Fitch 1957). PND2 prairie vole pups do not exhibit independent locomotion and eye opening has not yet occurred. By PND9, pups are capable of independent locomotion and exhibit open eyes (Robison et al. 2016; Solomon 1991). By PND21, prairie vole pups are weaned in the lab and can function independently (Carter and Getz 1985). We utilized a social isolation paradigm to examine neuronal functional plasticity, because isolation during early development can have fatal consequences, and, therefore, is likely to induce rapid physiological and behavioral responses in vulnerable pups. Adult rodents have well-defined nonapeptide neural responses to chronic social isolation (e.g., increases in PVN VP and OT) (Gilles and Polston 2017; Grippo et al. 2007b; Pan et al. 2009); however, it is largely unknown how nonapeptide neurons function in response to social isolation in early development. We hypothesized that VP and OT neurons would respond differently to social isolation over postnatal development. To test this hypothesis, we exposed pups at different developmental stages representing the main neural growth stages above (PND2, 9, 21) to acute social isolation to examine functional differences in nonapeptide neural responses in an immediate early gene (IEG) experiment. We examined eight VP or OT cell groups throughout the brain. However, because the PVN and SON nonapeptide cell groups modulate both physiological processes and social behavior, we hypothesized that these cell groups would exhibit greater functional differences across development compared to accessory hypothalamic and amygdalar nonapeptide cell groups.
Materials and methods
Animals
All prairie voles used in this study were obtained from our breeding colony of F1 breeding pairs that were offspring of wild caught animals we captured in Champagne County, Illinois, USA. All animals were housed in standard polycarbonate rodent cages (29 × 18 × 13 cm) that contained Sanichip bedding and nesting material. Animals were kept on a 14L:10D cycle, and ambient temperature was maintained at 20 ± 2 °C. Water and rodent chow (Laboratory Rodent Diet 5001, LabDiet, St. Louis, MO, USA) were provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Cornell University (2013–0102).
Experimental design
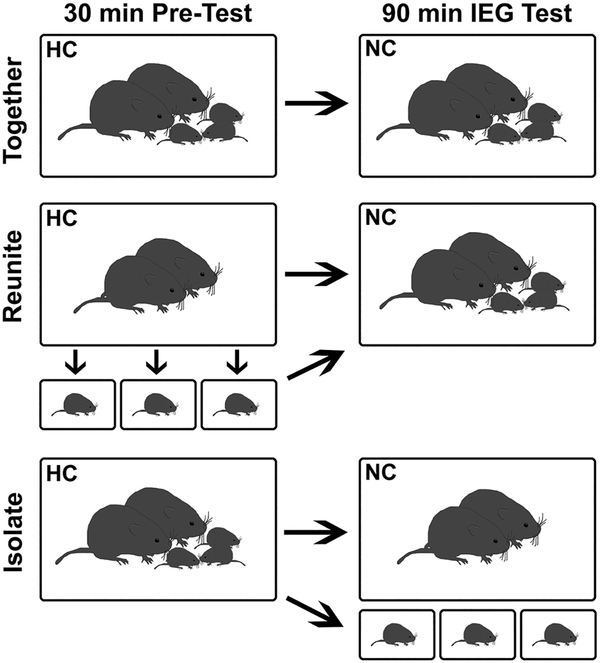
To determine if nonapeptide neurons exhibit differential responses to social condition based on age, we manipulated the family environment of pups at different developmental stages (PND2, 9, and 21). These ages represent the key benchmarks of neural maturation (Semple et al. 2013). We examined pup neural responses to reunion with and social isolation from their parents and siblings. Families (parents and their pups) were randomly assigned to one of three conditions (Fig. 1, and below): Isolate, Reunite, or Together.
Fig. 1.
Schematic representation of treatment groups. Family units were randomly assigned to one of three conditions: together, isolate, or reunite. The same procedure was used for family units with pups of each age (PND2, PND9, or PND21). See text for details. HC home cage, NC novel cage
Behavioral procedure
Ninety-four breeding pairs were established; only first litters were used for the experiment. Litter sizes average from 3 to 5 pups, and so to include the majority of breeding pairs and their pups in this study, litters were culled to three pups on the day of birth. Litter number was kept consistent to avoid social environmental differences across development (i.e., parental care and sibling relations may differ based on litter size). This also increased the likelihood of having 1 male and 1 female in each litter. We created three social conditions to assess the functional reaction of OT- and VP-immunoreactive (-ir) neurons in pups of different ages following interactions with or separation from their parents and siblings. The ‘Isolate’ condition examined pups’ neural responses to being housed apart from their family. The ‘Reunite’ condition was included to determine if nonapeptide cells exhibit a distinct functional profile following social isolation followed by reunion with parents and siblings. Our control condition allowed parents and pups to remain together (‘Together’).
To assess IEG responses (see below), we designed the experiment to have two phases (see Fig. 1): a 30-min home cage phase before an IEG test (pre-test), and a 90-min novel cage phase (IEG test). In the Isolate condition, parents and pups were handled and returned to their home cage in the first phase. After 30 min, parents were transferred to a novel cage together, while each pup was transferred to a different novel cage, at which time any functional changes measured by the IEG activation would have been initiated. Thus, pups were isolated from their parents and siblings for the 90 min IEG test. In the Reunite condition, pups and parents were handled as in the Isolate condition. However, while parents were returned to the home cage for the pre-test, the pups were individually isolated in novel cages during the 30 min pre-test. For the 90 min IEG test, the pups and parents were all transferred into the same novel cage (hence, reunited). In the Together condition, parents and pups were all handled as above, but all were returned to the home cage for the pre-test, and then transferred to the same novel cage for the 90-min IEG test.
For all tests, the degree to which each animal was handled was mimicked to control for the total quality and quantity of handling. For all trials, the same experimenter wore clean nitrile gloves and used a clean plastic beaker to transfer animals. All tests were conducted within an 8 h window of the light cycle, with morning and afternoon testing times counterbalanced across groups. All novel cages contained new wood chip bedding, new shredded nestlet material, contained no food or water, and had a clear Plexiglas lid with air holes for top–down video recording. All animals were video recorded for the full 2 h of testing. Immediately after the 90 min IEG test, all subjects were sacrificed and brains of parents and pups were collected (see below).
Group sizes
All pups in a litter were assigned to the same social condition and underwent behavioral testing and perfusion. After the sex of pups was confirmed via PCR (see below), 1 male and 1 female brain (if both were available) were randomly selected from each litter for immunocytochemistry.
Group sizes for each age group for the Isolate condition were: PND2, n = 10 males and 11 females (from 11 litters); PND9, n = 9 males and 9 females (from 11 litters); PND21, n = 8 males and 8 females (from 10 litters). Group sizes for each age group for the Reunite condition were: PND2, n = 9 males and 8 females (from 10 litters); PND9, n = 10 males and 10 females (from 10 litters); PND21, n = 9 males and 9 females (from 10 litters). Group sizes for each age group for the Together condition were: PND2, n = 10 males and 10 females (from 10 litters); PND9, n = 11 males and 11 females (from 12 litters); PND21, n = 8 males and 9 females (from 10 litters).
Accounting for thermoregulatory differences
We controlled for differences in thermoregulatory abilities between PND2, 9, and 21 pups that were isolated in the Isolate and Reunite conditions by adjusting the temperature in each testing cage using electric heating pads. We used a veterinary grade infrared thermometer (Nasco Product B01377N) to determine how much a novel test cage needed to be heated, so that a pup could maintain a temperature consistent with the temperature of pups in a nest with their family. By taking the average temperature from a sample of 5 families at each pup age, we found that the novel empty cage needed to be heated to 36 °C for PND2 pups, 34 °C for PND9 pups, and 30 °C for PND21 pups.
Sex determination
All pups were sexed visually after perfusions. Because it can be difficult to locate gonads in PND2 and 9 pups, we confirmed visual sexing with PCR amplification for the detection of the male-specific SRY gene. After being deeply anesthetized with isoflurane, and immediately prior to perfusion, punches were taken from the lung and the spleen for DNA extraction. Genomic DNA was extracted according to the manufacturer’s protocol using a DNEasy Blood and Tissue Kit (Qiagen, USA). All PCR reactions were performed in a thermal cycler (Eppendorf realplex4) in 25 μl for 35 cycles (94 °C 30 s, 55 °C 1 min, and 72 °C 30 s). The amplified PCR products were separated on 2% agarose gels, GelRed (Biotium, USA) stained, and visualized under a UV transilluminator. The sequences for SRY primers were forward 5’-TTATGCTGTGGTCTCGTGGTC-3’; and reverse 5’-GCA GTC TCT GTG CCT CTT GG-3’.
Histology and immunocytochemistry
We assessed age differences in VP or OT immunoreactive neuronal number and differences in neuronal activation of nonapeptide cells as assessed by co-expression of the IEG c-Fos (Fos hereafter). To visualize OT, VP, and Fos, subjects were sacrificed by isoflurane overdose and transcardially perfused with 0.1 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde. Brains were extracted, post-fixed overnight, and cryoprotected in 30% sucrose in PBS for 48 h prior to sectioning on a cryostat. Tissue was sectioned into three 40μ m series for PND21 subjects. To account for smaller brain size, tissue was sectioned into two 40 μm series for PND2 and PND9 subjects. Prior to the sectioning of subject brains, pilot brains representing our experimental age groups were sectioned to determine the best method to compensate for differences in brain size. Sectioning PND2 and 9 brains into two 40 μm series, and PND21 brains into the standard three 40 μm series, resulted in each series of tissue for all ages containing 5–7 sections that encompassed the VP and OT cell groups in the PVN and SON; we used these cell groups as a metric for making certain that quantification was similar between the ages because of their expansive size. Sectioning the brains with the aim of obtaining extremely similar coverage of the PVN and SON VP and OT cell groups resulted in similar anterior–posterior VP and OT neuron distribution for all cell groups quantified for all ages.
One series of tissue from each subject was immunofluorescently triple labeled for VP, OT, and Fos. Subjects from different groups were counterbalanced across immunocytochemical runs. Tissue was rinsed 5x for 10 min in 0.1M PBS (pH 7.4), incubated for 1 h in block (PBS + 10% normal donkey serum + 0.03% Triton-X-100), and then incubated for approximately 48 h in primary antibodies diluted in PBS containing 5% normal donkey serum + 0.03% Triton-X-100. Primary antibodies used were guinea pig anti-VP (1:1000; Peninsula Laboratories Cat# T-5048.0050 RRID:AB_518680), mouse anti-OT (1:325; Millipore Cat# MAB5296 RRID:AB_2157626), and rabbit anti-Fos (1:200; Santa Cruz Biotechnology Cat# sc-253 RRID:AB_2231996). The primary incubation was followed by two 30 min rinses in PBS. Tissue was incubated for 1 h in a biotinylated donkey anti-guinea pig secondary (1:125; Jackson ImmunoResearch Labs Cat# 706–065–148 RRID:AB_2341097), rinsed twice for 15 min in PBS, and incubated for 2 h at room temperature in streptavidin conjugated to Alexa Fluor 488 (1:325; Molecular Probes Cat# S32354 also S32354 RRID:AB_2315383), donkey anti-mouse secondary conjugated to Alexa Fluor 680 (1:165; Thermo Fisher Scientific Cat# A10038 RRID:AB_2534014), and donkey anti-rabbit secondary conjugated to Alexa Fluor 594 (1:200; Molecular Probes Cat# A-21207 also A21207 RRID:AB_141637). All secondary antibodies were diluted in PBS containing 5% normal donkey serum + 0.03% Trion-X-100. Following two 30 min rinses in PBS, sections were mounted on microscope slides and cover-slipped with Prolong Gold antifade containing a DAPI nuclear stain (Thermo Fisher Scientific).
Quantification and analysis
Photomicrographs were captured using a Zeiss AxioImager II microscope outfitted with an AxioCam MRm, z-drive, and an Apotome optical dissector (Carl Zeiss Inc., Gottingen, Germany). Flattened z-stack images were used by observers blind to treatment condition to conduct cell counts in Photoshop CS6 (Adobe Systems, RRID:SCR_014199) and ImageJ (ImageJ, RRID:SCR_003070) as previously described (Goodson and Wang 2006; Kelly and Goodson 2014a; Kelly et al. 2017).
We quantified the total number of VP-ir and OT-ir neurons, the total number of Fos-ir cells, and the number VP-ir and OT-ir neurons expressing Fos. We examined the proportion of VP and OT cells that were doubled labeled for Fos (hereafter referred to as VP-Fos or OT-Fos co-localization) to account for individual differences in the number of nona-peptide neurons. Cell counts for VP cell groups were quantified in the PVN, SON, AH, and suprachiasmatic nucleus (SCN). Cell counts for OT cell groups were quantified in the PVN, SON, MPO, and BST. For visual representations of these primary hypothalamic and accessory nonapeptide nuclei in rodents, see (Rood and De Vries 2011; Wang et al. 1996). We intended to quantify the BST VP cell group but could not, because VP-ir neurons were virtually absent in this region in our subjects. This is likely because VP production in the BST is strongly regulated by steroids (De Vries and Panzica 2006), and our subjects were not reproductively mature. Furthermore, a lack of borax in the paraformaldehyde used for perfusions may also result in a lack of visualization of this cell group (pers. comm. AH Veenema). The PVN and SON VP and OT cell groups were quantified at two levels given the extensive size of these cell groups. Although the magnocellular and parvocellular organization of the PVN has not been characterized in prairie voles, the rostral level of quantification primarily encompasses the magnocellular component, whereas the caudal level quantified encompasses primarily parvocellular components as described in rats by (Swanson and Kuypers 1980; Swanson and Sawchenko 1983).
Statistics
Neural data were analyzed using Linear Mixed Models (LMM) in SPSS 24 (IBM Analytics, USA). Sex of the pup, pup age, and treatment condition were analyzed as fixed factors, while litter number was assigned as a random factor to control for genetic variation in nonapeptide anatomy and function. Non-normally distributed data for VP-ir, OT-ir, or Fos-ir were log transformed. Neural activity data (VP-Fos and OT-Fos co-labeling) were analyzed using the arcsine transformation of the proportion of the number of double labeled neurons out of the total number of neurons in the cell group. Because this experiment was designed to examine differential responses to social condition based on age, we present data for Age × Condition interactions. We found no significant interactions for Sex × Age × Condition for any analysis, and thus, higher order interactions involving Sex are not discussed. See supplementary materials for data on anatomical development (VP-ir and OT-ir cell number) unrelated to social condition, and for sex or social condition differences in neural activity (Fos responses and VP/OT-Fos co-localization). All post hoc pairwise comparisons were adjusted using the Bonferonni correction. All data are presented as means ± standard errors.
Results
The objective of this experiment was to elucidate differential nonapeptide functioning in response to reunion with or isolation from families at different stages of early development. Thus, here, we focus on Age × Condition interactions.
Fos responses to social condition differ across development
Fos is an immediate early gene, from which neural activation can be inferred when neurons express Fos approximately 90 min after a specific experience. We quantified Fos-ir to provide a general characterization of neural activation at three stages of postnatal development under three distinct social contexts. We focused exclusively on brain regions known to contain VP or OT neurons because of the importance of nonapeptide neurons in social behavior.
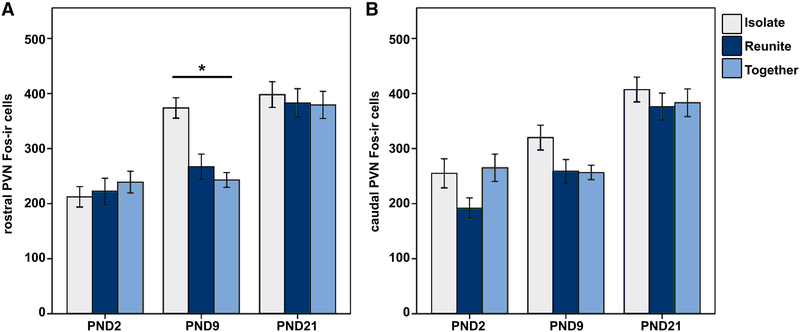
We observed significant differences in PVN Fos-ir expression indicating a pattern of rapid changes in PVN activation. Analysis of total (rostral and caudal levels combined) PVN Fos-ir yielded a significant Age × Condition interaction [F(4,150) = 2.427; p = 0.05; LMM], with only the Isolated PND9 pups exhibiting more Fos-ir than subjects of the same age in the Together (mean diff. = 194.298; p = 0.001) and Reunite (mean diff. = 168.089; p = 0.009) conditions.
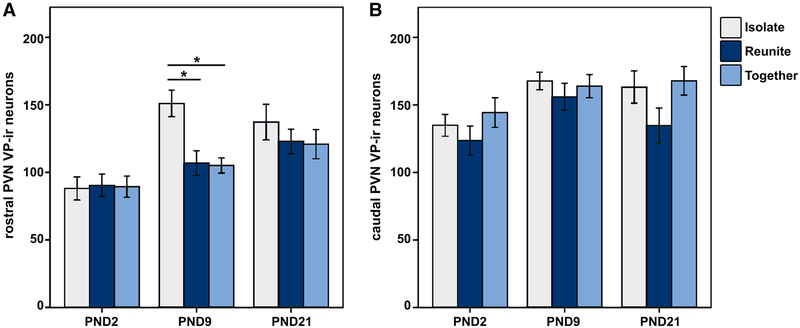
Due to the large size of the PVN, we quantified labeling at two levels (rostral and caudal). Although the magnocellular and parvocellular organization of the PVN has not been characterized in prairie voles, the rostral level of quantification primarily encompasses the magnocellular component, whereas the caudal level quantified encompasses primarily parvocellular components as described in rats by (Swanson and Kuypers 1980; Swanson and Sawchenko 1983). To determine if the effect observed for total PVN Fos-ir was driven by effects of the rostral or caudal levels, we also analyzed Fos-ir for the rostral-caudal subdivisions of the PVN. When subdivided, we observed a significant Age × Condition interaction for rostral PVN Fos-ir [F(4,150) = 4.165; p = 0.003; LMM; Fig. 2a], with PND9 pups that were Isolated exhibiting more Fos-ir than subjects in the Together (Mean diff. = 130.889; p < 0.001) and Reunite (Mean diff. = 106.789; p = 0.001) conditions. However, we found no significant Age × Condition interaction for caudal PVN Fosir [F(4,150) = 1.092; p = 0.363; LMM; Fig. 2b), demonstrating differential functioning within subdivisions of the PVN.
Fig. 2.
a Mean (± SEM) number of Fos immunoreactive (ir) neurons in the rostral paraventricular nucleus of the hypothalamus (PVN) for PND2, PND9, and PND21 pups that were Isolated (light blue), Reunited (dark blue), or Together (gray) for 1.5 h. *Indicates p ≤ 0.05. b Mean (± SEM) number of Fos immunoreactive (ir) neurons in the caudal PVN for PND2, PND9, and PND21 pups that were isolated, reunited, or together for 1.5 h
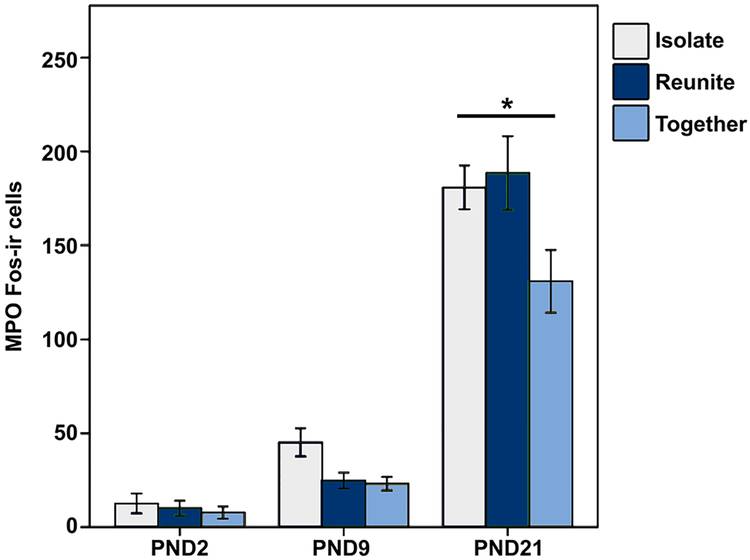
We also found a significant Age × Condition interaction for Fos-ir expression in the MPO [F(4,154) = 3.153, p = 0.016, LMM], with PND21 pups that were Isolated (Mean diff. = 49.897; p = 0.003) or Reunited (Mean diff. = 57.556; p < 0.001) having more Fos-ir than PND21 Together pups (Fig. 3). Finally, we found a significant Age × Condition interaction for Fos-ir expression in the caudal SON [F(4,152) = 2.662, p = 0.035, LMM]; however, post hoc analyses yielded no significant pairwise comparisons. These differences in Fos activity indicate that the developing vole brain responds to social isolation or reunion differentially based on age. We found no differences in Fos expression in the AH [F(4,147) = 0.279, p = 0.891, LMM], SCN [F(4,148) = 0.674, p = 0.611, LMM], or BST [F(4,150) = 0.374, p = 0.827, LMM].
Fig. 3.
Mean (± SEM) number of Fos immunoreactive (ir) neurons in the medial preoptic nucleus (MPO) for PND2, PND9, and PND21 pups that were isolated (light blue), reunited (dark blue), or together (gray) for 1.5 h. *Indicates p ≤ 0.05
VP and OT neuronal activity (Fos co-localization) in response to social condition does not differ across development
Surprisingly, we found no significant Age × Condition interactions for VP-Fos or OT-Fos co-labeling for any of the VP and OT cell groups examined. These data suggest that nonapeptide-dependent neural activity, as assessed by Fos co-expression, does not differ throughout early development in response to social isolation or familial reunion.
PVN VP and OT cell groups exhibit rapid protein synthesis in PND9 pups
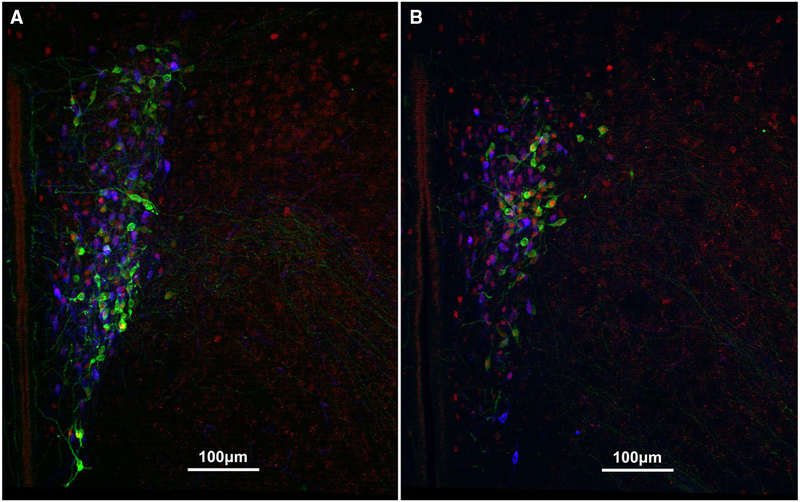
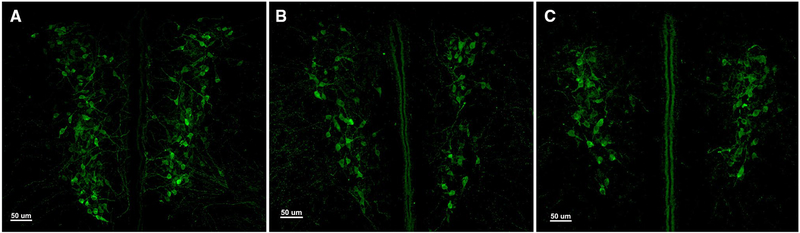
Finally, we quantified the total number of VP-ir and OT-ir neurons in the brain to assess how nonapeptide expression changes at each of these three critical stages of development. As discussed above, we grossly partitioned the PVN into rostral (Fig. 4a; presumably captures the magnocellular component) and caudal (Fig. 4b; presumably captures parvocellular components) subdivisions. Interestingly, analyzing the PVN VP cell group in this way yielded different results for each subdivision. Unexpectedly, we found that VP-ir appeared to be altered on the same timeline as Fos expression, with our results showing a robust Age × Condition interaction for VP-ir neurons in the rostral PVN [F(4,150) = 2.416; p = 0.003; LMM]. Specifically, the PND9 animals showed a unique VP expression pattern, in which pups that were Isolated exhibited significantly more VP-ir neurons compared to subjects that were in the Together (Mean diff. = 45.919; p < 0.001) and Reunite (Mean diff. = 44.206; p = 0.001) conditions (Fig. 5). This result demonstrates a profoundly rapid synthesis of VP in response to social isolation (Fig. 6a). Analysis of the caudal PVN VP-ir neurons yielded no significant Age × Condition interaction [F(4,150) = 0.519; p = 0.722; LMM; Fig. 6b], indicating that this unprecedented rapid synthesis is specific to rostral PVN VP neurons that are located primarily in the magnocellular component of the PVN.
Fig. 4.
Representative immunocytochemical staining of VP (green; Alexa Fluor 488), OT (pseudo-colored blue; Alexa Fluor 680), and Fos (red; Alexa Fluor 594) in the a rostral PVN and b caudal PVN of a PND9 male in the Together condition. Scale bar = 100 μm
Fig. 5.
Representative immunocytochemical staining of VP (green; Alexa Fluor 488) in the rostral PVN of PND9 male subjects in the a isolate, b reunite, and c together conditions
Fig. 6.
a Mean (± SEM) number of vasopressin (VP) immunoreactive (ir) neurons in the rostral portion of the paraventricular nucleus of the hypothalamus (PVN) for PND2, PND9, and PND21 pups that were Isolated (light blue), Reunited (dark blue), or Together (gray) for 1.5 h. *Indicates p ≤ 0.05. b Mean (± SEM) number of VP-ir neurons in the caudal portion of the PVN for PND 2, PND9, and PND21 pups that were isolated, reunited, or together for 1.5 h
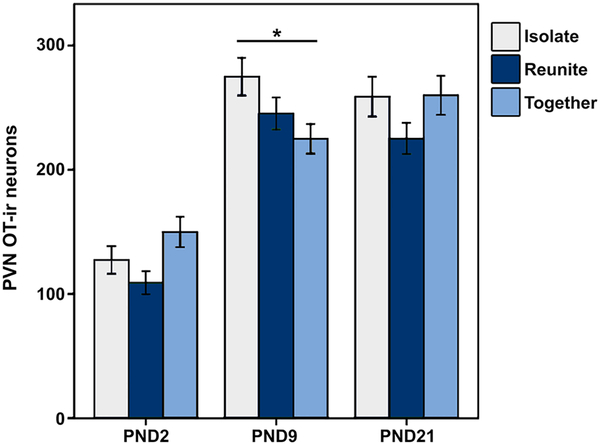
Separate analysis of PVN OT at the rostral and caudal levels did not yield significant Age × Condition interactions for OT-ir [rostral: F(4,150) = 1.979; p = 0.101; LMM; caudal: F(4,150) = 2.403; p = 0.084; LMM]. However, combining the two levels for a total measurement of PVN OT-ir yielded a significant interaction of Age × Condition [F(4,150) = 2.748; p = 0.030; LMM; Fig. 7]. Just as we found with the rostral PVN VP-ir neurons, pups that were Isolated exhibited significantly more OT-ir neurons in the PVN than pups in the Together condition (Mean diff. = 49.581; p = 0.019) in only PND9 subjects.
Fig. 7.
Mean (± SEM) number of oxytocin (OT) immunoreactive (ir) neurons in the paraventricular nucleus of the hypothalamus (PVN; rostral and caudal levels combined) for PND2, PND9, and PND21 pups that were Isolated (light blue), Reunited (dark blue), or Together (gray) for 1.5 h. *Indicates p ≤ 0.05
SON and accessory VP and OT cell groups do not exhibit rapid VP or OT synthesis or release
We observed no significant Age × Condition interactions for VP-ir cell groups [SON: F(4,152) = 1.324; p = 0.264; LMM; AH: VP: F(4,147) = 1.714; p = 0.415; LMM; SCN: VP: F(4,148) = 1.193; p = 0.316; LMM] or OT-ir cell groups [SON: F(4,152) = 0.294; p = 0.881; LMM; MPO: OT: F(4,154) = 2.062; p = 0.094; LMM; BST: OT: F(4,150) = 1.472; p = 0.219; LMM] outside of the PVN. This lack of significance, along with a lack of main effects of Condition for these cell groups, highlights the unique rapid synthesis of VP and OT exhibited by the PVN.
Discussion
We sought to characterize the functional responses of VP and OT neurons across different stages of early development following different parent-offspring social contexts. We observed no functional differences across development in nonapeptide responses to reunion with or isolation from pups’ families as assessed by VP/OT-Fos co-labeling. However, we did observe a striking increase of nonapeptides in response to social isolation only in the PVN and only in PND9 pups. These changes were concomitant with a similar increase in Fos activation in the PVN of isolated PND9 pups. While Fos changes are known to be rapid, increases in immunoreactivity of peptides in response to social environment typically take several days to weeks to express. Our data showing short-order increases of VP in the rostral region of the PVN and OT across the PVN suggest that nonapeptide neurons of the PVN exhibit hyper-responsivity during a neurodevelopmental period characterized by profound changes in the brain (Semple et al. 2013).
Consequences of social isolation
Social isolation is a stressful experience at any age, and mechanisms closely associated with the HPA axis might explain why PND9 socially isolated pups showed the most dramatic effects in our study. Indeed, the increased PVN Fos-ir in our study might be attributed to cell types that we did not measure like corticotropin-releasing hormone (CRH). CRH is the major neuropeptide that controls pituitary ACTH secretion in response to stress in adult animals (Beurel and Nemeroff 2014; Lightman 2008). Thus, elevated Fos-ir expression might relate to PVN CRH-neuron activation. Importantly, however, magnocellular VP is also involved in the control of secretion of ACTH (Antoni 1993; Sivukhina and Jirikowski 2016), and studies suggest that VP may assume an even more pronounced role in ACTH release in developing animals. For example, in response to a brief restraint stress, PND12 rat pups that were maternally deprived for 24 h exhibited elevated levels of ACTH and CORT, and an up-regulation of VP and CRH mRNA. Notably, these mRNA increases were observed on rapid time-scales, with increases observed after only 15 min in PND12 pups, whereas mRNA increases in PND18 pups were not observed until 4 h after the stressor (Dent et al. 2000). These findings, together with our results, suggest that neuropeptides of the PVN involved in regulating the HPA axis exhibit distinct cellular properties during early development. Indeed, despite differences in experimental design between our study and (Dent et al. 2000), VP appears to consistently play a critical role in regulating the HPA axis in response to stress during early development (Levine 2002). Our findings showing a rapid response of VP-ir synthesis to social isolation in PND9 pups support this conclusion.
Surprisingly, a few studies have examined the consequences of acute social isolation on nonapeptide functional responses at any age. The overwhelming majority of studies examining the effects of social isolation on nonapeptides involve chronic social isolation. Consequently, the effects of acute social isolation on IEG activity in nonapeptide cells have not been described. Studies in prairie voles demonstrate that adult females isolated for 60 days exhibit more PVN OT-ir neurons compared to socially housed females (Grippo et al. 2007a), and males isolated for 6 weeks after weaning exhibit more VP and OT mRNA in the PVN compared to socially housed males (Pan et al. 2009). We also found increases in PVN VP-ir and OT-ir in response to social isolation, which is consistent with these studies in adults; however, the increases we observed were on a considerably shorter timescale.
Changes in immunoreactivity are interpreted as either synthesis (for increases) or release (for decreases) of the peptide. We believe that our results indicate rapid synthesis of PVN nonapeptides in isolated PND9 pups. Studies examining extracellular VP and OT in the PVN following acute stressors show elevated peptide, suggesting that acute stress induces peptide release in the PVN (Bosch et al. 2005; Wigger et al. 2004; Kojima et al. 2012). Thus, it is likely that the rapid PVN VP and OT up-regulation observed here was a consequence of these neurons compensating for sudden releases of these peptides in response to isolation. Our data suggest that a rapid compensation for peptide release in response to a stressor may only be possible at a time (~ PND9) when the brain is experiencing substantial growth (Gottlieb et al. 1977; Semple et al. 2013). An alternative interpretation of the results here could be that PND9 pups may express extraordinarily high levels of VP and OT in the PVN, and that PND9 animals in the Together and Reunite conditions rapidly released large quantities of peptides, whereas Isolated pups did not. However, the aforementioned nonapeptide increases to chronic isolation in adults, and VP and OT release in response to acute stressors argue against this interpretation. The feature unifying Together and Reunite PND9 subjects, and distinguishing them from Isolate subjects, is sustained social contact (i.e., 90 min or more with the family unit). If the PND9 Together and Reunite groups did indeed release VP and/or OT, it was likely a tonic release due to social experiences during the 90 min of the IEG phase. However, Isolated animals would have experienced time with family units just prior to the IEG test, and, therefore, also would have experienced this (potentially chronic) release of nonapeptides. Thus, rapid synthesis would still be necessary for the nonapeptide levels in Isolated pups to reach the height that they did. Whatever the cause (synthesis resulting from isolation or recovery from cessation of tonic release due to social contact), the best explanation for the difference which we found is that VP and OT appear to be synthesized in Isolated PND9 pups on a profoundly short time scale.
Interestingly, we only observed increased VP-ir expression in the rostral portion of the PVN, which is populated with more magnocellular neurons than the caudal portion. This implies that the magnocellular PVN VP neurons, which exert direct effects on the periphery through a single axonal projection to the posterior pituitary (Ludwig and Leng 2006), are selectively responsive to stress at PND9. In contrast, we only observed a significant increase in PVN OT-ir when both rostral and caudal measures were combined, suggesting that both peripheral and central OT are important at PND9 in response to isolation. Notably, OT attenuates HPA axis activity as a coping reaction to stress both centrally and peripherally by lowering the basal and/or stress-induced concentrations of adrenocorticotropic hormone (ACTH) and corticosterone (CORT) (Neumann et al. 2000; Sivukhina and Jirikowski 2016). Our findings might, therefore, suggest that PVN OT plays a significant role in rapidly mediating the stress response to social isolation during a unique and vulnerable time in development.
The lack of rapid PVN VP and OT synthesis in isolated PND2 and PND21 pups may reflect a distinct parent-off-spring bond in PND9 animals. Given that PND9 prairie voles have open eyes and can independently locomote (Robison et al. 2016; Solomon 1991), they may be more attuned to their social environment compared to PND2 pups; PND9 offspring have also had more time to develop a more substantial bond with their parents and siblings. Meanwhile, PND21 pups are at an age where they can survive without their parents, whereas PND9 pups still depend on their mother for sustenance (Solomon 1991). Notably, parents also spend significantly less time in contact with PND21 pups compared to PND2 and PND9 pups, likely reflecting a transition of promoting offspring independence in weaning aged offspring (Kelly et al. 2017). Thus, the combination of a well-developed parent-offspring bond with a dependence on the mother for survival may underlie the unique cellular functions of neuropeptides in the PVN observed in PND9 animals. Future studies should be directed at honing the age range of which this unique rapid synthesis of PVN VP and OT occurs. Furthermore, it is feasible that we would have also observed rapid synthesis in PND9 pups that were Reunited with their families if the pre-IEG isolation period were longer. The length of isolation needed to induce rapid PVN nonapeptide synthesis also warrants future investigation.
In addition to significant differences observed within the PVN, we also found that neural activation (Fos-ir) in response to social condition in the MPO differed by age. Our findings suggest that, unrelated to VP neuronal function, neural activity within the MPO increases in response to social isolation only in weaning aged pups (PND21). Although the MPO is best known for modulating reproductive behavior (Hull 2010), studies have elucidated a role of the MPO to indirectly affect the HPA axis via connections through the PVN (Williamson and Viau 2007, 2008). MPO effects on the HPA axis are androgen mediated (Williamson et al. 2010), which may explain why we do not see significant differences in response to isolation in the younger, significantly less reproductively developed pups. In addition, the MPO also has neuronal projections to regions of the forebrain that regulate numerous behaviors and physiological mechanisms related to homeostasis (Simerly and Swanson 1988). Our findings in the MPO, together with the significant interaction observed for SON Fos-ir (despite the lack of significance in post hoc analyses), suggest that numerous areas of the hypothalamus are sensitive to stressful environments, and how regions of the hypothalamus respond to stressors is influenced by the developmental stage of an animal.
Although of interest, the ecological relevance of these findings for prairie voles in the wild is difficult to discern. Prairie vole pups in the wild are reared in three types of social groups: (1) communal groups, (2) male–female pairs with offspring, and (3) single-female with offspring (Getz et al. 1993; McGuire and Lowell 1995). Previous laboratory studies have shown that prairie vole parents coordinate nest attendance rather than coming and going independently (Ahern et al. 2011). Thus, our findings in relation to social isolation may be most reflective of isolation events that may occur with greater frequency for prairie vole pups reared by single-females in the wild. However, resource availability, predation, and other ecological variables are often unstable (Clutton-Brock 1991; Winkler 1987), and it is possible that pups in the wild may experience isolation from parents in more than just single-female families. Therefore, we would expect all prairie voles to exhibit the PVN nonapeptide rapid synthesis capabilities at critical periods of development observed here. Nonetheless, it is interesting to consider that the degree of this rapid plasticity may vary based on environmental experiences and differences in family structure in the wild.
When peptides respond like Fos: the uniqueness of PND9 rapid VP-OT synthesis in the PVN
Fos gene expression is rapidly induced in neurons, with its protein product peaking 60–90 min after stimulation (Hoffman et al. 1993). As a result, Fos is commonly used to assess changes in neuronal activity. Importantly, although Fos usually indicates neuronal firing, it does not always indicate protein synthesis (Hoffman and Lyo 2002). It is common for IEG studies to find nonapeptide functional responses (VP-or OT-Fos co-localization) 1–2 h after an acute stressor, but, such studies do not also report rapid changes in protein expression. For instance, sheep that were sacrificed 1.5 h after an acute social isolation stressor exhibited increased PVN VP-Fos co-localization compared to non-isolated animals, but no changes in VP-ir were observed (Rivalland et al. 2007). Adult California mice that underwent a social defeat stress test exhibited greater PVN VP- and OT-Fos co-localization 1 h later, but no changes in VP- or OT-ir neuron number (Steinman et al. 2015, 2016). Moreover, in response to 1.5 h of offspring separation, adult male and female prairie voles exhibited changes in VP- and OT-Fos co-localization within several cell groups, including the PVN, but no changes in VP- or OT-ir were observed (Kelly et al. 2017).
Work such as this has led the assumption that nonapeptide-producing neurons are stable, but IEGs change rapidly, making colocalized IEG experiments using immunocytochemistry useful to assess activation of neurons containing particular peptides of interest. In other words, co-localization is based on a proportion, in which the numerator is expected to be relatively dynamic and the denominator is expected to be relatively static. However, our results for the PVN VP and OT cell groups appear to violate this basic assumption given that we observed both Fos activation and an unprecedented change in protein (i.e., VP-ir and OT-ir) expression. This change in both Fos and peptide might explain why our co-localization data did not differ across groups in this region; the rate of Fos expression appears to increase concomitantly with that of PVN OT and VP. In this case, the lack of differences in VP-Fos and OT-Fos co-localization across ages and conditions reflects a proportional scaling of both Fos and OT or VP. That is, if the total number of Fos and VP or OT cells increase at the same rate as each other, then the number of cells that co-express Fos and either VP or OT should be proportional to those that did not show the concurrent increase of nonapeptide and Fos overall. It is worth noting that Fos and nonapeptides need not have scaled proportionately; the rate of Fos increases could have just as easily outpaced or under-paced rates of VP and OT, or could have changed in different directions (one increases, while the other decreases). Nevertheless, our study demonstrates a proportional and linear scaling of nonapeptide and Fos increases (both in terms of timing and number) in the PVN of PND9 pups following social isolation.
Changes in neuropeptide immunoreactivity are typically not observed until at least several days to weeks after manipulation in adult animals (Grippo et al. 2007a; Li et al. 2016; Pan et al. 2009; Bamshad et al. 1994; Song et al. 2010), and rapid responses to environmental changes (e.g., stressors, social interactions, etc.) are typically assessed using either IEGs or mRNA quantification. For example, in adult rats, increases in PVN VP mRNA can be seen 2 h after restraint stress (Ma et al. 1997). However, induction of mRNA does not guarantee induction of protein (Maier et al. 2009; Lodish et al. 2007). It is extremely rare to observe such rapid changes in peptide expression, and, to our knowledge, this is the first report of PVN VP-ir or OT-ir changes occurring on the same timescale as Fos (but see Wang et al. 2014; Cayetanot et al. 2005).
The rapid up-regulation of VP-ir and OT-ir that we observed questions whether nonapeptide expression should be considered as ‘anatomy.’ Studies often refer to nonapeptide neuron number as ‘neuroanatomy’ and it has been contrasted within and between species (Goodson et al. 2009; Kabelik et al. 2010; Mendonca et al. 2013). However, VP and OT expression can change after 1 week or more of environmental manipulation (e.g., Grippo et al. 2007b; Song et al. 2010; Steinman et al. 2015; Li et al. 2016), suggesting that nonapeptide neuronal expression can be quite malleable. Furthermore, our findings of rapid change on the timescale of hours emphasizes that caution should be exercised when characterizing nonapeptide neuronal number in terms of anatomy. Perhaps, it would be better to think of peptide expression in the brain in terms of the extent to which peptide expression is limited in the degree to which it can vary, rather than as a relatively stable and unchanging feature, particularly when it is used to differentiate behavioral phenotypes.
The plastic PVN
We originally hypothesized that we would observe functional differences across development in the largest and most dynamic nonapeptide cell groups (i.e., PVN and SON). However, upon examination of eight nonapeptide cell groups, our results yielded only significant nonapeptide differences in the PVN. Surprisingly, although we did not find significant differences in PVN VP-Fos or OT-Fos co-localization across development, we observed a rapid synthesis of PVN VP and OT in response to acute social isolation. The PVN is one of the most important sites for the integration of neuroendocrine and autonomic mechanisms (Swanson and Sawchenko 1980). Neurons in the PVN play essential roles in controlling stress, metabolism, growth, reproduction, and immune and cardiovascular functions (Ferguson et al. 2008). Furthermore, the PVN nonapeptide neuronal populations play crucial roles in the modulation of numerous social behaviors and, in fact, have been implicated in social behavior more than any other region containing VP and OT neurons, including the SON (Kelly and Goodson 2014b). The profound role of PVN nonapeptide neurons in physiology and behavior may explain their hyper-responsivity at a critical period of development.
Interestingly, as discussed above, PVN CRH and VP mRNA biosynthesis in PND12 rat pups has also been found to exhibit rapid induction properties. In response to an acute mild stressor after maternal deprivation, pups exhibited significant increases in CRH and VP mRNA in the PVN after only 15 min (Dent et al. 2000). To our knowledge, CRH and VP mRNA have not been reported to occur before 1 h, and more commonly increases in gene expression take 2–3 h, and only under severe stressors (Levine 2002). These findings, together with our results, suggest that neuropeptides of the PVN involved in regulating the HPA axis exhibit distinct cellular properties during early development.
The capability of the PVN to rapidly synthesize nonapeptides implicates this brain region as exhibiting a high degree of plasticity that can allow organisms to rapidly adapt to changing environments during critical periods of development. Further studies are required to determine the extent of this plasticity. Overall, our results suggest a unique functional role of the PVN and highlight unique synthesis properties of PVN nonapeptide neurons in response to social isolation at a critical period during development of the prairie vole.
Supplementary Material
Acknowledgements
We are grateful for statistical help from the Statistics Consulting Center at Cornell University, to Chang Kim for assistance with PCR, and to Jeanne Powell for assistance with cell counts.
Conflict of interest The authors acknowledge the support from the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development HD081959 to AMK and HD079573 to AGO). The authors declare no competing financial interests and no conflicts of interest
Footnotes
Ethical approval All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures were approved by and were in compliance with the Institutional Animal Care and Use Committee of Cornell University (2013–0102).
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00429-018-1640-2) contains supplementary material, which is available to authorized users.
References
- Ahern TH, Hammock EAD, Young LJ (2011) Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster). Dev Psychobiol 53(2):118–131. 10.1002/dev.20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni FA (1993) Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol 14(2):76–122. 10.1006/frne.1993.1004 [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, de Vries GJ (1994) Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster). Physiol Behav 56(4):751–758 [DOI] [PubMed] [Google Scholar]
- Beurel E, Nemeroff CB (2014) Interaction of stress, corticotropin-releasing factor, arginine vasopressin and behaviour. Curr Top Behav Neurosci 18:67–80. 10.1007/7854_2014_306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhorst KH, Narayana PA, Liu R, Ahobila-Vijjula P, Ramu J (2008) Early postnatal development of rat brain: in vivo diffusion tensor imaging. J Neurosci Res 86:1520–1528. 10.1002/jnr.21607 [DOI] [PubMed] [Google Scholar]
- Boer GJ, Buijs RM, Swaab DF, De Vries GJ (1980) Vasopressin and the developing rat brain. Peptides 1:203–209 [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID (2005) Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci 25(29):6807–6815. 10.1523/JNEUROSCI.1342-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Bains JS, Ludwig M, Stern JE (2013) Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms. J Neuroendocrinol 25(8):678–710. 10.1111/jne.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Getz LL (1985) Social and hormonal determinants of reproductive patterns in the prairie vole. Neurobiology 18–36 [Google Scholar]
- Cayetanot F, Bentivoglio M, Aujard F (2005) Arginine-vasopressin and vasointestinal polypeptide rhythms in the suprachiasmatic nucleus of the mouse lemur reveal aging-related alterations of circadian pacemaker neurons in a non-human primate. Eur J Neurosci 22(4):902–910. 10.1111/j.1460-9568.2005.04268.x [DOI] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL (2007) Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinfo 5(1):79–94 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH (1991) The evolution of parental care Princeton University Press 352 10.1046/j.1420-9101.1992.5040719.x [DOI] [Google Scholar]
- De Vries GJ, Panzica GC (2006) Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience 138(3):947–955. 10.1016/j.neuroscience.2005.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JM, Moravec MD, Grafe M, Abend N, Ren J, Gong X, Volpe JJ, Jensen FE, Hohimer AR, Back SA (2011) Strain-specific differences in perinatal rodent oligodendrocyte lineage progression and its correlation with human. Dev Neurosci 33(3–4):251–260. 10.1159/000327242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaban AS (1987) Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol 4:345–356 [DOI] [PubMed] [Google Scholar]
- Dent GW, Okimoto DK, Smith MA, Levine S (2000) Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology 71(6):333–342 [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH (2017) Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol. 10.1002/cne.24216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT (2004) The hypothalamic-neurohy pophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol 25(3–4):132–149. 10.1016/j.yfrne.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ, Samson WK (2008) The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12(6):717–727. 10.1517/14728222.12.6.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch HS (1957) Aspects of reproduction and development in the prairie vole. University of Kansas Publications, Museum of Natural History 10 (4):129–161 [Google Scholar]
- Fox WM (1965) Reflex-ontogeny and behavioural development of the mouse. Anim Behav 13(2):234–241 [DOI] [PubMed] [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann JE, Frase B (1993) Social organization of the prairie vole (Microtus ochrogaster). J Mammal 74:44–58 [Google Scholar]
- Gilles YD, Polston EK (2017) Effects of social deprivation on social and depressive-like behaviors and the numbers of oxytocin expressing neurons in rats. Behav Brain Res 328:28–38. 10.1016/j.bbr.2017.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy D, Dominici C, Hardin-Pouzet H, Anouar Y, Melik-Parsadaniantz S, Rostene W, Reaux-Le Goazigo A (2017) Three-dimensional distribution of tyrosine hydroxylase, vasopressin and oxytocin neurones in the transparent postnatal mouse brain. J Neuroendocrinol. 10.1111/jne.12551 [DOI] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR (2010) Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol 20(6):784–794. 10.1016/j.conb.2010.08.020 [DOI] [PubMed] [Google Scholar]
- Goodson JL, Wang Y (2006) Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA 103(45):17013–17017. 10.1073/pnas.0606278103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM (2009) Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav 55(1):197–202. 10.1016/j.yhbeh.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb A, Keydar I, Epstein HT (1977) Rodent brain growth stages: an analytical review. Biol Neonate 32(3–4):166–176 [DOI] [PubMed] [Google Scholar]
- Grinevich V, Desarmenien MG, Chini B, Tauber M, Muscatelli F (2014) Ontogenesis of oxytocin pathways in the mammalian brain: late maturation and psychosocial disorders. Front Neuroanat 8:164 10.3389/fnana.2014.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS (2007a) Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med 69(2):149–157. 10.1097/PSY.0b013e31802f054b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS (2007b) Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinol 32(8–10):966–980. 10.1016/j.psyneuen.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA (2015) Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology 40(1):24–42. 10.1038/npp.2014.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D (2002) Anatomical markers of activity in neuroendocrine systems: are we all ‘fos-ed out’? J Neuroendocrinol 14(4):259–268 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG (1993) c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 14(3):173–213. 10.1006/frne.1993.1006 [DOI] [PubMed] [Google Scholar]
- Hull EM (2010) Male sexual behavior. Encycl Behav Neurosci Acad Press; 154–162 [Google Scholar]
- Kabelik D, Morrison JA, Goodson JL (2010) Cryptic regulation of vasotocin neuronal activity but not anatomy by sex steroids and social stimuli in opportunistic desert finches. Brain Behav Evol 75(1):71–84. 10.1159/000297522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL (2014a) Personality is tightly coupled to vasopressin-oxytocin neuron activity in a gregarious finch. Front Behav Neurosci 8 (55). 10.3389/fnbeh.2014.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL (2014b) Social functions of individual vasopressin-oxytocin cell groups in vertebrates: What do we really know? Front Neuroendocrinol 35(4):512–529. 10.1016/j.yfrne.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hiura LC, Saunders AG, Ophir AG (2017) Oxytocin neurons exhibit extensive functional plasticity due to offspring age in mothers and fathers. Integr Comp Biol 57(3):603–618. 10.1093/icb/icx036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Saunders AG, Ophir AG (2018) Mechanistic substrates of a life history transition in male prairie voles: developmental plasticity in affiliation and aggression corresponds to nonapeptide neuronal function. Horm Behav 99:14–24. 10.1016/j.yhbeh.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Stewart RA, Demas GE, Alberts JR (2012) Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference. J Neuroendocrinol 24(5):831–840. 10.1111/j.1365-2826.2012.02280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch B (1980) Electron microscopic immunocytochemical investigation on the postnatal development of the vasopressin system in the rat. Cell Tissue Res 205:453–471 [DOI] [PubMed] [Google Scholar]
- Lagerspetz KYH (1966) Postnatal development of thermoregulation in laboratory mice. Helgol Mar Res 14(1):559–571 [Google Scholar]
- Landgraf R, Neumann ID (2004) Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 25(3–4):150–176. 10.1016/J.Yfrne.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Levine S (2002) Regulation of the hypothalamic-pituitary-adrenal axis in the neonatal rat: the role of maternal behavior. Neurotox Res 4(5–6):557–564. 10.1080/10298420290030569 [DOI] [PubMed] [Google Scholar]
- Li J, Li HX, Shou XJ, Xu XJ, Song TJ, Han SP, Zhang R, Han JS (2016) Effects of chronic restraint stress on social behaviors and the number of hypothalamic oxytocin neurons in male rats. Neuropeptides 60:21–28. 10.1016/j.npep.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Lightman SL (2008) The neuroendocrinology of stress: a never ending story. J Neuroendocrinol 20(6):880–884. 10.1111/j.1365-2826.2008.01711.x [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, Kaiser CA, Krieger M, Scott MP, Bretscher A, Ploegh H, Matsudaira P (2007) Molecular Cell Biology, 6th edn. WH Freeman and Company, New York, NY [Google Scholar]
- Ludwig M, Leng G (2006) Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7(2):126–136. 10.1038/nrn1845 [DOI] [PubMed] [Google Scholar]
- Ma XM, Levy A, Lightman SL (1997) Rapid changes in heteronuclear RNA for corticotrophin-releasing hormone and arginine vasopressin in response to acute stress. J Endocrinol 152(1):81–89 [DOI] [PubMed] [Google Scholar]
- Maier T, Guell M, Serrano L (2009) Correlation of mRNA and protein in complex biological samples. FEBS Lett 583(24):3966–3973. 10.1016/j.febslet.2009.10.036 [DOI] [PubMed] [Google Scholar]
- McGuire B, Lowell LL (1995) Communal nesting in prairie voles (Microtus ochrogaster)—an evaluation of costs and benefits based on patterns of dispersal and settlement. Can J Zool 73:383–391 [Google Scholar]
- Mendonca R, Soares MC, Bshary R, Oliveira RF (2013) Arginine vasotocin neuronal phenotype and interspecific cooperative behaviour. Brain Behav Evol 82(3):166–176. 10.1159/000354784 [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R (2000) Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol 12(3):235–243 [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z (2009) Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett 454(1):67–71. 10.1016/j.neulet.2009.02.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Zhu C, Wang X, Xu F, Eriksson PS, Nilsson M, Cooper-Kuhn CM, Kuhn HG, Blomgren K (2007) Less neurogenesis and inflammation in the immature than in the juvenile brain after cerebral hypoxia-ischemia. J Cereb Blood Flow Metab 27(4):785–794. 10.1038/sj.jcbfm.9600385 [DOI] [PubMed] [Google Scholar]
- Rabhi M, Ugrumov MV, Goncharevskaya OA, Bengelloun W, Calas A, Natchin YV (1996) Development of the hypothalamic vasopressin system and nephrons in Meriones shawi during ontogenesis. Anat Embryol (Berl) 193(3):281–296 [DOI] [PubMed] [Google Scholar]
- Rabhi M, Stoeckel ME, Calas A, Freund-Mercier MJ (1999) Historadioautographic localisation of oxytocin and vasopressin binding sites in the central nervous system of the merione (Meriones shawi). Brain Res Bull 48(2):147–163 [DOI] [PubMed] [Google Scholar]
- Rivalland ET, Clarke IJ, Turner AI, Pompolo S, Tilbrook AJ (2007) Isolation and restraint stress results in differential activation of corticotrophin-releasing hormone and arginine vasopressin neurons in sheep. Neuroscience 145(3):1048–1058. 10.1016/j.neuroscience.2006.12.045 [DOI] [PubMed] [Google Scholar]
- Robison WT, Myers MM, Hofer MA, Shair HN, Welch MG (2016) Prairie vole pups show potentiated isolation-induced vocalizations following isolation from their mother, but not their father. Dev Psychobiol 58(6):687–699. 10.1002/dev.21408 [DOI] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ (2011) Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol 519(12):2434–2474. 10.1002/cne.22635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ (2013) Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106–107: 1–16. 10.1016/j.pneurobio.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW (1988) Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol 270(2):209–242. 10.1002/cne.902700205 [DOI] [PubMed] [Google Scholar]
- Sivukhina EV, Jirikowski GF (2016) Magnocellular hypothalamic system and its interaction with the hypothalamo-pituitary-adrenal axis. Steroids 111:21–28. 10.1016/j.steroids.2016.01.008 [DOI] [PubMed] [Google Scholar]
- Smith JA, Pati D, Wang L, de Kloet AD, Frazier CJ, Krause EG (2015) Hydration and beyond: neuropeptides as mediators of hydromineral balance, anxiety and stress-responsiveness. Front Syst Neurosci 9:46 10.3389/fnsys.2015.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH (2017) Age and sex differences in oxytocin and vasopressin V1a receptor bindign densities in the rat brain: focus on the social decision-making network. Brain Struct Funct 222(2):981–1006. 10.1007/s00429-016-1260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NG (1991) Current indirect fitness benefits associated with philopatry in juvenile prairie voles. Behav Ecol Sociobiol 29(4):277–282. 10.1007/bf00163985 [DOI] [Google Scholar]
- Song Z, Tai F, Yu C, Wu R, Zhang X, Broders H, He F, Guo R (2010) Sexual or paternal experiences alter alloparental behavior and the central expression of ERalpha and OT in male mandarin voles (Microtus mandarinus). Behav Brain Res 214(2):290–300. 10.1016/j.bbr.2010.05.045 [DOI] [PubMed] [Google Scholar]
- Sperelakis N (2001) Cell physiology sourcebook: a molecular approach, 3rd edn. Academic Press, San Diego [Google Scholar]
- Steinman MQ, Laredo SA, Lopez EM, Manning CE, Hao RC, Doig IE, Campi KL, Flowers AE, Knight JK, Trainor BC (2015) Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinol 51:122–134. 10.1016/j.psyneuen.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, Laman-Maharg A, Manning CE, Doig IE, Lopez EM, Walch K, Bales KL, Trainor BC (2016) Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol Psychiatry 80(5):406–414. 10.1016/j.biopsych.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Ter Borg JP (1981) Development of peptidergic systems in the rat brain. Ciba Found Symp 86:271–294 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG (1980) The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194(3):555–570. 10.1002/cne.901940306 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE (1980) Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31(6):410–417 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE (1983) Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6:269–324. 10.1146/annurev.ne.06.030183.001413 [DOI] [PubMed] [Google Scholar]
- Tamborski S, Mintz EM, Caldwell HK (2016) Sex differences in the embryonic development of the central oxytocin system in mice. J Neuroendocrinol. 10.1111/jne.12364 [DOI] [PubMed] [Google Scholar]
- Wang Z, Young LJ (1997) Ontogeny of oxytocin and vasopressin receptor binding in the lateral septum in prairie and montane voles. Brain Res Dev Brain Res 104(1–2):191–195 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR (1996) Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol 366 (4):726–737. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang W, Wu R, Kong L, Feng W, Cao Y, Tai F, Zhang X (2014) Neuroendocrine responses to social isolation and paternal deprivation at different postnatal ages in mandarin voles. Dev Psychobiol 56(6):1214–1228. 10.1002/dev.21202 [DOI] [PubMed] [Google Scholar]
- Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R (2004) Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology 29(1):1–14. 10.1038/sj.npp.1300290 [DOI] [PubMed] [Google Scholar]
- Williamson M, Viau V (2007) Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol 503(6):717–740. 10.1002/cne.21411 [DOI] [PubMed] [Google Scholar]
- Williamson M, Viau V (2008) Selective contributions of the medial preoptic nucleus to testosterone-dependant regulation of the paraventricular nucleus of the hypothalamus and the HPA axis. Am J Physiol Regul Integr Comp Physiol 295(4):R1020–R1030. 10.1152/ajpregu.90389.2008 [DOI] [PubMed] [Google Scholar]
- Williamson M, Bingham B, Gray M, Innala L, Viau V (2010) The medial preoptic nucleus integrates the central influences of testosterone on the paraventricular nucleus of the hypothalamus and its extended circuitries. J Neurosci 30(35):11762–11770. 10.1523/JNEUROSCI.2852-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler DW (1987) A general model for parental care. Am Nat 103:526–543 [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS (2004) Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 125(4):947–955. 10.1016/j.neuroscience.2004.02.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.