Abstract
Heart size is an important factor in cardiac health and disease. In particular, increased heart weight is predictive of adverse cardiovascular outcomes in multiple large community-based studies. We use two cohorts of Diversity Outbred (DO) mice to investigate the role of genetics, sex, age, and diet on heart size. DO mice (n = 289) of both sexes from generation 10 were fed a standard chow diet, and analyzed at 12–15 weeks of age. Another cohort of female DO mice (n = 258) from generation 11 were fed either a high-fat, cholesterol-containing (HFC) diet or a low-fat, high-protein diet, and analyzed at 24–25 weeks. We did not observe an effect of diet on body or heart weight in generation 11 mice, although we previously reported an effect on other cardiovascular risk factors, including cholesterol, triglycerides, and insulin. We do observe a significant genetic effect on heart weight in this population. We identified two quantitative trait loci for heart weight, one (Hwtf1) at a genome-wide significance level of p ≤ 0.05 on MMU15 and one (Hwtf2) at a genome-wide suggestive level of p ≤ 0.1 on MMU10, that together explain 13.3% of the phenotypic variance. Hwtf1 contained collagen type XXII alpha 1 chain (Col22a1), and the NZO/HlLtJ and WSB/EiJ haplotypes were associated with larger hearts. This is consistent with heart tissue Col22a1 expression in DO founders and SNP patterns within Hwtf1 for Col22a1. Col22a1 has been previously associated with cardiac fibrosis in mice, suggesting that Col22a1 may be involved in pathological cardiac hypertrophy.
Introduction
Post-natal heart growth occurs primarily through hypertrophy of its constituent cardiomyocytes. The size of the heart, or the extent to which it hypertrophies, has important implications for cardiovascular health. Numerous large population-based studies have indicated that cardiac hypertrophy is a strong predictor of adverse outcomes including myocardial infarction, heart failure, stroke, and death (Levy et al. 1989, 1990; Bikkina et al. 1994; Gardin et al. 2001). Heart size in mature mammals is a highly complex polygenic trait and is influenced by extracardiac factors such as diet and body composition. Despite extensive study, the genetic determinants of cardiac hypertrophy remain incompletely understood.
Heart weight is heritable in both mice and humans, with narrow-sense heritability (h2) for mouse heart weight estimated between 0.07 and 0.59 (Deschepper et al. 2002; Leamy et al. 2005; Philip et al. 2011). Human genome-wide association studies (GWAS) have associated multiple loci with cardiac mass using non-invasive metrics such as echocardiography and electrocardiography (Vasan et al. 2009; Shah et al. 2011; Fox et al. 2013; Wild et al. 2017). However, none of these studies has directly measured heart weight. Mice are a widely used model system for heart development and hypertrophy (Van Vliet et al. 2012; Houser et al. 2012). Genetic approaches to the investigation of hypertrophy in mice have either deleted or overexpressed a single gene in an inbred mouse strain, which is an artificial model and does not incorporate the effects of variation in other genes. Previous studies have used a variety of rodent stocks in an effort to identify novel heart weight loci (Tsujita et al. 2000; Sugiyama et al. 2002; Rocha et al. 2004; Llamas et al. 2005, 2007), but these approaches have been limited by modest genetic variation for genetic mapping. The influence of diet as a co-determinant of heart weight has not been evaluated in mouse populations.
The Diversity Outbred (DO) population contains a large amount of genetic diversity contributed by five classical strains, A/J, C57BL/6J, 129S1/SvlmJ, NOD/ShiLtJ, NZO/HILtJ, and three wild-derived strains, CAST/EiJ, PWK/PhJ, WSB/EiJ. DO mice have an elevated minor allele frequency across the genome and high levels of recombination that allows for high-resolution genetic mapping (Churchill et al. 2012; Svenson et al. 2012). The structured genetic diversity in the DO allows for the association of founder haplotypes with the trait of interest, which can then be connected with the DO founder strain’s genome. The DO has been used to identify quantitative trait loci (QTL) for a wide variety of traits, including behavior, prostate cancer, cocaine self-administration, pain sensitivity, and atherosclerosis (Logan et al. 2013; Recla et al. 2014; Smallwood et al. 2014; Dickson et al. 2015; Tyler et al. 2017; Winter et al. 2016). The structured genetic diversity, and previous mapping successes make the DO research population ideal for exploring the genetic architecture of complex health-related traits such as heart size.
Here, we measured heart weight of DO adult mice at different ages exposed to different diets across two cohorts representing outbreeding generations 10 and 11 of DO mice. The diet conditions did not appear to alter heart weight or overall body weight, though data from a previous study in this population identified a diet-induced change in risk for development of atherosclerosis (Smallwood et al. 2014). We performed QTL mapping for heart weight and identified two QTL. We partially validated a candidate gene, collagen type XXII alpha 1 chain (Col22a1), centered at the QTL peak using DO founder mice, confirming a consistent haplotype effect from NZO/HlLtJ and WSB/EiJ genotypes.
Materials and methods
Animals
DO G10 cohort: male DO mice (n = 159; J:DO, JAX stock number 009376) and female DO mice (n = 158) were obtained from the Jackson Laboratory (Bar Harbor, ME) as 148 sibling pairs (each pair of male and female are from same sire/dams but different litters) at 4 weeks of age. Mice were from outbreeding generation 10 (G10; received May 2012). The mice were group housed (n = 5 mice per cage) with non-irradiated pine bedding and provided with high-efficiency particulate air-filtered air and free access to food and water in a climate-controlled facility under a 12 h light/ dark cycle at 22.2 ± 1.1 °C.
DO G11 cohort: female DO mice (n = 292) were obtained from the Jackson Laboratory (Bar Harbor, ME) as 146 full sibling pairs at 4 weeks of age and at outbreeding generation 11 (G11; received September 2012). The mice were group housed (n = 5 mice per cage) with non-irradiated pine bedding and provided with high-efficiency particulate air-filtered air and free access to food and water in a climate-controlled facility under a 12 h light/dark cycle at 22.2 ± 1.1 °C.
In 2008, DO founders were obtained from the laboratory of Gary Churchill at the Jackson Laboratory and reared at the Pardo-Manuel de Villena laboratory at the University of North Carolina—Chapel Hill. Since then, mice were bred at the University of North Carolina Hillsborough facility from 2008 to 2010 and bred at the University of North Carolina Genetics Medicine Building facility from 2010 to 2012. Pups were weaned and sex was determined at approximately 3 weeks of age. Animals were kept on a 14 h/10 h light/dark schedule with lights turned on at 6:00 AM; temperature was maintained at 20–24 °C with relative humidity between 40 and 50%. Mice were group housed (n = 5) in standard 20 × 30 cm ventilated polysulfone cages with standard laboratory grade Bed-O-Cob bedding. Water and Purina Prolab RMH 3000 were available ad libitum.
Diet
G10 cohort mice were maintained on a defined synthetic diet upon arrival and throughout the study (D10001, Research Diets, New Brunswick, NJ). G11 cohort mice were maintained on a defined synthetic diet upon arrival and through 6 weeks of age (D10001, Research Diets, New Brunswick, NJ); subsequently, 146 mice were transferred to a synthetic high-fat, cholic acid (HFC) diet that contained 20% fat, 1.25% cholesterol, and 0.5% cholic acid, to induce atherosclerotic lesions, and 146 mice were maintained on a high-protein diet [low-fat, high-protein (LFHP)] that contained 5% fat and 20.3% protein, which is not atherogenic (D12109C and D12083101, respectively; Research Diets, New Brunswick, NJ). One sibling from each of the 146 sibling pairs was randomly assigned to each one of the diets. The source of fat from the diets varied between the baseline diet (corn oil) fed to the mice from 4 to 6 weeks of age and the dietary treatment groups (soybean oil plus cocoa butter) fed to the mice from 6 to 24 weeks of age. All mice were maintained on their respective diets until 24–25 weeks of age, for a total of 18–19 weeks.
Phenotyping
G10 cohort mice were euthanized and analyzed at 12–15 weeks of age, while G11 cohort mice were euthanized and analyzed at 24–25 weeks. Mice were weighed and then euthanized by CO2 asphyxiation followed by cervical dislocation. The heart was dissected from the chest cavity. Atria and all adherent tissue were carefully excised prior to weighing the ventricles, then hearts were flash frozen in liquid nitrogen and stored at − 80 °C. Right hind legs were removed and fixed in 10% formalin. Subsequently, the femur was dissected and measured two independent times to 0.01 mm using digital calipers.
Genotyping
Tail biopsies were taken from mice at 6 weeks of age, and DNA was extracted from the tissue using the QIAGEN DNeasy kit per manufacturer’s instructions. The MegaMUGA SNP array [GeneSeek (Neogen), Lincoln, NE] was used to genotype all mice used in this study (Welsh et al. 2012). The MegaMUGA array, from the Illumina Infinium platform, was designed with 77,800 SNP markers with an average spacing of 33 Kb across the genome. Using a filtering step that removes poor performing markers from tiers three and four with a call rate of under 10% (Morgan et al. 2016), the total number of markers was pruned to 68,268 SNP markers. We used the Sanger Mouse Genomes Project REL-1505 to impute founder SNPs onto DO genomes during association mapping.
QTL mapping
We performed QTL mapping using the R package Rqtl2 (Broman 2014) and DOQTL version 1.10.0 (Gatti et al. 2014) for identifying SNP effects based on haplotype. Rqtl2 performs QTL mapping through a regression of the phenotype on the founder haplotype probabilities estimated with a hidden Markov model (HMM) designed for multi-parental populations. DOQTL allows for merge analysis based on association mapping of DO founder SNPs. Diet, age, and sex were conditionally included as covariates for trait mapping. We accounted for genetic similarity between the mice using a kinship matrix based on the leave-one-chromosome-out (LOCO) method. LOCO was chosen as the traditional kinship calculations that include the causative marker produce overly conservative mapping results (Yang et al. 2014; King and Long 2017). QTL significance intervals were defined by the 95% Bayesian credible interval, calculated by normalizing the area under the QTL curve (Sen and Churchill 2001). Log of the odds ratio (LOD) was the reported mapping statistic. The significance thresholds for QTL were calculated independently for autosomes and chromosome X using 1000 permutations. To determine statistical significance of a QTL peak, a genome-wide p value of 0.05 from permutation testing was used. Similarly, a suggestive QTL was determined using a p value of 0.1. The founder allelic effect was identified using a regression of the phenotype on the founder genotype probabilities at each locus.
mRNA expression analysis of candidate genes within QTL regions
We measured gene expression of 12 genes within or near the two QTL regions identified for heart weight using qRT-PCR. Intron-spanning primers were designed using https://lifescience.roche.com/en_us/brands/universal-probe-library.html#assay-design-centre. Primer efficiencies were confirmed using serial dilutions of standard cDNA. Total RNA was isolated from heart tissue (Qiagen RNeasy Plus Mini kit #74134) and analyzed using a NanoDrop (Thermo Scientific). For qRT-PCR, 1 μg of RNA was reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Life Technologies #4368814). Two-step qRT-PCR reactions contained 2% of the cDNA product. All reactions were performed in triplicate in a Roche 480 Light Cycler. Relative quantitation of PCR products used the ΔΔCt method relative to two validated reference genes (Tbp and Polr2a). Similar efficiencies were confirmed for all primers. All probes and primers were from Roche. To identify differential expression patterns across the DO founder strains, we used ANOVA statistical tests using JMP 12 software.
Data availability
Genotypes for the DO mice used in this study can be found at http://churchill.jax.org/research/cc/do_data/megamuga/raw/194_Pomp_DO/ and probability files can be found at http://churchill.jax.org/research/cc/do_data/megamuga/allele_genomes/. All genome coordinates are on GRCm38. Primers used for differential expression analysis can be found in Table S1. Raw phenotypes for the mice can be found in Table S2.
Results
Heart size in DO mice is primarily affected by age and sex, but not diet
We observed a large variation in heart size of DO mice (mean = 0.131 g, SD = 0.024 g, range 0.070–0.270 g) (Table S2). Age had a large effect on heart size (F5,566 = 31.6, p value < 0.0001) between mice from generation 10 (G10) and 11 (G11) of the DO. This is likely due to differences in body mass at the age of sacrifice of the two cohorts. In G10 mice, we see significant sex differences in heart size between male (0.123 g + 0.0013) and female mice (0.118 g + 0.0013) (t300 = 2.77, p value = 0.0059). The G11 cohort contains only female mice with diet as the major experimental variable. For diet effects in G11, there is no significant difference between the high-fat, cholic acid (HFC), and high-protein (LFHP) diets for either body weight (t251 = 0.16, p value = 0.873) or heart weight (t256 = − 0.15, p value = 0.879). Body weight across the two generations was significantly correlated with heart weight as expected (R2 = 0.32, p value < 0.0001). Femur length is also correlated with heart weight (R2 = 0.18, p value < 0.0001).
Identification of two QTL for heart size in DO mice
We aimed to identify QTL that influenced heart weight independent of body size and age. Indexing heart weight to long bone length is a standard approach to assessing cardiac hypertrophy in mice as body weight, composition, and systemic metabolism have direct effects on cardiac mass (Woodiwiss et al. 2008; Sweeney 2010; Kolwicz et al. 2013; Neeland et al. 2013; Cuspidi et al. 2014). Hence indexing heart weight to body weight may confound the analysis, particularly in studies that evaluate diet as a variable.
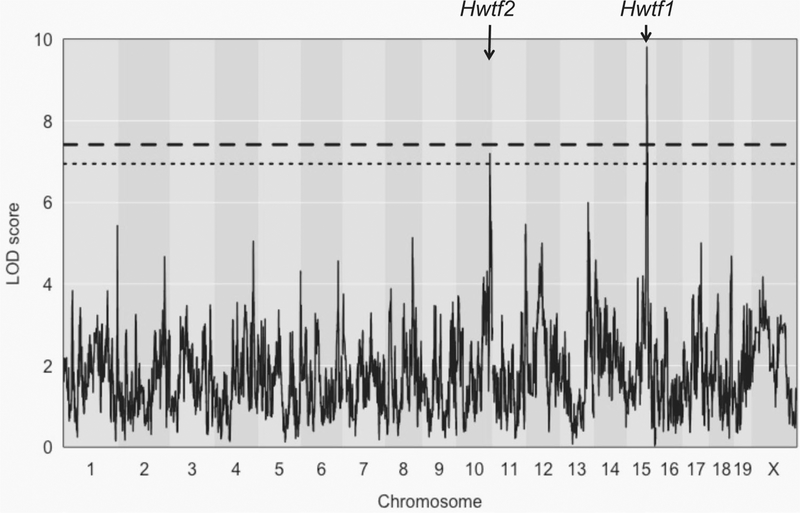
We performed QTL mapping for heart weight as a function of femur length, with age, sex, and diet as covariates for 547 DO mice. Mice that died over the course of the experiment or could not provide a full set of phenotypes were excluded from QTL mapping. We identified one QTL (heart weight by femur, Hwtf1) that was significant at a genome-wide p value of ≤ 0.05 on chr 15 at 72.47 Mb (LOD score = 9.812) that explains 7.1% of the variance and another on chr 10 (Hwtf2) at 120 Mb (LOD score = 7.199) that was significant at a genome-wide p value of ≤ 0.1 that explains 6.2% of the variance (Fig. 1). The confidence interval around the QTL peak on chr 15 is from 71.8 to 73 Mb and contains 11 candidate genes. For the QTL peak on chr 10, the confidence interval around the QTL peak is from 120 to 122 Mb and contains 36 candidate genes.
Fig. 1.
QTL mapping for heart weight in DO mice. Chromosome position is represented on the x-axis, and significance score (LOD) is represented on the y-axis. Horizontal lines represent significance threshold from permutation testing (dashed line at p value = 0.05, dotted line at p value = 0.1)
Though heart weight as a function of long bone length is considered the gold standard approach to account for variation in body size and composition (Woodiwiss et al. 2008; Sweeney 2010; Kolwicz et al. 2013; Neeland et al. 2013; Cuspidi et al. 2014), we present additional QTL analyses using only heart weight, as well as heart weight as a function of body weight (Fig. S1). These approaches have previously been used for QTL mapping of heart weight; however, they produce different results when compared to heart weight as a function of femur length in this population (Fig. S1). Interestingly, untransformed heart weight produces QTL that are much more similar to heart weight as a function of femur length than to heart weight as a function of body weight. We also present QTL mapping results in each generation (Fig. S2). We observe peaks at similar regions on chr 10 and 15 that are observed in the combined analysis; however, the smaller sample size (G10 n = 289, G11 n = 258) is underpowered for QTL detection of this trait at genome-wide significance levels.
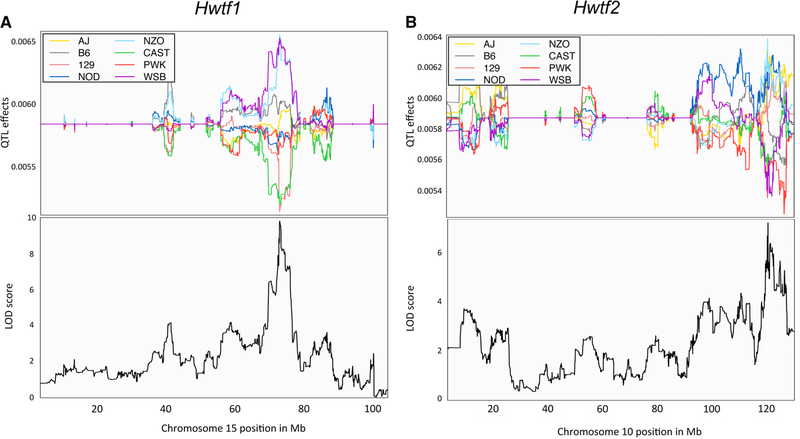
We estimated founder haplotype effects for each QTL (Fig. 2). For Hwtf1, we observed that the WSB/EiJ and NZO/HlLtJ founder haplotypes were associated with larger heart size while 129S1/SvImJ and CAST/EiJ founder haplotypes were associated with smaller heart size. This pattern of allele effects suggests that there are at least two distinct variants underlying Hwtf1. For Hwtf2, we found that the NZO/HiLtJ, NOD/ShiLtJ, A/J, and 129S1/SvImJ alleles were associated with increased heart size, and the PWK/PhJ, WSB/EiJ, CAST/EiJ, and C57BL6/J alleles were associated with a decreased size.
Fig. 2.
Haplotype effects at QTL for heart weight. The haplotype effects of the eight DO founders are plotted for the two chromosomes with QTL for heart weight. The x-axis is physical distance in Mb along the chromosome. The y-axis for the top panel is the effect coefficient, and the bottom panel is the LOD score. a The haplotype effects on chromosome 15 for Hwtf1, with the position of the QTL peak mapped below. b The haplotype effects on chromosome 10 for Hwtf2, with the position of the QTL peak mapped below
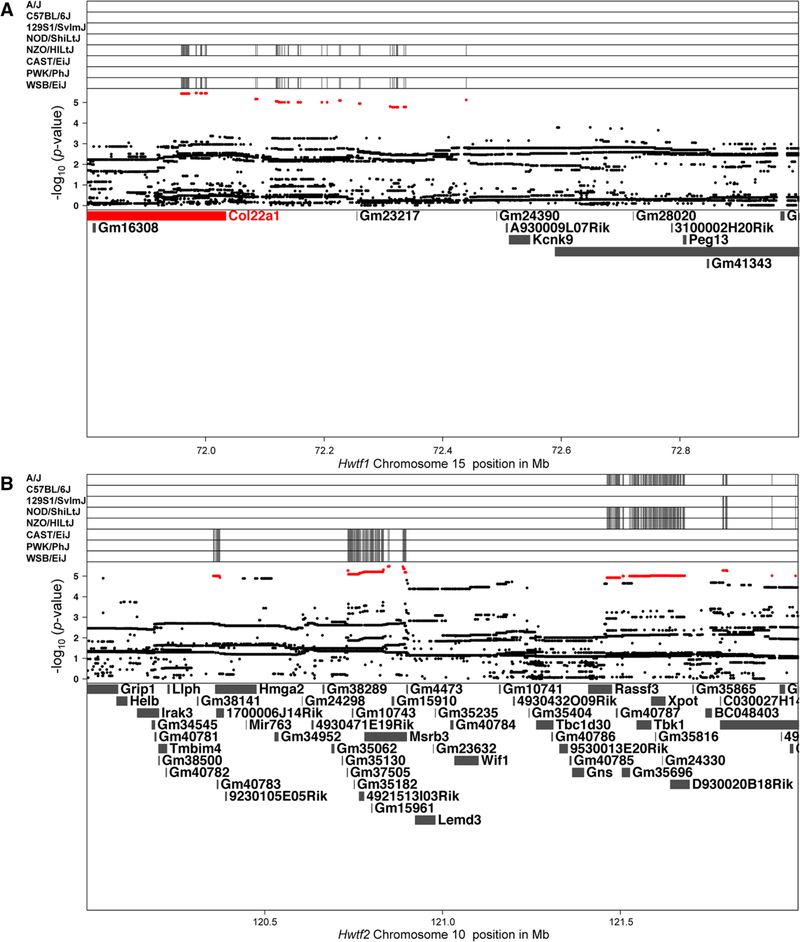
We searched for candidate genes under each QTL via high-resolution association mapping on chr 10 and 15 and identified SNPs associated with heart weight at the QTL peak (Fig. 3a, b). The most significant SNPs, over an arbitrary log10 p value score cutoff of 4 on chr 15 and 4.9 on chr 10 are highlighted in red. The top plot identifies the contributing minor allele for the high LOD score SNPs of the eight founders. On chr 15 at Hwtf1, 203 SNPs within the gene Col22a1 partition together by the “larger heart size” NZO/HlLtJ and WSB/EiJ haplotypes (Table S3). These 203 SNPs include both synonymous as well as missense variants (Keane et al. 2012). These 203 variants are biallelic, and segregate for the two founder haplotypes in the “high” group compared to the remaining six founder haplotypes, while Hwtf1 has three distinct groups. It is possible there are other variants outside of Col22a1 that contribute to the additional separation observed in the QTL. For Hwtf2, there is a complex pattern of SNPs across the QTL region that indicates polygenic effects from several of the DO founders. SNPs within multiple genes partition together by PWK/PhJ, WSB/EiJ, and CAST/EiJ haplotypes while another set of SNPs partition by the NZO/HiLtJ, NOD/ShiLtJ, A/J, and 129S1/SvImJ haplotypes. This grouping closely, but not entirely, mimics the allelic effects for Hwtf2.
Fig. 3.
High-resolution association mapping within QTL regions for heart weight. SNP marker associations of the eight DO founders labeled above. The x-axis shows the distribution along the chromosome in physical distance. The y-axis displays the minor allele frequency for SNPs colored in red, the LOD scores for all SNPs, and gene names in the interval. a The association mapping for SNPs near the QTL peak for Hwtf1 on chromosome 15. b The association mapping for SNPs near the QTL peak for Hwtf2 on chromosome 10
We also tested for the additive combination of single-locus additive effects between the Hwtf1 and Hwtf2. A single marker at each of the QTL peaks (UNC25866055, JAX00300487) was selected and incorporated into an additive model with the full haplotype effects of the eight founder haplotypes. An ANOVA was performed for each marker with a single-locus effect and a two-locus effect. For both markers, the ANOVA showed a significant interaction when comparing the single marker to the two-locus model (chr 10 additive and interaction model difference, p value = 0.0003; chr 15 additive and interaction model difference, p value < 0.0001). Thus, these two QTL have a significant epistatic effect on heart weight since both models show an improvement for total model fit when accounting for the other.
Differential expression of candidate genes for variation of heart size in DO founder strain mice
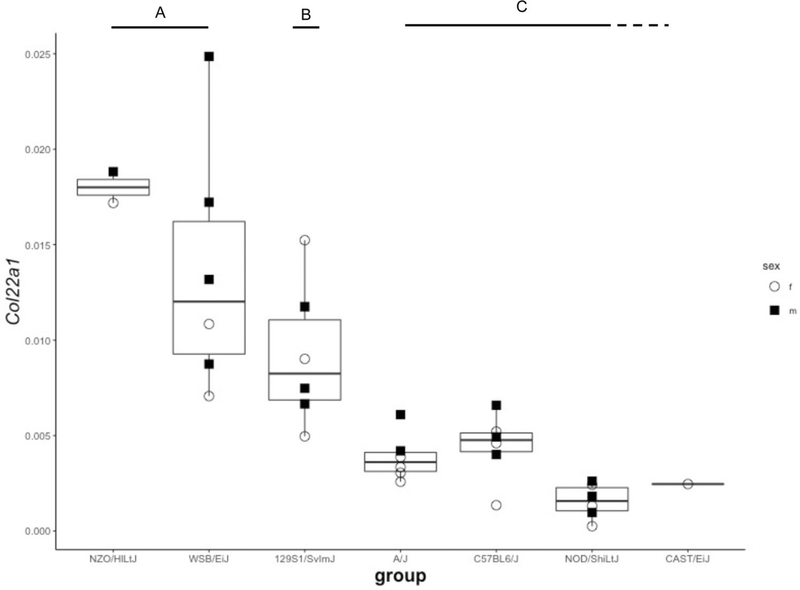
To assess if candidate gene expression patterns in the DO founders exhibited patterns that matched the allelic effects in the QTL and high-resolution association mapping for indexed heart weight, we performed quantitative reverse transcriptase PCR (qRT-PCR) on heart tissue from seven of the eight DO founders (PWK/PhJ heart tissue was not available). Based on genomic location relative to the QTL peak and biological plausibility (e.g., expressed in heart muscle), we selected 12 candidate genes for gene expression measurement: four within or near Hwtf2, and eight within or near Hwtf1 (Fig. S3). For Col22a1, we observe a pattern of expression from the DO founders similar to what we observe for the high-resolution association mapping on chr 15 (Fig. 4). The two haplotypes associated with increased heart size, NZO/HlLtJ and WSB/EiJ, displayed a significantly higher Col22a1 expression in qRT-PCR of founder heart tissue from these strains. This result matches the SNP marker partitioning of NZO/HlLtJ and WSB/EiJ for Col22a1 displayed in Fig. 3a. There may be other genetic variants within the Hwft1 QTL that explain the medium and low effects of other haplotypes, but those effects are outside of Col22a1. For the other candidates of Hwtf2 on chr 10, we did not observe expression patterns in the DO founders that mirrored QTL or high-resolution association mapping allelic effects.
Fig. 4.
Gene expression of Col22a1 from heart tissue of DO founder strains. Expression patterns of Col22a1 from seven DO founder mice. Open circles signify female and Closed squares signify male mice. ANOVA significance patterns were grouped into three categories (a–c). Expression was highest in NZO/HlLtJ and WSB/EiJ mice, intermediate in 129S1/SvlmJ, and low in A/J, C57BL6/J, CAST/EiJ, and NOD/ShiLtJ
Discussion
In this study, we used DO mice to investigate if genetic and diet effects are responsible for variation in heart size. We identified two novel QTL that acted independently of diet and collectively explain 13.3% of the variation for heart weight. We show that the NZO/HlLtJ and WSB/EiJ haplotypes at Hwtf1 are associated with larger heart size. We used high-resolution association mapping to identify 203 SNPs that partitioned together NZO/HlLtJ and WSB/EiJ haplotypes at Hwtf1. The most significant SNPs partitioned together at a gene previously implicated in heart development, Col22a1. Finally, we performed differential expression analysis with DO founder heart tissue that showed that NZO/HlLtJ and WSB/EiJ are statistically distinct from the other DO founders for Col22a1. While the haplotype effects within Hwtf1 have three classes, we demonstrate that the most significant genetic variants are consistent for the “high” class within Col22a1. From these experiments, we believe that genetic variation in or near Col22a1 may be responsible for explaining a significant component of phenotypic heart weight variability in the DO population.
Significant genetic effects on heart weight from the founders of the DO population have been previously identified (Philip et al. 2011). The DO allows for genetic mapping to identify the pattern of haplotype effects driving heart weight differences. For heart weight as a function of femur length, we observe a QTL (Hwtf1) on chr 15 and a QTL on chr 10 (Hwtf2). For Hwtf1, we see haplotype effects of WSB/EiJ and NZO/HlLtJ associating with larger heart size while 129S1/SvImJ and CAST/EiJ are associated with a smaller heart (Fig. 2). The founder haplotype effects for Hwtf2 do not have as clear divisions as in Hwtf1, and differential expression analysis of candidate genes from the DO founders does not follow the QTL allelic effects in Hwtf2. It is likely that there are polygenic effects at the Hwtf2 locus based on the high-resolution association mapping, while Hwtf1 is mostly explained by a single gene. It is clear that heart weight is a polygenic trait as these QTL only explain a fraction of the genetic variance.
Our qRT-PCR results indicate that the two founder strains, NZO/HlLtJ and WSB/EiJ, both display a statistically similar pattern of expression for Col22a1 (Fig. 4). This pattern is consistent with the SNP pattern identified for Col22a1 in Fig. 3a and the founder haplotype effect for the Hwtf1 QTL. Col22a1 is a particularly interesting candidate gene. It is produced by muscle cells, including cardiomyocytes, is known to influence mammalian muscle development and exhibits restricted localization at the myotendinous junction in heart muscle (Koch et al. 2004). In a study on cardiac fibrosis in 28 inbred strains of mice, polymorphisms in Col22a1 were associated with the formation and severity of dystrophic cardiac calcinosis (Li et al. 2016). Col22a1 was also associated with cardiac fibrosis in another independent study on chronic stress-induced cardiac pathology in a large panel of inbred mice (Rau et al. 2015). Fibrosis is a defining characteristic of pathological cardiac hypertrophy (reviewed in Shimizu and Minamino 2016; Travers et al. 2016). It is likely that Col22a1 variants influence heart weight through affecting either cardiac development or postnatal heart hypertrophy, though these possibilities require further study.
Previous mapping studies in mice have found QTL associated with heart weight, using both different populations and alternative statistical approaches (Sugiyama et al. 2002; Rocha et al. 2004). Typically, heart weight is transformed using a log adjustment or is indexed to body weight. The indexed heart weights in our dataset were normally distributed; hence log correction was not appropriate. We indexed heart weight to femur length to avoid confounding by either diet-induced or genetically induced obesity, particularly as the DO founder strain, NZO/HlLtJ is prone to fat storage and used to model obesity. We present QTL mapping results from heart weight unadjusted, adjusted for body weight, and adjusted for femur length (Fig. S1). These three analyses produce different QTL mapping results, which may partly explain differences in QTL identified for heart weight in past studies. Using heart weight only for QTL mapping replicates the two QTL we identified using heart weight adjusted with femur length, but with a larger LOD score for Hwtf2 and a lower LOD score for Hwtf1. Using heart weight adjusted for body weight fails to produce any significant QTL, with the largest LOD score found in chr 1. While body weight and femur length are significantly correlated with heart weight, they have a different impact on the variance when treated as covariates.
A limited sample size within each generation drove us to combine results across two cohorts of DO mice that were initially designed to ascertain effects of genetics, diet, and sex on a variety of metabolic phenotypes (Smallwood et al. 2014). By restricting the analysis to each generation, we are unable to detect significant QTL for heart weight adjusted by femur length (Fig. S2). We observe peaks on chr 10 and 15 that correspond to those in the full dataset; however, they fail to pass the permutation threshold level. Diet and sex differences are partially confounded due to the unequal distribution across the two cohorts. Since each cohort tests for either a diet or a sex effect, we are unable to test for all possible interactions. For example, male DO mice may have a significant diet effect, but it cannot be tested. There may also be a male-specific peak for heart size, as we observe a peak in the G10 cohort on chr 10 disappear in G11 cohort, which is only female. Additionally, the first cohort of mice was euthanized at 12–15 weeks while the second cohort was euthanized at 24–25 weeks. These factors are accounted for as covariates for trait mapping. Despite the limitations of the diet–sex–age factors across the two cohorts, Col22a1 and Hwtf1 still emerge as significant making this result highly robust.
We observe that heart weight in the second DO cohort was unaffected by two non-standard diets (HFC or LFHP). These two diets were previously reported to alter other cardiovascular risk factors, including cholesterol, triglycerides, and insulin in this cohort of DO mice (Smallwood et al. 2014; Coffey et al. 2017). These diets were chosen to either induce atherosclerosis (HFC) or to be non-atherogenic (LFHP). While other health factors were affected by this dietary change, overall body weight did not differ between these groups. It may be possible that changes in diet at an earlier age could influence heart weight and body weight, but within the scope of this study we see no evidence for a diet-induced effect.
One factor to consider when using the DO for genetic mapping is that allele frequency distributions could be distorted at particular regions due to selection or other effects (Chesler et al. 2016). When a particular haplotype is low in frequency, random chance may cause that haplotype to have a significant effect and drive a QTL peak. At Hwtf1 there is a reduction in the 129S1/SvImJ haplotype to 4.8% from the expected 12.5% across both generations of DO tested. If the 129S1/SvImJ haplotype was the only one to display large deviations from the mean phenotype, then it would be difficult to distinguish a true effect of the haplotype on the trait versus a false effect caused by low allele frequency. However, for Hwtf1, we see multiple robust effects on other haplotypes that indicate that the QTL isn’t driven by the low frequency of 129S1/SvImJ.
A final consideration when using the DO is that new mutations will be invisible to genotyping arrays and high-resolution association mapping. The breeding scheme for the DO is specifically designed to minimize the impact of genetic drift and new mutations; however, the use of partially inbred CC lines to seed the DO may have introduced new variants not present in the sequenced genome of the founder strains (Chesler et al. 2016). Every CC strain has accumulated unique variation, primarily due to genetic drift, which is not present in the eight founder strains (Srivastava et al. 2017; Shorter et al. 2017). The frequency of these mutations is low; however, in functional mutations, there is an increase in phenotypic variance within a haplotype.Whole genomic sequencing studies will exploit this variation when sequencing costs decline. This factor, along with our sub-analyses of smaller mapping sizes (Fig. S2), illustrates that DO genetic mapping experiments for polygenic traits need to be large to have sufficient power.
Supplementary Material
Acknowledgements
This work was supported in part by NIH Grants DK076050 and DK087346 (DP) and the UAI Research Foundation (BCJ). Phenotypes were collected using the Animal Metabolism Phenotyping core facility within UNC’s Nutrition and Obesity Research Center funded by NIH DK056350. We also acknowledge George Weinstock and The Genome Institute (Washington University) for partial funding of the mouse purchase and husbandry costs for cohort 2 of the DO mice used in these studies. We thank Liyang Zhao and Kuo-Chen Jung for assistance with all mouse experiments, and Brian Bennett and Tangi Smallwood for assistance with the cohort 2 experiments. We thank Martin Ferris for analysis support. Charles Farber is gratefully acknowledged for performing the femur length phenotyping and providing those data for use in these studies.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00335–017-9730–7) contains supplementary material, which is available to authorized users.
References
- Bikkina M, Levy D, Evans JC, Larson MG, Benjamin EJ, Wolf PA, Castelli WP (1994) Left ventricular mass and risk of stroke in an elderly cohort: the Framingham Heart Study. JAMA 272(1):33–36. 10.1001/jama.1994.03520010045030 [DOI] [PubMed] [Google Scholar]
- Broman KW (2014) Fourteen years of R/qtl: just barely sustainable. J Open Res Softw 2(1):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Gatti DM, Morgan AP, Strobel M, Trepanier L, Oberbeck D et al. (2016) Diversity outbred mice at 21: maintaining allelic variation in the face of selection. G3: Genes Genomes Genet 6(12):3893–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GA, Gatti DM, Munger SC, Svenson KL (2012) The diversity outbred mouse population. Mamm Genome 23(9–10):713–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey AR, Smallwood TL, Albright J, Hua K, Kanke M, Pomp D et al. (2017) Systems genetics identifies a co-regulated module of liver microRNAs associated with plasma LDL cholesterol in murine diet-induced dyslipidemia. Physiol Genomics 49(11):618–629. 10.1152/physiolgenomics.00050.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuspidi C, Rescaldani M, Sala C, Grassi G (2014) Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens 32(1):16–25. 10.1097/HJH.0b013e328364fb58 [DOI] [PubMed] [Google Scholar]
- Deschepper CF, Boutin-Ganache I, Zahabi A, Jiang Z (2002) In search of cardiovascular candidate genes: interactions between phenotypes and genotypes. Hypertension 39:332–336 [DOI] [PubMed] [Google Scholar]
- Dickson PE, Ndukum J, Wilcox T, Clark J, Roy B, Zhang L et al. (2015) Association of novelty-related behaviors and intravenous cocaine self-administration in Diversity Outbred mice. Psychopharmacology 232(6):1011–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox ER, Musani SK, Barbalic M, Lin H, Yu B, Ogunyankin KO et al. (2013) Genome-wide association study of cardiac structure and systolic function in African Americans: the Candidate Gene Association Resource (CARe) Study. Cardiovasc Genet 6(1):37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardin JM, McClelland R, Kitzman D et al. (2001) M-mode echocardiographic disease predictors of six-to seven -year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol 87:1051–1057 [DOI] [PubMed] [Google Scholar]
- Gatti DM, Svenson KL, Shabalin A, Wu LY, Valdar W, Simecek P et al. (2014) Quantitative trait locus mapping methods for diversity outbred mice. G3: Genes Genomes Genet 4(9):1623–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS et al. (2012) Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111:131–150. 10.1161/RES.0b013e3182582523 [DOI] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K et al. (2012) Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477(7364):289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EG, Long AD (2017) The Beavis effect in next-generation mapping panels in Drosophila melanogaster. G3: Genes Genomes Genet 7(6):1643–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Schulze J, Hansen U, Ashwodt T, Keene DR, Brunken WJ et al. (2004) A novel marker of tissue junctions, collagen XXII. J Biol Chem 279(21):22514–22521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolwicz SC, Purohit S, Tian R (2013) Cardiac metabolism and its interactions with contraction, growth, and survival of the cardiomyocte. Circ Res 113(5):603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamy LJ, Elo K, Nielsen MK, Van Vleck LD, Pomp D (2005) Genetic variance and covariance patterns for body weight and energy balance characters in an advanced intercross population of mice. Genet Sel Evol 37(3):151–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1989) Left ventricular mass and incidence of coronary heart disease in an elderly cohort: the Framingham Heart Study. Ann Intern Med 110:101–107 [DOI] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD et al. (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322:1561–1566 [DOI] [PubMed] [Google Scholar]
- Li Q, Berndt A, Sundberg BA, Silva KA, Kennedy VE, Cario CL et al. (2016) Mouse genome-wide association study identifies polymorphisms on chromosomes 4, 11 and 15 for age-related cardiac fibrosis. Mamm Genome 27(5–6):179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamas B, Jiang Z, Rainville ML, Picard S, Deschepper CF (2005) Distinct QTLs are linked to cardiac left ventricular mass in a sex-specific manner in a normotensive inbred rat intercross. Mamm Genome 16:700–712 [DOI] [PubMed] [Google Scholar]
- Llamas B, Bélanger S, Picard S, Deschepper CF (2007) Cardiac mass and cardiomyocyte size are governed by different genetic loci on either autosomes or chromosome Y in recombinant inbred mice. Physiol Genomics 31:176–182. 10.1152/physiolgenomics.00072.2007 [DOI] [PubMed] [Google Scholar]
- Logan RW, Robledo RF, Recla JM, Philip VM, Bubier JA, Jay JJ et al. (2013) High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes Brain Behav 12(4):424–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AP, Fu CP, Kao CY, Welsh CE, Didion JP, Yadgary L et al. (2016) The Mouse universal genotyping array: from substrains to subspecies. G3: Genes Genomes Genet 6(2):263–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame EJ, Das SR et al. (2013) Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging 6(5):800–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip VM, Sokoloff G, Ackert-Bicknell CL, Striz M, Branstetter L, Beckmann MA et al. (2011) Genetic analysis in the collaborative cross breeding population. Genome Res 21(8):1223–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau CD, Wang J, Avetisyan R, Romay M, Martin L, Ren S et al. (2015) Mapping genetic contributions to cardiac pathology induced by Beta-adrenergic stimulation in mice. Circ Cardiovasc Genet 8(1):40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recla JM, Robledo RF, Gatti DM, Bult CJ, Churchill GA, Chesler EJ (2014) Precise genetic mapping and integrative bioinformatics in Diversity Outbred mice reveals Hydin as a novel pain gene. Mamm Genome 25(5–6):211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha JL, Eisen EJ, Van Vleck LD, Pomp D (2004) A large-sample QTL study in mice: II. Body composition. Mamm Genome 15:100–113 [DOI] [PubMed] [Google Scholar]
- Sen S, Churchill GA (2001) A statistical framework for quantitative trait mapping. Genetics 159(1):371–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Nelson CP, Gaunt TR, van der Harst P, Barnes T, Braund PS et al. (2011) Four genetic loci influencing electrocardiographic indices of left ventricular hypertrophy. Circ Cardiovasc Genet 4(6):626–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I, Minamino T (2016) Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 97:245–262 [DOI] [PubMed] [Google Scholar]
- Shorter JR, Odet F, Aylor DL, Pan W, Kao CY et al. (2017) Male infertility is responsible for nearly half of the extinction observed in the collaborative cross. Genetics 206:557–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood TL, Gatti DM, Quizon P, Weinstock GM, Jung KC, Zhao L et al. (2014) High-resolution genetic mapping in the diversity outbred mouse population identifies Apobec1 as a candidate gene for atherosclerosis. G3: Genes Genomes Genet 4(12):2353–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Morgan AP, Najarian M, Sarsani VK, Sigmon JS et al. (2017) The genomes of the collaborative cross. Genetics 206:537–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama F, Churchill GA, Li R, Libby LJ, Carver T, Yagami K, John SW, Paigen B (2002) QTL associated with blood pressure, heart rate, and heart weight in CBA/CaJ and BALB/cJ mice. Physiol Genomics 10:5–12 [DOI] [PubMed] [Google Scholar]
- Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ et al. (2012) High-resolution genetic mapping using the mouse diversity outbred population. Genetics 190(2):437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney G (2010) Cardiovascular effects of leptin. Nat Rev Cardiol 7:22–29 [DOI] [PubMed] [Google Scholar]
- Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC (2016) Cardiac fibrosis: the fibroblast awakens. Circ Res 118(6):1021–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita Y, Iwai N, Tamaki S, Nakamura Y, Nishimura M, Kinoshita M (2000) Genetic mapping of quantitative trait loci influencing left ventricular mass in rats. Am J Physiol Heart Circ Physiol 279:H2062–H2067 [DOI] [PubMed] [Google Scholar]
- Tyler AL, Ji B, Gatti DM, Munger SC, Churchill GA, Svenson KL, Carter GW (2017) Epistatic networks jointly influence phenotypes related to metabolic disease and gene expression in diversity outbred mice. Genetics 206(2):621–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet P, Wu SM, Zaffran S, Pucéat M (2012) Early cardiac development: a view from stem cells to embryos. Cardiovasc Res 96(3):352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan RS, Glazer NL, Felix JF, Lieb W, Wild PS, Felix SB et al. (2009) Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. JAMA 302(2):168–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh CE, Miller DR, Manly KF, Wang J, McMillan L et al. (2012) Status and access to the collaborative cross population. Mamm Genome 23:706–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild PS, Felix JF, Schillert A, Teumer A, Chen MH, Leening MJG et al. (2017) Large-scale genome-wide analysis identifies genetic variants associated with cardiac structure and function. J Clin Invest 127(5):1798–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JM, Gildea DE, Andreas JP, Gatti DM, Williams KA, Lee M, Hu Y et al. (2016) Mapping complex traits in a diversity outbred F1 mouse population identifies germline modifiers of metastasis in human prostate cancer. Cell Syst 4:31e6–45.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodiwiss AJ, Libhaber CD, Majane OH, Libhaber E, Maseko M, Norton GR (2008) Obesity promotes left ventricular concentric rather than eccentric geometric remodeling and hypertrophy independent of blood pressure. Am J Hypertens 21:1144–1151 [DOI] [PubMed] [Google Scholar]
- Yang J, Zaitlen NA, Goddard ME, Visscher PM, Price AL (2014) Advantages and pitfalls in the application of mixed-model association methods. Nat Genet 46:100–106__ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genotypes for the DO mice used in this study can be found at http://churchill.jax.org/research/cc/do_data/megamuga/raw/194_Pomp_DO/ and probability files can be found at http://churchill.jax.org/research/cc/do_data/megamuga/allele_genomes/. All genome coordinates are on GRCm38. Primers used for differential expression analysis can be found in Table S1. Raw phenotypes for the mice can be found in Table S2.