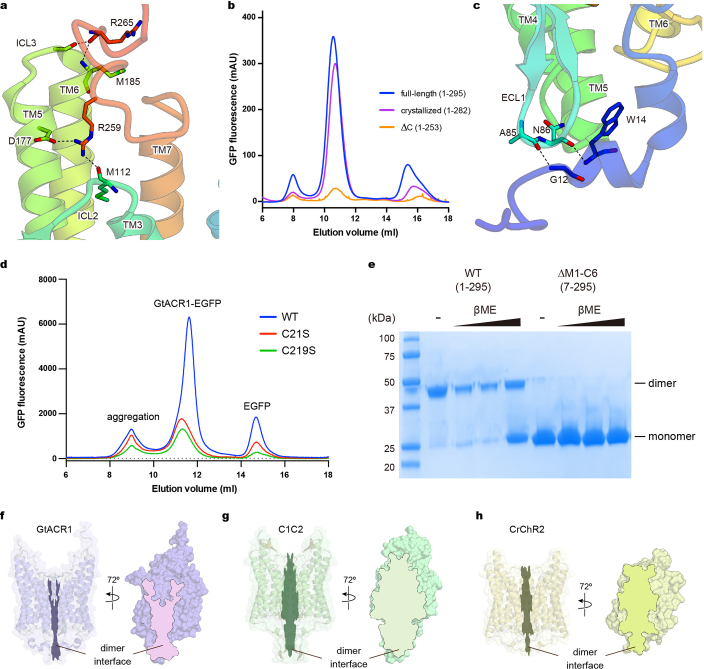
Extended Data Fig. 5. Interactions between N- and C-terminal regions and the 7-TM domain.
a, Interactions between the C-terminal region and the 7-TM domain. Hydrogen bonds are shown by dashed lines. b, Fluorescent size-exclusion chromatography traces of the full-length GtACR1 (1–295), the crystallized construct (1–282), and the C-terminal truncated construct (∆C: 1–253), showing possible importance of the C terminus in proper folding and/or stability. Similar results were observed in three independent experiments. c, Interactions between the N-terminal region and the ECL1. Hydrogen bonds are shown by dashed lines. d, Fluorescent size-exclusion chromatography traces of wild-type and C-to-S mutants of GtACR1. Labels indicate estimated elution positions of the aggregate, GtACR1–eGFP, and free eGFP; C-to-S mutants show decreased (<1/3) expression compared to the wild type. Similar results were observed in three independent experiments. e, Stained SDS–PAGE gel image of wild-type and N-terminal 6-amino-acid-truncated GtACR1 in the presence and absence of reducing reagent (β-mercaptoethanol); the wild type runs as a mixer of monomer and dimer in β-mercaptoethanol,whereas N-terminal-truncated GtACR1 stays monomeric even in the absence of β-mercaptoethanol. This experiment was performed once, but similar experiments with different concentrations of β-mercaptoethanol were performed three times, all with similar results. f–h, Dimer interfaces of GtACR1 (f), C1C2 (g) and CrChR2 (h) viewed at two angles from the side; note reduced interface area (outlined) for GtACR1. For gel source data, see Supplementary Fig. 1.