Abstract
Hydronephrosis is a sign of advanced stage disease in patients with cervical cancer. Its presence is believed to negatively affect the survival of patients. To date, however, consensus in this field is still lacking. The purpose of the present systematic review is to gather the available data and to provide directions for future research in the field. We systematically searched Medline, Scopus, Clinicaltrials.gov, EMBASE, Cochrane Central Register of Controlled Trials CENTRA and Google Scholar databases from inception till June 2018. Overall, 22 studies were included in the present systematic review that evaluated outcomes from 8521 patients with cervical cancer. The findings of our systematic review support that hydronephrosis negatively affects the overall survival of cervical cancer patients. Specifically, the reported 5- year OS hazards ratio for hydronephrosis ranged between 1.34 and 3.74. Outcomes concerning the disease-free survival of these patients were, however, less discrete. None of the included studies reported whether the decreased survival of patients with hydronephrosis was attributed to complications of obstructive uropathy such as uremia and sepsis. Thus, it remains, to date, unclear whether placement of ureteral stents or percutaneous nephrostomy may actually benefit these patients. More studies are needed to evaluate the actual impact of hydronephrosis on survival rates at the various stages of cervical cancer and to help establish consensus regarding the optimal mode of management of these patients.
Key words: Cervical cancer, hydronephrosis, uremia, ureteral obstruction, ureter
Introduction
Cervical cancer has gained significant attention during the last 30 years. Despite the advances in preventing strategies, which nowadays include the nine-valent vaccine and the introduction of the HPV DNA and mRNA tests along with the liquid phase cytology, its prevalence has reached a plateau that is still difficult to reduce.1 In developing countries, the incidence of cervical cancer is higher.2 Previous studies have associated age, smoking, presence of lymph node metastases, tumor histology, and serum squamous cell carcinoma levels with the OS of patients with advanced stage disease.3-5
The presence of ureteral obstruction and hydronephrosis is a sign of advanced stage disease as it indicates involvement of the parametria. It may be accompanied by electrolyte disorders and high blood urea nitrogen (BUN) and serum creatinine levels. Uraemia may often complicate these cases and can result in deterioration of the patient’s consciousness level and even death if left untreated. Ureteral stenting has been suggested as a potential method that could help alleviate obstructive symptoms and improve renal function; however, a previous study suggested that as the malignancy progresses, patients develop chronic kidney disease stage 4 an require further treatment.6 When stenting is not feasible, percutaneous nephrostomy has been suggested as an alternative mean to help renal function and seems to be promising as a mean that could help complete radiotherapy and chemotherapy. 7,8 Nevertheless, to date the method that is used to facilitate urinary diversion is highly dependent on physician`s preference as there is an absolute lack of recommendations concerning the management of cancer patients.9
Moreover, it remains unclear whether the presence of ureteral obstruction and hydronephrosis significantly alters the overall survival of patients with advanced stage cervical cancer. At 1988 Sinistrero et al where the first to investigate survival of patients that were treated with radiotherapy and observed that women with stage T3b cervical cancer (17 patients) and hydronephrosis had lower 5-year survival rates compared to those without hydronephrosis (26% vs 41%).10 Since then, a significant number of studies have been published in this field. However, despite the fact that hydronephrosis is unanimously considered as a sign that is associated with compromised survival, there is still lack of consensus concerning the management of these patients. The purpose of the present systematic review is to accumulate evidence related to the survival of patients with cervical cancer that have developed hydronephrosis and to provide directions for future research concerning their management.
Materials and methods
The present systematic review was designed according to the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guidelines.11
Information sources and search methods
We used the Medline (1966-2018), Scopus (2004-2018), Clinicaltrials.gov (2008-2018), EMBASE (1980-2018), Cochrane Central Register of Controlled Trials CENTRAL (1999-2018) and Google Scholar (2004-2018) databases in our primary search along with the reference lists of electronically retrieved full-text papers. The date of our last search was set at September 30th 2018. Our search strategy included the text words hydronephrosis; ureteral dilatation; cervical cancer; cervical carcinoma and is schematically presented in the PRISMA flow diagram (Figure 1).
Figure 1.
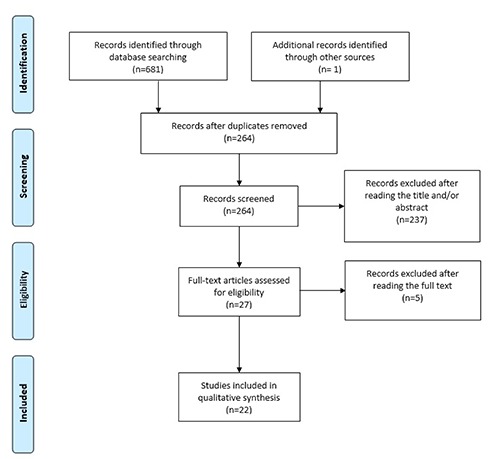
Search strategy.
The studies were selected in three consecutive stages. Following deduplication, the titles and abstracts of all electronic articles were screened by two authors (I.B. and G.D.) to assess their eligibility. The decision for inclusion of studies in the present systematic review was taken after retrieving and reviewing the full text of articles that considered as potentially eligible. Potential discrepancies in this latter stage were resolved by the consensus of all authors.
Study selection
Types of studies and patients
The eligibility criteria for the inclusion of studies were predetermined. No language restrictions were applied. All observational studies as well as randomized trials that evaluated the impact of hydronephrosis on survival of patients with cervical cancer were included in the present systematic review. Patients at any stage of the disease where considered as eligible for inclusion as hydronephrosis. Conference abstracts were also considered as eligible and tabulated. Case reports, small case series (<30 cases) as well as experimental animal studies and reviews were not included in the present systematic review.
Investigated outcomes
The 5-year OS rates were predefined as the primary outcome of the present systematic review; whereas OS rates for a time interval that was shorter were defined as a secondary outcome. Disease free survival (DFS) rates and absolute differences in mean survival were also considered as secondary outcomes.
Quality and risk of bias assessment
The risk of bias and methodological quality of the included studies was explored using the Newcastle-Ottawa Scale (NOS), which evaluates the selection of the study groups, the comparability of the groups and the ascertainment of the exposure or outcome of interest.12
Results
Overall, 22 studies were included in the present systematic review that evaluated outcomes from 8521 patients with cervical cancer.10,12-32 The majority of included studies was retrospective and their methodological quality was evaluated as moderate-high based on the Newcastle-Ottawa score (Table 1). Significant heterogeneity was noted in terms of tumor histology, stage of the disease, tumor size, presence of pelvic wall infiltration, lymph node status and implemented treatment (Table 2). The follow-up period of patient ranged significantly as depicted in Table 1, and 5-year overall survival rates were available in 12 studies (Table 3).
Table 1.
Study characteristics.
| Year; Author | Type of study | NOS score | Patient number | Exclusion criteria | Follow-up (months) |
|---|---|---|---|---|---|
| 2017; Hata | Retrospective | 6 | 28 | Stage other than IVA | 45 (3-116) |
| 2017; Ruiz | Retrospective | 6 | 449 | Age >35 years, not available medical history, no pathologic confirmation, pre-invasive lesions | 45 |
| 2016; Murakami | Retrospective | 7 | 209 | Distant metastasis other than para-aortic lymph node, follow-up <24 months | 32 (27-67.2) |
| 2016; Wakatsuki | Retrospective | 6 | 67 | Stage other than IVA, para-aortic lymph nodes ≥1 cm | 19 (2-235) |
| 2015; Patel | Retrospective | 7 | 279 | NR | 18 (0.24-64.8) |
| 2015; Goklu | Retrospective | 7 | 165 | Stage <IIIB, surgical treatment, no regular follow-up | NR |
| 2015; Kyung | Retrospective | 6 | 157 | Stage I, treatment other than surgery and/or CCRT | 55 (12-70) |
| 2015; Biewenga | Retrospective | 7 | 295 | Stage <IIB, distant metastasis, histologic type other than squamous, adenosquamous and adenocarcinoma | 61 (17-136) |
| 2014; Frumovitz | Retrospective | 7 | 3086 | Stage <IB, metastatic disease, cervical melanoma/sarcoma/lymphoma | 133 (0-366) |
| 2013; Pinn-Bingham | Retrospective | 6 | 116 | Distant metastasis | 35.1 (3.8-138.6) |
| 2013; Murakami | Retrospective | 6 | 34 | Stage other than IVA, para-aortic lymph node metastasis, surgery, palliative radiation <50 Gy | 50.9 (35.7-191) |
| 2012; Pérez-Regadera | Retrospective | 5 | 119 | Stage other than IIIB-IVA | 46.41 (1-143) |
| 2011; Pradhan | Retrospective | 7 | 197 | Stage <IIIB, not available hydronephrosis status | NR |
| 2011; Lapitan | Prospective | 7 | 205 | Age <18 years, stage other than IIB-IVA, absence of obstructive uropathy, history of supravesicular urinary diversion | 12 |
| 2010; Rose | Retrospective | 7 | 539 | Stage other than IIIB, serum creatinine >2 mg/dL | NR |
| 2010; Tseng | Prospective | 8 | 251 | Stage <IIB, distant metastasis, adenocarcinoma/adenosquamous carcinoma histologic types | 75.6 |
| 2006; Ogino | Retrospective | 7 | 352 | Stage <IIB, distant metastasis, adenocarcinoma/adenosquamous carcinoma histologic types | 53 (2-191) |
| 1999; Garipagaoglu | Retrospective | 6 | 166 | Stage <IIB or > III | 35 (3-119) |
| 1999; Logsdon | Retrospective | 6 | 1096 | Stage other than IIIB, history of prior hysterectomy or supracervical hysterectomy, recurrent disease, adenocarcinoma/adenosquamous carcinoma histologic types | 134 (2-386) |
| 1998; Chao | Retrospective | 7 | 297 | Stage other than IIIB | 156 |
| 1996; Sardi | Prospective | 7 | 155 | Stage other than IIIB, adenocarcinoma/adenosquamous carcinoma histologic types | 69.5 (15.4-131.4) |
| 1988; Sinistrero | Retrospective | 6 | 259 | Treatment other than radiation | 106.8 (30-174) |
NR, not reported; NOS, Newcastle-Ottawa score.
Table 2.
Patient characteristics.
| Year; Author | Age (years) | Histology | Tumor size (cm) | Pelvic wall infiltration Hydronephrosis vs Control | Lymph node status | Stage | Treatment |
|---|---|---|---|---|---|---|---|
| 2017; Hata | 72 (29–92) | Squamous: 25 (89%) Adeno: 3 (11%) |
6.1 (4.5–11.0) | Unilateral: 13 (46%) Bilateral: 13 (46%) |
Pelvic: 12 (42.9%) | IVA | Radiation |
| 2017; Ruiz | 32±3.4 | Squamous: 388 (84.9%) Adeno: 50 (11%) Adenosquamous: 11 (2.4%) |
4.98 | NR | Pelvic: 44 (9.7%) PAN: 18 (4%) |
I: 94 (20.9%) II: 219 (48.7%) III: 118 (26.2%) IV: 15 (3.3%) |
Surgery: 65 (14.5%) CCRT: 191 (42.5%) Radiation: 120 (26.9%) None: 56 (12.5%) |
| 2016; Murakami | 62 (42-82) | Squamous: 16 (80%) Adeno: 3 (15%) Adenosquamous: 1 (5%) |
7 (4-14) | NR | 13 (65%) | IIIA: 2 (10%) IIIB: 10 (50%) IVA: 1 (5%) |
CCRT: 17 (85%) Radiation: 3 (15%) |
| 2016; Wakatsuki | 70 (38–87) | Squamous: 61 (91%) Adeno: 6 (9%) |
≤6: 22 (32.8%) >6: 45 (67.2%) |
59 (88.1%) | IVB: 7 (35%) Pelvic: 27 (40.3%) |
IVA | CCRT: 11 (16.4%) Radiation: 56 (83.6%) |
| 2015; Patel | 54 (25-82) vs 47 (21-92) |
Squamous: 47 (72%) vs 122(58) Adeno: 10 (15%) vs 67 (32%) Other: 8 (12%) vs 23 (11%) |
NR | NR | NR | I: 21 (32%) vs 132 (63%) II: 9 (14%) vs 37 (18%) III: 18 (28%) vs 21 (10%) IV: 17 (26%) vs 21 (10%) |
Surgery: 30 (46%) vs 139 (65%) Chemotherapy: 45 (69%) vs 94 (44%) Radiation: 48 (74%) vs 3 (1%) |
| 2015; Goklu | ≥65: 128 (77.6%) <65: 37 (22.4%) |
Adeno: 81 (49.1%) Adenosquamous: 6 (3.6%) |
NR | Unilateral: 74 (44.8%) Bilateral: 74 (44.8%) |
NR | III: 131 (79.4%) IVA: 16 (9.7%) IVB: 18 (10.9%) |
Chemotherapy: 127 (77%) Radiation: 158 (95.8%) |
| 2015; Kyung | 56 (27-85) | Squamous: 124 (79%) Adeno/adenosquamous: 18 (11.5%) Small cell: 15 (9.5%) |
≤4: 61 (40.4%) 4-6: 60 (39.7%) >6: 30 (19.9%) |
NR | Pelvic: 65 (43.9%) PAN: 8 (5.4%) |
IIA: 55 (35.2%) IIB: 60 (38.5%) III: 17 (10.9%) IV: 24 (15.4%) |
CCRT: 76 (60.8%) Surgery + Chemotherapy: 26 (20.8%) Surgery + CCRT: 23 (18.4%) |
| 2015; Biewenga | 60 (32-83) | Squamous: 255 (86%) Adeno: 28 (10%) Adenosquamous: 12(45) |
5.0 (2.0-9.0) | 105 (36%) | Pelvic: 86 (29%) Pelvic + PAN: 37 (13%) |
IIB: 152 (51%) IIIA: 14 (5%) IIIB: 109 (37%) IVA: 20 (7%) |
Radiation: 94 (32%) CCRT: 97 (33%) CCRT + hyperthermia: 32 (11%) Radiation + hyperthermia: 66 (22%) Surgery + chemotherapy: 6 (2%) |
| 2014; Frumovitz | 45 (13-96) | Squamous: 2400 (77.8%) Adeno: 588 (19%) Other: 98 (3.2%) |
NR | NR | 652 (21.1%) | IB: 1999 (64.8%) II: 603 (19.6%) III: 451(14.6%) IVA: 32 (1%) |
Surgery: 468 (15.1%) Radiation: 2281 (74%) Surgery + radiation: 337 (10.9%) |
| 2013; Pinn-Bingham | 55 (20-89) | Squamous: 99 (85.3%) Adeno: 7 (6%) Adenosquamous: 7 (6%) Other: 3 (2.7%) |
≤6: 66 (58%) >6: 47 (42%) |
Unilateral: 20 (17.2%) Bilateral: 15 (12.9%) |
NR | IB: 10 (8.6%) II: 48 (41.4%) IIIA: 7 (6%) IIIB: 44 (38%) IVA: 7 (6%) |
CCRT: 61 (52.6%) Radiation: 55 (47.4%) |
| 2013; Murakami | 62 (32-80) | Squamous: 32 (94.1%) Adeno: 2 (5.9%) |
6.7 (3.9-10) | 34 (100%) | Pelvic: 12 (35.3%) | IVA | CCRT: 17 (50%) Radiation: 17 (50%) |
| 2012; Pérez-Regadera | NR | NR | NR | NR | NR | NR | CCRT |
| 2011; Pradhan | 56.2±14 vs 59±13.3 | Squamous: 64 (91.4%) vs 66 (90.4%) Adeno: 2 (2.9%) vs 5 (6.9%) Other: 4 (5.7%) vs 2 (2.7%) |
NR | Unilateral: 25 (35.7%) vs 46 (73%) Bilateral: 33 (47.1%) vs 20 (27.4%) |
Pelvic: 6 (8.6%) vs 9 (12.3%) PAN: 16 (22.9%) vs 10 (13.7%) Other: 5 (7.1%) vs 5 (6.8%) |
IIIB: 49 (70%) vs 61 (83.6%) IVA: 14 (20%) vs 7 (9.6%) IVB: 7 (10%) vs 5 (6.8%) |
CCRT: 116 (81.1%) Chemotherapy: 7 (4.9%) Radiation: 17 (11.9%) |
| 2011; Lapitan | 48.2 (26-71) | NR | NR | NR | NR | IIIB-IVA | NR |
| 2010; Rose | NR | Squamous: 223 (93.7%) vs 277 (92%) Adeno: 5 (2.1%) vs 10 (3.3%) Adenosquamous: 9 (3.8%) vs 12 (4%) |
≤2: 0 vs 2 (0.7%) 2-6: 94 (39.5%) vs 127 (42.2%) 6-10: 129 (54.2%) vs 152 (50.5%) ≥10: 13 (5.5%) vs 17 (5.6%) |
NR | 34 (14.3%) vs 43 (14.3%) | IIIB | CCRT |
| 2010; Tseng | 48.6±9.3 | Squamous | ≤4: 49 (19.5%) >4: 202 (80.5%) |
NR | Pelvic: 71 (28.3%) PAN: 38 (15.1%) |
IIB: 133 (52.9%) IIIA: 19 (7.6%) IIIB: 75 (29.8%) IVA: 24 (9.5%) |
CCRT |
| 2006; Ogino | 61 (28-90) | Squamous | NR | NR | 66 (19%) | IIB: 99 (28%) III: 239 (68%) IVA: 14 (4%) |
Radiation |
| 1999; Garipagaoglu | 52.5 (30-82) | Squamous: 153 (92.2%) Adeno: 8 (4.8%) Other: 5 (3%) |
NR | Unilateral: 36 (70%) Bilateral: 16 (30%) |
NR | IIB: 114 (68.7%) IIIB: 52 (31.3%) |
Radiation |
| 1999; Logsdon | 54 (19-99) | Squamous | ≤6: 35 (8.5%) 6-8: 97 (23.4%) ≥8: 282 (68.1%) |
Unilateral: 436 (40%) Bilateral: 331 (30%) |
Pelvis: 189 (32%) PAN: 42 (7%) |
IIIB | CCRT: 241 (24.5%) Radiation: 742 (75.5%) |
| 1998; Chao | NR | NR | NR | 281 (94.6%) | NR | IIIB | Radiation |
| 1996; Sardi | 48 | Squamous | NR | Bilateral: 59 (36.6%) | NR | IIIB | Radiation (33.5%) Neoadjuvant chemotherapy + radiation: 54 (33.5%) Surgery + chemotherapy + radiation: 53 (33%) |
| 1988; Sinistrero | 60 (18-88) | Squamous: 243 (93.8%) Adeno: 9 (3.5%) Undifferentiated: 4 (1.5%) Clear cell: 3 (1.2%) |
NR | NR | NR | T1a: 7 (2.7%) T1b: 30 (11.6%) T2a: 28 (10.8%) T3a: 98 (37.8%) T3b: 8 (3.1%) T4a: 73 (28.2%) T4b: 15 (5.8%) |
Radiation |
NR, not reported; PAN, para-aortic lymph node; CCRT, concurrent chemoradiation therapy.
Table 3.
Reported outcomes.
| Year; author | Outcome measure | Statistical test | Outcome |
|---|---|---|---|
| 2017; Hata | 3-year DFS | Chi-square P-value | P=0.127 |
| 3-year OS | Chi-square P-value | P=0.026 | |
| 2017; Ruiz | 5-year OS | Hazard ratio | HR: 1.6 (95%CI 1.0, 4.0) |
| 2016; Murakami | Disease relapse | Chi-square P-value | P=0.032 |
| 2016; Wakatsuki | 2-year DFS | Chi-square P-value | P=0.033 |
| 2015; Patel | 3-year OS | Hazard ratio | 4.00 (1.75, 8.01) |
| 2015; Goklu | Mean survival | Mean (95% CI) | 71.5 (58.2, 84.8) vs 42.2 (32.1, 52.3) vs 29.9 9 (21.8, 38.1)* |
| 2015; Kyung | 5-year OS | Hazard ratio | 3.740 (95% CI, 1.843, 7.589) |
| 2015; Biewenga | 5-year OS | Hazard ratio | 2.1 (95% CI, 1.5, 3.0) |
| 2014; Frumovitz | 5-year DSS | Hazard ratio | 1.34 (95% CI, 1.22, 1.73) |
| 2013; Pinn-Bingham | 3-year OS | Mean (95% CI) | 68% (46.3-83.0) vs 66% (51.0-77.6) |
| 5-year OS | Mean (95% CI) | 46% (10.0-76.5) vs 0% (40.4-74.3) | |
| Hazard ratio for OS/DFS | 1.65 (0.878-3.114) / 1.27 (0.571-2.806) | ||
| 2013; Murakami | 3-year OS/DFS | Chi-square P-value | P=0.091 / P=0.201 |
| 2012; Pérez-Regadera | 5-year OS/DFS | Chi-square | 66.02% vs 42.94% / 68.95 vs 46.89 |
| 2011; Pradhan | OS | Hazard ratio | 2.4 (95% CI, 1.5, 3.8) |
| 2011; Lapitan | 12-month OS | Hazard ratio | 3.26 (95% CI, 1.51, 7.01) |
| 2010; Rose | Median OS | Mean (95% CI) | 69.5% (39.5, 93.5) vs 31.5 (22.5, 40.4) |
| Median DFS | Mean (95% CI) | 46.6% (23.7, 73.8) vs 17.0 (13.9, 26.9) | |
| 2010; Tseng | 5-year OS | Multivariate Hazard ratio | 2.82 (1.89, 4.67) |
| 2006; Ogino | 5-year DFS | Chi-square P-value | P<0.001 |
| 1999; Garipagaoglu | 2-/5-year survival rates | Chi-square P-value | P=0.08 / P=0.21 |
| 1999; Logsdon | 5-year DSS | Chi-square P-value | P=0.6 |
| 1998; Chao | 5-year DFS | Wilcoxon log rank P-value | P=0.27 |
| 1988; Sinistrero | 5-year OS | Chi-square P-value | P=0.2 |
DSS, disease specific survival; *Survival compared among patients without hydronephrosis and those with unilateral, bilateral hydronephrosis.
Significant differences in terms of overall survival were noted in the majority of included articles as observed in Table 3. The reported 5-year OS hazards ratio for hydronephrosis ranged between 1.34 and 3.74. The severity of hydronephrosis and its impact on overall survival was not evaluated among the included articles, however, Goklu et al. observed that the mean survival of patients with unilateral hydronephrosis was significantly larger compared to that of patients with bilateral hydronephrosis (42.2 vs 29.9 months).18 Patel et al reported that the most prominent symptom of hydronephrosis was urinary tract infection (9 out of 17 patients), accompanied by pain (8 out of 17 patients).17 In their series they observed that 7 patients developed renal failure with creatinine levels that ranged between 1.7 and 5.6 mg/dL. None of the included studies reported, however, whether the decreased survival of patients with hydronephrosis was attributed to complications of obstructive uropathy such as uremia and sepsis.
Discussion
The findings of our systematic review support that hydronephrosis negatively affects the overall survival of cervical cancer patients. The statistical significance is more prominent when 5-year overall survival rates are investigated, as differences in terms of DFS rates are less obvious. On the other hand it remains, to date, unclear whether the survival of these patients is primarily affected by the presence of hydronephrosis as none of the included studies reported whether the survival rates were affected by the presence of acute renal failure and uremia. Moreover, the actual impact of the stage of the disease is a variable that may significantly contribute to the significant heterogeneity in outcomes that were reported in time intervals that were shorter than 5-years as patients with advanced stage disease are generally expected to have a short OS. At 2015 et al Patel et al reported that the presence of hydronephrosis is an ominous sign that predicts poor 3-year survival rates and that this effect is evident irrespective of the landmark time point that it is diagnosed.28
A significant aspect that also remains poorly investigated, to date, is whether treatment of hydronephrosis significantly benefits the OS of these patients. At 2005 Wilson et al observed that patients with an underlying gynaecological malignancy where more likely to benefit from percutaneous nephrostomy when ureteric obstruction was observed, compared to patients with primary bladder cancer.33 To date, the actual overall survival of patients with malignant obstructive uropathy remains extremely limited in patients with gynecological malignancy. At 2006, Radecka et al observed that the median survival of oncological patients (regardless of the underlying primary site of the disease) was 255 days when percutaneous nephrostomy was selected as a treatment optio.34 Dienstmann et al. investigated the impact of the procedure in 50 patients with recurrent cervical cancer and observed that 60% had an improvement of their renal function and a median decrease of creatinine that reached 2.7 mg/dL (from 6.4 pre-procedure to 3.7 mg/dL post-procedure).35 Twenty-nine patients (58%) died from renal failure and the median survival was 8.9 weeks. Current evidence remains compelling concerning the optimal route of urinary diversion in patients with malignant ureteral obstruction of gynecological origin as evidence in this field is extremely limited and mainly relies in population with malignant disease of various origin. In a retrospective analysis of patients with extrinsic ureteral obstruction due to advanced malignancy, Ku et al. observed that the possibility of failed diversion was higher when ureteral stenting was performed compared to percutaneous nephrostomy.36 For this reason, they suggested that patients should be carefully monitored, particularly when ureteral stenting is planned. On the other hand, complications of percutaneous nephrostomy seem to be high as the rates of urinary tract infections range between 20 and 50% and of postoperative ureteral obstruction due to advancing disease of catheter dislodgement between 10 and 40%.8,25,37-39 Nevertheless, in their majority they seem to be of mild to moderate severity and; thus, do not affect the quality of life of patients. However, since 1996, Emmert et al. suggested that patients with terminal cervical cancer cannot be served satisfactorily by nephrostomy.40 Gadducci et al. also suggested that the technique has little to offer to patients with recurrent disease; whereas patients with primary disease may be alleviated until definitive treatment with chemo- and radiotherapy is completed.41 Recently van Aardt confirmed this hypothesis as they also observed that nephrostomy may benefit patients with uremia and primary untreated locally advanced disease, as it may restore blood urea nitrogen levels to normal and; thus, permit the implementation of chemotherapy and radiotherapy that may prolong the survival of these patients.8 Taking this latter information into account it seems obvious that more information is needed to clarify if obstructive uropathy has the same impact among patients with stage IIb, III and IVa cervical cancer. To date, it is well known that these subgroups of patients have different 5-year survival outcomes and it would be of significant benefit if research would effectively designate whether this is primarily the result of obstructive uropathy, which is generally expected to be more prevalent in patients with stage ≥III disease. Moreover, future studies should investigate the actual impact of hydronephrosis in survival outcomes of patients with recurrent disease and determine if this differ compared to survival rates of patients with primary disease.
Conclusions
The presence of hydronephrosis in cervical cancer patients is a negative sign that predicts poor overall survival. However, to date, it remains unclear, whether treatment of the obstruction may benefit these patients. Limited data support that nephrostomy may restore blood urea nitrogen levels and permit the implementation of chemoradiotherapy, however, further studies are needed in this field to help establish consensuses regarding the optimal mode of treatment of these patients.
References
- 1.UK CR. Cervical cancer incidence statistics. Available from: https://www.cancerresearchuk.org/health-professional/cancerstatistics/statistics-by-cancer-type/cervical-cancer/incidence#heading-Two [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer. Available from: http://gco.iarc.fr/today/online-analysis-map?mode=population&mode_population=continents&population=900&sex=2&cancer=16&type=0&statistic=0&prevalence=0&color_palette=default&projection=natural-earth. 2012. [Google Scholar]
- 3.Carneiro SR, Fagundes MdA, do Rosário PdJO, et al. Five-year survival and associated factors in women treated for cervical cancer at a reference hospital in the Brazilian Amazon. PLoS One 2017;12:e0187579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mabuchi S, Isohashi F, Yoshioka Y, et al. Prognostic factors for survival in patients with recurrent cervical cancer previously treated with radiotherapy. Int J Gynecol Cancer 2010;20:834-40. [DOI] [PubMed] [Google Scholar]
- 5.Ayhan A, Al RA, Baykal C, et al. Prognostic factors in FIGO stage IB cervical cancer without lymph node metastasis and the role of adjuvant radiotherapy after radical hysterectomy. Int J Gynecol Cancer 2004;14:286-92. [DOI] [PubMed] [Google Scholar]
- 6.Song SH, Pak S, Jeong IG, et al. Outcomes of stent-change therapy for bilateral malignancy-related ureteral obstruction. Int Urol Nephrol 2015;47:19-24. [DOI] [PubMed] [Google Scholar]
- 7.Mishra K, Desai A, Patel S, et al. Role of percutaneous nephrostomy in advanced cervical carcinoma with obstructive uropathy: a case series. Indian J Palliat Care 2009;15:37-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Aardt MC, van Aardt J, Mouton A. Impact of percutaneous nephrostomy in South African women with advanced cervical cancer and obstructive uropathy. South Afr J Gynaecol Oncol 2017;9:6-10. [Google Scholar]
- 9.Netsch C, Becker B, Gross AJ. [Management of ureteral obstruction: value of percutaneous nephrostomy and ureteral stents]. Der Urologe Ausg A 2016;55:1497-510. [DOI] [PubMed] [Google Scholar]
- 10.Sinistrero G, Sismondi P, Zola P. Results of treatment of uterine cervix cancer by radiotherapy. Radiother Oncol 1988;13:257-65. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Regadera J, D’Ambrosi R, Martínez R, et al. PO-0708 Prognostic value of hydronephrosis in cervical cancer FIGO IIIB-IVA. Radiother Oncol 2012;103:S275-6. [Google Scholar]
- 13.Hata M, Koike I, Miyagi E, et al. Radiation therapy for stage IVA uterine cervical cancer: treatment outcomes including prognostic factors and risk of vesicovaginal and rectovaginal fistulas. Oncotarget 2017;8:112855-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz R, Serrano M, Ruiz EF, et al. Características clínicopatológicas y sobrevida en mujeres jóvenes con cáncer cervical: análisis retrospectivo del Instituto Nacional de Enfermedades Neoplásicas. Rev Peruana Med Exper Salud Publica 2017;34:218-27. [DOI] [PubMed] [Google Scholar]
- 15.Murakami N, Kobayashi K, Kato T, et al. The role of interstitial brachytherapy in the management of primary radiation therapy for uterine cervical cancer. J Contemp Brachyther 2016;8:391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakatsuki M, Kato S, Kiyohara H, et al. The prognostic value of rectal invasion for stage IVA uterine cervical cancer treated with radiation therapy. BMC Cancer 2016;16:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel K, Foster NR, Kumar A, et al. Hydronephrosis in patients with cervical cancer: an assessment of morbidity and survival. Support Care Cancer 2015;23:1303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goklu MR, Seckin KD, Togrul C, et al. Effect of hydronephrosis on survival in advanced stage cervical cancer. Asian Pacif J Cancer Prevent 2015;16:4219-22. [DOI] [PubMed] [Google Scholar]
- 19.Kyung MS, Kim HB, Seoung JY, et al. Tumor size and lymph node status determined by imaging are reliable factors for predicting advanced cervical cancer prognosis. Oncol Lett 2015;9:2218-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biewenga P, van der Velden J, Mol BW, et al. Prognostic model for survival in patients with early stage cervical cancer. Cancer 2011;117:768-76. [DOI] [PubMed] [Google Scholar]
- 21.Frumovitz M, Jhingran A, Soliman PT, et al. Morbid obesity as an independent risk factor for disease-specific mortality in women with cervical cancer. Obstetr Gynecol 2014;124:1098-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinn-Bingham M, Puthawala AA, Syed AM, et al. Outcomes of high-dose-rate interstitial brachytherapy in the treatment of locally advanced cervical cancer: long-term results. Int J Radiat Oncol Biol Physics 2013;85:714-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami N, Kasamatsu T, Morota M, et al. Radiation therapy for stage IVA cervical cancer. Anticanc Res 2013;33:4989-94. [PubMed] [Google Scholar]
- 24.Pradhan TS, Duan H, Katsoulakis E, et al. Hydronephrosis as a prognostic indicator of survival in advanced cervix cancer. Int J Gynecol Cancer 2011;21:1091-6. [DOI] [PubMed] [Google Scholar]
- 25.Lapitan MC, Buckley BS. Impact of palliative urinary diversion by percutaneous nephrostomy drainage and ureteral stenting among patients with advanced cervical cancer and obstructive uropathy: a prospective cohort. J Obstet Gynaecol Res 2011;37:1061-70. [DOI] [PubMed] [Google Scholar]
- 26.Rose PG, Ali S, Whitney CW, et al. Impact of hydronephrosis on outcome of stage IIIB cervical cancer patients with disease limited to the pelvis, treated with radiation and concurrent chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol 2010;117:270-5. [DOI] [PubMed] [Google Scholar]
- 27.Tseng JY, Yen MS, Twu NF, et al. Prognostic nomogram for overall survival in stage IIB-IVA cervical cancer patients treated with concurrent chemoradiotherapy. Am J Obstet Gynecol 2010;202:171-7. [DOI] [PubMed] [Google Scholar]
- 28.Ogino I, Nakayama H, Okamoto N, et al. The role of pretreatment squamous cell carcinoma antigen level in locally advanced squamous cell carcinoma of the uterine cervix treated by radiotherapy. Int J Gynecol Cancer 2006;16:1094-100. [DOI] [PubMed] [Google Scholar]
- 29.Garipagaoglu M, Yalvac S, Kose MF, et al. Treatment results and prognostic factors in inoperable carcinoma of the cervix treated with external plus high dose brachytherapy. Cancer Lett 1999;136:17-26. [DOI] [PubMed] [Google Scholar]
- 30.Logsdon MD, Eifel PJ. Figo IIIB squamous cell carcinoma of the cervix: an analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Physics 1999;43:763-75. [DOI] [PubMed] [Google Scholar]
- 31.Chao KS, Leung WM, Grigsby PW, et al. The clinical implications of hydronephrosis and the level of ureteral obstruction in stage IIIB cervical cancer. Int J Radiat Oncol Biol Physics 1998;40:1095-100. [DOI] [PubMed] [Google Scholar]
- 32.Sardi J, Giaroli A, Sananes C, et al. Randomized trial with neoadjuvant chemotherapy in stage IIIB squamous carcinoma cervix uteri: an unexpected therapeutic management. Int J Gynecol Cancer 1996;6:85-93. [Google Scholar]
- 33.Wilson JR, Urwin GH, Stower MJ. The role of percutaneous nephrostomy in malignant ureteric obstruction. Ann R Coll Surg Engl 2005;87:21-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radecka E, Magnusson M, Magnusson A. Survival time and period of catheterization in patients treated with percutaneous nephrostomy for urinary obstruction due to malignancy. Acta Radiol (Stockholm, Sweden: 1987) 2006;47:328-31. [DOI] [PubMed] [Google Scholar]
- 35.Dienstmann R, da Silva Pinto C, Pereira MT, et al. Palliative percutaneous nephrostomy in recurrent cervical cancer: a retrospective analysis of 50 consecutive cases. J Pain Sympt Manage 2008;36:185-90. [DOI] [PubMed] [Google Scholar]
- 36.Ku JH, Lee SW, Jeon HG, et al. Percutaneous nephrostomy versus indwelling ureteral stents in the management of extrinsic ureteral obstruction in advanced malignancies: are there differences? Urology 2004;64:895-9. [DOI] [PubMed] [Google Scholar]
- 37.Mishra K, Desai A, Patel S, et al. Role of percutaneous nephrostomy in advanced cervical carcinoma with obstructive uropathy: a case series. Indian J Palliat Care 2009;15:37-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plesinac-Karapandzic V, Masulovic D, Markovic B, et al. Percutaneous nephrostomy in the management of advanced and terminal-stage gynecologic malignancies: outcome and complications. Eur J Gynaecol Oncol 2010;31:645-50. [PubMed] [Google Scholar]
- 39.Souza AC, Souza AN, Kirsztajn R, Kirsztajn GM. Cervical cancer: renal complications and survival after percutaneous nephrostomy. Rev Assoc Med Brasil (1992) 2016;62:255-61. [DOI] [PubMed] [Google Scholar]
- 40.Emmert C, Rassler J, Kohler U. Survival and quality of life after percutaneous nephrostomy for malignant ureteric obstruction in patients with terminal cervical cancer. Archiv Gynecol Obstet 1997;259:147-51. [DOI] [PubMed] [Google Scholar]
- 41.Gadducci A, Madrigali A, Facchini V, Fioretti P. Percutaneous nephrostomy in patients with advanced or recurrent cervical cancer. Clin Exper Obstet Gynecol 1994;21:71-3. [PubMed] [Google Scholar]


