Abstract
Background
Depression is a common disorder linked with high levels of chronicity, psycho-social and physical problems, and suicide. Here, we assessed the antidepressant effects of the hydromethanolic extract of Taraxacum officinale and investigated the underlying mechanism.
Material/Methods
Antidepressant effects were examined by use of the tail suspension test (TST). Concentrations of corticosterone, dopamine, noradrenaline, and adrenaline were examined by biochemical assays. The mRNA expression was assessed by quantitative RT-PCR. Phytochemical analysis was performed by LC/MS.
Results
The results showed that the extract at the dosage of 50 and 100 mg/kg significantly (p<0.01) alleviated the TST-induced immobility in the mice, and the effects were comparable to the antidepressant drug Bupropion, which was used as the positive control. Investigation of the underlying mechanism revealed that the T. officinale extract exerts it effects by significantly (p<0.05) decreasing the levels of corticosterone and increasing the concentrations of dopamine, noradrenaline, and adrenaline. Further, the extract also increased the expression of brain-derived neurotrophic factor (Bdnf), which was associated with significant (p<0.05) decrease in the expression of mitogen-activated protein kinase phosphatase-1 (Mkp-1), indicative of the antidepressant potential of T. officinale. Finally, the active constituents of the extract, which include isoetin, hesperidin, naringenin, Kaempferol, sinapinic, and gallic acid, were also identified, which could potentially be responsible for its antidepressant effects.
Conclusions
In conclusion, T. officinale exerts significant antidepressant effects in a mouse model of depression by inhibition of corticosterone levels and modulation of Mkp-1 and Bdnf expression.
MeSH Keywords: Antidepressive Agents, Depression, Gene Expression Profiling
Background
Depression is a prevalent disorder that is often chronic, and which is associated with psycho-social and physical problems, as well as suicide [1]. The current treatment regimens used for the management of depression exhibit a number of adverse effects and negatively affect patient health [2]. As such, development of alternative therapeutics with higher efficacy and fewer adverse effects is urgently required [3]. The natural world has long been a source of extraordinary drugs used for the treatment of a wide array of deadly diseases such as cancer [4]. Among natural sources, plants have been used for the alleviation of human ailments since prehistoric times, and they have been used for curing many diseases, including depression [5]. Moreover, it is believed that herbal therapies that use edible plants for treatment of diabetes may prove beneficial owing to the negligible adverse effects associated with edible plants [6]. Taraxacum officinale, also known as the common dandelion, is an important medicinal herb belonging to the family Asteraceae [7]. A number of pharmacological activities have been attributed to this plant such as anti-inflammatory, anti-proliferative, and anti-oxidant effects [8]. It has been reported that T. officinale extract protects against lead-induced brain damage [9]. It has been also shown to exhibit hepatoprotective effects [10]. In a recent study, T. officinale has also been reported to show antidepressive effects [11,12]. However, the antidepressive effects of T. officinale have not been thoroughly investigated and the mechanism is unclear. Here, we examined the antidepressive effects of hydromethanolic extract of T. officinale and unveiled the probable mechanism of action. We also tentatively identified the active constituents of the extract that could be potentially responsible for its antidepressive effects. We believe that T. officinale may prove to be a vital and safer source of antidepressive agents and thus be used in the management of depression.
Material and Methods
Plant material and preparation of the extract
The fresh leaves of T. officinale were harvested from natural habitats. These harvested leaves were subjected to washing followed by shade-drying. The dried leaves the converted to a fine powder. The hydromethanolic extract of the powder was prepared as described previously. The extracts were then stored at 4°C in a vacuum until used for experimentation.
Animals and treatment groups
ICR male mice about 3 weeks old and weighing around 27 g were procured from the Animal Facility of the Mental Diseases Prevention and Treatment Institute of the Chinese PLA, PLA 91st Central Hospital, Jiaozuo 454000, China under approval by the Ethics Committee of the same institute (approval no. 897MDP/2018. The mice were housed separately and maintained under standard conditions with free accesses to food and water under a 12: 12 light/dark cycle. The mice were randomly grouped in 5 groups. Group 1 received distilled water without TST, Group II received distilled water plus TST, Group III received bupropion plus TST, Group IV received 50 mg/kg hydromethanolic extract plus TST, and Group V received 100 mg/kg hydromethanolic extract plus TST.
Tail suspension test (TST)
The TST was carried out as described in the literature with minor modifications. In brief, the mice were hung from the tail in a white box with their heads at least 30 cm from the bottom. The background noise was minimized and each mouse was kept in this position for 4 min. The mice were then sacrificed and the brains were isolated and stored at −80°C for further experimentation.
Determination of serum corticosterone level and brain dopamine (DOP), noradrenaline (NA), and adrenaline (ADR) levels
The blood serum was harvested by high-speed centrifugation for 15 min. We used Assay Max corticosterone ELSIA kits (Assaypro) for determination of corticosterone levels following the manufacturer’s guidelines. Next, 50 mg of the limbic area of the brain was extracted and homogenised and the NAD, DOP, and ADR levels were estimated using the 3-CAT ELSIA kit as per the manufacturer’s guidelines.
cDNA synthesis and quantitative RT-PCR
The RNA was isolated from the tissues by Trizol reagent and then transcribed into cDNA using RevertAid cDNA synthesis kits. The expression of Mpk1 and Bdnf was determined by qRT-PCR, as described previously [13]. The relative quantification (ΔΔ-CT) method was used to quantitatively evaluate variations between the samples examined. The reaction was performed in a total volume of 20 μl, which included 10 μl of 2 SYBR Green Master Mix, 0.2 μM gene-specific primers, and 100 ng of template cDNA. The cycling conditions were 95°C for 20 s, followed by 40 cycles of 95°C for 15 s and 58°C for 1 min. GAPDH was used as an endogenous control to normalize all data.
Phytochemical analysis
The phytochemical analysis of the hydromethanolic extract of T. officinale was carried out as described previously [14]. In brief, the sample was prepared by dissolving it in methanol followed by filtration through a 0.22-μM nylon HPLC filter. The LC-MS apparatus of Nexera UHPLC (130 MPa) equipped with MS-8030 (Shimadzu) was used for the study, and data were generated using Lab Solutions software. The Enable RP-C18 column (250×4.6 mm, 5 μM) was used. The injection volume was 5 μL and flow rate was 0.3 ml/min. Mobile phase A (water and acetonitrile ratio 1: 1) and mobile phase B (0.1% Acetic acid in water) were used in a linear gradient flow and the column temperature was set at 75°C initially. Nitrogen was used as the interface gas and MS was obtained under the following MS parameters: positive and negative ionization modes, scan range 100–1000 m/z, ESI MS source, DL temperature 250°C, nebulising gas flow 3 L/min, heat block temperature 400°C, and drying gas flow of 15 L/min.
Statistical analysis
The experiments were performed in triplicate and the data are expressed as mean ±SD. The Tukey’s test followed by one-way ANOVA was used for statistical analysis using GraphPad prism 7 software. A p value of >0.05 was considered statistically significant.
Results
T. officinale hydromethanolic extract affects the immobility time in TST in mice
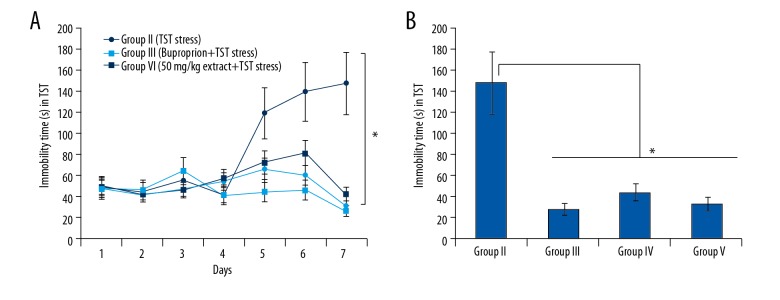
The antidepressant activity of the hydromethanolic extract of T. officinale was evaluated by TST-triggered stress in mice. It was found that the administration of the extract at the dosage of 50 and 10 mg/kg had no apparent effects on weight or hair color. Moreover, no deaths were observed following admiration of the extract. The immobility time remained constant in all the groups up to day 4. However, from the day 5, the immobility time in Group II (the Model group that received TST stress) increased significantly. In contrast, the extract significantly reduced the TST-induced immobility. The effect of the extract at 100 mg/kg (Group V) on the immobility was comparable to that of the positive control bupropion-treated mice (Group III) (Figure 1)
Figure 1.
(A) Effect of T. officinale extract on immobility time (seconds) for 7 days. (B) The immobility time of different groups on the final day. The experiments were performed in triplicate (* p<0.05).
T. officinale hydromethanolic extract affects the corticosterone levels in mice
The serum corticosterone levels of the TST-stressed Group II significantly increased relative to the Group I receiving distilled water. However, administration of the extract at the dosages of 50 mg/kg (group IV) and 100 mg/kg (Group V) resulted in considerable decline in the corticosterone levels, which was similar to that of the Bupropion-treated group (Group III) (Figure 2).
Figure 2.
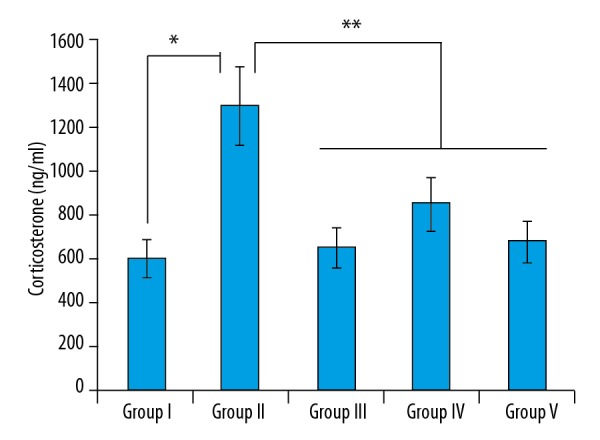
Effect of the T. officinale extract of corticosterone (ng/ml) in different groups. The experiments were performed in triplicate (Group I vs. Group II, * p<0.05 and ** P<0.01Group II vs. Group III, IV and V).
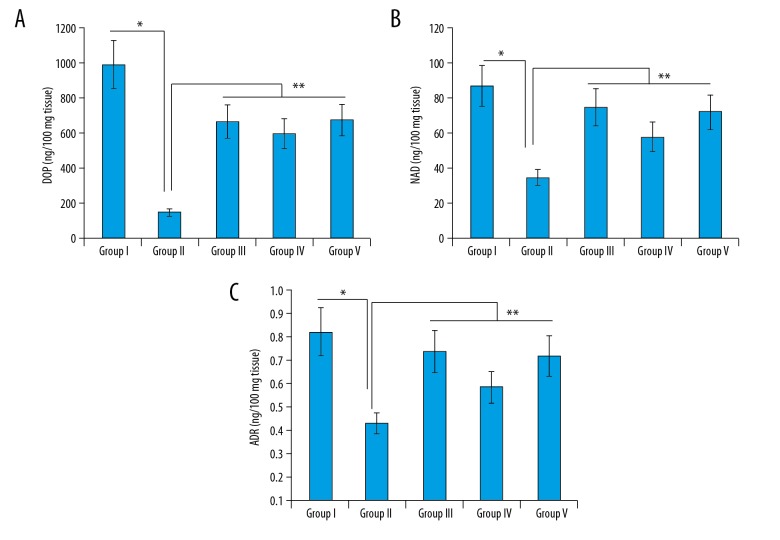
T. officinale hydromethanolic extract affects the DOP, ADR, and NAD levels in mice
We found that the TST stress induced in Group II decreased the levels of DOP, ADR, and NAD in comparison to the vehicle group I. However, administration of the extract at 50 mg/kg and 100 mg/kg (Group IV and V) significantly restored the levels of DOP, ADR, and NAD. The effects of the positive control bupropion (Group III) were less than that of extract at the dosage of 100 mg/k g(Figure 3A–3C).
Figure 3.
Effect of the T. officinale extract on (A) DOP (B) NAD (C) ADR levels in different groups. The experiments were performed in triplicate (Group I vs. Group II, * p<0.05 and Group II vs. Group III, IV and V, ** P<0.01).
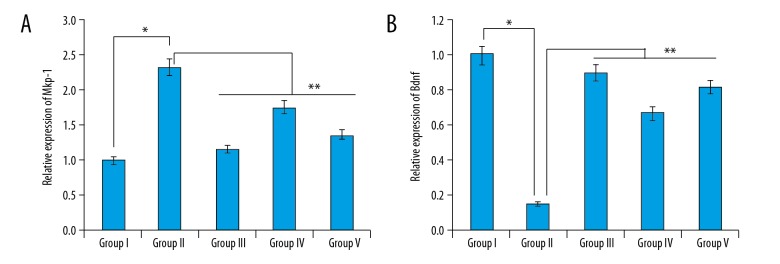
T. officinale hydromethanolic extract affects the expression of Mkp-1 and Bdnf in mice
The results revealed that TST stress (Group II) caused a significant increase in the expression of Mkp-1 in the limbic system, which was associated with decreased expression levels of Bdnf in comparison to the control group (Group I). However, administration of the extract at the dosage of 50 mg/kg and 100 mg/kg (Group IV and V) reversed the effects of TST stress on the expression of Mkp-1 and Bdnf. We found that extract at both these doses decreased the expression of Mkp-1 and increased the expression of Bdnf, but was lower than in the positive control bupropion group (Figure 4A, 4B).
Figure 4.
Effect of the T. officinale extract of the expression of (A) Mkp1 and (B) Bdnf in different groups. The experiments were performed in triplicate (Group I vs. Group II, * p<0.05 and Group II vs. Group III, IV and V, ** P<0.01).
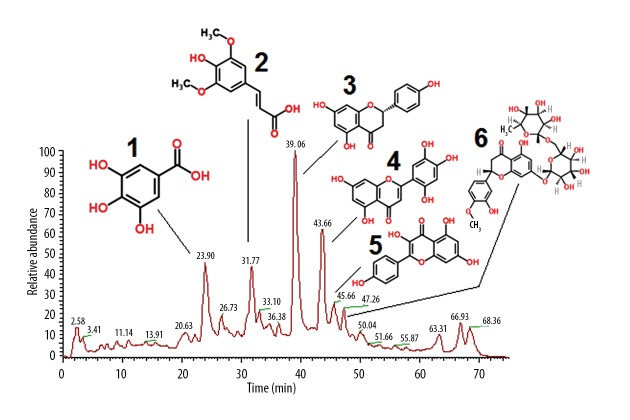
Phytochemical analysis of the hydromethanolic extract of T. officinale
To identify the phytochemical constituents of the hydromethanolic extract of the hydromethanolic extract of T. officinale, LC/MS analysis was carried out. Several of the constituents were identified in a literature search. The main compounds identified from the extract were isoetin, hesperidin, naringenin, Kaempferol, sinapinic, and gallic acid (Figure 5).
Figure 5.
Phytochemical profiling of T. officinale extract by LC/MS. The peaks represent the tentative identified compounds Peak 1: gallic acid, Peak 2: sinapinic acid, Peak 3: Naringenin, Peak 4: Kaempferol, Peak 5: Isotein, Peak 6: hesperidin.
Discussion
With advancements in the field of pharmacology, new drugs are being developed for treatment of diseases and disorders such as depression. However, even the newer antidepressants exhibit severe adverse effects on the overall health of the patients, which include elevated risk to heart attack, insomnia, and headache [16]. Depression is now becoming a huge threat to quality of life and is the second major cause of disease-related disability [17]. Because it has relatively fewer and less severe adverse effects, herbal medicine may prove beneficial in the treatment of depression. T. officinale is an important medicinal herb and has been reported to exhibit antidepressant effects [11,12]. However, the antidepressant effects of this plant are not fully explored and its mechanism of action is not known. Herein, the antidepressant effects of the hydromethanolic extract of T. officinale was confirmed by TST, showing that the extract can significantly alleviate the TST-induced immobility and the effects were comparable to the positive control bupropion which is an antidepressant. It has been reported that stresses such as the TST in mice results in human depression-like condition and initiates corticosterone production, which ultimately causes a chain of endocrine events [18]. Here, we for the first time report that the hydromethanolic extract of T. officinale exerts antidepressant effects by decreasing corticosterone levels and by increasing the ADR, NAD, and DOP levels. In addition, the hydromethanolic extract of the T. officinale can increase the expression of the Brain-derived neurotrophic factor (Bdnf), which is associated with decreased expression of mitogen-activated protein kinase phosphatase-1 (Mkp-1). The increase in the expression of Bdnf and decrease in the expression of Mkp-1 has been reported to exert protective effects in TST-stressed mice, further complementing our findings [19]. Finally, the active constituents that are potentially responsible for the antidepressant effects of the T. officinale were identified: isoetin, hesperidin, naringenin, Kaempferol, sinapic, and gallic acid. Previous studies have also reported the presence of these compounds in T. officinale or other related species [20,21]. Some of these metabolites have even been shown to exhibit antidepressant effects.
Conclusions
The hydromethanolic extract of T. officinale exerts antidepressant effects in a mouse models of depression, mainly due to inhibition of corticosterone production, activation of DOP, ADA, and NAD production, and modulation of Bdnf and Mapk1 expression. Hence, T. officinale may prove beneficial in the management of depression.
Footnotes
Source of support: We acknowledge the funding support from Jiading District Mental Health Key Specialty Construction Projects (No. JDYXZDZK-3) and the Key Scientific and Technological Project of Henan Province (No. 132102310084)
References
- 1.dos Santos RG, Osório FL, Crippa JA, et al. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): A systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol. 2016;6(3):193–213. doi: 10.1177/2045125316638008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polyakova M, Schroeter ML, Elzinga BM, et al. Brain-derived neurotrophic factor and antidepressive effect of electroconvulsive therapy: Systematic review and meta-analyses of the preclinical and clinical literature. PLoS One. 2015;10(11):e0141564. doi: 10.1371/journal.pone.0141564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apazoglou K, Farley S, Gorgievski V, et al. Antidepressive effects of targeting ELK-1 signal transduction. Nature Med. 2018;24(5):591–98. doi: 10.1038/s41591-018-0011-0. [DOI] [PubMed] [Google Scholar]
- 4.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–61. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 5.Mhalla D, Zouari Bouassida K, Chawech R, et al. Antioxidant, hepatoprotective, and antidepression effects of rumex tingitanus extracts and identification of a novel bioactive compound. BioMed Res Int. 2018;3:5–11. doi: 10.1155/2018/7295848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saki K, Hassanzad-Azar H, Naghdi N, et al. Ginkgo biloba; An effective medicinal plant on neurological disorders. J Prev Epidemiol. 2016;27:1–7. [Google Scholar]
- 7.Martinez M, Poirrier P, Chamy R, et al. Taraxacum officinale and related species – an ethnopharmacological review and its potential as a commercial medicinal plant. J Ethanopharmacol. 2015;169:244–62. doi: 10.1016/j.jep.2015.03.067. [DOI] [PubMed] [Google Scholar]
- 8.Astafieva AA, Enyenihi AA, Rogozhin EA, et al. Novel proline-hydroxyproline glycopeptides from the dandelion (Taraxacum officinale Wigg.) flowers: De novo sequencing and biological activity. Plant Sci. 2015;238:323–29. doi: 10.1016/j.plantsci.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Gargouri M, Magné C, Ben Amara I, et al. Dandelion enriched diet of mothers alleviates lead-induced damages in liver of newborn rats. Cell Mol Biol. 2017;63(2):67–75. doi: 10.14715/cmb/2017.63.2.10. [DOI] [PubMed] [Google Scholar]
- 10.Hfaiedh M, Brahmi D, Zourgui L. Hepatoprotective effect of Taraxacum officinale leaf extract on sodium dichromate-induced liver injury in rats. Environ Toxicol. 2016;31(3):339–49. doi: 10.1002/tox.22048. [DOI] [PubMed] [Google Scholar]
- 11.Lee BR, Lee JH, An HJ. Effects of Taraxacum officinale on fatigue and immunological parameters in mice. Molecules. 2012;17(11):13253–65. doi: 10.3390/molecules171113253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YC, Shen JD, Li YY, et al. Antidepressant effects of the water extract from Taraxacum officinale leaves and roots in mice. Pharm Biol. 2014;52(8):1028–32. doi: 10.3109/13880209.2013.876432. [DOI] [PubMed] [Google Scholar]
- 13.Kroh EM, Parkin RK, Mitchell PS, et al. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50(4):298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baba SA, Malik AH, Wani ZA, et al. Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. S Afr J Bot. 2015;99:80–87. [Google Scholar]
- 15.Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: A systematic review. Lancet. 2003;361:653–61. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- 16.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–66. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho AF, Sharma MS, Brunoni AR, et al. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: A critical review of the literature. Psychother Psychosom. 2016;85(5):270–88. doi: 10.1159/000447034. [DOI] [PubMed] [Google Scholar]
- 18.van Donkelaar EL, Vaessen KR, Pawluski JL, et al. Long-term corticosterone exposure decreases insulin sensitivity and induces depressive-like behaviour in the C57BL/6NCrl mouse. PLoS One. 2014;9(10):e106960. doi: 10.1371/journal.pone.0106960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo S, El Omri A, Han J, et al. Antidepressant-like effects of rosmarinic acid through mitogen-activated protein kinase phosphatase-1 and brain-derived neurotrophic factor modulation. J Funct Foods. 2015;14:758–66. [Google Scholar]
- 20.Huber M, Triebwasser-Freese D, Reichelt M, et al. Identification, quantification, spatiotemporal distribution and genetic variation of major latex secondary metabolites in the common dandelion (Taraxacum officinale agg.) Phytochem. 2015;115:89–98. doi: 10.1016/j.phytochem.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Lee C, Kim YH, et al. Chemical constituents of the aerial part of Taraxacum mongolicum and their chemotaxonomic significance. Nat Prod Res. 2017;31(19):2303–7. doi: 10.1080/14786419.2017.1292511. [DOI] [PubMed] [Google Scholar]