The proband (patient 1), a 25-year-old woman, was the product of normal pregnancy and delivery. Her parents were first cousins from Lebanon. She sat at 6 months and crawled at 12 months. At age 3, she was noted to have developmental delay, hypotonia, and ataxia. The following year, she had a febrile illness and suspected absence seizure. EEG showed 4–5 Hz spike and slow wave complexes and she was treated with sodium valproate. At age 6, seizure frequency increased but improved with the addition of ethosuximide. Brain MRI at age 10 revealed increased T2 signal in both thalami and upper brainstem (figure). Serum and urinary amino acids, white blood cell lysosomal enzymes, serum lactate and pyruvate, liver function, and ammonia were normal. CSF neurotransmitters, glucose, and lactate were normal, although GABA was not measured. Her condition then remained static with occasional absence seizures. At age 22, she developed paroxysmal episodes of chorea in the neck, arms, and trunk associated with drowsiness, triggered by fever or hot weather (video 1). These occurred 4–5 times a year, lasting from 1 to 10 minutes. In all episodes, she remained responsive to verbal stimuli. Prolonged interictal video EEGs showed intermittent 4–6 Hz generalized epileptiform discharges, although no motor events were recorded.
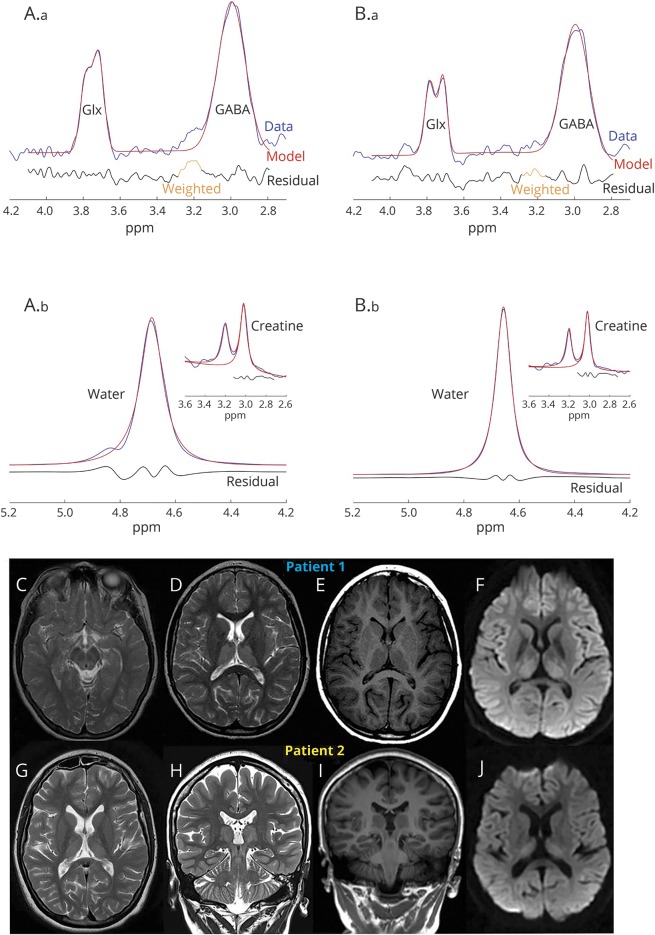
Figure. 1H magnetic resonance spectroscopy (MRS) quantification of GABA and MRI of the patients with GABA transaminase deficiency.
(A.a, B.a) (Patients 1 and 2, respectively) modeled data (red), raw GABA + signal (blue), and residuals (black) exemplifying the accuracy of the GABA spectroscopy data fit. Representative water data (insert: unedited raw data at 3.0 ppm highlighting creatine and choline peaks) (A.b, B.b). The 1H-MRS quantification of GABA (not shown in figure) showed increased levels in the thalamus (patient 1, GABA = 3.99 mM; patient 2, GABA = 4.46 mM) and occipital lobe (patient 1, GABA = 3.72 mM; patient 2, GABA = 2.79 mM) in both patients (normal range 0.5–2.0 mM). See also data supplemental material (doi.org/10.5061/dryad.d6140fj) for 1H-MRS acquisition and analysis protocol. The MRI of patient 1 at age 10 years shows T2 sequence upper midbrain (C) and thalamic hyperintensities (D) and T1 hypointensity (E). Diffusion-weighted imaging (DWI) restriction is seen in the thalamus (F). MRI of patient 2 at age 19 years demonstrates similar findings with symmetric hyperintense signals in thalamus (G) and midbrain (H) in the T2 sequences that are also hypointense in T1 sequences (I). DWI sequences show diffusion restriction in the thalamus (J).
Paroxysmal dyskinesias in GABA transaminase deficiency. The video shows patient 1 during an episode of chorea concomitant to a febrile illness. In the supine position, she had generalized intermittent truncal, neck, and limb choreic movements associated with mild drowsiness; however, alertness remains preserved.Download Supplementary Video 1 (6.2MB, mp4) via http://dx.doi.org/10.1212/006744_Video_1
The index patient's sister (patient 2), aged 32, was diagnosed at age 2 with mild motor developmental delay and had 2 febrile illnesses with chorea and fluctuating drowsiness. Following recovery from these episodes, she had gait and limb ataxia. At age 3, she had episodes of abnormal posturing of her right limbs and head lasting 5–10 minutes, precipitated by emotional distress or physical fatigue. These episodes resolved spontaneously. Between ages 7 and 12, she had absence seizures that were controlled with sodium valproate. EEG and MRI brain findings were similar to those of her sister (figure). Both patients achieved reading and writing and attended a vocational school. Interictally, both had hypotonia, hyporeflexia, and ataxia (videos 2–3 ). Nerve conduction studies were normal.
Patient 1. The interictal examination of patient 1 shows no rest or postural tremor. There is only mild tremor during finger-to-nose task bilaterally. Alternating hand movements are uncoordinated and ballistic tracking task demonstrates hypermetria. During gait, the base is mildly broad but tandem gait is performed with errors.Download Supplementary Video 2 (9.6MB, mp4) via http://dx.doi.org/10.1212/006744_Video_2
Patient 2. Interictal examination of patient 2. With the limbs resting, there is no tremor. The patient has mild upper limb tremor during finger-to-nose task and during ballistic tracking there is hypermetria of the upper limbs. The tandem gait is also abnormal. Oculomotor examination shows hypometric vertical saccades more than horizontal but no nystagmus.Download Supplementary Video 3 (8.1MB, mp4) via http://dx.doi.org/10.1212/006744_Video_3
Whole exome sequencing in both sisters revealed c.275G>A (p.Arg92Gln) pathogenic variants in the ABAT gene (supplemental material, doi.org/10.5061/dryad.d6140fj) that were confirmed as homozygous on Sanger sequencing, whereas their parents were heterozygous carriers. 1H magnetic resonance spectroscopy (MRS) demonstrated elevated brain GABA levels (figure), confirming a functional consequence of their mutations (supplemental material).
Discussion
Recessive mutations in the ABAT gene cause GABA transaminase (GABA-T) deficiency, the neurochemical hallmark of which is increased brain GABA levels.1 Our patients have a distinctive phenotype with mild developmental delay, absence epilepsy, paroxysmal dyskinesia, and nonprogressive ataxia. To date, only 10 infants with GABA-T deficiency have been described, with the longest survivor aged 10.1,2 Universal findings are severe epileptic encephalopathy, developmental delay, drowsiness, hypotonia, and cortical visual impairment. Secondary hypomyelination, T2 hyperintensities, or diffusion-weighted imaging restriction in the internal capsule or brainstem tegmentum are seen on MRI. The T2 thalamic hyperintensities in our patients have not previously been reported. The occurrence of paroxysmal dyskinesia with drowsiness in our patients is an unreported feature of GABA-T deficiency. Drowsiness may be a diagnostic clue as this feature is present in 90% of reported patients.1 Three cases with an otherwise classical presentation of ABAT mutations have also had persistent chorea.1 One of these cases had gradual stabilization of symptoms over a 10-year follow-up despite sustained high GABA levels.2 Movement disorders in other conditions of GABA metabolism have been reported. Patients with succinic semialdehyde dehydrogenase deficiency develop choreoathetosis, dystonia, myoclonus, and paroxysmal dyskinesias.3 Furthermore, patients treated with vigabatrin—a GABA-T inhibitor—rarely develop hyperkinetic movement disorders and thalamic and pallidal T2 hyperintensities.4 Enhanced GABA neurotransmission has been implicated in absence epilepsy pathophysiology; however, how this may predispose to hyperkinetic disorders remains unknown.
Our patients were homozygous for the c.275G>A (p.Arg92Gln) variant, previously reported as pathogenic as part of a compound heterozygous state with a c.199-231del, causing a severe phenotype, although interestingly with the longest survival reported thus far in GABA-T deficiency.5 Each of these alleles individually conferred enzymatic activity of 63% and 15%, respectively.6 Thus the relatively milder phenotype in our patients might be attributed to higher residual enzymatic function.
The diagnosis of GABA-T deficiency has potential therapeutic implications because GABAergic medications are potentially harmful and treatment with flumazenil has been shown to improve neurologic function.1,7 In addition to GABA levels and 1H MRS,5 2-pyrrolidinone is a surrogate biomarker for GABA-T deficiency and can be detected in plasma and CSF.7 Our patients show that GABA-T deficiency can cause paroxysmal dyskinesia with epilepsy and should be added to the differential diagnosis of this syndrome.
Acknowledgment
The authors thank the patient's family for participation in this project and consent for video publication and Michael Tchan for critical review of the manuscript.
Footnotes
CME Course: NPub.org/cmelist
Author contributions
Dr. Morales-Briceño: study concept and design, acquisition and interpretation of data, writing first draft and final version of the manuscript. F. Chang: study concept and design, acquisition and interpretation of data, writing first draft, critical revision of final manuscript for intellectual content. Dr. Wong: study concept and design, acquisition and interpretation of data, writing first draft, critical revision of final manuscript for intellectual content. A. Mallawaarachchi: study concept and design, acquisition and interpretation of data, writing first draft, critical revision of final manuscript for intellectual content. N. Wolfe: study concept and design, acquisition and interpretation of data, writing first draft, critical revision of final manuscript for intellectual content. Dr. Pellegrino da Silva: study concept and design, acquisition and interpretation of data, writing first draft, critical revision of final manuscript for intellectual content. Dr. Hakonarson: study concept and design, acquisition and interpretation of data, writing first draft, critical revision of final manuscript for intellectual content. S.A. Sandaradura: study concept and design, interpretation of data, study supervision, critical revision of final manuscript for intellectual content. Dr. Guo: study concept and design, interpretation of data, study supervision, critical revision of final manuscript for intellectual content. Dr. Christodoulou: study concept and design, interpretation of data, study supervision, critical revision of final manuscript for intellectual content. Dr. Lagopouloss: study concept, interpretation of data, critical revision of final manuscript for intellectual content. P. Grattan-Smith: study concept, interpretation of data, critical revision of final manuscript for intellectual content. Dr. Fung: study concept and design, interpretation of data, study supervision, critical revision of final manuscript for intellectual content.
Study funding
The research conducted at the Murdoch Children's Research Institute was supported by the Victorian Government's Operational Infrastructure Support Program. Guo Yiran received funding for the research from the Center for Applied Genomics, Children's Hospital of Philadelphia.
Disclosure
H. Morales-Briceño reports no disclosures relevant to the manuscript. F. Chang was recipient of the Neville-Brown Scholarship from the University of Sydney. C. Wong, A. Mallawaarachchi, N. Wolfe, R. Pellegrino da Silva, H. Hakonarson, S. Sandaradura, Y. Guo, J. Christodoulou, J. Lagopouloss, P. Grattan-Smith, and V. Fung report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology June 3, 2018. Accepted in final form October 2, 2018.
References
- 1.Koenig MK, Hodgeman R, Riviello JJ, et al. Phenotype of GABA-transaminase deficiency. Neurology 2017;88:1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichikawa K, Tsuji M, Tsuyusaki Y, Tomiyasu M, Aida N, Goto T. Serial magnetic resonance imaging and (1)H-magnetic resonance spectroscopy in GABA transaminase deficiency: a case report. JIMD Rep Epub 2018 Feb 25. [DOI] [PMC free article] [PubMed]
- 3.Leuzzi V, Di Sabato ML, Deodato F, et al. Vigabatrin improves paroxysmal dystonia in succinic semialdehyde dehydrogenase deficiency. Neurology 2007;68:1320–1321. [DOI] [PubMed] [Google Scholar]
- 4.Dill P, Datta AN, Weber P, Schneider J. Are vigabatrin induced T2 hyperintensities in cranial MRI associated with acute encephalopathy and extrapyramidal symptoms? Eur J Paediatric Neurol 2013;17:311–315. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji M, Aida N, Obata T, et al. A new case of GABA transaminase deficiency facilitated by proton MR spectroscopy. J Inherit Metab Dis 2010;33:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besse A, Wu P, Bruni F, et al. The GABA transaminase, ABAT, is essential for mitochondrial nucleoside metabolism. Cell Metab 2015;21:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenig MK, Bonnen PE. Metabolomics profile in ABAT deficiency pre- and post-treatment. JIMD Rep Epub 2018 Feb 27. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Paroxysmal dyskinesias in GABA transaminase deficiency. The video shows patient 1 during an episode of chorea concomitant to a febrile illness. In the supine position, she had generalized intermittent truncal, neck, and limb choreic movements associated with mild drowsiness; however, alertness remains preserved.Download Supplementary Video 1 (6.2MB, mp4) via http://dx.doi.org/10.1212/006744_Video_1
Patient 1. The interictal examination of patient 1 shows no rest or postural tremor. There is only mild tremor during finger-to-nose task bilaterally. Alternating hand movements are uncoordinated and ballistic tracking task demonstrates hypermetria. During gait, the base is mildly broad but tandem gait is performed with errors.Download Supplementary Video 2 (9.6MB, mp4) via http://dx.doi.org/10.1212/006744_Video_2
Patient 2. Interictal examination of patient 2. With the limbs resting, there is no tremor. The patient has mild upper limb tremor during finger-to-nose task and during ballistic tracking there is hypermetria of the upper limbs. The tandem gait is also abnormal. Oculomotor examination shows hypometric vertical saccades more than horizontal but no nystagmus.Download Supplementary Video 3 (8.1MB, mp4) via http://dx.doi.org/10.1212/006744_Video_3