Abstract
Objective
To study the relationship between endothelial dysfunction, HIV infection, and stroke in Malawians.
Methods
Using a cross-sectional design, we measured plasma levels of intercellular adhesion molecule-1 (ICAM-1), plasminogen activator inhibitor-1 (PAI-1), vascular endothelial growth factor (VEGF), and soluble thrombomodulin (sTM) in stroke patients and controls, stratified by HIV status. These biomarkers were measured using ELISA. After dichotomization, each biomarker was used as the dependent variable in a multivariable logistic regression model. Primary independent variables included HIV and stroke status. Adjustment variables were age, sex, hypertension, diabetes mellitus, tobacco and alcohol consumption, personal/family history of stroke, antiretroviral therapy status, and hypercholesterolemia.
Results
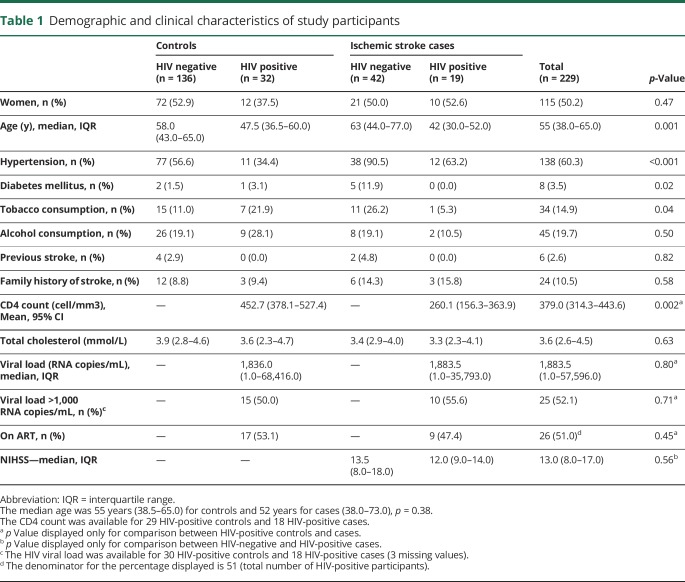
Sixty-one stroke cases (19 HIV+) and 168 controls (32 HIV+) were enrolled. The median age was 55 years (38.5–65.0) for controls and 52 years (38.0–73.0) for cases (p = 0.38). The median CD4+ T-cell count was 260.1 cells/mm3 (156.3–363.9) and 452 cells/mm3 (378.1–527.4) in HIV-infected cases and controls, respectively. HIV infection was independently associated with high levels of ICAM-1 (OR = 3.6, 95% CI: 1.3–10.6, p = 0.018) in controls but not in stroke cases even after excluding patients with a viral load >1,000 RNA copies/mL (OR = 4.1, 95% CI: 1.3–13.1, p = 0.017). There was no association between the clinical profiles of HIV-positive controls or HIV-positive stroke and high levels of PAI-1, VEGF, and sTM.
Conclusions
HIV infection is associated with endothelial activation despite antiretroviral treatment. Our findings underscore the need for larger clinical cohorts to better understand the contribution of this perturbation of the endothelial function to the increasing burden of cardiovascular diseases in sub-Saharan Africa.
The vascular endothelium is a critical regulator of cell adhesion, inflammation, coagulation, and vascular remodeling. Perturbation of one or more of these functions, also known as endothelial dysfunction, is induced by classical cardiovascular risk factors. Endothelial dysfunction is a major component of local and systemic inflammation. At the early stage of endothelial activation, there is increased expression of cell adhesion molecules (e.g., intercellular adhesion molecule-1 [ICAM-1]) on the surface of endothelial cells to facilitate leukocyte transendothelial migration. The occurrence of endothelial damage leads to the secretion of other biomarkers, notably plasminogen activator inhibitor-1 (PAI-1) and soluble thrombomodulin (sTM) responsible for the perturbation of the coagulation process and vascular endothelial growth factor (VEGF) responsible for angiogenesis.1 The interaction of these biomarkers with other proinflammatory cytokines, as well as white blood cells and smooth muscle cells, leads to vessel wall remodeling and ultimately to various types of vasculopathy.
Several studies conducted on HIV populations from Western countries have reported an association between high levels of biomarkers of endothelial dysfunction and death and/or cardiovascular endpoints.2 However, it is unknown whether the same association is observed in a sub-Saharan African setting, where antiretroviral therapy (ART) has become more widespread. This knowledge gap provides the rationale for this study conducted to evaluate the relationship between endothelial dysfunction, HIV infection, and stroke in Malawi.
Methods
Standard protocol approvals, registrations, and patient consents
This work was conducted as part of the study of Biomarkers of Stroke and Arterial Diseases in Malawian Adults, which was approved by the Research Ethics Committees of the Liverpool School of Tropical Medicine and the University of Malawi College of Medicine. The study used plasma samples available in the biobank of the Malawi START study, a case-control study designed to identify risk factors of stroke in Malawi, where the prevalence of HIV infection is 10.3%.3 All patients enrolled in the Malawi START study provided a written informed consent that allowed their blood specimens to be reused for subsequent ethically cleared studies within a 5-year period from the time of collection.
Patient selection
As previously described,3 stroke cases enrolled in the original Malawi START study were adult residents of Blantyre presenting at the Queen Elizabeth Central Hospital within 7 days of symptom onset, meeting the WHO's clinical definition of stroke, and having a brain MRI confirming the diagnosis. Two community controls were selected for each case with the goal of obtaining an age/sex frequency-matched random sample of the population, with a geographical distribution in proportion to the population density. The subsequent patient selection (or more accurately “plasma specimen selection”) for the current study is described in figure.
Figure. Flow diagram of case and control selection.
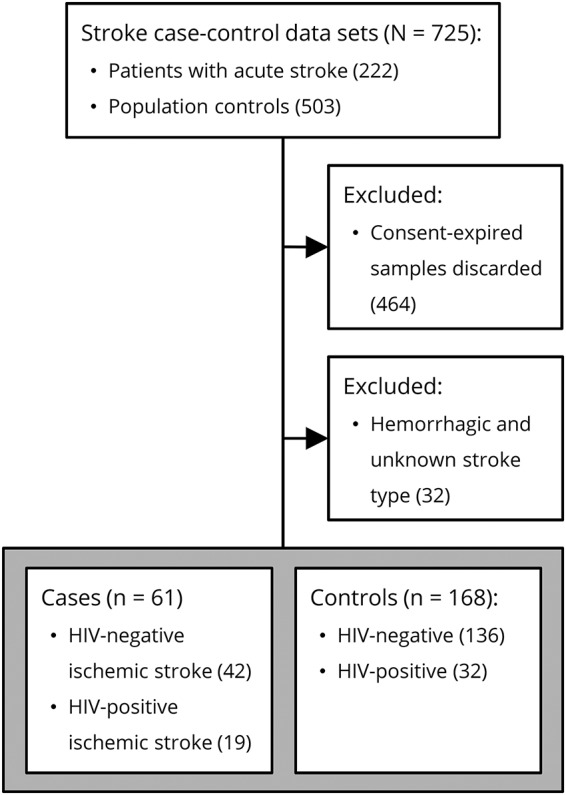
Demographic and clinical data of cases and controls included age, sex, hypertension, diabetes status, tobacco and alcohol consumption status, personal/family history of stroke, CD4+ T-cell count, HIV viral load, hypercholesterolemia, ART status, recent infection, and National Institute of Health Stroke Score for stroke cases only.3 The stroke type and etiology were defined using MRI brain imaging and the trial of Org 10172 in acute stroke treatment criteria, respectively.3
Selection of biomarkers of endothelial dysfunction
The biomarkers measured in this study were selected to assess the different stages of endothelial dysfunction as described in the introduction. The major cellular source of ICAM-1, PAI-1, and thrombomodulin is activated endothelial cells.4 VEGF, an essential growth factor for endothelial cells, is produced by macrophages, platelets, and keratinocytes. There is no validated normal range for the plasma concentration of biomarkers of endothelial dysfunction. Higher concentrations correlate well with the magnitude of local or systemic inflammation and have been consistently associated with an increased cardiovascular risk. Because HIV-infected individuals have persistent systemic inflammation,5 we hypothesized that they would have higher levels of biomarkers of endothelial dysfunction, explaining their greater risk of stroke.
Measurement of biomarkers of endothelial dysfunction
The plasma concentrations of ICAM-1, PAI-1, VEGF, and sTM were measured using ELISA kits (Abcam, Cambridge, United Kingdom). SimpleStep ELISA kits were used for ICAM-1, PAI-1, and sTM, whereas the standard kit was used for VEGF. All samples were run in duplicates. Samples with an individual coefficient of variability (CV) greater than 25% were reanalyzed.
All the ELISA tests were performed according to the manufacturer’s instructions with the exception of the washing steps that were performed with an automated washer (Wellwash; ThermoFisher Scientific, Waltham, MA) using a mixture of Tween 20 and phosphate-buffered saline (0.05% vol/vol). The plates were read at 450 nm using an automated microplate reader (Multiskan; ThermoFisher Scientific). The standard curves were constructed using a 4-parameter logistic regression fitting equation (online software: elisaanalysis.com). The mean intra-assay and inter-assay CV for ICAM-1, PAI-1, and VEGF was <10% and <15%, respectively.
Statistical analysis
The statistical analyses were performed using the software STATA (version 13; StataCorp, College Station, TX). Based on the stroke and HIV status, 4 clinical profiles were defined: HIV-negative controls, HIV-positive controls, HIV-negative stroke, and HIV-positive stroke. Demographic and clinical parameters were summarized as proportions for categorical variables and median with interquartile range (IQR) for numerical variables. For comparisons across groups, Fisher exact and Kruskall-Wallis tests were used for categorical and numerical variables, respectively.
The third tertile of the distribution of plasma concentration values was considered as the threshold for the distinction between high and low levels for each biomarker. To identify the predictors of endothelial dysfunction, the plasma level of each biomarker was included as a binary dependent variable (low/high levels) in a separate multivariable logistic regression analysis. Age, sex, hypertension, diabetes mellitus, tobacco and alcohol consumption, personal/family history of stroke, ART status, and hypercholesterolemia were included in the model as potential confounders. We also explored the plasma levels of the biomarkers as a continuous variable in a separate multiple linear regression analysis.
To further explore the effect of ART, the regression analyses described above were repeated after excluding HIV-positive patients with a viral load greater than 1,000 RNA copies/mL (25 of 48 HIV-positive participants with viral load available). The cutoff thresholds to distinguish between high and low levels of biomarkers were recalculated to adjust for the change in the population structure and size. The ART status was not included as a confounder in multivariable analyses because 87% of patients with a low or undetectable viral load were on ART.
All the statistical tests performed were 2-tailed, and statistical significance was defined as p < 0.05.
Data availability
The database of the Malawi START study is available for consultation or reuse on request to the principal investigator (L.B.). Sharing of all or part of this database is subject to prior authorization by the University of Malawi College of Medicine Research Ethics Committee.
Results
Participants' characteristics
The 229 participants (50.2% women) included in this study were distributed as follows: 136 HIV-negative controls, 32 HIV-positive controls, 42 HIV-negative cases, and 19 HIV-positive cases. The median age of the participants was 55 years (IQR: 38.0–65.0). Participants' baseline characteristics are summarized in table 1.
Table 1.
Demographic and clinical characteristics of study participants
The etiologies of 60 of 61 ischemic strokes were as follows: 7 (12%) atherosclerotic strokes, 5 (8%) cardio-thromboembolic strokes, 16 (27%) strokes with other determined causes, and 32 (53%) strokes with an undetermined cause.
Factors associated with endothelial dysfunction
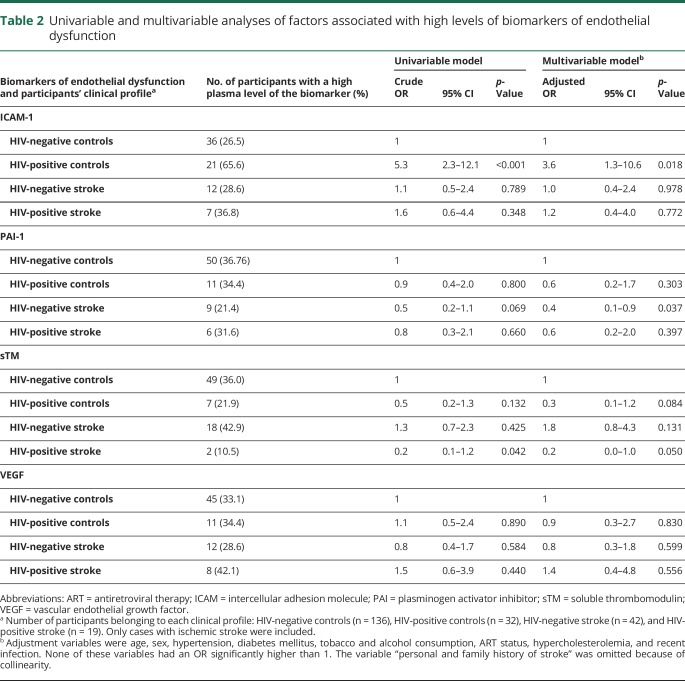
In the logistic regression analysis, the adjusted OR for having high plasma levels of ICAM-1 was 3.6 (95% CI: 1.3–10.6, p = 0.018) for the profile “HIV-positive controls” and 1.2 (95% CI: 0.4–4.0, p = 0.772) for the profile “HIV-positive stroke” (table 2). The adjusted OR for having high plasma levels of PAI-1, sTM, or VEGF was not significant for HIV-positive controls and HIV-positive stroke. The adjusted OR for having high plasma levels of PAI-1 was 0.4 (95% CI: 0.1–0.9, p = 0.037) for the profile “HIV-negative stroke” (table 2). Linear regression did not modify the associations reported here.
Table 2.
Univariable and multivariable analyses of factors associated with high levels of biomarkers of endothelial dysfunction
After excluding HIV-positive patients with a viral load greater than 1,000 RNA copies/mL, the adjusted OR for having high plasma levels of ICAM-1 was 4.1 (95% CI: 1.3–13.1, p = 0.017) for the profile “HIV-positive controls,” whereas the OR for having high plasma levels of PAI-1 was 0.5 (95% CI: 0.2–1.3, p = 0.183).
Discussion
Our results show that HIV infection is independently associated with high plasma levels of ICAM-1, a biomarker of endothelial activation. There was no association between stroke status and high levels of ICAM-1 or between HIV infection and high levels of PAI-1, sTM, and VEGF (table e-1, links.lww.com/NXI/A94).
The association between HIV infection and high levels of ICAM-1 reported here expands the limited literature on sub-Saharan Africa6 and is consistent with findings from high-income countries.2 Although other risk factors, such as diabetes, hypercholesterolemia, and ART, can contribute to endothelial activation, we found that this association is independent of those factors. Chronic systemic inflammation induced by HIV infection is a trigger for endothelial activation,7 which is an important component of endothelial dysfunction. In turn, endothelial activation and dysfunction are both risk factors for atherogenesis. For example, high levels of ICAM-1 have been associated with increasing carotid intima-media thickness, a surrogate marker of atherosclerosis and a predictor of cardiovascular events.2,8 In our cohort, more than 50% of the HIV-positive controls were on ART, and their CD4+ T-cell count was >400 cells/mm3. This means that despite having a relatively stable infection, HIV-infected individuals still have an ongoing endothelial activation as demonstrated by previous studies conducted in Western countries.9 Therefore, ART alone might not be enough to suppress cardiovascular risk in people living with HIV, and additional interventions to control HIV-related inflammation might be needed. One potential intervention might be the use of statins as an adjunct therapy to attenuate endothelial dysfunction and vascular inflammation.
Unexpectedly, we showed no association between HIV and endothelial dysfunction among patients with stroke. The most likely explanation is that our sample size was too small, with only 19 HIV-positive stroke cases. Furthermore, despite restricting the analysis to ischemic stroke, the cohort remained heterogeneous, thus reducing our statistical power further and our ability to explore the varied etiologies of cerebrovascular diseases. However, the independent association between HIV infection and high levels of ICAM-1 in the control group suggests that a cardiovascular risk does exist. Consequently, further evaluation in a larger clinical cohort is warranted.
PAI-1 is a molecule that inhibits fibrinolysis. High and low plasma levels are expected in ischemic stroke and hemorrhagic stroke, respectively. A decrease in the plasma concentration of PAI-1 with increasing age has been described.10 Therefore, we suspect that the association between levels of PAI-I and the profile “HIV-negative stroke” is due to a residual confounding effect of age.
The absence of a fixed cutoff to distinguish between low and high plasma levels of the biomarkers considered in this study represents a limitation and continues to be a challenge in this field. However, there is good evidence that high plasma concentrations are related to increased cardiovascular risk.2,11,12
Overall, this study confirms the association between HIV infection and endothelial activation despite ART in a sub-Saharan African setting. Larger well-phenotyped cohort studies are needed to clarify the contribution of HIV-related endothelial activation to the long-term stroke morbidity and mortality in the ART era.
Acknowledgment
The authors are grateful to (1) all the patients and their families for consenting to take part in this study, (2) the staff of the Institute of Infection and Global Health and the Wellcome Trust Centre for Global Health Research for their administrative support, and (3) the staff at Malawi-Liverpool-Wellcome Clinical Research Programme and Queen Elizabeth Central Hospital.
Glossary
- ART
antiretroviral therapy
- CV
coefficient of variability
- ICAM
intercellular adhesion molecule
- IQR
interquartile range
- PAI
plasminogen activator inhibitor
- sTM
soluble thrombomodulin
- VEGF
vascular endothelial growth factor
Author contributions
L. Benjamin and J. Kamtchum-Tatuene conceived the study. L. Benjamin acquired the samples and the clinical data. J. Kamtchum-Tatuene and Z. Al-Bayati performed the laboratory analyses under the supervision of J. Flatley and M. Griffiths. J. Kamtchum-Tatuene performed the statistical analyses under the supervision of L. Benjamin and H. Mwandumba. J. Kamtchum-Tatuene drafted the first version of the manuscript that was edited by L. Benjamin. H. Mwandumba, Z. Al-Bayati, J. Flatley, M. Griffiths, and T. Solomon revised the subsequent versions of the manuscript. All authors approved the final version.
Study funding
This research was funded by the Wellcome Trust [106829/Z/15/Z to JKT and CNR11146 to L.B.]. L.B. was also supported by an NIHR Clinical Lectureship.
Disclosure
J. Kamtchum-Tatuene received research support from Geneva University Hospital Fund for Research and Development and the Wellcome Trust. H. Mwandumba received research support from the Bill and Melinda Gates Foundation and MRC. Z. AL-Bayati and J. Flatley report no disclosures. M. Griffiths received travel funding from British Paediatric Neurology Association and Royal College of Physicians of Edinburgh; received research support from Fast Track Diagnostics Research Limited, Department of Health UK NIHR, MRC, European Commission, Ministry of France, and Republic of Indonesia. T. Solomon served on a commercial data safety monitoring board for the study of Ebola vaccine; served on a clinical advisory board of Siemens Healthineers; received travel funding from World Congress of Neurology, South East Asia Encephalitis, European Commission, and WHO; and received research support from NIHR HPRU. L. Benjamin received research support from GlaxoSmithKline. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NN.
References
- 1.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol 2012;11:878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross AC, Rizk N, O'Riordan MA, et al. . Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009;49:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin LA, Corbett EL, Connor MD, et al. . HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology 2016;86:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Defelice AF, Hanig JP, Colatsky T. Biomarkers of endothelial cell activation serve as potential surrogate markers for drug-induced vascular injury. Toxicol Pathol 2010;38:856–871. [DOI] [PubMed] [Google Scholar]
- 5.Sereti I, Krebs SJ, Phanuphak N, et al. . Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis 2017;64:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham SM, Rajwans N, Jaoko W, et al. . Endothelial activation biomarkers increase after HIV-1 acquisition: plasma vascular cell adhesion molecule-1 predicts disease progression. AIDS 2013;27:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melendez MM, McNurlan MA, Mynarcik DC, Khan S, Gelato MC. Endothelial adhesion molecules are associated with inflammation in subjects with HIV disease. Clin Infect Dis 2008;46:775–780. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 9.de Larrañaga GF, Bocassi AR, Puga LM, Alonso BS, Benetucci JA. Endothelial markers and HIV infection in the era of highly active antiretroviral treatment. Thromb Res 2003;110:93–98. [DOI] [PubMed] [Google Scholar]
- 10.Surjawan Y, Setiabudy RD, Suryaatmadja M, Ranakusuma TAS. The association of plasminogen activator inhibitor-1 level with ischemic stroke (preliminary study). Med J Indones 2009;19:158–163. [Google Scholar]
- 11.Knudsen A, Katzenstein TL, Benfield T, et al. . Plasma plasminogen activator inhibitor-1 predicts myocardial infarction in HIV-1-infected individuals. AIDS 2014;28:1171–1179. [DOI] [PubMed] [Google Scholar]
- 12.Musselwhite LW, Sheikh V, Norton TD, et al. . Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS 2011;25:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database of the Malawi START study is available for consultation or reuse on request to the principal investigator (L.B.). Sharing of all or part of this database is subject to prior authorization by the University of Malawi College of Medicine Research Ethics Committee.