Abstract
Objective
To characterize peri-ictal apnea and postictal asystole in generalized convulsive seizures (GCS) of intractable epilepsy.
Methods
This was a prospective, multicenter epilepsy monitoring study of autonomic and breathing biomarkers of sudden unexpected death in epilepsy (SUDEP) in patients ≥18 years old with intractable epilepsy and monitored GCS. Video-EEG, thoracoabdominal excursions, nasal airflow, capillary oxygen saturation, and ECG were analyzed.
Results
We studied 148 GCS in 87 patients. Nineteen patients had generalized epilepsy; 65 had focal epilepsy; 1 had both; and the epileptogenic zone was unknown in 2. Ictal central apnea (ICA) preceded GCS in 49 of 121 (40.4%) seizures in 23 patients, all with focal epilepsy. Postconvulsive central apnea (PCCA) occurred in 31 of 140 (22.1%) seizures in 22 patients, with generalized, focal, or unknown epileptogenic zones. In 2 patients, PCCA occurred concurrently with asystole (near-SUDEP), with an incidence rate of 10.2 per 1,000 patient-years. One patient with PCCA died of probable SUDEP during follow-up, suggesting a SUDEP incidence rate 5.1 per 1,000 patient-years. No cases of laryngospasm were detected. Rhythmic muscle artifact synchronous with breathing was present in 75 of 147 seizures and related to stertorous breathing (odds ratio 3.856, 95% confidence interval 1.395–10.663, p = 0.009).
Conclusions
PCCA occurred in both focal and generalized epilepsies, suggesting a different pathophysiology from ICA, which occurred only in focal epilepsy. PCCA was seen in 2 near-SUDEP cases and 1 probable SUDEP case, suggesting that this phenomenon may serve as a clinical biomarker of SUDEP. Larger studies are needed to validate this observation. Rhythmic postictal muscle artifact is suggestive of post-GCS breathing effort rather than a specific biomarker of laryngospasm.
The landmark Mortality in Epilepsy Monitoring Units Study (MORTEMUS) study1 found sudden unexpected death in epilepsy (SUDEP) and near-SUDEP incidences to be 7.5 per 1,000 and 2.2 per 1,000 patient-years, respectively, in the intractable epilepsy population undergoing epilepsy monitoring unit evaluation. Profound postictal cardiorespiratory dysfunction was found in all monitored SUDEP cases, with terminal central apnea preceding terminal asystole in all cases. Six of 25 cases with SUDEP and near-SUDEP had previously noted postictal central apnea, postictal cardiorespiratory arrest, or ictal asystole,1 suggesting that these features may be implicated in SUDEP pathophysiology. Ictal respiratory dysfunction during epileptic seizures is well described.2–6 Less is known about postictal breathing, particularly after generalized convulsive seizures (GCS). Apnea is a particular concern; ictal central apnea (ICA) is usually transient and benign in partial seizures but poses danger in a minority when prolonged and associated with profound hypoxemia. In particular, postictal apnea, whether obstructive7 or central,1,2,4,6,8 may be associated with SUDEP.8 Obstructive breathing dysfunction from laryngospasm is rarely reported7 but may be more frequent.9 Recent studies have re-examined muscle artifact from terminal breathing effort in the MORTEMUS study, as well as in an animal model of laryngospasm, leading to speculation about its potential as a SUDEP biomarker,10 although immediately after GCS, there is increased muscle tone that may account for this.11 There is general consensus that ictal asystole is likely benign,12 although postictal asystole may not be.13 We set out to characterize both central and obstructive breathing dysfunction and the occurrence of postictal asystole in GCS, the seizure type most associated with SUDEP.14
Methods
Patient selection
All patients were prospectively consented participants in the National Institute of Neurological Disorders and Stroke (NINDS) Center for SUDEP Research's Autonomic and Imaging Biomarkers of SUDEP multicenter project (U01-NS090407) and its preliminary phase, the Prevention and Risk Identification of SUDEP Mortality (PRISM) project (P20NS076965). Patients with intractable epilepsy (failure of adequate trials of ≥2 antiepileptic medications)15 who were ≥18 years of age and were undergoing video-EEG (VEEG) evaluation in the adult epilepsy monitoring units of participating centers from September 2011 until December 2017 were studied. Follow-up was conducted until April 30, 2018, with 6-month telephone calls or clinic visits. We included patients with recorded GCS, including generalized tonic-clonic seizures, focal to bilateral tonic-clonic seizures, and focal-onset motor bilateral clonic seizures.16 Exclusion criteria were status epilepticus and obscured or unavailable video. Demographic and clinical data were collected, including phenotypic epilepsy characteristics, epilepsy duration, seizure type and frequency, semiologic seizure features, awake or asleep states, and presence of major cardiac or respiratory disease. Epileptogenic zone was classified as generalized (genetic generalized epilepsy in all cases), focal, both, or unknown.17 GCS duration was defined as time from onset of bilateral motor signs of tonicity or clonicity to clinical seizure end. Early nursing intervention was defined as oxygen administration or suction applied during the seizure or within 5 seconds of seizure termination.18
Data collection
Cardiorespiratory monitoring and VEEG monitoring
All patients underwent prolonged surface VEEG monitoring with the 10–20 international electrode system. EEG and ECG were acquired with Nihon Kohden (Tokyo, Japan). Peripheral capillary oxygen saturation (Spo2) and heart rate were monitored with pulse oximetry and plethysmography (Nellcor OxiMax N-600x, Covidien, MN). Chest wall and abdominal excursions were recorded with inductance plethysmography (Ambu, Ballerup, Denmark; and Sleepmate or Perfect Fit 2, Dymedix, St. Paul, MN). In a subset of 21 seizures (14 patients), oral and nasal airflow was also recorded with a nasal pressure transducer (BiNAPS, Salter Labs, Lake Forest, IL) and an oral/nasal thermistor (ThermiSense, Salter Labs), with simultaneous transcutaneous CO2 (tcCO2) recordings with the Sen TecDigital Monitoring System (SenTec AG, Therwil, Switzerland).
A board certified pulmonologist (K.S.) oversaw breathing analysis. Breathing assessments (breathing rate, O2 saturation, tcCO2) were made 2 minutes pre-ictally and 3 minutes after clinical seizure end1 through careful composite analysis of inductance plethysmography, EEG breathing artifact, visually inspected thoracoabdominal excursions and synchrony with airflow, auditory breathing information, and nasal pressure signal when available. Postictal tcCO2 was analyzed up to 5 minutes. Baseline interictal Spo2 was defined as mean Spo2 over a 15-second nonapneic, artifact-free epoch. Hypoxemia was defined as Spo2<95% (mild [Spo2 90%–94%], moderate [Spo2 75%–89%], or severe [Spo2 <75%]). When baseline Spo2 was already <95% (38 of 135 seizures), a >1% drop was considered significant.
Postconvulsive breathing rate was measured and averaged in 15-second epochs. Because breathing rate after GCS is usually significantly increased compared to baseline,5 we classified abnormal postconvulsive breathing rate according to deviations from the mean in our cohort. Thus, seizures were divided into 3 groups: postconvulsive bradypnea (breathing rate 2 SD below the mean in any epoch), postconvulsive tachypnea (breathing rate 2 SD above the mean in any epoch), and eupnea (postconvulsive breathing rate within 2 SD of the mean). Postconvulsive breathing was classified as stertorous (audible, deep, snoring-like or snuffling-like sound in active inspiration or active expiration) or quiet.19 Inspiratory stridor for laryngospasm was stringently analyzed with auditory analysis, tracheal/neck movements, and nasal airflow when available.
Central apnea was defined as ≥1 missed breaths without any other explanation (i.e., speech, movement, or intervention). ICA referred to apnea that occurred before GCS. Postconvulsive central apnea (PCCA) was considered immediate, if no breath was taken for at least 5 seconds after seizure end, or delayed, when apnea occurred after at least 1 breath was detected after GCS end. Asystole was defined as an R-R interval of >3 seconds. We defined near-SUDEP as simultaneous cardiac (asystole) and breathing (central apnea) cessation, with or without cardiopulmonary resuscitation (CPR).
Presence and duration of postictal generalized EEG suppression (PGES)20 were determined by a validated automated EEG suppression detection tool21 and supplemented by visual analysis by 2 epilepsy neurophysiologists when the tool gave no solution. Presence and duration of postictal EEG burst suppression were also determined. Combined PGES and burst suppression made up the EEG recovery duration. Finally, we analyzed the presence of postictal EEG muscle artifact in a 3-minute postictal period using previously described methodology10 (time constant 0.03–0.001) in 60-second epochs.
Statistical analysis
Statistical analysis was performed with SPSS (version 24, IBM Corp, Armonk, NY). Summary statistics were reported as mean ± SD (median, range). Statistical significance for intergroup differences was assessed with Pearson χ2 test and Fisher exact tests for dichotomous or nominal variables. The Mann-Whitney U test was used for continuous variables because they did not follow a normal distribution (Kolmogorov-Smirnov test). Binary logistic regressions were used to assess associations between dichotomous variables and other variables and combinations. The Kruskal-Wallis H test was used to determine differences in continuous variables. The Spearman correlation was used to determine correlation between continuous variables. The level of significance was set at p < 0.05. Central tendency and dispersion measures of breathing rate were obtained in each 15-second interval after clinical seizure end. Outliers for breathing rate were identified when values were above or below 2 SD of the mean.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on request.
Results
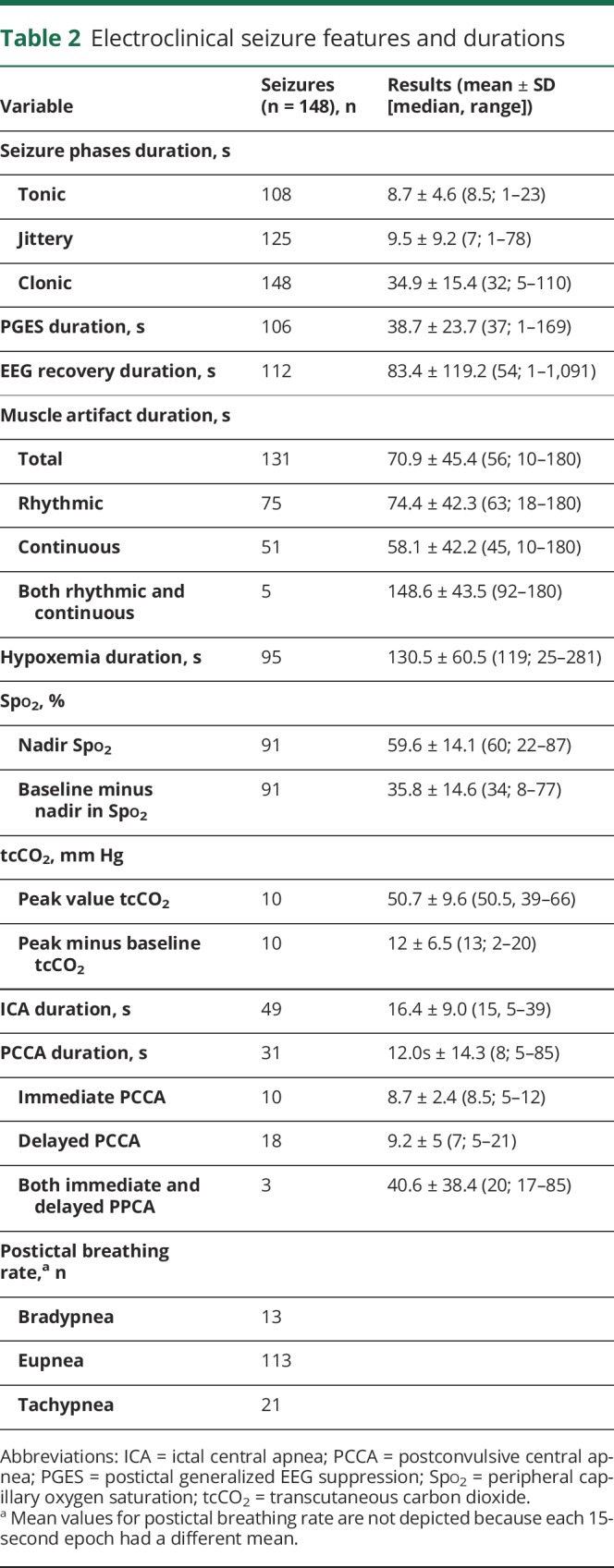
One hundred sixty-four GCS were identified in 94 patients. Reliable VEEG recordings that met study criteria were available in 148 seizures in 87 patients (47 female). Of the148, 108 were tonic-clonic GCS; the remainder were clonic GCS. Mean follow-up time was 27.7 ± 15.2 months (26.1; 5.1–84.9 months); total cohort follow-up was 195 person-years. Mean age was 37.9 ± 13.7 years (36; 18–77 years). Mean body mass index was 28.7 ± 6.6 kg/m2 (28.1; 15.1–45.8 kg/m2). Mean age at epilepsy onset was 21.4 ± 16.8 years (17; 1–67 years), and mean epilepsy duration was 16.4 ± 12.7 years (12 years; 1 month–45 years). Seventy-two seizures occurred during wakefulness, 73 during sleep, and 3 (1 patient) during postictal stupor due to a seizure cluster. PGES was present in 106 of 148 seizures (71.6%). Epilepsy phenotypic details are shown in table 1, and seizure characteristics are given in table 2.
Table 1.
Epilepsy phenotypic and MRI neuroimaging details
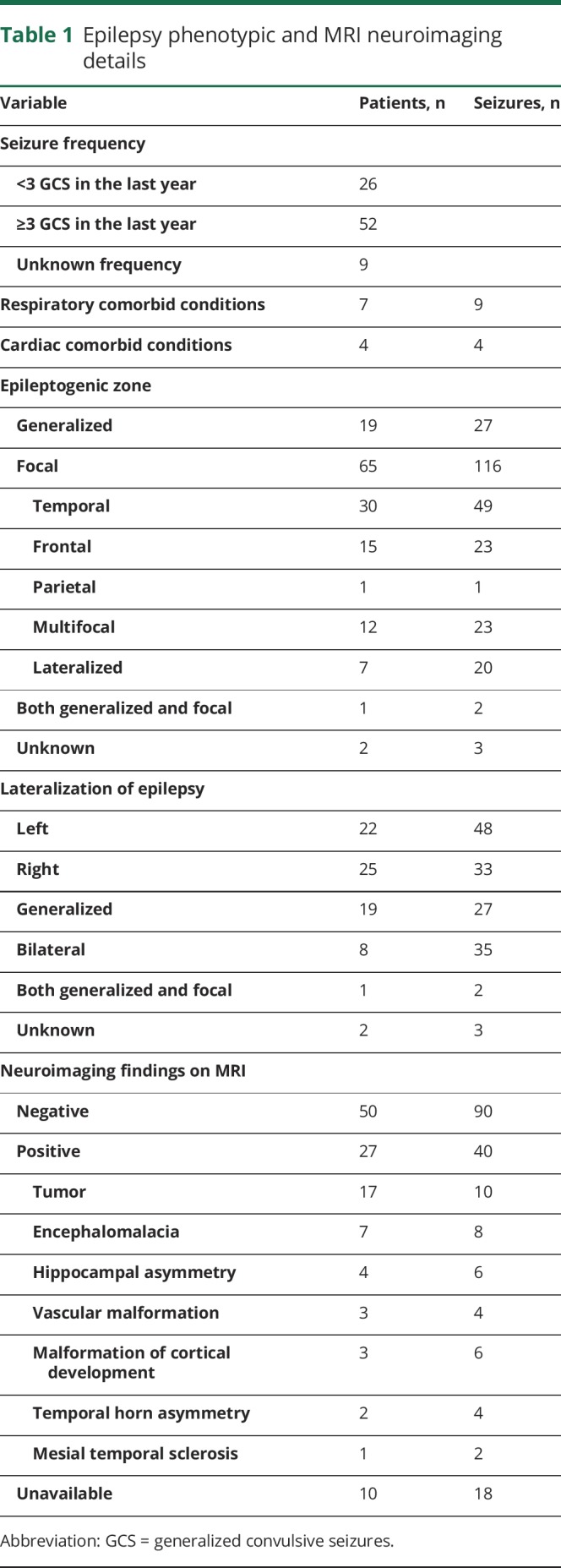
Table 2.
Electroclinical seizure features and durations
Peri-ictal apnea
In 27 of 148 seizures in 16 of 87 patients (3 with focal epilepsy, 13 with generalized epilepsy), movement or acquisition artifact prevented accurate analysis of ictal apnea. In the remaining 121 seizures, ICA was detected in 49 of 121 (40.4%) seizures in 23 of 71 (32.4%) patients. All patients with ICA had focal epilepsy (temporal 12 [23 seizures], frontal 4 [7 seizures], lateralized 4 [14 seizures], multifocal 3 [5 seizures]).
In 8 of 148 seizures in 6 patients (3 generalized epilepsy, 3 focal epilepsy), artifact contaminated the immediate postictal period. PCCA (figure 1) was found in 31 of 140 (22.1%) seizures in 22 of 81 (27.1%) patients and observed in generalized (5 of 16 patients [6 seizures], 31.2%) and focal (15 of 62 patients [22 seizures], 24.2%) epilepsies (frontal 6 [7 seizures], multifocal 4 [6 seizures], temporal 4 [6 seizures], lateralized left hemisphere 1 [3 seizures]) and in unknown (2 [3 seizures]). PCCA duration was 12.0 ± 14.3 seconds (8; 5–85 seconds), longer in male patients (14.4 ± 4.8 vs 11.4 ± 16 seconds, p = 0.005) and in focal epilepsy (13.7 ± 16.5 vs 6.5 ± 1.4 seconds, p = 0.020).
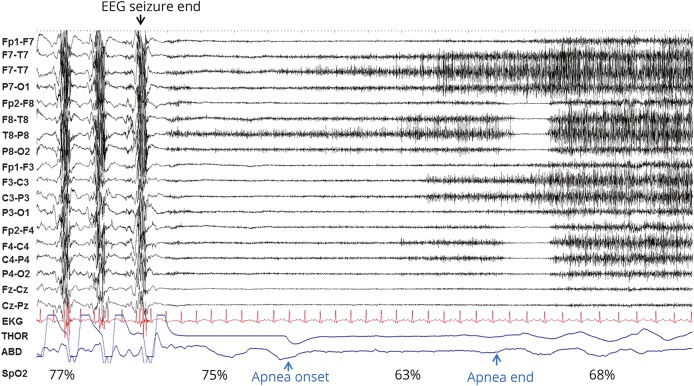
Figure 1. Postconvulsive central apnea.
Generalized convulsive seizure end and subsequent postictal phase are shown in a 20-second page: EEG sensitivity 7 μV, time constant 0.1, high frequency filter 70 Hz. After seizure end, 2 noticeable breaths are followed by postconvulsive central apnea of 6 seconds, with pulse artifact identifiable in the plethysmography signal during this period. Oxygen desaturation and subsequent ongoing recovery are seen as the patient resumes breathing. ABD = abdominal; THOR = thoracic.
PCCA was immediate in 10 of 140 (7.1%) seizures in 8 of 81 (9.8%) patients and delayed in 18 of 140 (12.8%) seizures in 14 of 81 (17.2%) patients. In 3 of 140 seizures in 3 patients, both types were found. Two of these 3 patients had subsequent seizures, 1 with only delayed PCCA and the other patient with immediate and delayed PCCA separately. In 11 of 31 (35.4%) PCCA seizures (9 of 22 [40.9%] patients), PCCA was accompanied by an ongoing postconvulsive electrographic seizure discharge. Of the 10 seizures with immediate PCCA, 9 had continuation of EEG seizure compared to 2 of 18 seizures with delayed PCCA (p = 0.001). PGES duration was longer in delayed PCCA (40.7 ± 15.1 seconds) than in immediate PCCA (27.22 ± 15.3, p = 0.045). In 5 delayed PCCA seizures (5 patients), apnea was recurrent in the same seizure. In the 22 patients with PCCA, 13 patients had 43 subsequent seizures, 22 of them with PCCA. Five patients had PCCA in all their seizures.
In the subset of 21 seizures with available tcCO2 recordings, complete data were available in 10 seizures in 8 patients. In 9 of 10 seizures, there was an increase in tcCO2, with the peak occurring after clinical seizure end. Four seizures had PCCA (2 immediate and 2 delayed), with a mean change of tcCO2 of 15.5 ± 3.87 mm Hg (16.5, 10–19 mm Hg) compared to 9.6 ± 7.2 mm Hg (7; 2–20 mm Hg) in patients without PCCA, although those CO2 values did not reach statistical significance (p = 0.238). Obstructive apnea/hypopnea was not found in the small subset of patients who underwent airflow monitoring.
ICA presence did not predict PCCA (p = 0.356). PCCA was more common in female patients (p = 0.003), in shorter seizure duration (p = 0.033), when PGES was present (87% in patients with PCCA vs 67.8% in patients without PCCA, p = 0.041), and with nadir Spo2 in the postictal (rather than ictal) phase (p = 0.031). Respiratory comorbid conditions were present in 3 patients (3 seizures) with PCCA (all with uncomplicated obstructive sleep apnea) and in 3 patients (5 seizures) without PCCA (asthma 1, chronic sinusitis 1, obstructive sleep apnea 1). PCCA was not associated with GCS frequency, epilepsy age at onset, or epilepsy duration data (table e-1 available from Dryad, doi.org/10.5061/dryad.1k7d35q). After binomial regression analysis, female sex remained an independent predictor for PCCA (p = 0.007, odds ratio [OR] 7.8, 95% confidence interval [CI] 1.7–34.8).
Patients with near-SUDEP and SUDEP
Two patients with PCCA each had a monitored near-SUDEP seizure (incidence rate 10.2 per 1,000 patient-years), and another with immediate PCCA had an out-of-hospital probable SUDEP (incidence rate 5.1 per 1,000 patient-years).
Patient 1 (video 1 and figure 2) was a 58-year-old right-handed woman with 3 years of intractable right mesial frontal lobe epilepsy and 3 to 4 GCS per year due to a meningioma. Two GCS arising were recorded; the first arose from wakefulness and lasted 47 seconds, ending with PCCA and asystole. After clinical seizure end, the EEG seizure continued for 14 seconds. During this postconvulsive period, immediate PCCA occurred for 10 seconds, followed by 1 breath and 3 delayed PCCA periods of 12, 32, and 31 seconds (total PCCA 85 seconds). Concurrent with immediate PCCA, she had bradytachycardia preceding an initial asystole of 40-second duration, followed by 2 more asystole periods of 12 and 7 seconds (total asystole period 59 seconds). Oxygen administration, alerting stimuli, and repositioning of the patient were performed. PGES lasted 169 seconds, and EEG recovery duration was 1,091 seconds (13 times the mean value of our study). The patient refused both epilepsy surgery and pacemaker implantation and remains under follow-up with sporadic seizures.
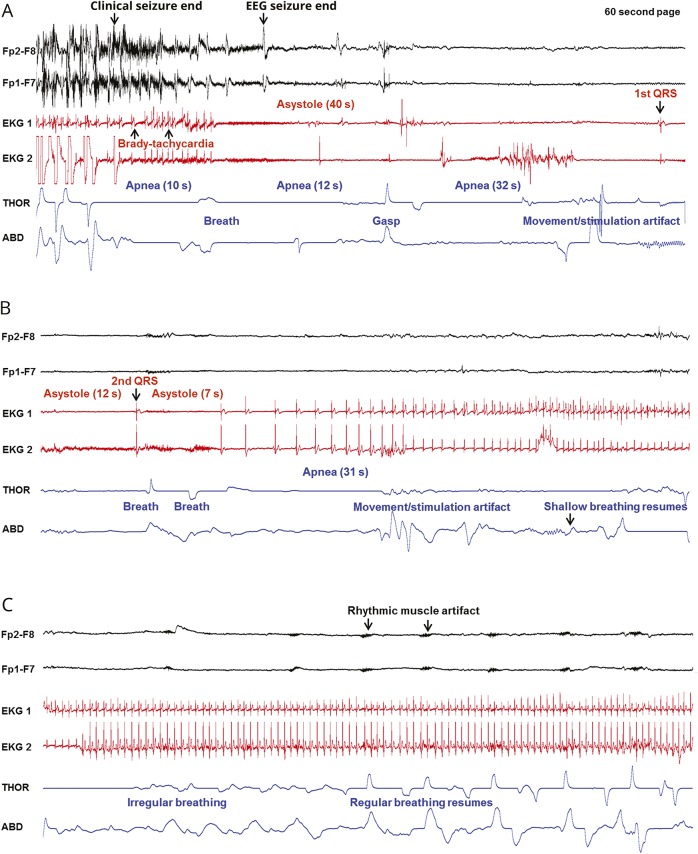
Figure 2. Sequence of EEG, ECG, and breathing changes in the near-SUDEP event of patient 1.
(A–C) Clinical seizure end and onset of the postictal convulsive phase are shown in 3 consecutive 60-second pages. Two channels of the EEG recording are displayed, along with 2 ECG channels and thoracic (THOR) and abdominal (ABD) belts. (A) After clinical seizure end, apnea is noted, accompanied by bradytachycardia that progresses into asystole. Three apneic periods are seen. The first QRS complex is seen at the end of the page. (B) Apnea and asystole continue from panel A until a second QRS complex and 2 breaths, followed by further apnea and asystole. Regular cardiac rhythm is progressively restored, although apnea persists until several seconds later. (C) Cardiac rhythm is re-established, and breathing excursions become more regular and increase in amplitude. Rhythmic muscle artifact becomes more evident on EEG. SUDEP = sudden unexpected death in epilepsy.
Near sudden expected death in epilepsy (SUDEP) event in patient 1, a 58-year-old woman with intractable right mesial frontal lobe epilepsy. Shown are generalized convulsive seizure and the postictal period with video-EEG, ECG, and thoracic and abdominal excursions. After the last clonic jerk at clinical seizure end, the EEG seizure continues for 14 seconds. During this period, the patient has immediate postconvulsive central apnea (PCCA) for 10 seconds, followed by 1 breath and 3 delayed PCCA periods of 12, 32, and 31 seconds, for a total of 85 seconds of PCCA. Concurrent with immediate PCCA, there is bradytachycardia preceding an initial asystole of 40 seconds’ duration, which is followed by 2 more asystole periods of 12 and 7 seconds, making up a total asystole period of 59 seconds. Cardiac rhythm is restored, and breathing excursions become progressively more regular and increase in amplitude. (Audio has been edited to protect the patient's identity.)Download Supplementary Video 1 (19.7MB, mp4) via http://dx.doi.org/10.1212/006785_Video_1
Patient 2 (video 2 and figure 3) was a 53-year-old right-handed man with intractable epilepsy of unknown etiology of 45 years’ duration with 1 GCS per year. He had mild obstructive sleep apnea and coronary artery disease with stenting. He had 1 GCS during admission, out of sleep, that lasted 78 seconds. After clinical seizure end, the EEG seizure continued for 5 seconds. During the clonic and postconvulsive phase, the patient had progressive bradycardia. Asystole occurred 3 seconds after EEG seizure end. Asystole durations were 8 and 10 seconds. Cardiac rhythm was progressively restored with bradytachycardia and then normal sinus rhythm. Concurrently with asystole, the patient had delayed PCCA that recurred, with durations of 5 and 16 seconds each (total apnea duration 21 seconds). Oxygen was administered, and the patient was repositioned. PGES duration was 75 seconds. The patient is currently seizure free.
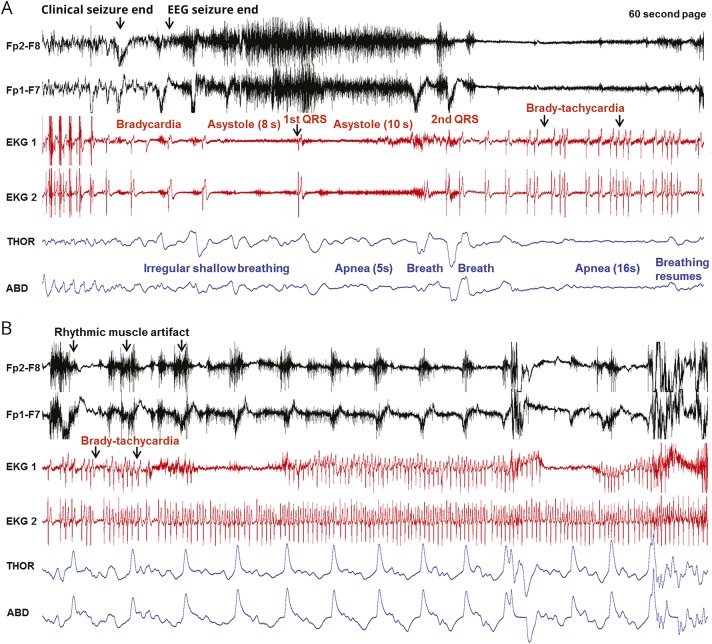
Figure 3. Sequence of EEG, ECG, and breathing changes in the near-SUDEP event of patient 2.
Seizure end and onset of the postconvulsive phase are shown in 2 consecutive 60-second pages. Two channels of the EEG recording are displayed, along with 2 ECG channels and thoracic (THOR) and abdominal (ABD) excursions. (A) After clinical end, progressive bradycardia is noted progressing to asystole after EEG end. After the first QRS complex, a brief period of apnea is noted. There are some isolated breaths followed by a longer period of apnea, even as cardiac activity is re-established, although in an arrhythmic fashion. Toward the end of the page, apnea ends and 2 breaths are noted. (B) A bradytachyarrhythmic pattern on the ECG is still present at the beginning of the page. Breathing excursions increase and become more regular accompanied by prominent rhythmic muscle artifact on EEG. SUDEP = sudden unexpected death in epilepsy.
Near sudden unexpected death in epilepsy (SUDEP) event in patient 2, a 53-year-old man with intractable epilepsy with unknown epileptogenic zone. Shown are generalized convulsive seizure and the subsequent postictal period with video-EEG, ECG, and thoracic and abdominal excursions. After clinical seizure end, the EEG seizure continues for 5 seconds. During the clonic and postconvulsive phase, the patient has progressive bradycardia. Asystole occurs 3 seconds after EEG seizure end. The first asystole duration is 8 seconds, and the second is 10 seconds. Cardiac rhythm is progressively restored, with a combination of bradycardia-tachycardia. Concurrently with asystole, the patient has delayed postconvulsive central apnea that recurs, with durations of 5 and 16 seconds each, for a total apnea duration of 21 seconds (Audio has been edited to protect the patient's identity.)Download Supplementary Video 2 (20.4MB, mp4) via http://dx.doi.org/10.1212/006785_Video_2
Patient 3 was a 21-year-old woman with genetic generalized epilepsy since 8 years of age with intractable absence seizures and 3 to 4 GCS per year. She was admitted with a 4-seizure cluster in 24 hours. One GCS of 58 seconds’ duration was captured, arising from sleep. PGES duration was 42 seconds. She had 8 seconds of immediate PCCA, during which EEG seizure was evident. Breathing was restored spontaneously after EEG seizure end. No bradycardia or asystole was noted. Oxygen was administered. Seizures remained intractable. Two years after admission, the patient was found in cardiorespiratory arrest at home. CPR achieved recovery of pulse, but she died several hours later in hospital. Autopsy was declined, and death was classified as probable SUDEP.
Postictal breathing rate and hypoxemia
Breathing rate was available in 147 of 148 seizures (table 2). Bradypneic seizures had longer epilepsy duration, usually occurred in sleep, and were associated with severe hypoxemia and the presence of PCCA (p < 0.05). There was no association with age at study or at epilepsy onset or with seizure frequency and other epilepsy phenotypic or seizure characteristics (table e-2 available from Dryad, doi.org/10.5061/dryad.1k7d35q). Complete Spo2 data were available in 91 seizures. All had at least moderate hypoxemia (moderate in 14 of 91 [15%] seizures, severe in 77 of 91 [85%] seizures). Nadir Spo2 was detected postictally in 63 of 91 (69%) seizures and ictally in the rest (table 2). Early oxygen administration was related to shorter hypoxemia duration, with a mean in the early oxygen administration group of 120.33 ± 60.1 seconds vs 146.6 ± 57.4 seconds (p = 0.018).
Postictal breathing effort and muscle artifact
Patients exhibited stertorous breathing in 109 of 147 (74.1%) seizures in 69 of 87 (79.3%) patients. Breathing sounds were expiratory in 60, both inspiratory and expiratory in 40, and solely inspiratory in 9. None exhibited ictal or postictal inspiratory stridor suggestive of laryngospasm. In 1 patient (smoker) with no known cardiorespiratory comorbidity, stereotypic wheezing occurred after clinical seizure end in all 3 recorded GCS, suggesting distal airway obstruction.
Postictal muscle artifact was present in 131 of 147 (89%) seizures (1 was excluded due to excessive electrode artifact), with a mean duration of 70.9 ± 45.4 seconds (56; 10–180 seconds). Muscle artifact was unrelated to epilepsy phenotypic features (p > 0.05), but longer hypoxemia duration (p = 0.005), lower mean Spo2 nadir values (p = 0.001), higher decreases in Spo2 (p = 0.001), stertorous breathing (p = 0.005), and presence of PGES (p < 0.001) were associated (table e-3 available from Dryad, doi.org/10.5061/dryad.1k7d35q). After binominal regression analysis, the presence of PGES remained an independent association (OR 11.630, 95% CI 1.631–82.945, p = 0.014). Duration of muscle artifact was longer in patients in the sleep state, those with tonic phases in GCS, and patients with stertorous breathing and correlated with PGES duration (r = 0.286, p = 0.004) and EEG recovery duration (r = 0.405, p = 0.001).
Two different muscle artifact patterns were noted: a rhythmic, intermittent pattern, synchronous with breathing, in 75 of 147 (51%) seizures in 53 patients (figure 4A) and a continuous, nonrhythmic pattern in 51 of 147 (34.6%) seizures in 35 patients (figure 4B). Five seizures (3.4%) in 5 patients successively exhibited both types of patterns.
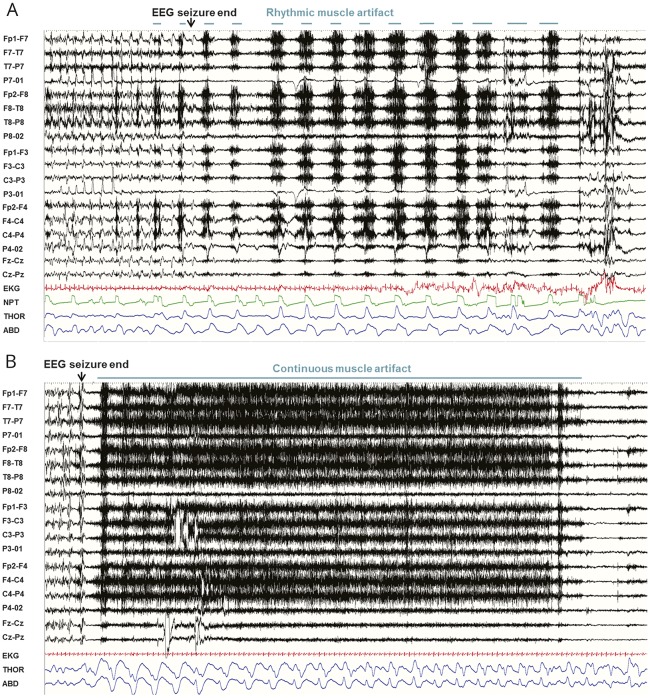
Figure 4. Types of muscle artifact.
(A) Rhythmic muscle artifact in a 60-second page. Note the one-to-one correspondence with thoraco (THOR)-abdominal (ABD) excursions and nasal flow assessed by nasal pressure transducer (NPT) (sensitivity 7 μV, time constant 0.03, high frequency filter 70). (B) Continuous muscle artifact in a 60-second page (sensitivity 7 μV, time constant 0.03, high frequency filter 70).
Rhythmic muscle artifact was associated with older age at study, higher body mass index, longer seizure duration, and loud breathing (p < 0.050), whereas PCCA was less frequent in this group. After binominal regression analysis, presence of stertorous breathing (OR 3.856, 95% CI 1.395–10.663, p = 0.009), age at study (OR 1.032, 95% CI 1.001–1.064, p = 0.040), and longer clinical seizure duration (OR 1.026, 95% CI 1.001–1.051, p = 0.045) remained independently associated with rhythmic muscle artifact. It was also correlated with EEG recovery duration (r = 0.303, p = 0.020).
Discussion
Our study suggests that PCCA is a possible SUDEP biomarker, occurring in only 22% of GCS. Consistent with MORTEMUS, PCCA was associated with near-SUDEP phenomena as well as SUDEP, the latter in a prospectively ascertained case. Its incidence was approximately half that of ICA, which appears more likely to be a benign semiologic feature of focal seizures unless prolonged (>60 seconds) or associated with severe hypoxemia (<75% Spo2).6 PCCA lasted 5 to 85 seconds, and 1 patient with only 8 seconds of PCCA in 1 recorded GCS went on to have an at-home, unmonitored SUDEP, suggesting that its presence may be a predictor of death. Prolonged PCCA may be especially dangerous. Our data suggest a distinction between the pathophenomenology of ICA and PCCA. The former is a preconvulsive phenomenon in focal epilepsy, likely driven by seizure discharges in cortical sites that modulate breathing6,22,23; on multivariate analysis, we found no association between ICA with PCCA. The latter, on the other hand, occurs in either focal or generalized epilepsy regardless of electrographic seizure discharge, suggesting that it is driven by brainstem mechanisms akin to a pontomedullary, rather than cortical, Todd paresis–like phenomenon. Whereas PCCA may be immediate or delayed, either can occur without postconvulsive electrographic seizure discharge, and the same patient may exhibit either or both types of PCCA. Thus, the 2 phenomena either are the same or share a common anatomic substrate of a compromised brainstem. Thus, in our patients with near-SUDEP/SUDEP, both immediate and delayed apnea occurred in patient 1, delayed apnea was seen in patient 2, and immediate apnea occurred in patient 3. Blunted CO2 chemoreceptor responses due to preexisting obstructive sleep apnea in patient 2 may have played a role in his near-SUDEP, although his sleep apnea was uncomplicated and unlikely to have contributed.
ICA incidence (40.4%) was concordant with previous publications.6 No patients with generalized epilepsy had preconvulsive ICA, supporting the contention that preconvulsive ICA is a semiologic phenomenon unique to focal epilepsies.22 On the other hand, PCCA occurred in both generalized and focal epilepsies, consistent with findings that SUDEP occurs in both categories. PCCA was more frequent in women and in the presence of PGES. However, PCCA duration was longer in men. Confirmed in multivariate analysis, female predilection is not easily explained because SUDEP is reported to more likely afflict men14; longer PCCA duration in men may be more indicative of risk. Imaging studies in our cohort with intractable epilepsy and GCS indicate sex-specific cortical changes in autonomic and breathing control sites such as preferential, lateralized orbitofrontal cortex thinning in women.24 Bradypnea occurred more often in seizures arising from sleep, when most SUDEP occurs. Postconvulsive bradypnea or PCCA accompanied by asystole appears to be particularly rare, occurring in only 1 seizure each in the 2 near-SUDEP cases (<1% of all GCS), and thus may represent an especially important biomarker.
The apnea/bradycardia combination after seizures leading to death has been described in animal25,26 and human1,2 SUDEP, although its mechanisms are unclear. Apnea and the consequent hypoxemia and hypercapnia stimulate carotid body chemoreceptors, which may trigger bradycardia through vagal efferent activation.27 However, in a Dravet syndrome animal model,26 selective peripheral muscarinic receptor blockade did not prevent postictal apnea, bradycardia, and death. On the other hand, selective central, medullary muscarinic receptor blockade (intravenous methylatropine or intracerebro-ventricular N-methylscopolamin) did, suggesting that prevention of central apnea, rather than vagal output blockade, is more relevant. Medullary muscarinic receptors can stimulate or inhibit breathing28,29 and are involved in central CO2 chemoreception.30 Another critical brainstem component is the periaqueductal gray (PAG), which is an integrative center for breathing responses. Its afferents, including orbito-insular and dorsomedial projections, amygdalo-hippocampal complex, and thalamus, presumably participate in GCS seizure discharge. PAG functional and structural segregation can determine deep breathing, tachypnea, or apnea.31 PAG efferents project to multiple nuclei, including medullary neurons involved in respiratory rhythm and pattern generation, shaping final breathing output. Stimulation of ventrolateral caudal PAG in the cat decreases spontaneous activity and responsiveness to surrounding stimuli and elicits irregular breathing, hypotension, and bradycardia.32 Whereas significant GCS-driven disruption of brainstem-mediated breathing function may result in brief PCCA, severe and prolonged, fatal or near fatal PCCA may involve structures such as the PAG,33 causing bradyarrhythmia, asystole, and hypotension.34–36 Possible ineffective breathing during a seizure, along with a high metabolic load, could set the hypoxic stage for more catastrophic PCCA effects.
Postictal spreading depolarization in dorsal medulla after seizures, shown to produce cardiorespiratory arrest, preceded by EEG suppression and apnea, constitutes another potential mechanism.37 Longer PGES duration was found in patients with delayed compared to immediate PCCA, suggesting a possible relationship between cerebral shutdown and brainstem dysfunction. Establishment of nonseizure SUDEP as an entity38 suggests that brainstem dysfunction may initiate the agonal event independently of cortical seizures.39,40 Whereas spreading depression in cardiorespiratory suppression is unproven in humans, increasing evidence from imaging41,42 and pathologic studies43 suggests that key cardiorespiratory control structures are significantly damaged in patients with intractable epilepsy who succumb to SUDEP. Imaging studies42 have shown increased right hippocampal and parahippocampal gray matter volume in patients experiencing SUDEP and high-SUDEP-risk patients, setting the stage for possible autonomic and respiratory output dysregulation.44 Gray matter volume decline in the posterior thalamus and brainstem (superior colliculi, PAG, mesencephalic reticular formation, and raphe nuclei) in patients experiencing SUDEP and high-SUDEP-risk patients suggests damage to structures essential for breathing response modulation. Neuropathologic examination of brainstems of patients experiencing SUDEP43 has shown structural injury in neuropeptidergic and monoaminergic systems, specifically somatostatin and neurokinin 1 receptor neurons in medullary raphe and ventrolateral medulla, where the pre-Botzinger complex45 nucleus is located. The pre-Botzinger complex nucleus is an important site for inspiratory rhythm generation and a key PAG efferent. These findings provide crucial anatomic substrates for breathing and cardiac dysfunction seen in patients with PCCA and those experiencing near-SUDEP/SUDEP.
Recent studies9,10 describe potentially greater ictal and postictal laryngospasm incidence in SUDEP phenomenology than previously reported in the literature.7,46 Rodent laryngospasm models point to distinctive potential SUDEP biomarkers based on breathing effort and cardiorespiratory coupling.10 One such is postconvulsive rhythmic breathing EMG artifact evident in ECG and EEG channels. Another is an abrupt increase in RR interval variance, indicating increased breathing effort. Further analysis of MORTEMUS SUDEP records has suggested the presence of 1 or more of these biomarkers, pointing to a role for laryngospasm and obstructive apnea in death. However, despite careful analysis of breathing in our study, aided by an expert pulmonologist, no convincing laryngospasm or obstructive apnea was seen despite the presence of rhythmic muscle artifact in 51% of GCS. The absence of such breathing compromise was further confirmed in the small subset of patients with airflow monitoring, in whom rhythmic muscle artifact occurred without airflow impediment (figure 4A). This suggests that the rarity of reported laryngospasm in the literature is deserved. Thus, rhythmic muscle artifact is more likely to represent enhanced, nonobstructive breathing effort in the aftermath of the physically vigorous seizure that is a GCS. Accordingly, its presence was related to older age, longer seizure duration, and stertorous breathing, reflecting strenuous seizure-related physical impact. On the other hand, rhythmic muscle artifact was not associated with objective markers of breathing drive such as oxygen desaturation or hypercarbia. PGES correlated with the presence of muscle artifact overall, although not with rhythmic artifact alone or continuous artifact alone. This finding suggests that PGES does not correlate with enhanced breathing effort but may correlate with increased muscle tone in the postictal state.11
PCCA, found in our cases of near-SUDEP/probable SUDEP and reported in all MORTEMUS SUDEP, may help stratify SUDEP risk. However, larger prospective studies are needed to confirm this and to standardize procedures of response. Whereas pacemaker implantation in ictal asystole may prevent fall-related injuries,47,48 its role in postictal asystole with PCCA is uncertain. The accompanying apnea and centrally driven asystole may or may not render pacemakers futile. Optimization of seizure control, prevention of GCS, and potentially stimulation of ventilation remain critical.
Our study has limitations. Our near-SUDEP estimate (10.2 per 1,000 patient-years) may underestimate true incidence in the severely intractable population (but likely overestimates it in the general epilepsy population) because we could comment only on monitored seizures in this preliminary report and similar events at home may pass unnoticed. On the other hand, our definition of near-SUDEP did not include the institution of CPR49; the distinction between those with and those without CPR may be artificial because there is overreliance on the resuscitator's judgment as to its necessity. There is strong argument that all patients who have combined asystole and central apnea, regardless of CPR, be included in the definition. Our apnea definition may overestimate incidence because previous studies are based on sleep-study criteria. Our definition is more sensitive to brief disturbances of breathing that would otherwise be missed. While there was combined use of airflow and thoracoabdominal descriptions, precise distinction between obstructive and nonobstructive apnea can be made only by direct measures of chest wall/diaphragm EMG or measures of esophageal pressure. However, we assumed that even brief absences of ventilation in the postconvulsive phase, when most patients have enhanced breathing rates, could have important biological significance in the SUDEP context. Multiple apneic exposures may damage hypoxia-sensitive neural systems such as the long axons in the hippocampus and climbing fibers of the cerebellum; both structures serve significant breathing and blood pressure control roles, and progressive damage has the potential to lead to system collapse. Another limitation is that our study is based on surface EEG, and persistence of intracranial seizure cannot be completely ruled out in all patients with PCCA.50 Exquisitely focal postconvulsive electrographic seizures in cortical sites that influence breathing such as the amygdala and hippocampus may account for sustained apnea, although it seems relatively unlikely to account for intermittent PCCA periods that were collectively as long as 85 seconds in our patients. Nasal outflow recordings were available in only a small subset, which may underestimate the true incidence of obstructive apnea/hypopnea. Limited airflow and tcO2 datasets in this study reflect the clinical epilepsy monitoring unit setting, where data acquisition can be variable. However, it appears clear that clinically significant laryngospasm is probably rare in GCS.
PCCA is a relatively uncommon phenomenon. Its occurrence, regardless of epileptogenic zone or demonstrable seizure discharge, and an association with asystole indicate a functional substrate in the brainstem akin to Todd paresis or even self-limited spreading depolarization. PCCA may serve as a SUDEP biomarker, and further follow-up of this large prospective cohort may further clarify its potential role. This study further highlights the role of polygraphic, multimodal recording in the epilepsy monitoring unit in peri-ictal breathing dysfunction diagnosis. We found that seizure-related laryngospasm is probably extremely rare, although it remains a potential mechanism of death. Breathing related rhythmic muscle artifact is more indicative of breathing effort than obstructed breathing and thus may not be a particularly useful biomarker for SUDEP.
Acknowledgment
The authors thank Sarah J. Delozier (University Hospitals Cleveland Medical Center, OH) for her assistance with the statistical analysis.
Glossary
- CI
confidence interval
- CPR
cardiopulmonary resuscitation
- GCS
generalized convulsive seizures
- ICA
ictal central apnea
- MORTEMUS
Mortality in Epilepsy Monitoring Units Study
- NINDS
National Institute of Neurological Disorders and Stroke
- OR
odds ratio
- PAG
periaqueductal gray
- PCCA
postconvulsive central apnea
- PGES
postictal generalized EEG suppression
- PRISM
Prevention and Risk Identification of SUDEP Mortality
- Spo2
peripheral capillary oxygen saturation
- SUDEP
sudden unexpected death in epilepsy
- tcCO2
transcutaneous CO2
- VEEG
video-EEG
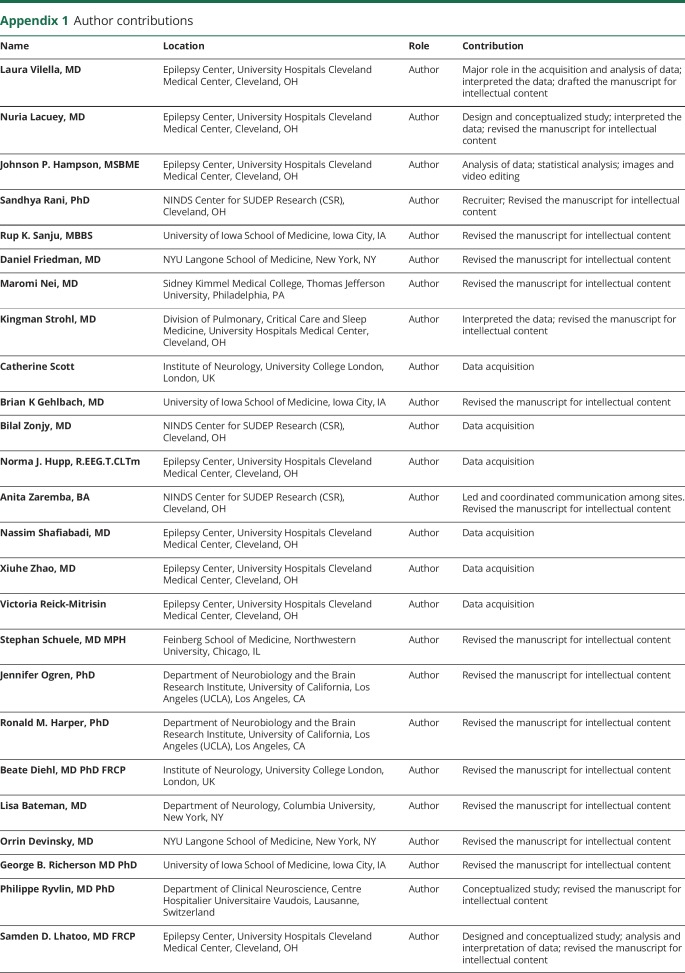
Appendix 1. Author contributions
Footnotes
Editorial, page 115
Study funding
No targeted funding reported.
Disclosure
L. Vilella, N. Lacuey, J. Hampson, M. Sandhya Rani, R. Sanju, D. Friedman, M. Nei, K. Strohl, C. Scott, B. Gehlbach, B. Zonjy, N. Hupp, A. Zaremba, N. Shafiabadi, X. Zhao, V. Reick-Mitrisin, S. Schuele, J. Ogren, R. Harper, B. Diehl, L. Bateman, and O. Devinsky report no disclosures relevant to the manuscript. G. Richerson is funded by the Center for SUDEP Research: NIH/NINDS U01-NS090414. P. Ryvlin reports no disclosures relevant to the manuscript. S. Lhatoo is funded by the Center for SUDEP Research: NIH/NINDS U01-NS090405 and NIH/NINDS U01-NS090407. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology May 18, 2018. Accepted in final form August 29, 2018.
References
- 1.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 2.Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 1996;60:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum AS, Ives JR, Goldberger AL, et al. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia 2000;41:536–541. [DOI] [PubMed] [Google Scholar]
- 4.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 2008;131:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyal M, Bateman LM, Albertson TE, Lin TC, Li CS. Respiratory changes with seizures in localization-related epilepsy: analysis of periictal hypercapnia and airflow patterns. Epilepsia 2010;51:1359–1364. [DOI] [PubMed] [Google Scholar]
- 6.Lacuey N, Zonjy B, Hampson JP, et al. The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 2018;59:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavee J, Morris H III. Severe postictal laryngospasm as a potential mechanism for sudden unexpected death in epilepsy: a near-miss in an EMU. Epilepsia 2008;49:2113–2117. [DOI] [PubMed] [Google Scholar]
- 8.So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia 2000;41:1494–1497. [DOI] [PubMed] [Google Scholar]
- 9.Nakase K, Kollmar R, Lazar J, et al. Laryngospasm, central and obstructive apnea during seizures: defining pathophysiology for sudden death in a rat model. Epilepsy Res 2016;128:126–139. [DOI] [PubMed] [Google Scholar]
- 10.Stewart M, Kollmar R, Nakase K, et al. Obstructive apnea due to laryngospasm links ictal to postictal events in SUDEP cases and offers practical biomarkers for review of past cases and prevention of new ones. Epilepsia 2017;58:e87–e90. [DOI] [PubMed] [Google Scholar]
- 11.Gastaut H, Broughton R. Epileptic Seizures: Clinical and Electrographic Features, Diagnosis and Treatment. Springfield: Charles C Thomas; 1972. [Google Scholar]
- 12.Schuele SU, Bermeo AC, Alexopoulos AV, Burgess RC. Anoxia-ischemia: a mechanism of seizure termination in ictal asystole. Epilepsia 2010;51:170–173. [DOI] [PubMed] [Google Scholar]
- 13.van der Lende M, Surges R, Sander JW, Thijs RD. Cardiac arrhythmias during or after epileptic seizures. J Neurol Neurosurg Psychiatry 2016;87:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hesdorffer DC, Tomson T, Benn E, et al. Combined analysis of risk factors for SUDEP. Epilepsia 2011;52:1150–1159. [DOI] [PubMed] [Google Scholar]
- 15.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 16.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522–530. [DOI] [PubMed] [Google Scholar]
- 17.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandre V, Mercedes B, Valton L, et al. Risk factors of postictal generalized EEG suppression in generalized convulsive seizures. Neurology 2015;85:1598–1603. [DOI] [PubMed] [Google Scholar]
- 19.Azar NJ, Tayah TF, Wang L, Song Y, Abou-Khalil BW. Postictal breathing pattern distinguishes epileptic from nonepileptic convulsive seizures. Epilepsia 2008;49:132–137. [DOI] [PubMed] [Google Scholar]
- 20.Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol 2010;68:787–796. [DOI] [PubMed] [Google Scholar]
- 21.Theeranaew W, McDonald J, Zonjy B, et al. Automated detection of postictal generalized EEG suppression. IEEE Trans Biomed Eng 2018;65:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacuey N, Zonjy B, Londono L, Lhatoo SD. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology 2017;88:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dlouhy BJ, Gehlbach BK, Kreple CJ, et al. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci 2015;35:10281–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogren JA, Tripathi R, Macey PM, et al. Regional cortical thickness changes accompanying generalized tonic-clonic seizures. Neuroimage Clin 2018;20:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston SC, Siedenberg R, Min JK, Jerome EH, Laxer KD. Central apnea and acute cardiac ischemia in a sheep model of epileptic sudden death. Ann Neurol 1997;42:588–594. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Bravo E, Thirnbeck CK, et al. Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest 2018;128:1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Brain Res Rev 2005;49:555–565. [DOI] [PubMed] [Google Scholar]
- 28.Muere C, Neumueller S, Miller J, et al. Atropine microdialysis within or near the pre-Botzinger complex increases breathing frequency more during wakefulness than during NREM sleep. J Appl Physiol 2013;114:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol 1993;264:R544–R554. [DOI] [PubMed] [Google Scholar]
- 30.Dev NB, Loeschcke HH. A cholinergic mechanism involved in the respiratory chemosensitivity of the medulla oblongata in the cat. Pflugers Arch 1979;379:29–36. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci 2008;28:12274–12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res 1993;58:27–47. [DOI] [PubMed] [Google Scholar]
- 33.UEDA H. Arrhythmias produced by cerebral stimulation. Jpn Circ J 1962;26:225–230. [DOI] [PubMed] [Google Scholar]
- 34.Bozorgi A, Chung S, Kaffashi F, et al. Significant postictal hypotension: expanding the spectrum of seizure-induced autonomic dysregulation. Epilepsia 2013;54:e127-e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hampel KG, Jahanbekam A, Elger CE, Surges R. Seizure-related modulation of systemic arterial blood pressure in focal epilepsy. Epilepsia 2016;57:1709–1718. [DOI] [PubMed] [Google Scholar]
- 36.Hampel KG, Elger CE, Surges R. Impaired baroreflex sensitivity after bilateral convulsive seizures in patients with focal epilepsy. Front Neurol 2017;8:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 2015;7:282ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ba-Armah DM, Donner EJ, Ochi A, et al. “Saved by the bell”: near SUDEP during intracranial EEG monitoring. Epilepsia Open 2018;3:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devinsky O, Friedman D, Duckrow RB, et al. Sudden unexpected death in epilepsy in patients treated with brain-responsive neurostimulation. Epilepsia 2018;59:555–561. [DOI] [PubMed] [Google Scholar]
- 40.Lhatoo SD, Nei M, Raghavan M, et al. Nonseizure SUDEP: sudden unexpected death in epilepsy without preceding epileptic seizures. Epilepsia 2016;57:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller SG, Bateman LM, Laxer KD. Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. Neuroimage Clin 2014;5:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wandschneider B, Koepp M, Scott C, et al. Structural imaging biomarkers of sudden unexpected death in epilepsy. Brain 2015;138:2907–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patodia S, Somani A, O'Hare M, et al. The ventrolateral medulla and medullary raphe in sudden unexpected death in epilepsy. Brain 2018;141:1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology 1992;42:1727–1732. [DOI] [PubMed] [Google Scholar]
- 45.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 2006;7:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacuey N, Vilella L, Hampson JP, Sahadevan J, Lhatoo SD. Ictal laryngospasm monitored by video-EEG polygraphy: a potential SUDEP mechanism. Epileptic Disord 2018;20:146–150. [DOI] [PubMed] [Google Scholar]
- 47.Moseley BD, Ghearing GR, Munger TM, Britton JW. The treatment of ictal asystole with cardiac pacing. Epilepsia 2011;52:e16–e19. [DOI] [PubMed] [Google Scholar]
- 48.Hampel KG, Thijs RD, Elger CE, Surges R. Recurrence risk of ictal asystole in epilepsy. Neurology 2017;89:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012;53:227–233. [DOI] [PubMed] [Google Scholar]
- 50.Altenmüller DM, Schulze-Bonhage A, Elger CE, Surges R. Local brain activity persists during apparently generalized postictal EEG suppression. Epilepsy Behav 2016;62:218–224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Near sudden expected death in epilepsy (SUDEP) event in patient 1, a 58-year-old woman with intractable right mesial frontal lobe epilepsy. Shown are generalized convulsive seizure and the postictal period with video-EEG, ECG, and thoracic and abdominal excursions. After the last clonic jerk at clinical seizure end, the EEG seizure continues for 14 seconds. During this period, the patient has immediate postconvulsive central apnea (PCCA) for 10 seconds, followed by 1 breath and 3 delayed PCCA periods of 12, 32, and 31 seconds, for a total of 85 seconds of PCCA. Concurrent with immediate PCCA, there is bradytachycardia preceding an initial asystole of 40 seconds’ duration, which is followed by 2 more asystole periods of 12 and 7 seconds, making up a total asystole period of 59 seconds. Cardiac rhythm is restored, and breathing excursions become progressively more regular and increase in amplitude. (Audio has been edited to protect the patient's identity.)Download Supplementary Video 1 (19.7MB, mp4) via http://dx.doi.org/10.1212/006785_Video_1
Near sudden unexpected death in epilepsy (SUDEP) event in patient 2, a 53-year-old man with intractable epilepsy with unknown epileptogenic zone. Shown are generalized convulsive seizure and the subsequent postictal period with video-EEG, ECG, and thoracic and abdominal excursions. After clinical seizure end, the EEG seizure continues for 5 seconds. During the clonic and postconvulsive phase, the patient has progressive bradycardia. Asystole occurs 3 seconds after EEG seizure end. The first asystole duration is 8 seconds, and the second is 10 seconds. Cardiac rhythm is progressively restored, with a combination of bradycardia-tachycardia. Concurrently with asystole, the patient has delayed postconvulsive central apnea that recurs, with durations of 5 and 16 seconds each, for a total apnea duration of 21 seconds (Audio has been edited to protect the patient's identity.)Download Supplementary Video 2 (20.4MB, mp4) via http://dx.doi.org/10.1212/006785_Video_2
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on request.