Abstract
Introduction:
Generalized anxiety disorder (GAD), a chronic illness, often begins during adolescence or early adulthood and persists throughout the lifespan. Randomized controlled trials support the efficacy of selective serotonin and selective norepinephrine reuptake inhibitors (SSRIs and SNRIs, respectively), as well as benzodiazepines, azapirones, anti-adrenergic medications, melatonin analogues, second-generation antipsychotics, kava and lavender oil in GAD. However, psychopharmacologic treatment selection requires clinicians to consider multiple factors, including age, co-morbidity and prior treatment.
Areas covered:
The authors review the literature concerning pharmacotherapy for pediatric and adult patients with GAD with specific commentary on the efficacy and tolerability of selected agents in these age groups. The authors describe an algorithmic approach to the pediatric and adult patient with GAD and highlight considerations for the use of selected medications in these patients.
Expert opinion:
In adults with GAD, SSRIs and SNRIs represent the first-line psychopharmacologic treatment while second-line pharmacotherapies may include buspirone, benzodiazepins, SGAs and pregabalin. In pediatric patients with GAD, SSRIs should be considered the first line pharmacotherapy and psychotherapy enhances antidepressant response.
Keywords: Selective Serotonin Reuptake Inhibitor (SSRI), Selective Norepinephrine Reuptake Inhibitor (SNRI), antidepressants, pediatric, adult, benzodiazepine, buspirone
1. Introduction
Generalized anxiety disorder (GAD), a chronic illness [1,2], often begins during adolescence or early adulthood [3,4], and persists throughout the lifespan [5]. Characterized by pervasive, diffuse anxiety related to multiple domains, GAD affects up to 5% of children and adolescents and between 3–6% of adults. In addition to inflicting significant individual, societal and economic costs [6,7], untreated GAD increases the likelihood of developing secondary disorders, including major depressive disorder (MDD) [8,9], other anxiety disorders and increases the risk of suicide attempt and completed suicide across the lifespan [10,11].
Treatment of GAD and other anxiety disorders in children and adults frequently consists of both psychotherapy and pharmacotherapy. In fact, numerous studies suggest concurrent psychotherapy amplifies the benefits of pharmacotherapy (e.g., sertraline, fluoxetine, etc.) [12–15]. Additionally, multimodal approaches (e.g., psychotherapy and pharmacotherapy) may differentially target specific symptoms (e.g., cognitive vs. somatic) and the addition of psychotherapy increases treatment adherence and decreases reported side effects of pharmacotherapy.
The last 4 decades saw rapid progress in the systematic evaluation of numerous psychopharmacologic interventions for GAD in pediatric and adult populations. To date, randomized controlled trials, open-label studies, and other data support the efficacy of selective serotonin and selective norepinephrine reuptake inhibitors (SSRIs and SNRIs, respectively), as well as benzodiazepines, monoamine oxidase inhibitors, tricyclics, azapirones, anti-adrenergic medications, antihistamines, melatonin analogues, second generation antipsychotics [16] in addition to trazodone. Herein the evidence and clinical use of these medications and their related classes will be reviewed.
2. Psychopharmacologic interventions
2.1. Selective serotonin reuptake inhibitors
The SSRIs inhibit serotonin (5-hydroxytryptamine, 5-HT) reuptake transporter and in some cases weakly inhibit dopamine and norepinephrine reuptake mechanisms. Inhibition of 5-HT reuptake increases the concentrations of synaptic 5-HT, which in turn increases extra-synaptic diffusion. The SSRIs (Figure 1) differ significantly in terms of the potency and selectivity for the 5-HT transporter relative to norepinephrine and dopamine transporters, as well as in their ability to interact with other synaptic and extra-synaptic receptors, which subtends their variable efficacy and side effect profiles. Furthermore, these compounds all differ in their metabolism, side effect profiles, and duration of action. The use of SSRIs in the treatment of GAD is endorsed by both the 2014 Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders [17] and the British Association for Psychopharmacology 2014 Guidelines [18]. The latter notes that the SSRIs “have ‘broad spectrum’ efficacy in both short-term and long-term treatment, and are generally well tolerated; and for these reasons are widely considered to be the first-line pharmacological approach in patients with anxiety disorders” [18].
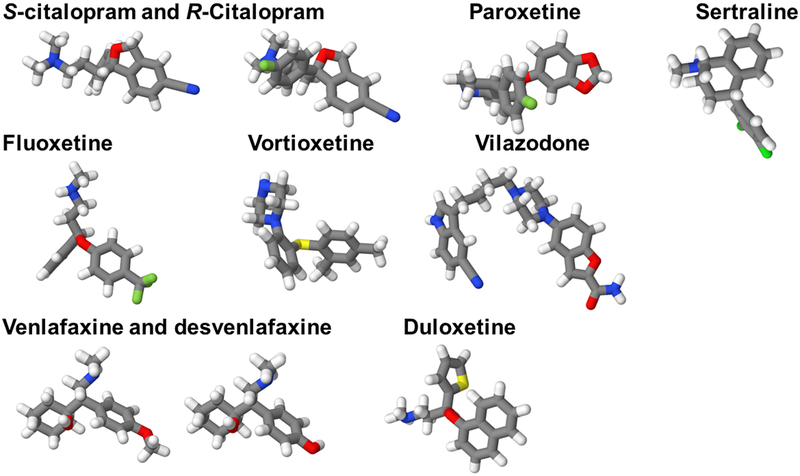
Figure 1: Structures of antidepressant medications that have been systematically evaluated in patients with generalized anxiety disorder (GAD).
Structurally, SSRIs and SNRIs share many similarities and contain halogen atoms at specific positions, which are key determinants of their specificity for the serotonin transporter. The position and type of the specific substitution on an aromatic component of the antidepressant molecule determines the specificity at the serotonin transporter. Halogen substitutions at this ring are associated with the most binding specificity at the serotonin transporter and are mediated through binding within the halogen binding pocket (HBP). Colors represent specific atoms: nitrogen (blue); oxygen (red); halogens (fluorine and chloride) (green); sulfur (yellow); carbon (gray).
2.1.1. Fluoxetine
Originally synthesized in the early 1970s, fluoxetine became the first SSRI to be introduced in the United States in 1987. Fluoxetine increases central serotonergic transmission, but it also has noradrenergic and dopaminergic effects which putatively underlie its therapeutic efficacy. Fluoxetine, which is primarily metabolized through the CYP2D6 system, also inhibits CYP2D6 activity; and its active metabolite, norfluoxetine, inhibits CYP3A4 [19]. Compared to its parent compound fluoxetine, which has a half-life of 4–6 days, norfluoxetine has a half-life in the order of two weeks [19]. Additionally, fluoxetine is highly protein bound, and because of its extensive CYP2D6-dependent metabolism may, exhibit considerable intra-individual variability in tolerability and response [20].
While relatively few studies have evaluated the efficacy of fluoxetine in adults with GAD, fluoxetine has been studied in a large randomized controlled trial (RCT) of pediatric patients with generalized, separation and/or social anxiety disorders [21]. In this study, youth, aged 7–17 years of age were randomized to fixed dose fluoxetine (20 mg/day, n=37) or placebo (n=37) for 12 weeks; 61% of patients fluoxetine-treated youth (compared to 35% of those receiving placebo) responded in terms of CGI categorical outcome and fluoxetine was well tolerated [21].
2.1.2. Sertraline
Sertraline is slowly absorbed from the gastrointestinal tract with peak serum levels occurring between 6 and 8 hours [22] after administration. Its absorption is significantly influenced by co-administration with food, which may hasten absorption by 1–2 hours. Sertraline is primarily metabolized by CYP2C19 and CYP2B6 [23] and has a half-life of 26–32 hours in adults, although this half-life varies as a function of CYP2C19 phenotype [24]. In children, the half-life of sertraline is significantly lower, and some pharmacokinetic studies have suggested the need for BID dosing in pre-pubertal patients [25].
In pediatric patients with GAD (age 6–17 years), sertraline was evaluated in a fixed-dose randomized controlled trial that was conducted by Rynn and colleagues [26]. In this study, sertraline was initiated at 25 mg and titrated to 50 mg/day and improvements in sertraline-treated patients were observed by the fourth week of treatment. Also, despite a fixed-dose administration, improvement increased over time, as is generally observed in meta-analyses of antidepressants in anxious youth. In a subsequent study of sertraline in anxious youth, of whom nearly 40% met DSM-IV criteria for GAD, flexibly dosed sertraline significantly reduced symptoms, and was associated with a similar decrease in anxiety symptoms compared to placebo. As has been observed in studies of adults with GAD, the effects of sertraline were amplified by CBT with response rates to CBT being superior to monotherapy and superior to placebo [12] In this study, potential side effects were extensively explored and sertraline was found to be relatively well-tolerated. Specifically, no differences were detected in the double-blinded conditions for total physical and psychiatric side effects, or any one physical or psychiatric AE. However, the total somatic symptom-related side effects were more common in patients who received sertraline monotherapy compared to those who received CBT or CBT + sertraline (p’s <0.01), while the total number of psychiatric adverse events was greater in children compared to adolescents. Importantly, sertraline-associated adverse events were generally found to decrease over time [27].
2.1.3. Paroxetine
The third SSRI to be introduced in the United States, paroxetine potently inhibits 5-HT reuptake and also blocks some reuptake of norepinephrine. As an FDA-approved treatment for GAD in adults, immediate release paroxetine has been studied extensively and multiple studies support its efficacy in adults with GAD. In the first double-blind, placebo-controlled study, adults with a primary diagnosis of GAD were randomized to paroxetine (n=161; 20–50 mg/day) or placebo (n=163) for 8 weeks, and improvement in both anxiety symptoms and daily functioning was reported in paroxetine-treated patients relative to placebo [28]. In a second double-blind, placebo-controlled trial, Rickels and colleagues examined the efficacy of paroxetine (20 mg or 40 mg) in adults with GAD (N=566) over an 8-week double-blind trial and noted significantly greater improvement in HAM-A scores for both doses relative to placebo, and also found paroxetine to be generally well-tolerated [29].
Several head-to-head studies have evaluated the comparative efficacy and tolerability of paroxetine and other SSRIs. In the first, sertraline and paroxetine were studied in a head-to-head study in adults with GAD (N=55); both SSRIs improved anxiety symptoms and no differences were detected in response or remission rates nor in the tolerability profiles of the two medications. A second and longer study (24 weeks) involved double-blind treatment with escitalopram (n=60; 10–20 mg/day) or paroxetine (n=61; 20–0 mg/day) and, while no differences were observed with regard to improvement in anxiety symptoms, treatment-emergent adverse events were more frequent in paroxetine-treated patients compared to those randomized to escitalopram (88.7% vs. 77.0%). Additionally, significantly fewer escitalopram- compared to paroxetine-treated patients discontinued due to adverse events (6.6% vs. 22.6%; p=0.02) [30]. In another trial of paroxetine, adults with GAD were randomized to imipramine or diazepam; patients treated with the benzodiazepine improved earlier in the course of their treatment although both paroxetine and imipramine were associated with significant improvement in anxiety symptoms. Importantly, the improvement associated with paroxetine was greater than the decreases anxiety associated with benzodiazepines by week for the same trial.
The side effect profile of paroxetine is relatively unique among SSRIs. In this regard, paroxetine blocks both serotonin and norepinephrine reuptake and has significant muscarinic cholinergic receptor antagonism and may have some antihistamine effects as well. Additionally, paroxetine undergoes significant first pass metabolism, is highly protein-bound, and is extensively metabolized through the CYP2D6 system. Moreover, paroxetine has a very short half-life and its variability in steady-state plasma concentrations may be further affected by numerous CYP2D6 polymorphisms. Because of its short half-life, SSRI withdrawal symptoms may be more common in paroxetine-treated patients relative to those treated with other SSRIs, and these symptoms may be especially more pronounced in younger patients.
2.1.4. Citalopram
Citalopram and its pharmacologically-active s-enantiomer, escitalopram, represent the newest SSRIs on the US market, released in 1998 and 2002 respectively, and are among the most selective for the serotonin transporter of all the SSRIs. Interestingly, “a blunting effect of R-citalopram on escitalopram is presumed to explain the therapeutic differences between citalopram and escitalopram. Additionally, the r-enantiomer may have greater histamine affinity and associated antihistaminergic effects as well as more QTc-prolongation compared to escitalopram [31] and compared to other SSRIs [32]. Rapidly absorbed from the gastrointestinal system with a bioavailability of 80%, citalopram levels generally peak in 2–4 hours following oral administration and, unlike several other SSRIs, citalopram does not undergo substantial first-pass metabolism [33]. The antidepressant, like other SSRIs, is also highly protein-bound, and metabolized primarily through the CYP2C19 system. Recently, large studies suggested that drug level is influenced by CYP2C19 genotype and that levels are also greater than expected in patients older than approximately 65 years of age, although drug level was not separately evaluated in this age group.
Escitalopram has been approved by the FDA for the treatment of GAD in adults, and both escitalopram and citalopram have been evaluated in adults with GAD. To date, three 8-week trials have demonstrated that escitalopram is superior to placebo in the acute treatment of GAD, and that the medication is generally well tolerated [34]. In these three trials, adults with GAD (age 18–80 years; approximate N=850) were randomized to escitalopram or placebo and, in each individual study, escitalopram was found to be significantly superior to placebo (p<0.05) in terms of improvement in HAM-A scores over the course of each trial [34]. In a double-blind, placebo-controlled maintenance study, patients were treated with fixed-dose escitalopram (20 mg/day) over the course of 24–76 weeks, and re-randomization to placebo was associated with a significantly higher risk of relapse (OR: 4; 56% vs. 19%; log-rank test, p<0.001). Also, during this study, discontinuation-emergent symptoms were assessed and included dizziness, nervousness and dyssomnia [35].
In pediatric patients, escitalopram is approved by the FDA for the treatment of MDD in adolescents aged 12–17, but no studies have evaluated its efficacy in pediatric patients with GAD (although one single-site, double-blind placebo controlled trial is ongoing).
2.2. Selective serotonin-norepinephrine reuptake inhibitors
2.2.1. Venlafaxine
Venlafaxine is a selective serotonin and norepinephrine reuptake inhibitor (SNRI) that is FDA-approved for the treatment of GAD, MDD, panic disorder, and social anxiety disorder. Its active metabolite, o-desmethylvenlafaxine, inhibits the serotonin and norepinephrine reuptake transporters albeit with greater potency at the norepinephrine transporter. Desvenlafaxine is itself marketed as an antidepressant, and both venlafaxine and desvenlafaxine weakly inhibit dopamine reuptake. In general, dose-related increases in blood pressure are associated with treatment and are likely mediated by increases in central and peripheral noradrenergic tone (see Effexor XR package insert, Wyeth Pharmaceuticals, a subsidiary of Pfizer, Philadelphia, 2015). Additionally, venlafaxine is converted to its active metabolite by CYP2D6, which potentially contributes to variability in efficacy and tolerability profiles, as individuals who are rapid metabolizers for CYP2D6 may have a higher concentration of desvenlafaxine to venlafaxine, which is associated with more serotonergic effects. Others who are poor metabolizers with regard to CYP2D6, however, may have a higher ratio of venlafaxine to desvenlafaxine, resulting in more 5-HT relative to norepinephrine reuptake blockade. This is another example that underscores the complex pharmacology of these medications.
In adults with GAD, venlafaxine (75–225 mg/day) effectively reduces anxiety symptoms [36–38] and was the first FDA-approved antidepressant for the treatment of GAD in this age group. In the second double-blind, placebo-controlled study of venlafaxine, fixed doses of extended-release venlafaxine were evaluated (N=377) with venlafaxine being superior to placebo in terms of categorical response criteria and continuous measures of anxiety [37]. The first study of venlafaxine in adults with GAD examined the efficacy of venlafaxine XR (75 mg/day, 150 mg/day, or 225 mg/day; N=124) compared to placebo (N=127) over a 6-month period. Response rates in patients receiving venlafaxine XR were 69% compared to 46% in patients randomized to placebo (p<0.001) [38]. Across trials in adults with MDD, the most common treatment-emergent adverse events were nausea, somnolence, and xerostomia; and increases in systolic blood pressure and heart rate have also been observed. Finally, two studies suggest that the efficacy of venlafaxine in patients with GAD and one suggests that this efficacy is maintained over long treatment periods of 6–12 months [39]. Importantly, these longer-term studies suggest that 12 months of treatment may be preferable to 6 months in decreasing the risk of relapse; however, they also highlight that in patients who have experienced relapse upon discontinuation of venlafaxine, response can be recaptured by re-initiation of treatment [39].
One randomized placebo-controlled trial evaluated venlafaxine XR in pediatric patients aged 6–17 years of age with GAD and suggested efficacy [40]. In this study, venlafaxine was initiated at 37.5 mg and flexibly titrated. Over the course of 8 weeks, both GAD symptoms and HAM-A scores improved compared to placebo. In general, venlafaxine was well tolerated but associated with asthenia, pain, somnolence, anorexia, and weight loss [40].
2.2.2. Duloxetine
Duloxetine, a selective 5-HT and norepinephrine reuptake inhibitor, is FDA-approved for the treatment of GAD, MDD, diabetic peripheral neuropathic pain, fibromyalgia, and chronic musculoskeletal pain. In multiple randomized placebo-controlled trials of adults with GAD, duloxetine is associated with significant improvements in anxiety symptoms and functional outcomes. These trials have recently been subjected to a meta-analysis which revealed superior remission rates for duloxetine (n=2,399, RR = 1.60, 95% CI: 1.43–1.80) and greater improvement in HAM-A scores (n=1,135, MD = 3.34, 95% CI: 2.37–4.32), while finding no differences in discontinuation rates between placebo and duloxetine [41]. Also, duloxetine, like escitalopram and several other pharmacotherapies, has been evaluated as a relapse-prevention treatment for adults with GAD. In this double-blind, placebo-controlled trial, flexibly-dosed duloxetine reduced relapse more frequently than placebo (41.8% vs. 13.7%, p<0.001) and was generally well-tolerated [42]. Duloxetine has been evaluated in older adults with GAD and, in this double-blind, placebo-controlled study, adults aged 65 years or older were randomized to duloxetine (N=151) or placebo (N=140) and, over the course of 10 weeks of treatment, reductions in HAM-A total scores were significantly greater than those in patients receiving placebo [43]. In this population, constipation, dry mouth, and somnolence were cited as adverse effects that were more common with duloxetine compared to placebo [43].
In children and adolescents, duloxetine is the only antidepressant that has received FDA approval for the treatment of GAD. This approval is based on a randomized trial of flexibly dosed duloxetine in youth aged 7 through 17 years with a primary diagnosis of GAD. In this study, youth were treated with duloxetine (30–120 mg/day, N=135) or placebo (N=137) for 10 weeks and the acute treatment phase was followed by 18 weeks of open-label duloxetine treatment (30–120 mg/day) [44]. Improvement for the Pediatric Anxiety Rating Scale (PARS) [45] score was greater for duloxetine (−9.7) compared with placebo (−7.1, p≤0.001, Cohen’s d: 0.5). Furthermore, symptomatic response (50% improvement on the PARS severity for GAD), remission (PARS severity for GAD ≤8), and functional remission (CGAS >70) rates for the duloxetine group (59%, 50%, 37%, respectively) were greater than for the placebo group (42%, 34%, 24%, respectively, p≤0.05) during acute treatment. Of note, dose-independent hemodynamic side effects were observed in the duloxetine group with regard to systolic blood pressure and heart rate as well as a small, but statistically-significant weight loss in this age group [44].
2.3. Atypical antidepressants
2.3.1. Vilazodone
Vilazodone, a 5-HT transporter inhibitor and a partial agonist at the 5-HT1A receptor, is approved by the FDA for the treatment of MDD in adults [46,47]. To date, one double-blind, parallel-group, placebo-controlled, fixed-dose study evaluated two fixed doses of vilazodone (20 mg/day, N=230; 40 mg; n=227) compared to placebo (n=223) and observed a statistically significant improvement in patients treated with 40 mg/day (vs placebo; p=0.031) but did not observe a drug placebo difference. An additional study evaluated flexibly dosed vilazodone (20–40 mg/day) over the course of 8-weeks and observed a statistically significant decrease in anxiety symptoms in vilazodone-treated patients compared to those receiving placebo (p=0.005) [47]. In both studies, The most commonly reported side effects, which occurred at twice the rate in patients being treated with vilazodone compared to placebo, was nausea, diarrhea, dizziness, vomiting, delayed ejaculation, and erectile dysfunction as well as fatigue [46,47]. To our knowledge, vilazodone has not been systematically evaluated in youth with GAD.
2.3.3. Mirtazapine
Mirtazapine, is an inhibitor of the pre-synaptic α2-adrenergic autoreceptor and has affinity for both the 5-HT2A and 5-HT2C receptors. Mitrazapine has no substantial impact on dopamine and 5-HT reuptake machinery, and very little activity at the norepinephrine transporter. It is a potent histamine H1 receptor antagonist in as much as in vivo cortical histamine H1 receptor binding occupancy reaches 80–90% following a 15 mg oral dose [48]. Because of its unique combination of antihistaminergic and serotonergic effects, there has been considerable interest in this compound as a potential treatment for GAD and other anxiety disorders. However, this interest has not been realized in the clinic, with only two known studies published to date of mirtazapine in adults with GAD. In the first study, open-label mirtazapine treatment in adults with MDD and co-occurring GAD was associated with improvement in depressive symptom severity and in anxiety symptoms (HAM-A), which was noted following the first week and continuing through the following 8 weeks of treatment [49]. In a second open-label study, fixed-dose mirtazapine (30 mg/day) was associated with improvement in the HAM-A in adults with GAD (N=44), and that at the week 12 endpoint, nearly 80% of the sample had met response criteria, with one third meeting criteria for remission [50].
2.4. Multimodal Antidepressants
2.4.1. Vortioxetine
Vortioxetine, a medication with a multimodal mechanism of action including inhibition of the 5-HT transporter and 5-HT1D receptor, in addition to modulation of 5-HT1A reports 5-HT1B, 5-HT1D, and 5-HT7 receptors [51], has been evaluated in four double-blind placebo-controlled trials of adults with GAD [52–55]. In the first of these trials, patients were randomized to vortioxetine or placebo for 8 weeks and modest, but statistically-significant improvements were noted for HAM-A scores in the vortioxetine group [54]. In a subsequent study, patients (N=457) were randomized to vortioxetine (2.5 mg or 10 mg) or placebo (1:1:1) and no differences were observed [55]. A final study utilized an interesting design to examine the risk of relapse following stabilization on vortioxetine, and found the risk of relapse following randomization to vortioxetine (5 or 10 mg/day) to be significantly lower than for those randomized to placebo. Finally, a meta-analysis of vortioxetine in adults with GAD revealed vortioxetine to be significantly more effective than placebo in reducing anxiety symptoms (standardized mean difference: −0.118, p=0.007) and suggested that the benefit might be strongest in those with more severe GAD. In this meta-analysis, odds ratios for response and remission were 1.221 (95% CIs, 1.027 to 1.452, p=0.024) and 1.052 (95% confidence interval: 0.853 to 1.296, p=0.637), respectively [56]. In general, vortioxetine is well tolerated with the most common side effects being nausea, diarrhea, constipation, vomiting, xerostomia, and headache.
Vortioxetine has not been evaluated in any double-blind placebo-controlled trials in youth with GAD but has been evaluated in 2 prospective pediatric trials of patients with mixed depressive or anxiety disorders. In an acute phase pharmacokinetic study of youth aged 7–17 years with depressive and/or anxiety disorders, vortioxetine was safe and well tolerated (dose range: 5–20 mg/day) however, an open-label extension study (N=41), suggest that vortioxetine was not well tolerated; 85% of participants reported adverse events including headache, nausea, dysmenorrhea, and vomiting [57].
2.5. Benzodiazepines
Benzodiazepines bind to the GABAA receptor and serve as allosteric modulators, potentiating the effects of endogenous GABA. Prior to the introduction of SSRIs and SNRIs, benzodiazepines represented the mainstay of GAD pharmacotherapy. These medications vary in their duration of action, abuse liability, lipophilicity, and metabolism. They also vary in their therapeutic onset; although, unlike antidepressant medications, the therapeutic effects associated with benzodiazepines typically emerge shortly after administration.
While benzodiazepines have been studied in patients with GAD, many of these compounds were introduced and evaluated as treatments for ‘anxiety disorders’ rather than for GAD specifically, which emerged as a diagnosis in adults with the publication of the DSM-III, and in children and adolescents with the publication of the DSM-IV-TR in 1994. As such, alprazolam is the only benzodiazepine to be formally approved by the FDA for the treatment of GAD. However, clonazepam, lorazepam, and diazepam are often used to treat GAD in adults and have been studied in pediatric patients with mixed anxiety disorders, which include GAD. Benzodiazepines such as diazepam and clonazepam (both of which are long-acting agents) may be efficacious in treating GAD, although many clinicians choose to limit their use due to concerns about the risks regarding possible misuse and dependence. Current prescribing guidelines suggest that benzodiazepine therapy for adults should be short-term (3–6 months), although it has been noted that this time frame falls short of addressing the typically chronic nature of GAD. Of note, numerous specialists have voiced the opinion that benzodiazepines can be a reasonable option for certain patients, such as those at a low risk for substance abuse, when other treatments are contraindicated or proven ineffective, so long as they are administered with consistent, close monitoring [58,59]. In fact, while SSRIs an SNRIS are the most common pharmacotherapy for GAD, a recent meta-analysis involving 12,655 patients (κSSRI =16, κSNRI =17, κbenzodiazepine =23), the Hedges’ g for SSRIs and SNRIs were 0.33 and 0.36, respectively while the Hedges g for benzodiazepines was 0.5 [60]. These findings suggest that benzodiazepines are more effective than SSRIs or SNRIs in adults with GAD [60].
It is of interest that benzodiazepines have been evaluated as adjunctive interventions in adults with anxiety disorders who were prescribed SSRIs and that this may circumvent the transient increase in anxiety that, in some patients, occurs shortly after initiation of SSRI treatment. In this regard, a double-blind, placebo-controlled trial of clonazepam in adults with panic disorder initiating sertraline treatment observed more rapid stabilization when paired with clonazepam combined initiation with placebo [61]. Similarly, in a 10-week trial of adjunctive clonazepam or placebo in adults with generalized social anxiety disorder, 79% of patients who received paroxetine and clonazepam combined improved in terms of CGI response compared to 43% of those treated with paroxetine and placebo, although this narrowly missed the threshold for statistical significance (p=0.06) [62]. In adults with anxiety disorders, the most common side effects of clonazepam and other benzodiazepines are somnolence, fatigue, increased sweating, jitteriness. Additionally, standardized withdrawal assessments suggest that the following are more common with discontinuation from a benzodiazepines compared to placebo: anxiety, diaphoresis and tremor [62].
Studies of benzodiazepines in youth with GAD are limited. In one study of children and adolescents with GAD (mean age: 12.6 years, N=30), alprazolam did not result in a statistically significant improvement compared to placebo in terms of CGI [63]. Similarly, a study of clonazepam in youth with social anxiety disorder and/or GAD (aged: 7–13 years, N=15) found decreases in anxiety symptoms (standardized, continuous measure of anxiety not reported) but no statistically significant differences on the categorical CGI-I response rate between placebo and clonazepam were detected. Finally, in youth, the side effects of benzodiazepines are reported to include irritability, drowsiness, and ‘oppositional behavior’ [64] as well as dry mouth and sedation [63], although relatively aggressive dosing of benzodiazepines was used in the two double-blind studies that have suggested poor tolerability of this class of medications in anxious youth.
2.6. Non-benzodiazepine hypnotics
To date, one interesting study has evaluated the non-benzodiazepine soporific, eszopiclone, in patients with GAD [65]. This study addressed an important limitation to antidepressant treatments—that some patients experience worsening anxiety and insomnia associated with the initiation of treatment—and specifically addressed the need for a targeted insomnia treatment, given that insomnia is frequently a symptom of GAD. In this double-blind, randomized, placebo-controlled, parallel-group, add-on therapy 10-week trial, adults with GAD received 10 mg of escitalopram oxalate for 10 weeks and were randomized to receive adjunctive eszopiclone (n=294) or placebo (n=301). The combination of eszopiclone and escitalopram significantly improved sleep and daytime functioning (p<0.05) and there was no evidence of tolerance. Furthermore, the addition of eszopiclone to escitalopram resulted in greater improvement in HAM-A scores at each week, even when the insomnia item of the HAM-A was removed, and also enhanced the likelihood of response and remission [65]. The most common side effects were reported to be unpleasant taste, headache, dry mouth, and somnolence [65].
2.7. Tricyclic Antidepressants
The tricyclic antidepressants (TCAs) were first developed in the 1950s as antidepressants and represented the mainstay of psychopharmacotherapy prior to the introduction of the SSRIs in the late 1980s. The TCAs are categorized into tertiary amine (e.g., amitriptyline, clomipramine, doxepin, imuipramine, and trimipramine) and secondary amines (e.g., desipramine, nortriptyline, protriptyline). Secondary amines are more effective in blocking the norepinephrine transporter, whereas the tertiary amines are more potent inhibitors of 5-HT reuptake (Table 1). The TCAs are largely absorbed in the small intestine, which occurs relatively rapidly with peek concentrations achieved within 2–4 hours (Table 2). Additionally, like the SSRIs, and SNRIs other than venlafaxine, the TCAs are highly protein bound and are metabolized through the cytochrome system [66].
Table 1:
Comparison of tricyclic antidepressants (TCAs)
| Potency reuptake blockade | |||
|---|---|---|---|
| Drug | 5-HT | Norepinephrine | Dopamine |
| Amitriptyline | • | • | |
| Clomipramine | ••• | • | |
| Desipramine | • | ••• | |
| Imipramine | •• | • | |
| Nortriptyline | • | ••• | • |
Table 2:
Dosage, half-life and apparent therapeutic plasma concentrations of tricyclics and tetracyclics
| Drug | T½
(hours) [134–136] |
Dosage (mg/day) [137] |
Plasma concentration (ng/mL) [137] |
|---|---|---|---|
| Tertiary tricyclics | |||
| Amitriptyline | 13-36 | 100-300 | |
| Clomipramine | 19-40 | 100-250/300 | >150* |
| Imipramine | 8-21 | 100-300 | >200* |
| Secondary tricyclics | |||
| Desipramine | 15-24 | 75-300 | >125 |
| Nortriptyline | 26 | 50-150 | 50-150 |
Total plasma concentration + metabolite
While the use of TCAs has largely been supplanted by the use of SSRIs for the treatment of GAD, they still represent a viable option for patients who fail to respond after multiple trials of SSRIs, SNRIs, and adjunctive psychopharmacologic interventions. Mechanistically, the TCAs bind to norepinephrine and 5-HT transporters and increase synaptic concentrations of these biogenic amines, although they have some affinity for histaminergic, cholinergic, and α1 adrenergic receptors which lead to the common side-effects of dry mouth, constipation, and weight gain [67]. Additionally, QTc prolongation with TCAs is more pronounced compared to SSRIs (7.1 msec; 95% CI 3.84–10.27)[32] and TCAs may be fatal in overdose, largely secondary to TCA-related ventricular arrhythmia and seizures [68].
Numerous studies have demonstrated the efficacy of TCA’s in the treatment of patients with GAD, so we will review only a selection of the more notable randomized controlled trials here. Rickels and colleagues a study of multiple TCAs, assigning treatment with imipramine, trazodone, diazepam, or placebo over the course of 8 weeks to adults with GAD with moderate to severe severity (HAM-A score ≥18) [69]. Diazepam was found to be superior to imipramine and trazodone within the first 2 weeks, although by week 4, the salutary effects of imipramine statistically eclipsed both trazodone and diazepam. In this study response rates were 73% for imipramine-treated patients, compared to 69% for patients receiving trazodone and 66% for those receiving diazepam, and 47% for the placebo group. Imipramine was relatively well-tolerated, with the most common side effects being the expected anticholinergic outcomes, including dry mouth and constipation, with less drowsiness reported compared to either of the other studied medications [69]. In another double-blind placebo-controlled, parallel group study of imipramine and alprazolam, the latter was found to be more effective in reducing somatic symptoms, whereas imipramine was more effective in ameliorating psychic symptoms (e.g., dysphoria, negative anticipatory thinking). Based on this trial, the authors suggested that in adults with GAD, somatic symptoms and hyperarousal respond best to GABAergic medications, while psychic symptoms respond best to medications targeting the noradrenergic or serotonergic systems [70]. Imipramine has also been evaluated in tapering benzodiazepeines in patients with GAD [71]. In this study, 83% of long-term benzodiazepine-treated adults (average duration: 8.5 years) who had previously failed at least three tapers, were successfully tapered when treated with imipramine [71].
2.8. Monoamine Oxidase Inhibitors
The monoamine oxidase inhibitors inhibit the mitochondrial enzyme monoamine oxidase, which metabolizes and inactivates dopamine serotonin and norepinephrine. Thus, inhibition of monoamine oxidase increases the synaptic concentrations of these monoamines. The MAOIs may inhibit one or both isomers of MAO [72]. MAO-A is primarily expressed in gastrointestinal and central nervous system, whereas MAO-B is found mainly in platelets in those CNS regions that are heavily innervated by dopaminergic neurons [72]. Currently, in the United States, two non-selective MAOIs are commonly prescribed: tranylcypromine and phenalzine, while the MAO-B inhibitor, selegiline is also available [73], each with different side effect profiles and rates of effectiveness. To date, most trials of MAOIs in anxious adults have focused on the treatment of panic disorder and social anxiety disorder, although they may still represent a viable option for patients with refractory GAD in whom SSRIs, SNRIs, and alternative pharmacotherapies are not viable treatment options.
2.9. Azapirones
2.9.1. Buspirone
Buspirone, a non-benzodiazepine anxiolytic and 5-HT1A receptor agonist, is approved for the treatment of GAD in adults and has been evaluated in multiple double-blind placebo-controlled trials of children and adolescents with GAD. Recently, a Cochrane review of randomized controlled trials of azapirones (κ=36) in adults with GAD suggested that this class of medications is relatively well tolerated and effective, particularly in patients who have not been previously treated with benzodiazepines [74]. though interestingly, the comparative analysis conducted by this review suggested that azapirones may be less effective compared to benzodiazepines [74].
Buspirone has also been evaluated as a treatment for pediatric patients with GAD. The results of these trials, which were recently published, suggest that buspirone is relatively well tolerated in the pediatric population, although two randomized controlled trials were too underpowered to detect small effect sizes (Cohen’s d <0.15) [75].
2.10. Antihistamines
2.10.1. Hydroxyzine
Anti-histamines have long been used as abortive interventions in pediatric and adult patients with GAD, and yet systematic analyses of antihistamines have only recently been undertaken. The H1 receptor antagonist, hydroxyzine, a centrally acting antihistamine with mild anti-cholinergic effects was first introduced in 1955 and repeatedly systematically evaluated in the following four decades for the treatment of anxiety disorders. This antihistamine was recently examined for tolerability and efficacy in a Cochran review of 39 studies, of which five met inclusion criteria [76], which found hydroxyzine to be more effective than placebo in the treatment of adults with GAD. In general, across studies, hydroxyzine was relatively well tolerated and there were no statistically significant differences in the number of patients experiencing adverse effects between hydroxyzine treated patients and those receiving placebo [76]. Overall, hydroxyzine was more effective than placebo in reducing symptoms of GAD; however, the quality of these studies was generally deemed to be low [76].
2.11. GABA-related interventions
2.11.1. Pregabalin
The γ-aminobutyric acid (GABA) analogue pregabalin binds with high affinity to the α2δ subunit of brain voltage-gated calcium channels (N-type), and in doing so decreases presynaptic calcium currents [77] and subsequently decreases calcium-dependent vesicle docking at the presynaptic membrane. However, unlike benzodiazepines which exert their therapeutic effects through binding to GABAA, and benzodiazepine receptors, pregabalin does not bind directly to these receptors, augment currents or affect GABA metabolism. Moreover, pregabalin does not appear to have functional activity at 5-HT, dopamine, or norepinephrine receptors. This highly lipophilic compound is rapidly absorbed from the gastrointestinal tract and has a high bioavailability and is renally-cleared.
Nearly a dozen studies have examined the efficacy of pregabalin in adults with GAD [78]. In the first of these studies, Pande and colleagues [79] evaluated the efficacy of pregabalin over four weeks with a one-week placebo lead in. Additionally, in this study, as in similar trials of pregabalin in adult GAD, active benzodiazepine comparison arms were included. In most studies, separation from placebo occurred relatively early and statistically significant improvements were observed relative to placebo over the course of treatment. Additionally, the efficacy of pregabalin was, in general, consistent with the efficacy of active benzodiazepine comparators. Furthermore, in adult outpatients randomized to fixed-dose venlafaxine (75 mg/day) or pregabalin, treatment effects emerged earlier for pregabalin (week 2) compared to the SNRI venlafaxine [80].
In adults with GAD, the most common treatment emergent adverse effects associated with pregabalin-included dizziness, somnolence, headache, xerostomia, amblyopia and diarrhea. Importantly, these treatment emergent adverse events were generally more common with higher doses (600 mg/day) compared to lower doses (150 mg/day). Given the known potential neurocognitive and psychomotor effects of pregabalin, its neuropsychiatric side effect profile has additionally been evaluated in comparison with the benzodiazepine alprazolam, to which it was found to compare favorably [81]. The most significant treatment limiting adverse event appears to be the weight gain associated with pregabalin. Across studies, significant weight gain was associated with this medication in nearly all trials, though the effect does appear to be dose-dependent [78].
Pregabalin is reported to be generally well-tolerated in adult patients with GAD and has a rapid onset of action compared to many other pharmacotherapies, approximately one week, as well as comparable efficacy to benzodiazepines [82,83]. Additionally, it has been evaluated in older adults with GAD and found to be efficacious and well-tolerated both as monotherapy [84] and as an adjunctive intervention in antidepressant-resistant GAD [85]. Discontinuation rates in double-blind, placebo-controlled trials of pregabalin in the treatment of GAD have generally been lower than those observed for both benzodiazepines [79,83] and 75 mg/day venlafaxine [86]. However, pharmacovigilance data suggest that both pregabalin (and gabapentin) have abuse liability and are associated with overdose fatalities [87]. A recent systematic review of abuse liability suggests that pregabalin may be more addictive than gabapentin regarding the magnitude of behavioral dependence symptoms and transitions from prescription to self-administration” [87]. Additionally, in this review overdoses “appeared relatively safe but can become lethal (pregabalin > gabapentin)” which is relevant in that lethal overdoses frequently include concomitant medications (e.g., opioids and sedative-hypnotics) [87].
Finally, it is of interest that more recent studies, including a meta-analysis, suggest that the effect size of pregabalin may be lower than was first appreciated. Specifically, this meta-analysis of seven studies suggested an Hedges’ g of 0.364, with effect sizes of 0.349 and 0.24 for psychic and somatic symptoms, respectively [88].
2.11.2. Tiagabine
Tiagabine is a specific GABA-reuptake inhibitor and exerts its action via the presynaptic GAT-1 transporter blockade [89]. Open label studies in adults with GAD [90] as well as case series of adult outpatients with treatment refractory GAD [91,92], have found tiagabine to be generally effective and well tolerated in the treatment of GAD, although results from double-blind placebo-controlled trials have produced mixed results. In an 8-week open-label study of tiagabine in SSRI-treated adults with GAD (N=17, mean dose: 13 mg/day), nearly 80% experienced at least a 50% reduction in anxiety symptoms as assessed by the HAM-A, with nearly 60% achieving remission [90]. In an analysis of three placebo-controlled trials, tiagabine demonstrated no superiority to placebo on the HAM-A for any dose in two flexible-dose studies. However, tiagabine-treated patients did improve, but this improvement only reached significance in study completers [93]. Common side effects of tiagabine in adults with GAD include dizziness, headache, nausea, fatigue, and somnolence [93]. To date, no studies have evaluated tiagabine in pediatric patients with GAD.
2.12. Second-generation antipsychotics (SGAs)
2.12.1. Olanzapine
Olanzapine, a D2/5-HT2A antagonist with significant H1 and α1 antagonism [94], is rapidly absorbed with peak concentrations occurring in 4–6 hours [95] and is primarily metabolized through CYP1A2 [96]. Olanzapine has been evaluated in adults with GAD who continued to experience significant symptoms during the course of treatment with fixed-dose fluoxetine (20 mg/day) [97]. In this study, patients who failed to respond completely to fluoxetine were randomized to olanzapine or placebo (N=24) for six weeks (mean dose 8±7 mg/day). Response rates were significantly higher in patients treated with olanzapine on both the CGI [98] and HAM-A [80], while remission rates were numerically greater in patients treated with olanzapine, but this effect only trended towards significance (p=0.1). As is common with SGAs, treatment emergent weight gain was associated with olanzapine [97]. No studies have evaluated olanzapine in pediatric patients with GAD.
2.12.2. Ziprasidone
Snyderman and colleagues evaluated low-dose ziprasidone in patients with treatment-resistant GAD and observed that in adults with GAD (N=13), ziprasidone (20 to 80 mg/day) resulted in significant improvement in anxiety symptoms [99]. To date, no studies have evaluated ziprasidone in pediatric patients with GAD.
2.12.3. Risperidone
This high potency SGA with D2/5-HT2A/α1 antagonism is rapidly absorbed with peak concentrations occurring in 1–2 hours, and is primarily metabolized through CYP2D6 to the active metabolite, 9-OH-risperidone (paliperidone) [100]. Several studies have evaluated open-label risperidone treatment in adults with refractory anxiety, including GAD [101]. One double-blind, placebo controlled study in adults with GAD who were already treated with an anti-anxiety agent but with only partial response has evaluated risperidone as adjunctive therapy. In the controlled study, patients with GAD (N=40) were randomized to 5 weeks of double-blind adjunctive treatment with placebo or risperidone (0.5–1.5 mg/day) and greater reductions in HAM-A total scores (p=0.034) and HAM-A psychic anxiety factor scores (p=0.047) were observed in patients treated with risperidone compared to those who received placebo [102]. To date, no studies have evaluated risperidone in pediatric patients with GAD.
2.12.4. Aripiprazole
Aripiprazole is a partial dopamine D2 and 5HT1A receptor agonist that also binds to D3 dopamine and 5HT2A receptors. This SGA is well absorbed with peak plasma concentrations typically occurring 3–5 hours after administration and has a long half-life, approximately 75 hours in adults [103]. Two open-label studies of low-dose aripiprazole in adults with treatment-resistant GAD suggest that adjunctive aripiprazole is associated with significant improvement in terms of CGI and is generally well-tolerated, although a minority of patients discontinued treatment due to side effects [104,105]. To date, no studies have evaluated aripiprazole in pediatric patients with GAD.
2.12.5. Quetiapine
The high affinity for 5-HT2A, α1 and H1 receptors and D2 antagonism that characterize the quetiapine neuropharmacology only partially account for its clinical effects. In this regard, quetiapine, despite its D2 receptor potency, rapidly disassociates from the D2 receptor [106] conferring a low risk of extrapyramidal symptoms that is equivalent to placebo—including akathisia, which may be particularly problematic in patients with GAD. To date, five open-label studies have evaluated quetiapine (XR formulation: κ=2; immediate release: κ=3) as an adjunctive treatment adults with GAD [107] and this compound has also been evaluated for GAD in geriatric patients [108]. In these studies, quetiapine was generally efficacious and well tolerated. Two of the double-blind trials of quetiapine in adults with GAD require additional discussion. Statistically significant improvement was observed over an 8-week treatment period, and dosing was relatively lower than is typically used for bipolar disorder or schizophrenia. Among patients randomized to fixed-dose treatment (quetiapine XR: 50 mg/day, n=234; 150 mg/d, n=241; 300 mg/d, n=241) or placebo (n=235) quetiapine was statistically superior to placebo except at the 300 mg/day dose, wherein side effects were significantly greater [109]. An additional study evaluated the efficacy of quetiapine XR in the maintenance treatment of adults with GAD. In this double-blind, placebo-controlled study, patients were stabilized with quetiapine XR (50–300 mg/day) over a period of 4–8 weeks and then randomized to continue quetiapine XR (n=216) or switch to placebo (n=216); and significantly fewer patients who had been randomized to quetiapine XR experienced recurrence of their anxiety symptoms compared to placebo [110]. There are no studies of quetiapine in pediatric patients with GAD.
2.13. Other agents
2.13.1. Agomelatine
Agomelatine (β-methyl-6-chloromelatonin) is a melatonin structural homologue that potently agonizes melatonin-1 (MT1) and MT2 receptors in addition to antagonizing 5-HT2C receptors [111]. This compound, which has been found to normalize sleep in some studies without daytime sedation, and which has established efficacy for the treatment of MDD in adults, has been evaluated in several double-blind, placebo controlled studies for its efficacy in adults with GAD. In the first study of agomelatine in adults with GAD, Stein and colleagues observed that agomelatine (25 to 50 mg/day) was associated with a greater reduction in anxiety symptoms compared to placebo and similar improvements were observed for clinical response, insomnia, and associated disability [112]. In an international multi-site study, agomelatine was compared to escitalopram and placebo in adults with GAD. Comparable reductions were observed between both active treatments, and agomelatine was well tolerated whereas escitalopram was associated with increased rates of side effects compared to placebo [113]. The most recent acute efficacy study of agomelatine observed improvement in HAM-A scores in adults with GAD who were treated with agomelatine (10 mg/day and 25 mg/day) over a 12-week period and observed a dose-response relationship for this effect. Additionally, response (51% to 70%) and remission (25% to 40%) were also found to be significantly superior in agomelatine treated patients compared to those treated with placebo [114]. Finally, a relapse-prevention study found that over a 6 month follow-up period, patients subsequently randomized to agomelatine had lower rates of relapse compared to those randomized to placebo (19.5% vs. 30.7%, p=0.046) [115].
2.13.2. Guanfacine
Strawn and colleagues [116] examined the efficacy of the α2A-adrenergic receptor agonist, guanfacine (extended-release) in pediatric patients (N=83) with generalized, separation and social anxiety disorders (95% met DSM-IV criteria for GAD). At the α2A receptor, guanfacine may decrease synaptic release of norepinephrine which is relevant to anxiety in that agents that decrease norepinephrine release or dampen its postsynaptic effects may attenuate fear responses [117]. Patients were randomized (3:1) to guanfacine (n=62) or placebo (n=21) and extended release guanfacine was relatively well tolerated with the most common side effects being headache, somnolence, fatigue, abdominal pain, dizziness and constipation [116]. No differences were observed between medication and placebo for the improvement in PARS or SCARED scores (PARSguanfacine: −6.9 ± 6.6; PARSplacebo: −5.6 ± 6.3), although the number of patients who responded to treatment as measured by CGI-I score was statistically significantly higher in youth who received guanfacine relative to those who received placebo.
2.13.3. Lavender oil
Long used in aromatherapy, silexan, an essential oil derived from lavender has been evaluated in adults with GAD. In one trial comparing the benzodiazepine lorazepam to silexan, comparable improvements were observed between lorazepam and silexan [118]. Additionally, in a randomized, double-blind, double-dummy trial (N=539) that was referenced to paroxetine, silexan (160 or 80 mg/day) was superior to placebo in reducing symptoms of anxiety (HAM-A) total score, p<0.01); paroxetine trended towards significance (p=0.10). Silexan was well-tolerated and was associated with fewer adverse events compared to paroxetine.[119] Additionally, in a met-analysis of silexan in patients with sub-threshold anxiety (K=3), a significant effect was observed “for observer- and self-rated anxiety” in addition to sleep and quality of life and this compound was well-tolerated [120]. To date, there are no reports of silexan in pediatric patients with GAD, although there may be specific tolerability concerns in this population, particularly in boys. In this regard, lavender oils have been associated with sex-steroid signaling disruption that may result in unopposed estrogen action on breast tissue producing gynecomastia in pre-pubertal youth [121].
2.13.4. Kava
Kava (Piper methysticum), has been used for centuries and over the last several decades was evaluated as a treatment for anxiety and in adults with GAD. Among trials in adults with GAD, several [122], but not all [123], suggest efficacy. Kava was banned in the UK and in several European studies in the early 2000s secondary to approximately 100 reports of hepatotoxicity; however, it has recently ben re-introduced. Interestingly, some have suggested that as the compound became increasingly used in the late 1990s and early 2000s, kava “prices were high and kava was in short supply. Thus kava cultivars (over 80 different cultivars exist) and parts (e.g., peelings of the stump instead of the rhizome), were used” and these may have been associated with hepatotoxicity [124]. As such, routine transaminase monitoring appears to be indicated should clinicians choose to recommend treatment with kava. To date, there are no trials of kava in pediatric patients.
3. Conclusions
The extant literature suggests that multiple classes of medications are available to clinicians treating GAD in children, adolescents, and adults. Collectively, these medications comprise a pharmacologic armamentarium rather than an interchangeable list, although guidelines and evidence suggest that—across age groups—SSRIs should be considered the first line interventions, followed by SNRIs [17,18]. Additionally, guidelines for the treatment of GAD in adults, including both the Canadian and US guidelines [17,18] as well the International Psychopharmacology Algorithm Group recommendations (www.ipap.org) are generally convergent with regard to second line pharmacotherapies [125]. However, the International Psychopharmacology Algorithm Group recommendations for GAD also note the importance of co-morbidity in guiding treatment selection (e.g., adjunctive mood stabilizer in a patient with bipolar disorder + GAD, non-benzodiazepine GABAergic hypnotic or benzodiazepine or trazadone in a patient with GAD and prominent insomnia) [125]. Ultimately, the choice of medication for the treatment of GAD will be based on numerous individual patient considerations, including target symptoms, patient age, co-morbidities, genotypes for CYP450 enzymes, and other genetic polymorphisms of clinical relevance. As such, this review of the available pharmacotherapy for GAD underscores the tenet that psychopharmacologic treatment with all its intricacies is still just emerging. Finally, regardless of the treatment—whether psychopharmacologic, psychotherapeutic or a combination—early treatment response and remission should be the most important goal. In this regard, large prospective studies of patients with GAD consistently find early treatment response is a significant predictor of long-term remission and overall functional improvement [126].
4. Expert opinion
4.1. Synthesis of data
SSRIs are considered to be the first line psychopharmacological interventions in adults with GAD [17,18], although there is considerable evidence supporting the efficacy and tolerability of SNRIs in these patients as well. The classification of these two medications as first-line interventions is primarily related to the substantial body of evidence supporting their efficacy and, importantly, their tolerability and side effect profile and is consistent with international guidelines [17,18]. Second line interventions in adults with GAD are also supported by numerous randomized controlled trials; however, these medications, which include benzodiazepines, buspirone, SGAs (primarily quetiapine), and some antiepileptic medications (e.g., pregabalin) may be associated with less favorable side effect profiles relative to antidepressant medications. Third-line interventions include tricyclic antidepressants, which are associated with substantial efficacy but require more monitoring given their clinically-significant, class-related tolerability concerns, which tends to limit their use by non-psychiatrists.
In pediatric patients, SSRIs are considered to be the first line psychopharmacological intervention, and SNRIs may be considered second line. This distinction between SSRIs and SNRIs arise with regard to first and second line treatment stems not from differences in tolerability, but rather from accumulating data suggesting that SSRIs may be associated with greater magnitude of treatment response and a more rapid treatment response compared to SNRIs [127]. In pediatric patients with GAD and related anxiety disorders, SSRIs and SNRIs differ in their response trajectories and magnitude. For SNRIs, the response rate at week 8 was reported to be only 40% of that typically observed for SSRI treatment, and this difference in trajectory was apparent by the second week of treatment. This is of interest in light of three recent meta-analyses of SNRIs and SSRIs in pediatric anxiety disorders [127–129]. These meta-analyses suggest an advantage to SSRIs, compared to SNRIs, and one meta-analysis specifically suggests a relationship between serotonergic selectivity and the weighted effect size of the antidepressant at endpoint [129]. In youth, SNRIs may be associated with class-specific tolerability concerns and, in fact, venlafaxine was associated with increased treatment-emergent suicidality in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) study [130] and in a recent meta-analysis of antidepressants in pediatric patients with MDD [131]. Additionally, the 5-HT system matures earlier than the noradrenergic system; this developmental lag may underlie differences in the effectiveness of antidepressants that mechanistically target both norepinephrine and 5-HT reuptake (e.g., SNRIs and tricyclic antidepressants) vs. those that more selectively target 5-HT reuptake (i.e., SSRIs) between youth and adults [132]. Lastly, GAD pathophysiology may involve more serotonergic dysfunction relative to noradrenergic dysfunction [133] which could relate to the greater effectiveness of SSRIs relative to noradrenergic agents.
Compared to the literature for the treatment of GAD in adults, there is considerably less evidence for “next step” interventions for youth. Additionally, many of the trials responsible for generating evidence for these “next step” interventions are complicated by methodologic and sample size concerns. Nonetheless, there remains some data to suggest that, in some patients, adjunctive benzodiazepines or buspirone may have a role to play during the course of treatment. Finally, the emergence of agomelatine as a potential therapeutic agent with a low side effect profile in GAD in adults merits its potential investigation in youth with GAD, especially in the adolescent population where co-morbid circadian rhythm disorders prevail.
4.2. What research or knowledge is required to achieve goals and biggest challenges?
The last four decades have seen a number of studies establishing the efficacy for numerous psychopharmacologic interventions in both pediatric and adult patients with GAD. However, relatively few studies have examined the optimal treatment duration, strategies for discontinuation of medication, and adjunct of interventions for inpatients with GAD. We also desperately need (1) more treatment studies; (2) studies that evaluate psychotherapeutic interventions in parallel with psychopharmacologic treatments; (3) head-to-head trials of pharmacologic treatments; and (4) studies that identify predictors of differential treatment response to guide patient-specific treatment selection. An unfortunate inconvenience of many of our treatments is the requirement that patients wait weeks in order to determine whether or not a particular treatment is going to work for them. This underscores the need to identify predictors of treatment response. Similarly, we need to understand how treatment might be tailored based on a given patient, how the disorder manifests in them, and other factors that affect symptom severity or course of illness. For example, might medications be titrated more slowly in some patients versus others, and in what patients should pharmacotherapy occur in tandem with, or precede, or follow psychotherapy? Unfortunately, despite being nearly two decades into this millennium, the process of selecting an antidepressant remains largely a trial and error process. For this reason, a better understanding of the clinical as well as demographic predictors of treatment response, as well as potential pharmacogenetic or other biological predictors of treatment response, are desperately needed.
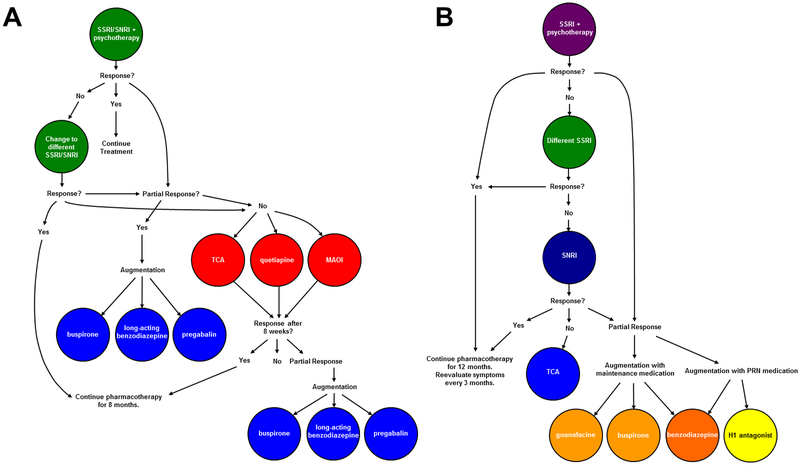
Figure 2: Psychopharmacologic Treatment algorithm for moderate to severe generalized, anxiety disorder in adults (A) and in children and adolescents (B).
Cooler colors represent higher levels of evidence and reflect greater evidence of tolerability and safety. Trials of SSRI and SNRIs should be ≥8 weeks, although if there is no response by week 8, there is a low likelihood of additional improvement. In the pediatric population, clinicians should note that for buspirone, guanfacine, antihistamines, and maintenance administration of benzodiazepines, the evidence base is very weak or conflicting. SSRI, selective serotonin reuptake inhibitor; SNRI, selective serotonin norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
Article Highlights Box.
In adults with GAD, SSRIs and SNRIs represent the first-line psychopharmacologic treatment.
Second-line pharmacotherapies in adults with GAD include buspirone, benzodiazepins, SGAs and pregabalin.
The selection of second-line psychopharmacologic treatment in adults with GAD should be driven by specific characteristics of the medication being considered (e.g., abuse liability, side effect profile) as well as patient characteristics.
In adults with GAD, the optimal, minimal duration of treatment to reduce the risk of relapse is 12 months.
In pediatric patients with GAD, SSRIs should be considered the first line pharmacotherapy.
In pediatric patients with GAD, psychotherapy augments antidepressant response.
Acknowledgments
Funding:
This manuscript is funded by the National Institute of Mental Health.
Footnotes
Declaration of Interest:
JR Strawn has received research support from the National Institutes of Health as well as Edgemont, Eli Lilly and Company, Forest Laboratories, Shire, Lundbeck and Neuronetics. He has also received material support and consulted for Genesight/Assurex Health as well as having received royalties from publications from Springer. Finally, this author has served as an author for UpToDate and is an Associate Editor for Current Psychiatry. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Contributor Information
Jeffrey R. Strawn, University of Cincinnati College of Medicine, Cincinnati, Ohio 45267
Laura Geracioti, University of Cincinnati College of Medicine, Cincinnati, Ohio 45267.
Neil Rajdev, University of Cincinnati College of Medicine, Cincinnati, Ohio 45267.
Kelly Clemenza, Columbia University, New York City, New York.
Amir Levine, Columbia University, New York City, New York.
REFERENCES
- [1].Ramsawh HJ, Chavira DA, Stein MB. Burden of anxiety disorders in pediatric medical settings: prevalence, phenomenology, and a research agenda. Arch. Pediatr. Adolesc. Med 2010;164:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baxter AJ, Vos T, Scott KM, et al. The global burden of anxiety disorders in 2010. Psychol. Med 2014;44:2363–2374. [DOI] [PubMed] [Google Scholar]
- [3].Beesdo-Baum K, Knappe S. Developmental Epidemiology of Anxiety Disorders. Child Adolesc. Psychiatr. Clin. N. Am 2012;21: 457–478. [DOI] [PubMed] [Google Scholar]
- *[4].Beesdo K, Pine DS, Lieb R, et al. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch. Gen. Psychiatry 2010;67:47–57. [DOI] [PubMed] [Google Scholar]; • This study describes the course of generalized anxiety disorder and its categorization.
- [5].Nutt DJ, Wittchen HU, Ballenger JC, et al. Generalized anxiety disorder: Nature and course. J. Clin. Psychiatry 2001:15–21. [PubMed] [Google Scholar]
- [6].Wittchen HU. Generalized anxiety disorder: Prevalence, burden, and cost to society. Depress. Anxiety 2002;16:162–171. [DOI] [PubMed] [Google Scholar]
- [7].Hoffman DL, Dukes EM, Wittchen H-U. Human and economic burden of Generalized Anxiety Disorder. Depress. Anxiety 2008;25:72–90. [DOI] [PubMed] [Google Scholar]
- [8].Wittchen HU, Kessler RC, Pfister H, et al. Why do people with anxiety disorders become depressed? A prospective-longitudinal community study. Acta Psychiatr. Scand. Suppl 2000;14–23. [PubMed] [Google Scholar]
- **[9].Pine DS, Cohen P, Gurley D, et al. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch. Gen. Psychiatry 1998;55:56–64. [DOI] [PubMed] [Google Scholar]; • This study links the risk of depressive disorders with the presence of anxiety disorders earlier in life.
- [10].Husky MM, Olfson M, He J, et al. Twelve-month suicidal symptoms and use of services among adolescents: results from the National Comorbidity Survey. Psychiatr. Serv. [Internet]. 2012;63:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nepon J, Belik SL, Bolton J, & Sareen J The Relationship Between Anxiety Disorders and Suicide Attempts: Findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Depress. Anxiety 2010;27:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[12].Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N. Engl. J. Med 2008;359:2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study, the Child/Adolescent Anxiety Multimodal Study is the largest evaluation of pharmacotherapy, combined pharmacotherapy and cognitive behavioral therapy in youth.
- [13].Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc.Psychiatr.Clin. N Am 2012;21:527–539. [DOI] [PubMed] [Google Scholar]
- [14].Beidel DC, Turner SM, Sallee FR, et al. SET-C versus fluoxetine in the treatment of childhood social phobia. J. Am. Acad. Child Adolesc. Psychiatry 2007;46:1622–1632. [DOI] [PubMed] [Google Scholar]
- [15].Wetherell JL, Petkus AJ, White KS, et al. Antidepressant medication augmented with cognitive-behavioral therapy for generalized anxiety disorder in older adults. Am. J. Psychiatry 2013;170:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hidalgo RB, Tupler LA, Davidson JRT. An effect-size analysis of pharmacologic treatments for generalized anxiety disorder. J. Psychopharmacol 2007;21:864–872. [DOI] [PubMed] [Google Scholar]
- [17].Katzman MA, Bleau P, Blier P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. J. Psychopharmacol 2014;28:403–439. [DOI] [PubMed] [Google Scholar]
- [19].Altamura AC, Moro AR, Percudani M. Clinical Pharmacokinetics of Fluoxetine. Clin. Pharmacokinet 1994;201–214. [DOI] [PubMed] [Google Scholar]
- [20].Leonard BE. Pharmacological Differences of Serotonin Reuptake Inhibitors and Possible Clinical Relevance. Drugs. 1992;43:3–10. [DOI] [PubMed] [Google Scholar]
- [21].Birmaher B, Axelson DA, Monk K, et al. Fluoxetine for the treatment of childhood anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry 2003;42:415–423. [DOI] [PubMed] [Google Scholar]
- [22].Warrington SJ. Clinical implications of the pharmacology of sertraline. Int. Clin. Psychopharmacol 1991;6 Suppl 2:11–21. [DOI] [PubMed] [Google Scholar]
- [23].Rudberg I, Hermann M, Refsum H, et al. Serum concentrations of sertraline and N-desmethyl sertraline in relation to CYP2C19 genotype in psychiatric patients. Eur. J. Clin. Pharmacol 2008;64:1181–1188. [DOI] [PubMed] [Google Scholar]
- [24].Wang JH, Liu ZQ, Wang W, et al. Pharmacokinetics of sertraline in relation to genetic polymorphism of CYP2C19. Clin. Pharmacol. Ther 2001;70:42–47. [DOI] [PubMed] [Google Scholar]
- [25].Findling RL, McNamara NK, Stansbrey RJ, et al. The relevance of pharmacokinetic studies in designing efficacy trials in juvenile major depression. J. Child Adolesc. Psychopharmacol 2006;16:131–145. [DOI] [PubMed] [Google Scholar]
- [26].Rynn MA, Siqueland L, Rickels K. Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am. J. Psychiatry 2001;158:2008–2014. [DOI] [PubMed] [Google Scholar]
- [27].Rynn MA, Walkup JT, Compton SN, et al. Child/Adolescent Anxiety Multimodal Study: Evaluating Safety. J. Am. Acad. Child Adolesc. Psychiatry 2015;54:180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pollack MH, Zaninelli R, Goddard A, et al. Paroxetine in the treatment of generalized anxiety disorder: results of a placebo-controlled, flexible-dosage trial. J. Clin. Psychiatry 2001;62:350–357. [DOI] [PubMed] [Google Scholar]
- [29].Rickels K, Zaninelli R, McCafferty J, et al. Paroxetine treatment of generalized anxiety disorder: A double-blind, placebo-controlled study. Am. J. Psychiatry 2003;160:749–756. [DOI] [PubMed] [Google Scholar]
- [30].Bielski RJ, Bose A, Chang C-C. A double-blind comparison of escitalopram and paroxetine in the long-term treatment of generalized anxiety disorder. Ann. Clin. Psychiatry 2005;17:65–69. [DOI] [PubMed] [Google Scholar]
- [31].Roseboom PH, Kalin NH. Citalopram and S-citalopram Am. Psychiatric Publishing. Textbook of Psychopharmacol. (4th ed.). 2013. [Google Scholar]
- [32].Beach SR, Kostis WJ, Celano CM, et al. Meta-analysis of selective serotonin reuptake inhibitor-associated QTc prolongation. J. Clin. Psychiatry 2014;75. [DOI] [PubMed] [Google Scholar]
- [33].Baumann P. Clinical pharmacokinetics of citalopram and other selective serotonergic reuptake inhibitors (SSRI). Int. Clin. Psychopharmacol 1992;6 Suppl 5:13–20. [PubMed] [Google Scholar]
- [34].Goodman WK, Bose A, Wang Q. Treatment of generalized anxiety disorder with escitalopram: Pooled results from double-blind, placebo-controlled trials. J. Affect. Disord 2005;87:161–167. [DOI] [PubMed] [Google Scholar]
- [35].Allgulander C, Florea I, AKT H. Prevention of Relapse in Generalized Anxiety Disorder by Escitalopram Treatment. Int. J. Neuropsychopharmacol 2006;9:495–505. [DOI] [PubMed] [Google Scholar]
- [36].Sheehan DV. Attaining remission in generalized anxiety disorder: Venlafaxine extended release comparative data. J. Clin. Psychiatry 2001;62:26–31. [PubMed] [Google Scholar]
- [37].Rickels K, Pollack MH, Sheehan DV., et al. Efficacy of extended-release Venlafaxine in nondepressed outpatients with generalized anxiety disorder. Am. J. Psychiatry 2000;157:968–974. [DOI] [PubMed] [Google Scholar]
- [38].Gelenberg AJ, Lydiard RB, Rudolph RL, et al. Efficacy of Venlafaxine Extended-Release Capsules in Nondepressed Outpatients With Generalized Anxiety Disorder. JAMA [Internet]. 2000;283:3082. [DOI] [PubMed] [Google Scholar]
- **[39].Rickels K, Etemad B, Khalid-Khan S, et al. Time to relapse after 6 and 12 months’ treatment of generalized anxiety disorder with venlafaxine extended release. Arch. Gen. Psychiatry 2010;67:1274–1281. [DOI] [PubMed] [Google Scholar]; • This study is the only trial that has evaluated the optimal treatment duration for pharmacotherapy in adults with GAD.
- [40].Rynn MA, Riddle MA, Yeung PP, et al. Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: two placebo-controlled trials. Am. J. Psychiatry 2007;164:290–300. [DOI] [PubMed] [Google Scholar]
- [41].Zhang Y, Huang G, Yang S, et al. Duloxetine in treating generalized anxiety disorder in adults: A meta-analysis of published randomized, double-blind, placebo-controlled trials. Asia. Pac. Psychiatry 2016;8:215–225. [DOI] [PubMed] [Google Scholar]
- [42].Davidson JR, Wittchen HU, Llorca PM, et al. Duloxetine treatment for relapse prevention in adults with generalized anxiety disorder: a double-blind placebo-controlled trial. Eur Neuropsychopharmacol . 2008;18:673–681. [DOI] [PubMed] [Google Scholar]
- [43].Alaka KJ, Noble W, Montejo A, et al. Efficacy and safety of duloxetine in the treatment of older adult patients with generalized anxiety disorder: a randomized, double-blind, placebo-controlled trial. Int. J. Geriatr. Psychiatry 2014;29:978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:283–293. [DOI] [PubMed] [Google Scholar]
- [45].The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J. Am. Acad. Child Adolesc. Psychiatry 2002;41:1061–1069. [DOI] [PubMed] [Google Scholar]
- [46].Gommoll C, Durgam S, Mathews M, et al. A double-blind, randomized, placebo-controlled, fixed-dose phase iii study of vilazodone in patients with generalized anxiety disorder. Depress. Anxiety 2015;32:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Durgam S, Gommoll C, Forero G, et al. Efficacy and safety of vilazodone in patients with generalized anxiety disorder: A randomized, double-blind, placebo-controlled, flexible-dose trial. J. Clin. Psychiatry 2016;77:1687–1694. [DOI] [PubMed] [Google Scholar]
- [48].Sato H, Ito C, Tashiro M, et al. Histamine H1 receptor occupancy by the new-generation antidepressants fluvoxamine and mirtazapine: A positron emission tomography study in healthy volunteers. Psychopharmacology (Berl). 2013;230:227–234. [DOI] [PubMed] [Google Scholar]
- [49].Goodnick PJ, Puig A, DeVane CL, et al. Mirtazapine in major depression with comorbid generalized anxiety disorder. J. Clin. Psychiatry 1999;60:446–448. [DOI] [PubMed] [Google Scholar]
- [50].Gambi F, De Berardis D, Campanella D, et al. Mirtazapine treatment of generalized anxiety disorder: A fixed dose, open label study. J. Psychopharmacol 2005;19:483–487. [DOI] [PubMed] [Google Scholar]
- [51].Orsolini L, Tomasetti C, Valchera A, et al. New advances in the treatment of generalized anxiety disorder: The multimodal antidepressant vortioxetine. Expert Rev. Neurother 2016;16. [DOI] [PubMed] [Google Scholar]
- [52].Bidzan L, Mahableshwarkar AR, Jacobsen P, et al. Vortioxetine (Lu AA21004) in generalized anxiety disorder: Results of an 8-week, multinational, randomized, double-blind, placebo-controlled clinical trial. Eur. Neuropsychopharmacol 2012;22:847–857. [DOI] [PubMed] [Google Scholar]
- [53].Rothschild AJ, Mahableshwarkar AR, Jacobsen P, et al. Vortioxetine (Lu AA21004) 5mg in generalized anxiety disorder: Results of an 8-week randomized, double-blind, placebo-controlled clinical trial in the United States. Eur. Neuropsychopharmacol 2012;22:858–866. [DOI] [PubMed] [Google Scholar]
- [54].Christensen MC, Loft H, Florea I, et al. Efficacy of vortioxetine in working patients with generalized anxiety disorder. CNS Spectr. 2017;1–9. [DOI] [PubMed] [Google Scholar]
- [55].Mahableshwarkar AR, Jacobsen PL, Chen Y, et al. A randomised, double-blind, placebo-controlled, duloxetine-referenced study of the efficacy and tolerability of vortioxetine in the acute treatment of adults with generalised anxiety disorder. Int. J. Clin. Pract 2014;68:49–59. [DOI] [PubMed] [Google Scholar]
- [56].Pae C-U, Wang S-M, Han C, et al. Vortioxetine, a multimodal antidepressant for generalized anxiety disorder: A systematic review and meta-analysis. J. Psychiatr. Res 2015;64:88–98. [DOI] [PubMed] [Google Scholar]
- [57].Findling RL, Robb AS, DelBello M, et al. Pharmacokinetics and Safety of Vortioxetine in Pediatric Patients. J. Child Adolesc. Psychopharmacol 2017;cap.2016.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[58].Stein MB, Sareen J. Clinical Practice. Generalized Anxiety Disorder. N. Engl. J. Med 2015;373:2059–2068. [DOI] [PubMed] [Google Scholar]; • A recent review of the diagnosis and treatment of generalized anxiety disorder.
- [59].Reinhold JA, Rickels K. Pharmacological treatment for generalized anxiety disorder in adults: an update. Expert Opin. Pharmacother 2015;16:1669–1681. [DOI] [PubMed] [Google Scholar]
- [60].Gomez AF, Barthel AL, Hofmann SG. Comparing the efficacy of benzodiazepines and serotonergic anti-depressants for adults with generalized anxiety disorder: a meta-analytic review. Expert Opin. Pharmacother 2018;883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Goddard a W, Brouette T, Almai A, et al. Early coadministration of clonazepam with sertraline for panic disorder. Arch. Gen. Psychiatry 2001. [DOI] [PubMed] [Google Scholar]
- [62].Seedat S, Stein MB. Double-blind, placebo-controlled assessment of combined clonazepam with paroxetine compared with paroxetine monotherapy for generalized social anxiety disorder. J. Clin. Psychiatry 2004;65:244–248. [PubMed] [Google Scholar]
- [63].Simeon JG, Ferguson HB, Knott V, et al. Clinical, cognitive, and neurophysiological effects of alprazolam in children and adolescents with overanxious and avoidant disorders. J. Am. Acad. Child Adolesc. Psychiatry 1992;31:29–33. [DOI] [PubMed] [Google Scholar]
- [64].Graae F, Milner J, Rizzotto L, et al. Clonazepam in childhood anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry 1994;33:372–376. [DOI] [PubMed] [Google Scholar]
- [65].Pollack M, Kinrys G, Krystal A, et al. Eszopiclone coadministered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Arch. Gen. Psychiatry 2008;65:551–562. [DOI] [PubMed] [Google Scholar]
- [66].Nelson JC. Tricyclic and tetracyclic drugs Essentials Clin. Psychopharmacol (3rd ed). 2013. p. 5–28. [Google Scholar]
- [67].Feighner JP. Mechanism of action of antidepressant medications. J. Clin. Psychiatry 1999;4–13. [PubMed] [Google Scholar]
- [68].Bailey B, Buckley NA, Amre DK. A meta-analysis of prognostic indicators to predict seizures, arrhythmias or death after tricyclic antidepressant overdose. J. Toxicol. - Clin. Toxicol 2004. p. 877–888. [DOI] [PubMed] [Google Scholar]
- [69].Rickels K, Downing R, Schweizer E, et al. Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch. Gen. Psychiatry 1993;50:884–895. [DOI] [PubMed] [Google Scholar]
- [70].Hoehn-Saric R, McLeod DR, Zimmerli WD. Differential effects of alprazolam and imipramine in generalized anxiety disorder: Somatic versus psychic symptoms. J. Clin. Psychiatry 1988;49:293–301. [PubMed] [Google Scholar]
- [71].Rynn M, García-España F, Greenblatt DJ, et al. Imipramine and buspirone in patients with panic disorder who are discontinuing long-term benzodiazepine therapy. J. Clin. Psychopharmacol 2003;23:505–508. [DOI] [PubMed] [Google Scholar]
- [72].Youdim MBH, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci 2006;7:295–309. [DOI] [PubMed] [Google Scholar]
- [73].Thase ME. The role of monoamine oxidase inhibitors in depression treatment guidelines. J. Clin. Psychiatry 2012;73 Suppl 1:10–16. [DOI] [PubMed] [Google Scholar]
- [74].Chessick CA, Allen MH, Thase M, et al. Azapirones for generalized anxiety disorder. Cochrane database Syst. Rev 2006;CD006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in Children and Adolescents with Anxiety: A Review and Bayesian Analysis of Abandoned Randomized Controlled Trials. J. Child Adolesc. Psychopharmacol 2018;28(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Guaiana G, Barbui C, Cipriani A. Hydroxyzine for generalised anxiety disorder. Cochrane Database Syst. Rev 2010; [DOI] [PubMed] [Google Scholar]
- [77].Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–236. [DOI] [PubMed] [Google Scholar]
- [78].Strawn JR, Geracioti TD. The treatment of generalized anxiety disorder with pregabalin, an atypical anxiolytic. Neuropsychiatr. Dis. Treat 2007;3:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pande AC, Crockatt JG, Feltner DE, et al. Pregabalin in generalized anxiety disorder: A placebo-controlled trial. Am. J. Psychiatry 2003;160:533–540. [DOI] [PubMed] [Google Scholar]
- [80].Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol 1959;32:50–55. [DOI] [PubMed] [Google Scholar]
- [81].Hindmarch I, Trick L, Ridout F. A double-blind, placebo- and positive-internal-controlled (alprazolam) investigation of the cognitive and psychomotor profile of pregabalin in healthy volunteers. Psychopharmacology (Berl). 2005;183:133–143. [DOI] [PubMed] [Google Scholar]
- [82].Feltner DE, Crockatt JG, Dubovsky SJ, et al. A randomized, double-blind, placebo-controlled, fixed-dose, multicenter study of pregabalin in patients with generalized anxiety disorder. J. Clin. Psychopharmacol 2003;23:240–249. [DOI] [PubMed] [Google Scholar]
- [83].Rickels K, Pollack MH, Feltner DE, et al. Pregabalin for treatment of generalized anxiety disorder: a 4-week, multicenter, double-blind, placebo-controlled trial of pregabalin and alprazolam. Arch. Gen. Psychiatry 2005;62:1022–1030. [DOI] [PubMed] [Google Scholar]
- [84].Montgomery S, Chatamra K, Pauer L, et al. Efficacy and safety of pregabalin in elderly people with generalised anxiety disorder. Br. J. Psychiatry 2008;389–394. [DOI] [PubMed] [Google Scholar]
- [85].Olivares JM, Álvarez E, Carrasco JL, et al. Pregabalin for the treatment of patients with generalized anxiety disorder with inadequate treatment response to antidepressants and severe depressive symptoms. Int. Clin. Psychopharmacol 2015;30:265–271. [DOI] [PubMed] [Google Scholar]
- [86].Montgomery SA, Tobias K, Zornberg GL, et al. Efficacy and safety of pregabalin in the treatment of generalized anxiety disorder: A 6-week, multicenter, randomized, double-blind, placebo-controlled comparison of pregabalin and venlafaxine. J. Clin. Psychiatry 2006;67:771–782. [DOI] [PubMed] [Google Scholar]
- [87].Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur. Neuropsychopharmacol 2017;1185–1215. [DOI] [PubMed] [Google Scholar]
- [88].Boschen MJ. A meta-analysis of the efficacy of pregabalin in the treatment of generalized anxiety disorder. Can. J. Psychiatry 2011;56:558–566. [DOI] [PubMed] [Google Scholar]
- [89].Schwartz TL, Nihalani N. Tiagabine in anxiety disorders. Expert Opin. Pharmacother 2006;7:1977–1987. [DOI] [PubMed] [Google Scholar]
- [90].Rosenthal M Tiagabine for the treatment of generalized anxiety disorder: a randomized, open-label, clinical trial with paroxetine as a positive control. J. Clin. Psychiatry 2003;64:1245–1249. [DOI] [PubMed] [Google Scholar]
- [91].Schaller JL, Thomas J, Rawlings D. Low-dose tiagabine effectiveness in anxiety disorders. MedGenMed. 2004;6:8. [PMC free article] [PubMed] [Google Scholar]
- [92].Schwartz TL. The use of tiagabine augmentation for treatment-resistant anxiety disorders: a case series. Psychopharmacol. Bull 2002;36:53–57. [PubMed] [Google Scholar]
- [93].Pollack MH, Tiller J, Xie F, et al. Tiagabine in adult patients with generalized anxiety disorder: Results from 3 randomized, double-blind, placebo-controlled, parallel-group studies. J. Clin. Psychopharmacol 2008;28:308–316. [DOI] [PubMed] [Google Scholar]
- [94].Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. [DOI] [PubMed] [Google Scholar]
- [95].Kassahun K, Mattiuz E, Nyhart E, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab. Dispos 1997;25:81–93. [PubMed] [Google Scholar]
- [96].Ring BJ, Catlow J, Lindsay TJ, et al. Identification of the human cytochromes P450 responsible for the in vitro formation of the major oxidative metabolites of the antipsychotic agent olanzapine. J. Pharmacol. Exp. Ther 1996;276:658–666. [PubMed] [Google Scholar]
- [97].Pollack MH, Simon NM, Zalta AK, et al. Olanzapine augmentation of fluoxetine for refractory generalized anxiety disorder: A placebo controlled study. Biol. Psychiatry 2006;59:211–215. [DOI] [PubMed] [Google Scholar]
- [98].Guy W CGI Clinical Global Impressions. ECDEU Assess. Man 1976. p. 217–222. [Google Scholar]
- [99].Snydermae SH, Rynn MA, Rickels K. Open-label pilot study of ziprasidone for refractory generalized anxiety disorder [2]. J. Clin. Psychopharmacol 2005. p. 497–499. [DOI] [PubMed] [Google Scholar]
- [100].Goff DC. Risperidone and paliperidone American Psychiatric Publishing Textbook of. Psychopharmacol. (4th ed.). 2009;627–640. [Google Scholar]
- [101].Simon NM, Hoge EA, Fischmann D, et al. An open-label trial of risperidone augmentation for refractory anxiety disorders. J. Clin. Psychiatry 2006;67:381–385. [DOI] [PubMed] [Google Scholar]
- [102].Brawman-Mintzer O, Knapp RG, Nietert PJ. Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 2005;66:1321–1325. [DOI] [PubMed] [Google Scholar]
- [103].Lieberman JA. Aripiprazole. American Psychiatric Publishing Textbook of Psychopharmacol. 2004;487–494. [Google Scholar]
- [104].Menza MA, Dobkin RD, Marin H. An Open-Label Trial of Aripiprazole Augmentation for Treatment-Resistant Generalized Anxiety Disorder. J. Clin. Psychopharmacol 2007; 207–210. [DOI] [PubMed] [Google Scholar]
- [105].Hoge EA, Worthington JJ III, Kaufman RE, et al. Aripiprazole as augmentation treatment of refractory generalized anxiety disorder and panic disorder. CNS Spectr. 2008;13. [DOI] [PubMed] [Google Scholar]
- [106].Kapur S, Zipursky R, Jones C, et al. A positron emission tomography study of quetiapine in schizophrenia: A preliminary finding of an antipsychotic effect with only transiently high dopamine D2receptor occupancy. Arch. Gen. Psychiatry 2000;57:553–559. [DOI] [PubMed] [Google Scholar]
- [107].Kreys T-JM, Phan SV. A literature review of quetiapine for generalized anxiety disorder. Pharmacotherapy. 2015;35:175–188. [DOI] [PubMed] [Google Scholar]
- [108].Mezhebovsky I, Mägi K, She F, et al. Double-blind, randomized study of extended release quetiapine fumarate (quetiapine XR) monotherapy in older patients with generalized anxiety disorder. Int. J. Geriatr. Psychiatry 2013;28:615–625. [DOI] [PubMed] [Google Scholar]
- [109].Merideth C, Cutler AJ, She F, et al. Efficacy and tolerability of extended release quetiapine fumarate monotherapy in the acute treatment of generalized anxiety disorder: a randomized, placebo controlled and active-controlled study. Int. Clin. Psychopharmacol 2012;27:40–54. [DOI] [PubMed] [Google Scholar]
- [110].Liebowitz M, Lam RW, Lepola U, et al. Efficacy and tolerability of extended release quetiapine fumarate monotherapy as maintenance treatment of major depressive disorder: A randomized, placebo-controlled trial. Depress. Anxiety 2010;27:964–976. [DOI] [PubMed] [Google Scholar]
- [111].Dubovsky SL, Warren C. Agomelatine, a melatonin agonist with antidepressant properties. Expert Opin. Investig. Drugs 2009;18:1533–1540. [DOI] [PubMed] [Google Scholar]
- [112].Stein DJ, Ahokas AA, De Bodinat C. Efficacy of agomelatine in generalized anxiety disorder: A randomized, double-blind, placebo-controlled study. J. Clin. Psychopharmacol 2008;28:561–566. [DOI] [PubMed] [Google Scholar]
- [113].Stein DJ, Ahokas A, Márquez MS, et al. Agomelatine in generalized anxiety disorder: An active comparator and placebo-controlled study. J. Clin. Psychiatry 2014;75:362–368. [DOI] [PubMed] [Google Scholar]
- [114].Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: A 12-week, double-blind, placebo-controlled study. Eur. Neuropsychopharmacol 2017;27:526–537. [DOI] [PubMed] [Google Scholar]
- [115].Stein DJ, Ahokas A, Albarran C, et al. Agomelatine prevents relapse in generalized anxiety disorder: A 6-month randomized, double-blind, placebo-controlled discontinuation study. J. Clin. Psychiatry 2012;73:1002–1008. [DOI] [PubMed] [Google Scholar]
- [116].Strawn JR, Compton SN, Robertson B, et al. Extended Release Guanfacine in Pediatric Anxiety Disorders: A Pilot, Randomized, Placebo-Controlled Trial. J. Child Adolesc. Psychopharmacol 2017;27(1):29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Soeter M, Kindt M. An Abrupt Transformation of Phobic Behavior after a Post-Retrieval Amnesic Agent. Biol. Psychiatry 2015;78:880–886. [DOI] [PubMed] [Google Scholar]
- [118].Woelk H, Schläfke S. A multi-center, double-blind, randomised study of the Lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder. Phytomedicine. 2010;17:94–99. [DOI] [PubMed] [Google Scholar]
- [119].Kasper S, Gastpar M, Müller WE, et al. Lavender oil preparation Silexan is effective in generalized anxiety disorder - A randomized, double-blind comparison to placebo and paroxetine. Int. J. Neuropsychopharmacol 2014;17:859–869. [DOI] [PubMed] [Google Scholar]
- [120].Möller HJ, Volz HP, Dienel A, et al. Efficacy of Silexan in subthreshold anxiety: meta-analysis of randomised, placebo-controlled trials. Eur. Arch. Psychiatry Clin. Neurosci 2017;1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Henley DV, Lipson N, Korach KS, et al. Prepubertal Gynecomastia Linked to Lavender and Tea Tree Oils. N. Engl. J. Med 2007;356:479–485. [DOI] [PubMed] [Google Scholar]
- *[122].Pittler MH, Ernst E. Kava extract versus placebo for treating anxiety. Cochrane Database Syst. Rev 2005;1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This Cochrane Review describes the evidence for kava in adults with anxiety disorders, including generalized anxiety disorder.
- [123].Connor KM, Davidson JRT. A placebo-controlled study of Kava kava in generalized anxiety disorder. Int. Clin. Psychopharmacol 2002;17:185–188. [DOI] [PubMed] [Google Scholar]
- [124].Ernst E. A re-evaluation of kava (Piper methysticum). Br. J. Clin. Pharmacol 2007;415–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Davidson J, Zhang W, Connor K, et al. Review: A psychopharmacological treatment algorithm for generalised anxiety disorder (GAD). J. Psychopharmacol 2010;24:3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bruce SE, Yonkers KA, Otto MW, et al. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: A 12-year prospective study. Am. J. Psychiatry 2005;162:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[127].Strawn JR, Mills JA, Sauley BA, et al. The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2018;57:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Meta-analysis of antidepressant trajectory of response in pediatric anxiety disorders revealing more rapid improvement and greater improvement for SSRIs compared to SNRIs.
- [128].Wang Z, SH W, Sim L, et al. Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: A systematic review and meta-analysis. JAMA Pediatr. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Strawn JR, Welge JA., Wehry AM, et al. Efficacy and Tolerability of Antidepressants in Pediatric Anxiety Disorders: a Systematic Review and Meta-Analysis. Depress. Anxiety 2015;32:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Brent DA, Emslie GJ, Clarke GN, et al. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am. J. Psychiatry 2009;166:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cipriani A, Zhou X, Giovane C Del, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;1263–1271. [DOI] [PubMed] [Google Scholar]
- [132].Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: Implications for differential drug effects on juveniles and adults. Biochem. Pharmacol 2007;73:1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety 2000. p. 2–19. [DOI] [PubMed] [Google Scholar]