Abstract
Major depressive disorder (MDD) is a common but serious neuropsychiatric affliction that comprises a diverse set of symptoms such as the inability to feel pleasure, lack of motivation, changes in appetite, and cognitive difficulties. Given the patient to patient symptomatic variability in MDD and differing severities of individual symptoms, it is likely that maladaptive changes in distinct brain areas may mediate discrete symptoms in MDD. The advent and recent surge of studies using viral-genetic approaches have allowed for circuit-specific dissection of networks underlying motivational behavior. In particular, areas such as the ventral tegmental area (VTA), nucleus accumbens (NAc), and ventral pallidum (VP) are thought to generally promote reward, with the medial prefrontal cortex (mPFC) providing top-down control of reward seeking. On the contrary, the lateral habenula (LHb) is considered to be the aversive center of the brain as it has been shown to encode negative valence. The behavioral symptoms of MDD may arise from a disruption in the reward circuitry, hyperactivity of aversive centers, or a combination of the two. Thus, gaining access to specific circuits within the brain and how separate motivational-relevant regions transmit and encode information between each other in the context of separate depression-related symptoms can provide critical knowledge towards symptom-specific treatment of MDD. Here, we review published literature emphasizing circuit- and cell type-specific dissection of depressive-like behaviors in animal models of depression with a particular focus on the chronic social defeat stress model of MDD.
1. Introduction
Major depressive disorder (MDD) affects about 10% of adults in the United States and twice as many suffer at least one depressive episode during their lifetime (Andrade et al., 2003; Ferrari et al., 2013). Despite this widespread prevalence and continued investment into identifying effective cures, long-term treatments are generally required for MDD and have variable effectiveness – some achieve complete remission while other patients exhibit little to no change in behavioral or cognitive symptoms (Berton and Nestler, 2006; Nestler, 1998). To date, it is unclear what molecular or genetic factors might confer positive or negative response to treatment.
Those experiencing MDD display a common but often variable set of symptoms including but not limited to anhedonia, psychomotor impairment, sleep impairment, loss of appetite, and retraction from social interaction (Nestler and Hyman, 2010; Russo and Charney, 2013). The DSM V criteria for MDD lists nine discrete symptoms including the aforementioned, with at least five required to be present daily for MDD diagnosis. This diversity in behavioral symptoms of depression suggests that different circuits, or discrete components of a single circuit, may underlie separate depressive-like phenotypes. This idea is a driving impetus for the many circuit-focused papers invading the field.
Some of the most common symptoms of MDD are characterized by decreases in appetite and motivation. As such, intense focus has been placed on understanding the aberrant signaling and changes of the reward and motivational circuitry in MDD. In particular, the mesolimbic dopaminergic circuitry comprising of the nucleus accumbens (NAc) and ventral tegmental area (VTA) have been extensively studied and will be emphasized in this review. In line with this reasoning, polymorphisms in dopaminergic receptors (in particular, D3 and D4) and catechol-O-methyltransferase, an enzyme critical in degrading dopamine (DA), have been identified in patients with MDD (Cravchik and Goldman, 2000; Dunlop and Nemeroff, 2007; Lopez Leon et al., 2005; Szegedi et al., 2005). Concentrations of DA metabolites in the cerebro-spinal fluid of MDD patients have also been measured to be lower than those of healthy individuals (Mendels et al., 1972; Roy et al., 1989). While dopamine and the reward circuitry have been identified as putative targets for MDD, the wide and varied behavioral symptoms patients present with in MDD still represents a significant barrier in both treatment and developing a unifying animal model that adequately captures the spectrum of phenotypes present in humans.
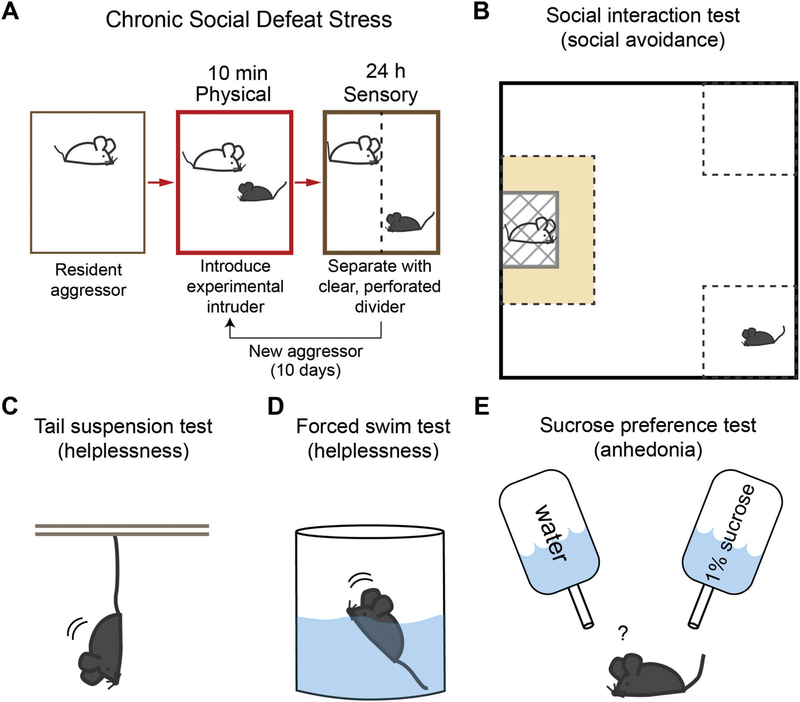
To date, the most common and reliable animal model in depression research is chronic social defeat stress (CSDS), although many other variations such as chronic variable stress (CVS) and chronic mild stress (CMS) are widely used (Golden et al., 2011). CSDS comprises of two distinct stages of defeat (Fig. 1). First, an experimental intruder animal is placed in the home cage of a resident, aggressive CD-1 male mouse and undergoes roughly 10 min of physical defeat. Immediately after physical defeat, the experimental animal is transferred to the opposite side of a clear, perforated divider placed in the resident cage. For 24 h, the intruder maintains sensory contact with the resident that had just defeated him. This process is repeated for 10 consecutive days. Animals experiencing social defeat exhibit several physiological changes including retarded growth, sensitivity to other environmental stressors, increased anxiety, and heightened levels of glucocorticoid activity (Meerlo et al., 1996; Tornatzky and Miczek, 1993). Repeated exposure to social defeat induces a number of depressive-like behaviors that parallel those seen in humans providing nice face validity. These include: helplessness/behavioral despair, as tested by reduced mobility in the forced swim test and tail suspension test; anhedonia, as tested by reduced preference for a sucrose solution in the sucrose preference test; and social dysfunction, as displayed by profound social avoidance in the social interaction test (Fig. 1) (Berton et al., 2006; Hollis et al., 2010). Most of these behavioral changes persist for at least 4 weeks and can be reversed by chronic, but not acute, antidepressant administration (Berton et al., 2006; Berton and Nestler, 2006; Rygula et al., 2006a, b; Tsankova et al., 2006), providing robust predictive validity as a model for depression in humans. Interestingly, a subset of animals subjected to CSDS appear unaffected by this protocol and do not display depressive-like behaviors. These animals are deemed to be resilient and are thought to represent an active coping mechanism in response to environmental stressors (Krishnan et al., 2007; Krishnan and Nestler, 2008, 2011). On the contrary, animals that undergo CSDS and show depression-related behaviors are called susceptible. Animals can be screened in an unbiased manner to differentiate resilience or susceptibility by a post-CSDS social interaction test (Krishnan et al., 2007). Since humans also display different levels of response and coping towards aversive external stimuli, comparing resilient and susceptible animals provides another level of investigation towards elucidating the circuits and molecular mechanisms underlying how heterogeneous responses to aversive events contribute to the development of depressive phenotypes. Due to its robust predictive and face validity, this review will focus heavily on studies utilizing the CSDS model of depression-related behaviors, but will also briefly touch on its shortcomings and other models.
Fig. 1.
Schematic diagram of chronic social defeat stress (CSDS) (A), and commonly used behavioral tests for different depressive behaviors, social interaction (B), helplessness (C, D), and anhedonia (E).
Overcoming the enormous complexity of brain area-, cell type-, projection-, and molecular-specific adaptations over the course of healthy individuals descending into depression represents a daunting undertaking. The advent of viral-genetic techniques such as viral-mediated circuit tracing and optogenetics in tandem with mouse lines driving Cre-recombinase in specific cell types have allowed for precise dissection and manipulation of neural circuits to begin to tackle this question in animal models of depression. In this review, we will summarize the existing literature of the motivational circuitries and their role in stress-induced depression with a particular emphasis on the CSDS model of depression and its effects on the NAc and VTA and their related circuitry, as well as the emerging role of the ventral pallidum (VP).
2. Circuit-specific roles of VTA dopaminergic neurons in depressive behaviors
Through multiple layers of results in past decades, the role of DA in reward processing and motivation-related behaviors has been well documented (Lammel et al., 2014). Since a hallmark of depression is loss of motivation and blunted ability to feel reward (anhedonia), it follows that the dopamine circuitry is likely involved in mediating depressive-like behaviors. Corroborating this theory, many studies have found that repeated aversive or traumatic experiences such as stress can induce several core symptoms of depression such as social dysfunction, anhedonia or helplessness that are driven by maladaptive function of mesolimbic DA signals (Berton et al., 2006; Chaudhury et al., 2013; Krishnan et al., 2007; Tye et al., 2013; Willner, 2005).
Previous studies indicate that phasic bursting and excitability of VTA DA neurons increases after social defeat stress in vivo and in ex vivo brain slice (Friedman et al., 2014; Razzoli et al., 2011). This increase is specific to animals susceptible to CSDS, as animals that underwent CSDS but did not display depressive-like phenotypes (resilient) did not have heightened VTA DA activity (Cao et al., 2010; Chaudhury et al., 2013). Enhanced VTA DA activity that was induced by CSDS was normalized by chronic antidepressant treatment, suggesting that antidepressants may exert their behavioral effects through regulation of VTA DA neuronal activity (Cao et al., 2010). Studies have also shown that VTA DA neurons from susceptible animals also display increased Ih current (Cao et al., 2010). Furthermore, local infusion of Ih channel inhibitors ZD7288 or DK-AH 269 into the VTA reversed CSDS-induced social withdrawal. Ih currents may also represent an important target by which selective serotonin reuptake inhibitors (SSRIs), commonly prescribed antidepressant medications in humans, exert their behavioral effects. Chronic SSRI treatment (fluoxetine) significantly reduced Ih current in susceptible animals which correlated with a reduction in their depressive-like behavioral symptoms (Cao et al., 2010). Paradoxically, resilient animals have an even larger Ih current which correlates with an increase in potassium channel currents (Friedman et al., 2014). Artificially increasing level of Ih current by local infusion of lamotrigine, an Ih channel potentiator that has previously been used as a mood stabilizer, promoted a resilient phenotype in animals subjected to CSDS. Taken together, we see that both reducing and enhancing Ih current levels after stress has antidepressive effects. Curiously, this may be due to a tightly regulated range of Ih current in VTA DA neurons which contributes to the development of depression-related behavior. Any deviation from this, whether it be an increase or decrease, promotes normal behavior. Understanding the precise contribution of abnormal electrophysiological profiles due to aberrant Ih channel activity, and how careful titering of Ih current can lead to separate behavioral outcomes, remain important future questions.
Changes in protein expression levels or secretion of neurotrophic factors have also been thought to contribute to the development of depression-related behaviors. In particular, brain derived neurotrophic factor (BDNF) has been proposed as a molecular signature of susceptibility to CSDS. Susceptible, but not resilient, animals show increased protein levels of BDNF specifically in the NAc (Krishnan et al., 2007). A naturally occurring single nucleotide polymorphism (SNP) in the BDNF gene in humans impairs BDNF secretion. Mice that have a similar polymorphism, and thus have an impaired ability to secrete BDNF, are less susceptible to CSDS than control animals (Krishnan et al., 2007). While increased BDNF secretion in the NAc, but not VTA, is characteristic of susceptible animals, enhanced VTA activity is an essential prerequisite for heightened BDNF NAc levels. Optogenetic activation of VTA DA neurons during CSDS was found to significantly increase levels of BDNF in the NAc as well as exacerbate behavioral deficits (Berton et al., 2006; Eisch et al., 2003; Wook Koo et al., 2016). Blockade of BDNF-TrkB signaling in the NAc reversed this effect (Wook Koo et al., 2016). However, optogenetically driving VTA DA activity by itself is not sufficient to induce susceptibility or increases in NAc BDNF levels. CSDS-induced susceptibility and secretion of corticotropin-releasing factor secretion (CRF), a hormone secreted when animals undergo stressful experiences, were required for elevated BDNF levels (Walsh et al., 2014). Infusion of a CRF antagonist prevented VTA DA-induced increases in BDNF and promoted resilience to CSDS in both mice and hamsters (Cooper and Huhman, 2007; Walsh et al., 2014). Thus, while elevated BDNF levels in the NAc correlate with increased VTA DA firing in susceptible animals, neither is sufficient by itself to induce susceptibility in stress naïve mice. External environmental stressors such as CSDS likely promote the release of stress hormones such as CRF which gate and promote the release of BDNF which ultimately induces susceptibility.
There is an increasing body of evidence propounding the idea that DA neurons within the VTA are anatomically distinct depending on their projection pattern and may have distinct functional roles (Lammel et al., 2008; Lammel et al., 2011). For example, cocaine administration induces alterations in AMPA/NMDA ratio in NAc-projecting VTA DA neurons, while cortex-projecting VTA DA neurons show no change. Conversely, cortex-projecting VTA DA neurons, but not NAc-projecting neurons, respond to aversive formalin injection into the paw (Lammel et al., 2011). Further, the authors showed that the medial and lateral shell of NAc exhibit differential responses to cocaine administration or aversive stimulation, further supporting the target specific roles of VTA DA neurons, even within subregions of the NAc (Lammel et al., 2011). As such, bulk manipulation of VTA DA neurons in animal models of depression may not be sufficient to fully capture the heterogeneity of these neurons.
Taking this heterogeneity into account, studies have used optogenetics to manipulate discrete components of the VTA DA circuitry in an attempt to parse out discrete contributions to depressive-like behaviors. Phasic channelrhodopsin (ChR2) mediated stimulation of NAc projecting VTA DA neurons (VTA-NAc) during a subthreshold social defeat paradigm that does not normally induce depressive-like behaviors is sufficient to generate social withdrawal and anhedonia (Chaudhury et al., 2013). Thus, aversive environmental stressors in parallel with increased VTA-NAc activity are sufficient to induce depression-related behaviors, but not VTA-NAc activity by itself. However, the same manipulation of VTA-medial prefrontal cortex (mPFC) projecting DA neurons had no effect. It was also found that optogenetic inhibition of VTA-NAc DA neurons acutely reverses CSDS-induced social withdrawal and anhedonia, while inhibition of VTA-mPFC DA neurons reduces social interaction (Chaudhury et al., 2013). In line with these results, another study found that optogenetic stimulation of the mPFC elicits antidepressant-like effects after CSDS (Covington III et al., 2010). These opposing effects of the VTA-NAc and VTA-mPFC circuits extend to the cellular level as well, as VTA-mPFC projecting DA neurons were measured to have reduced firing and no change in Ih current in susceptible animals (Chaudhury et al., 2013; Friedman et al., 2014). Conversely, VTA-NAc DA neurons from susceptible, but not resilient animals have significantly enhanced firing rates in slice and increased Ih current (Chaudhury et al., 2013; Razzoli et al., 2011). Collectively, these results suggest that VTA DA neurons have differential roles at the behavioral and cellular level in response to CSDS depending on their efferent target.
In addition to circuit-specific contributions to behavior, the type and duration of stress may also have profound effects on DA signaling in NAc. In mice exposed to chronic mild stress (CMS), a separate type of depressive-behavior induction protocol involving weeks of mild, unpredictable stressors, phasic VTA DA stimulation attenuated depression-related behavior in the tail-suspension test, forced swim test, and sucrose preference test, suggesting that increased VTA DA neuronal activity induces antidepressive effects (Tye et al., 2013). Silencing VTA DA neurons was also sufficient to drive depressive-like behaviors in the aforementioned assays (Tye et al., 2013). Moreover, separate studies in rats also exposed to CMS found a reduction in in vivo VTA DA neuronal firing rate (Chang and Grace, 2014; Moreines et al., 2017). Oppositely, in mice exposed to CSDS, phasic activation of VTA DA neurons induced persistent depressive-like symptoms, while inhibition attenuated these behaviors (Chaudhury et al., 2013). It is thus possible that the type and length of external stressors used as an induction protocol could have a profound effect on the physiological changes observed in the brain. Interestingly, this suggests that while the outward behavioral phenotypes may be similar, the underlying etiology precipitating these behaviors may be separate further emphasizing the complexity of studying conditions such as MDD.
In addition to differences in the stress protocol (CMS vs. CSDS), other factors may in part explain this discrepancy. Artificial stimulation of VTA DA neurons does not recapitulate the natural neural activity pattern in vivo. It is known that burst firing in vivo promotes increased DA release in target areas compared to slow frequency tonic firing (Grace et al., 2007). In line with this, Chaudhury et al. (2013) revealed that the pro-depressive behavioral effects caused by VTA DA optogenetic stimulation was dependent on the stimulation protocol. Driving phasic firing of 5 pulses at 20 Hz every 10 s for 10 min induced depression-related behavior, however a tonic stimulation protocol of continuous 0.5 Hz stimulation did not have any effect. While Tye et al. (2013) also used a phasic stimulation protocol, VTA DA neurons were activated slightly differently at 5 pulses at 30 Hz every 5 s for 3 min. Thus, the difference in stimulation pattern of VTA DA neurons could have a distinct impact on the behavioral effects seen.
Moreover, recent studies have suggested that separate depression-related behaviors could be mediated by distinct circuits (Knowland et al., 2017). As such, the behavioral assays selected to assess depressive-like behaviors could have profound effects on the behavioral outcomes due to optogenetic manipulation. Tye et al. (2013) predominantly focused on behavioral despair as assessed by the tail suspension test (TST), and anhedonia as assessed by the sucrose preference test (SPT). Chaudhury et al. (2013) used the social interaction test (SI) and the SPT. It remains possible that the disparate responses due to optogenetic stimulation could be due to using the TST or SI tests. The distinct activity pattern of VTA DA neurons might thus differentially mediate separate depressive-related behaviors. Moreover, since techniques like optogenetics provide precise temporal access to cell manipulation, whether ChR2-mediated stimulation or NpHR-mediated inhibition is given during the stress induction protocol (CMS or CSDS) or the depression-related behavioral assays (tail suspension test, social interaction test, etc.) could have differential functional consequences. While Tye et al. (2013) drove VTA DA activity for 30 min during the SPT, Chaudhury et al. (2013) optogenetically stimulated during subthreshold defeat, or during a 2.5 min window prior to the SPT. Collectively, these subtle differences may have a significant effect on a condition as complex to model in animals as MDD, and could account for some of the discrepancies reported in these studies.
Taken together, the VTA DA circuit contribution towards depressive-like behaviors can depend on its output targets or type of environmental stress. Some studies have shown that GABAergic neurons within the VTA can promote aversion, yet their contributions towards depression or CSDS-induced depression related phenotypes is still unclear (Tan et al., 2012; van Zessen et al., 2012). Additionally, recent studies have reported that transcriptional changes in the VTA, in particular, Otx2, can bidirectionally affect susceptibility or resilience to CSDS (Pena et al., 2017). Further studies requiring cell type- and projection-specific manipulation, recordings, and molecular analyses are necessary to comprehensively understand the VTA contribution towards depression.
3. Roles of different NAc cell types in CSDS-induced depressive behaviors
As previously discussed, DA signaling in the NAc emanating from the VTA have been implicated in depressive behaviors. Many studies have underscored the importance of the NAc in MDD. In humans, deep brain stimulation of the NAc has been shown to alleviate anhedonic symptoms of depression (Schlaepfer et al., 2008). However, the NAc is a heterogeneous structure comprising of different cell types, various dopamine receptors, and other neuromodulatory signaling (Francis and Lobo, 2017). These characteristics make it difficult to elucidate the precise role of the NAc circuitry in depressive behaviors.
D1-receptor expressing and D2-receptor expressing medium spiny neurons (D1-MSNs and D2-MSNs, respectively) comprise the predominant cell populations in the NAc. Emerging evidence shows that D1-MSNs and D2-MSNs in the NAc have distinct roles in stress-induced depressive behaviors. In mice susceptible to CSDS, D1-MSNs were measured to have increased intrinsic excitability and reduced mEPSC frequency, while D2-MSNs exhibit increased mEPSC frequency but no change in intrinsic excitability (Francis et al., 2015). Moreover, resilient animals displayed an upregulation of synaptic strength at large mushroom spines of D1-MSNs and a concomitant downregulation in D2-MSNs (Khibnik et al., 2016). In addition, excitatory inputs to D1-MSNs, not to D2-MSNs, showed long-term synaptic depression in mice with stress-induced anhedonia, a major symptom of depression. These alterations were mediated by alpha-melanocortin stimulating hormone (MSH) signaling (Lim et al., 2012).
Using optogenetics and chemogenetics to specifically manipulate D1- or D2-MSNs in vivo, Francis et al. (2015) demonstrated that driving an increase in D1-MSN activity rescues social interaction and sucrose preference deficits in susceptible animals, while inhibition of these neurons had the opposite effect. On the other hand, stimulation of D2-MSNs was sufficient to produce social avoidance, but no change in sucrose preference, following subthreshold social defeat stress (Francis et al., 2015). As evidenced by these results, it appears that distinct cell types in the NAc are differentially involved in stress-induced depressive behaviors. To this point, a recent report using in vivo fiber photometry to monitor neuronal dynamics in vivo revealed that heightened activity in D1-MSN activity during social interaction before CSDS was predictive of resilience, but not D2-MSN activity (Muir et al., 2018).
Based on the distinct roles of NAc MSNs in stress-induced depression, it is not surprising that different molecular mechanisms have been proposed to underlie separate cell-type neural adaptations in the NAc in response to stress. Indeed, Lobo et al. (2013) showed that ΔFosB expression is enhanced in D1-MSNs of resilient mice and enhanced in D2-MSNs of susceptible mice in NAc. This corroborates an earlier study reporting increased numbers of ΔFosB-positive neurons in the NAc and mPFC after stress (Nikulina et al., 2008). Furthermore, using engineered zinc-finger proteins to target the FosB gene, induction of histone acetylation in D2-MSNs, or histone methylation in D1-MSNs, promoted a susceptible phenotype in mice undergoing CSDS. Conversely, histone methylation of D2-MSNs or histone acetylation in D1-MSNs induced a pro-resilience (Hamilton et al., 2018). These results provide interesting insight into the role of cell-type specific epigenetic and histone modifications and their effect on the development of depressive-like phenotypes.
Moreover, increased protein levels of ΔFosB in the NAc, but not other isoforms, FosB and Δ2ΔFosB, negatively correlated with the level of defeat animals exhibited (Vialou et al., 2015). Paradoxically, chronic fluoxetine treatment, an FDA-approved selective serotonin reuptake inhibitor commonly prescribed as an antidepressant, reversed CSDS-induced behavioral deficits yet ΔFosB protein levels were found to be further increased from control and susceptible levels in the NAc (Vialou et al., 2015). Thus, levels of ΔFosB may be tightly regulated in which increased levels induce susceptibility, yet a further increase can promote resilience or reverse depressive behaviors in susceptible animals, similar to what has been seen with increasing Ih current in the VTA (Friedman et al., 2014). Furthermore, Slc6a15, a molecule linked to MDD susceptibility in humans, was specifically reduced in D2-MSNs after CSDS (Chandra et al., 2017). Artificially enhancing Slc6a15 in D2-MSNs promoted susceptibility to subthreshold defeat stress (Chandra et al., 2017).
While accumulating data highlights the differential roles of D1-MSNs and D2-MSNs, recent reports paradoxically show that the anatomical projections of these neurons are quite similar (Knowland et al., 2017; Kupchik et al., 2015). Thus, interpretation of these cell-type differences likely go beyond efferent circuitry disparities. Separate downstream synaptic adaptations from D1- and D2-MSNs to VP have begun to be reported (Creed et al., 2016), yet how NAc D1-MSNs or D2-MSNs separately modulate downstream circuitry in response to stress has not yet been explored and will be required for future studies.
In addition to the canonical D1- and D2-MSN cell-type dichotomy in the NAc, neurons can also be discriminated by their expression of dynorphin (it is of note that these neurons are not an independent sub-population, they co-express with predominantly D1-MSNs, but to a lesser degree D2-MSNs), the endogenous ligand of the kappa opioid receptor (KOR, Al-Hasani et al., 2015). Activation of KORs are well known to elicit aversive behaviors in humans and mice, and present a tractable pharmacologic target for MDD (Laman-Maharg et al., 2017; Mucha and Herz, 1985; Pfeiffer et al., 1986).In regards to depression-related behaviors, infusion of a KOR antagonist in both mice and rats reduced immobility time in the forced swim test which correlated with a selective increase in expression of the immediate early gene, cfos, in the NAc (Carr et al., 2010; Mague et al., 2003; McLaughlin et al., 2003). Similarly, KOR antagonist infusion into the NAc, but not hippocampus, attenuated depressive-like behaviors in mice exposed to the learned helplessness model of depression (Newton et al., 2002). KOR activation and inhibition has also been shown to modulate dopamine release in the NAc. Microdialysis and fast scan cyclic voltammetry studies in the NAc in response to KOR antagonist administration increased DA release in the NAc, while supra-threshold concentrations of KOR agonist treatment decreased phasic dopamine release concordant with a reduction in VTA DA activity (Di Chiara and Imperato, 1988; Ebner et al., 2010; Shippenberg and Rea, 1997). How then might this paradoxical situation arise, in which KOR agonists induce pro-depressive like behavioral effects, yet elicits a reduction in VTA DA activity and DA release in the NAc, contrary to previous reports on increased VTA DA activity projecting to NAc in models of depression (Cao et al., 2010; Chaudhury et al., 2013)? Curiously, optogenetic activation of dynorphin neurons induces either aversion and preference dependent on their anatomical localization within the NAc (Al-Hasani et al., 2015). Thus, future studies investigating whether subpopulations of VTA DA neurons projecting to distinct prodynorphin-rich subregions of the NAc may be differentially affected during depression-related behaviors are required. It has also been proposed that the timing of KOR antagonist administration, whether before, during, or after stressors are given to the animal, may be critical to specify its effects (Knoll and Carlezon, 2010).
Together, we see a convergence of putative cell-type specific factors capable of contributing to the expression or attenuation of depressive-like behaviors. Further identification of the interactions or overlap between these neurons using a Boolean viral intersectional strategy (Fenno et al., 2014) and the ideal temporal manipulation to target them for treatment remain important unanswered questions.
4. Roles of afferent connections to NAc in depressive behaviors
4.1. Medial prefrontal cortex (mPFC)
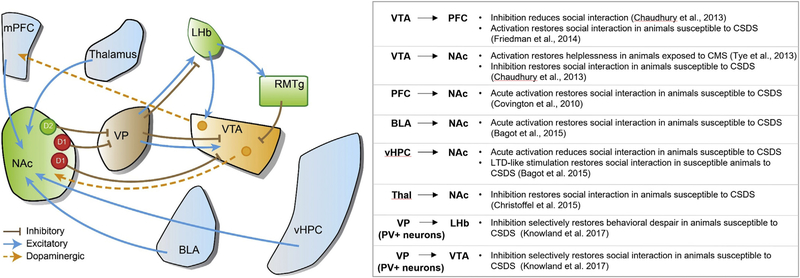
The nucleus accumbens is known to receive input from a broad array of brain areas. In addition to dopaminergic signaling from the VTA, afferents originating from the ventral hippocampus (vHPC), basolateral amygdala (BLA), and medial prefrontal cortex (mPFC) have received particular attention in both depression and addiction (Dumitriu et al., 2012; Khibnik et al., 2016; MacAskill et al., 2014; MacAskill et al., 2012). How these input-specific afferents generate diverse adaptations at D1- and D2-MSN synapses have been outlined in several motivational contexts. In humans, chronic deep brain stimulation (DBS) of the subgenual cingulate cortex (Cg25), the equivalent of the rodent vmPFC, restores and alleviates several symptoms of depression in treatment-resistant MDD patients (Dunlop and Mayberg, 2014; Mayberg et al., 2005). DBS in the vmPFC of rodents exposed to CSDS also reversed social withdrawal deficits as well as normalized aberrant structural and physiological changes in serotonergic dorsal raphe nuclei neurons (Veerakumar et al., 2014). Subsequent rodent studies have reinforced these results and shown that electrical and optogenetic stimulation of the mPFC in rats alleviated depressive-like symptoms (Hamani et al., 2010). Furthermore, precise optogenetic manipulation of mPFC neurons projecting to NAc promoted resiliency in animals exposed to CSDS (Covington III et al., 2010). Other studies using light delivery to manipulate precise circuits show that mPFC efferents to other brain areas including lateral habenula (LHb) and dorsal raphe nucleus (DRN) are also involved in several symptoms of depression (Warden et al., 2012). Interestingly, reduced dopaminergic activity in the mPFC correlates with reductions in social interaction in subthreshold defeat mice (Fig. 2) (Chaudhury et al., 2013).
Fig. 2.
Optogenetic manipulations of neural circuits in animal models of depression. Schematic diagram of the reward circuitry and its connections. Summary of accumulated optogenetic evidence of NAc afferent projections and dopaminergic outputs in animal models of depression.
The mPFC contribution to depression has also been thoroughly investigated at the epigenetic and molecular level. A comprehensive transcriptional profiling study identified Sdk1, among others, as a susceptible-specific transcriptional regulator. Sdk1 overexpression in the mPFC increased sociability in susceptible mice, while Sdk1 overexpression in the vHPC had the opposite effect (Bagot et al., 2016). This highlights the importance of brain region-specific manipulations and analyses, as the same protein may have drastically difference effects dependent on its regional localization. Epigenetic modifications in the mPFC have also been reported in depression. Inhibition of histone deacetylases in the mPFC induces anti-depressive-like effects after CSDS (Covington III et al., 2015). Furthermore, the histone demethylase, Phf8 is highly expressed in the mPFC. Knockout of Phf8 confers no developmental effects, however mice exhibit a significant increase in resilience to CSDS (Walsh et al., 2017). Specific deletion of Phf8 in mPFC induces a substantial upregulated of serotonin receptors (Htr1a, Htr1b, and Htr2a) in the area, suggesting that histone modifications are crucial regulators of depression. Interestingly, 5-Htr1b has been suggested to regulate the response to selective serotonin reuptake inhibitors and has been shown to be downregulated in the NAc in susceptible animals (Bagot et al., 2017). This downregulation was reversed after treatment with ketamine, a promising treatment for MDD (Bagot et al., 2017; Zanos et al., 2016). Further studies investigating subregional differences within the mPFC, and whether projection-specific epigenetic or transcriptional changes occur are required as technologies advance.
4.2. Ventral hippocampus (vHPC)
An abundance of evidence has also implicated the vHPC as another good candidate for differentially regulating NAc D1- and D2-MSNs. Interestingly, unlike mPFC-NAc afferents, attenuating synaptic strength of vHPC-NAc via an artificial long term depression induces resiliency to CSDS, while enhancement of these inputs induces a susceptible phenotype to stress (Fig. 2) (Bagot et al., 2015). Indeed, synaptic transmission from vHPC to NAc is increased in mice susceptible to CSDS whereas that from mPFC to NAc is decreased; supporting the notion of pathway specific neural adaptations in response to CSDS. Moreover, chronic optogenetic stimulation within the vHPC induced distinct changes in the expression of ΔFosB (Lobo et al., 2013). As previously mentioned, ΔFosB expression in the NAc D1-MSNs and D2-MSNs has separate roles in depressive behaviors, suggesting that these different cell types may receive different information from the vHPC in response to stress.
Studies have also reported transcriptional changes within the vHPC that underlie susceptibility to depression. Dkkl1, Neurod2, and Sdk1 were all identified as genes that were upregulated in the vHPC following CSDS. Overexpression of these molecules specifically in the vHPC all induced susceptibility (Bagot et al., 2016). Moreover, chronic imipramine treatment, a tricyclic antidepressant, induced a significant upregulation in CTLA4, a gene previously implicated in cohorts of Korean and Chinese MDD patients (Bagot et al., 2017; Jun et al., 2001; Liu et al., 2011). Circuit specific manipulations, in particular how NAc-projecting vHPC neurons differentially express these genes compared to other vHPC neurons, will be interesting to elucidate whether the efferent targets of neurons within a single brain region can affect gene expression in response to aversive environmental events.
4.3. Basolateral amygdala (BLA)
The BLA also sends strong projections to the NAc. While optogenetic stimulation of amygdala inputs to NAc in susceptible animals acutely reversed social withdrawal behavior, synaptic changes as tested by paired pulse ratio (PPR) were not as significant as those seen from vHPC and mPFC afferents (Fig. 2) (Bagot et al., 2015). Additionally, optogenetically inducing LTD at BLA-NAc synapses had no effect on behavior in contrast to vHPC afferents. Correspondingly, expression of the immediate early genes Arc and Egr1 showed no significant change in the BLA of depressed animals while a significant reduction in Arc was measured in susceptible, but not resilient animals in NAc-projecting mPFC neurons (Bagot et al., 2015). Other studies using extracellular in vivo recordings show that activity of the mPFC-to-BLA circuit is predictive of stress vulnerability (Kumar et al., 2014). Collectively, it appears that the NAc, mPFC, vHPC, and BLA circuits are all inextricably linked and it is likely that epigenetic, transcriptional, and cellular changes in one region induces adaptations in other regions that ultimately manifest as MDD. Studies analyzing how these separate regions integrate changes from other areas will be necessary in the future to comprehensively describe the etiology of depression.
5. Thalamic inputs
More recent NAc afferent studies have expanded focus to thalamic areas (Zhu et al., 2016). Christoffel et al. (2015) reported significantly increased excitatory synaptic strength of intralaminar thalamic (ILT) neurons projecting to the NAc in susceptible, but not resilient mice (Fig. 2). Artificial reduction of the ILT-NAc pathway induced a reversal in social withdrawal behavior, and caused structural changes in NAc MSNs as well as measured by spine density (Christoffel et al., 2015).
Together, the broad and varied input circuitry to the NAc beget diverse and separate roles for specific inputs. As evidenced here, different inputs to the same brain area may have opposing roles. Thus, it is increasingly imperative to consider specific afferents, and specific cell types within a single brain area to fully capture the heterogeneous roles in a condition as complex and nuanced as MDD.
6. Roles of LHb in depressive behaviors
The lateral habenula (LHb), a relatively small epithalamic structure, has received considerable preclinical and clinical attention in treating MDD in recent years. The LHb is broadly believed to signal aversion, with increases in activity resulting in behavioral avoidance in many animal studies (Proulx et al., 2014). The LHb also extensively connects with monoaminergic centers of the brain including the DRN containing serotonergic neurons and the previously mentioned VTA DA neurons, which have been heavily linked to development of MDD (Beier et al., 2015; Watabe-Uchida et al., 2012; Weissbourd et al., 2014).
These connections make the LHb anatomically poised to encode monoaminergic tone into motivational output. Indeed, previous reports have shown that inputs to the LHb from the entopeduncular nucleus (EP), the rodent correlate of the human globus pallidus interna, mediate aversion and negative affect associated with cocaine withdrawal (Meye et al., 2016; Shabel et al., 2014). While dopamine has no effect on EP-to-LHb synaptic transmission, serotonin suppressed both excitatory input to the LHb and the intrinsic excitability of LHb neurons (Shabel et al., 2012). Alteration of glutamate and GABA co-release at the EP-to-LHb synapse has been reported in the chronic learned helplessness (cLH) model of depression as well. In line with the hypothesis that increased activity in LHb encodes aversion, cLH animals exhibited reduced ratios of inhibitory/excitatory response at the EP-to-LHb synapse primarily driven by reduced GABAergic transmission. Furthermore, chronic citalopram (a commonly prescribed SSRI antidepressant) administration not only alleviated behavioral symptoms of cLH animals, but also increased GABA/AMPA ratios providing a possible mechanism for antidepressant action in the LHb (Shabel et al., 2014).
Other reports have corroborated these results and found that increased activity in VTA-projecting LHb neurons correlates with the severity of helplessness behavior exhibited by animals subjected to cLH (Li et al., 2011). This potentiation of excitatory activity in the LHb was reported to be due to an increase in presynaptic release probability. Furthermore, injection of the GABA agonist muscimol into LHb induced antidepressant-like effects in cLH rats (Winter et al., 2011). These effects do not appear limited to the cLH model of depressive-like symptoms, as chemogenetic inhibition of the LHb with DREADDs increased social approach behavior in animals that underwent CSDS (Sachs et al., 2015).
The plethora of preclinical work has underscored the LHb as a potential therapeutic target in humans. Promising preclinical work in rodents using DBS of the LHb has yielded significant reversal of depressive-like behaviors (Li et al., 2011). This has translated into promising nascent studies using LHb DBS in humans in treatment resistant depression. DBS resulted in complete remission of depressive-like symptoms in one patient, with relapses closely corresponding with malfunctions in the DBS pacer, and an ongoing second patient showing 50% improvement on a depressive symptom scale (Kiening and Sartorius, 2013; Sartorius et al., 2010). Collectively, the work generated from animal models and human clinical studies highlight the LHb as a critical mediator of depressive-like behaviors that merits further study.
7. Roles of distinct ventral pallidal circuits in CSDS-induced depressive behaviors
The ventral pallidum (VP) is known to receive dense inputs from both D1- and D2-MSNs in the NAc and transmits this information to downstream targets such as the LHb, VTA, and lateral hypothalamus (LH) (Kupchik et al., 2015; Root et al., 2015). Studies have also underscored the dopaminergic, glutamatergic, and GABAergic VTA neurons as major efferent targets of the VP (Beier et al., 2015; Faget et al., 2016). Thus, it follows that the VP is proposed to be an important convergent point at the interface of the motivational and reward circuitry implicated in drug addiction and depression. Its varied outputs and close connections with aversive centers such as the LHb, and reward-promoting centers such as the VTA, make it potentially well situated to encode diverse types of valence information (Root et al., 2015). Indeed, a recent study revealed differential cocaine-induced synaptic alterations in VTA-projecting VP neurons receiving input from either D1- or D2-MSNs (Creed et al., 2016). This synapse requires further investigation in the context of depression.
Additional studies have shown selective decreases in VP serotonin receptor binding in patients with MDD, and that the VP is critical for the antidepressant effects of ketamine (Murrough et al., 2011; Yamanaka et al., 2014). However, it remains largely unknown how the VP circuitry contributes to depression, and whether specific cell types within the VP are responsible for these effects, much like what has been described in the NAc (Chandra et al., 2017; Francis et al., 2015).
Recent studies have identified parvalbumin-positive (PV) neurons in the VP as key regulators of phenotypes induced by CSDS (Knowland et al., 2017). Chronic silencing of VP PV neuronal activity during the induction phase of CSDS promotes a pro-resilient phenotype in mice. VP PV neurons were found to send largely non-collateralized projections to the lateral habenula (LHb) or ventral tegmental area (VTA), but not both and exhibited different electrophysiological adaptations in response to CSDS. VP PV neurons that project to the VTA receive increased excitatory input in susceptible, but not animals resilient to CSDS (Fig. 2) (Knowland et al., 2017). Conversely, neurons projecting to the LHb from susceptible animals exhibited significant increases in intrinsic excitability, an effect not seen in resilient animals. Furthering their relevance towards depression, these cellular adaptations were normalized back to control levels when susceptible animals were given two weeks of chronic fluoxetine treatment (Knowland et al., 2017).
Interestingly, LHb-projecting VP PV neurons were found to be predominantly glutamatergic. Thus, increased activity in this population of neurons found in susceptible animals would ultimately drive increased LHb cellular activity, in line with what other studies have seen in animal models of depression (Proulx et al., 2014). On the other hand, the neurotransmitter identity of VTA-projecting VP PV neurons depended on their cellular target within the VTA. GABAergic VTA neurons received exclusively GABAergic input from VP PV neurons while DA VTA neurons received mixed excitatory and inhibitory input, with a slight bias towards glutamatergic innervation. Given that VTA-projecting VP PV neurons exhibited increased firing in animals susceptible to CSDS, it may be that increased GABAergic innervation onto local VTA GABAergic neurons results in net disinhibition of VTA DA neurons. Net disinhibition would increase VTA DA activity and be in line with previous studies describing hyperactivity of VTA DA neurons in the depressive state (Cao et al., 2010; Chaudhury et al., 2013). Furthermore, since glutamatergic VP neurons are biased towards VTA DA neurons, this may provide another, more direct mechanism by which susceptible animals exhibit enhanced VTA DA activity. Collectively, hyperactivity within the VP may lie upstream of aberrant VTA DA activity that has been highlighted as a hallmark of animals susceptible to CSDS, however more studies are required to support this hypothesis.
It was also found that selective chemogenetic or optogenetic silencing of LHb-projecting VP PV neurons attenuated behavioral despair in the tail suspension test, with no effect on VTA-projecting VP PV manipulation. Conversely, silencing of VTA-projecting VP PV neurons in susceptible animals reversed social withdrawal induced by CSDS (Knowland et al., 2017). Together, this suggests that separate circuits that originate from the same brain area and even same cell type can mediate discrete behavioral facets of depression and can exhibit separate cellular adaptations as well. Further studies are required to differentiate whether VP-to-VTA DA neurons or VP-to-VTA GABAergic neurons are selectively necessary or sufficient to engage a depressive circuit, or if coordinated activity between these two downstream areas is more important.
In contrast, another study in rats reported a reduction in VTA DA firing after CMS was given (Chang and Grace, 2014), paralleling other studies using the CMS model of stress-elicited depression-related behaviors (Tye et al., 2013). This CMS-induced reduction in VTA DA firing was dependent on VP activity, as blockade of glutamatergic inputs to the VP via local infusion of kynurenic acid reversed this effect (Chang and Grace, 2014). These inputs likely originated from the BLA, as attenuation of BLA activity recapitulated the effect on VTA DA neurons. While the direction of stress-induced changes in VTA DA activity remains to be parsed out on a model by model basis, these studies highlight the intimate relationship of the VP-VTA circuitry and its putative relevance towards MDD.
These studies begin to shed light on how the VP integrates with other major players in the depressive circuitry such as the NAc and VTA and how concerted activity between these areas may contribute to the diverse behavioral phenotypes that manifest in MDD. However there remains a dearth of studies examining the role of the VP in the context of depression. While a few human imaging studies also support this idea (Murrough et al., 2011; Yamanaka et al., 2014), further efforts to elucidate the underlying mechanisms must be undertaken to provide more useful insight for relevant and effective human clinical studies.
8. Monoamines
Alterations in monoaminergic tone have long been believed to be a critical etiology in MDD (Chaudhury et al., 2015; Heninger et al., 1996). Indeed, many available, approved therapeutic treatments such as serotonin and norepinephrine reuptake inhibitors (SSRIs) target these chemical pathways. DBS stimulation in the vmPFC in rodents reversed social deficits induced by CSDS while also reversing CSDS-induced serotonergic neuronal alterations (Veerakumar et al., 2014). Many animal studies have highlighted antidepressant serotonergic effects on the EP-LHb pathway (Li et al., 2011; Shabel et al., 2014; Shabel et al., 2012). Others have found significant effects of fluoxetine on neural activity in the VP which receives dense input from the major serotonergic nuclei in the brain, the dorsal raphe nucleus (DRN) (Knowland et al., 2017). Moreover, reports have delineated differential effects of fluoxetine, ketamine, and imipramine on gene expression in the mPFC, NAc, amygdala, and vHPC (Bagot et al., 2017; Vialou et al., 2015). Social defeat in rats resulted in a significant increase in cfos reactivity in serotonergic neurons in the DRN, suggesting that they play a critical role mediated social defeat-induced depressive-like behaviors (Paul et al., 2011). Tryptophan hydroxylase 2 knockin mice which display a 60–80% reduction in brain serotonin levels were more susceptible to CSDS and resistant to antidepressant treatment (Sachs et al., 2015).
Since aberrations in VTA DA neuronal activity have been heavily studied in MDD and the DRN is one of the largest input structures to these neurons, the DRNserotonin to VTAdopamine pathway remains an interesting, yet largely unexplored circuit. Norepinephrine (noradrenaline) may also contribute to the expression of depression-related behaviors, as chronic stimulation of norepinephrine terminals in the VTA originating from the locus coeruleus (LC) after CSDS promoted recovery of social withdrawal deficits. Similarly, chronic treatment of the α2noradrenergic receptor antagonist idazoxan which increases LC firing recapitulates the optogenetic antidepressant effects (Isingrini et al., 2016).
How interconnected or separate are monoaminergic pathways involved in the expression of depressive-like behaviors? Few studies have attempted to examine the concerted relative contributions of serotonin and dopamine towards discrete depressive-like behaviors. This is in spite of the fact that one of the main inputs to the VTA is the DRN (Beier et al., 2015; Faget et al., 2016; Watabe-Uchida et al., 2012) and alterations of this circuitry have been shown to mediate antidepressant efficacy (Adachi et al., 2017; Neumaier et al., 1996). Understanding how serotonergic inputs to the VTA can affect animals’ response to stress, especially in the context of depression, and how this input may modulate different downstream targets such as the LHb or dopaminergic signaling in the NAc or mPFC remain important questions to answer in the future.
Beyond monoamines, as previously described in detail in this review, other studies have honed in on aberrant dopamine signaling in the VTA-NAc and VTA-mPFC circuit or NAc dynorphinergic activity as a critical driver of depressive-like behaviors (Chaudhury et al., 2013; Knoll and Carlezon, 2010; Tye et al., 2013). Additional studies have highlighted the antidepressant effects of increased oxytocin in CSDS (Lukas et al., 2011), further revealing the complex etiology and path towards an effective treatment for MDD.
9. Modelling depression-related behaviors in females
MDD and related affective disorders are nearly twice as prevalent in females than males (Kessler et al., 1993, 2005). In spite of this significant disparity, male-centric preclinical animal studies of MDD predominate the literature. In particular, CSDS relies upon male on male intruder-resident aggressive behavior which is absent in female C57BL6 mice. Thus, significant efforts to develop and adopt new models of MDD, or adapt existing models, with sufficient predictive and face validity are currently underway.
California mice, as opposed to the widely used C57BL6 laboratory strain, are territorial creatures that will actively attack intruders encroaching on their home. This unbiased aggression allows for social defeat in both males and females. Curiously, CSDS in California mice induces social avoidance only in females (Trainor et al., 2011) and results in an increase in the number of tyrosine hydroxylase, a marker for putative dopaminergic neurons, positive neurons in the VTA (Greenberg et al., 2015). In line with studies in male C57BL6 mice, social defeat stress in both male and female California mice also elicited increases in dopamine in the NAc and that stress-induced social withdrawal ins dependent on D1 receptors in the NAc (Campi et al., 2014). While useful, a significant drawback using California mice is the substantial lack of genetic lines expressing Cre-recombinase. Thus, circuit-based studies using cell type-specific expression of viral opsins remain severely limited at this point in time.
Bypassing the need to use California mice, a recent study successfully adapted CSDS in C57BL6 female animals. Instead of the classical two part physical/sensory stage CSDS paradigm, females observe the physical defeat of a conspecific male intruder by a male CD1 aggressor. Aptly named vicarious defeat stress (VDS), females only observe physical defeat but never actually experience it. 10 consecutive days of VDS induces the typical phenotypes of social avoidance, anhedonia, and increased serum corticosterone levels normally found in CSDS (Iñiguez et al., 2018). Although the induction protocol is slightly different than regular CSDS and different circuits may be involved, VDS offers a close correlate by which investigators can utilize the myriad viral-genetic approaches in C57BL6 female mice.
As previously mentioned, there also exist models such as CMS or learned helplessness that are capable of inducing depressive-like phenotypes in both males and females. Despite this, many studies still choose to focus strictly on male subjects (Chang and Grace, 2014; Li et al., 2013; Moreines et al., 2017; Tye et al., 2013). This may be, in large part, due to the fact that studies which incorporate both sexes find significant sex-dependent molecular and physiological differences (Brancato et al., 2017; Hodes et al., 2015; Labonté et al., 2017). Ironically, this also underscores the pressing need to not only adopt sex-inclusive models of depression, but to compare both sexes in a study.
An adaptation of CMS, the subchronic variable stress model (SCVS) which relies upon roughly a week of daily variable unpredictable stressors, induces depressive-like behaviors only in females, which may mirror the increased susceptibility of females to MDD in humans (Hodes et al., 2015). Using SCVS, researchers found measured a more pronounced upregulation of DNA methyltransferase 3a (Dnmt3a) in the NAc in females than in males (Hodes et al., 2015). Interestingly, NAc-specific overexpression of Dnmt3a in females promoted resilience, while the same treatment in males promoted susceptibility (Hodes et al., 2015). Other studies have utilized to SCVS to uncover sex-specific synaptic and micro-RNA profile changes in the NAc as well (Brancato et al., 2017; Pfau et al., 2016). Females undergoing SCVS exhibited an increase in expression of the vesicular glutamate transporter-2, and a reduction in vesicular glutamate transporter-1 in the NAc (Brancato et al., 2017).
Beyond the NAc, region specific RNA-seq analysis after CVS (similar to SCVS but for 3 weeks and elicits depression-related behaviors in both sexes) also found a selective downregulation of Dusp6 in females in mPFC. Correspondingly, viral downregulation of mPFC Dusp6 also promoted anhedonia and increased latency to eat in the novelty-suppressed feeding test as well (Labonté et al., 2017). Contrarily, Emx1 was found to be selectively upregulated in males post CVS and viral over-expression promoted susceptibility to depression-related behaviors (Labonté et al., 2017).
Indeed, several useful models capable of inducing depression-related behaviors in strictly males, females, or both are being developed; each with their advantages and drawbacks. Careful consideration of the construct, face, and predictive validities of each in the context of interpreting results remain critical to effectively driving the field forward and to properly model the sex differences that exist in human MDD patients.
10. Conclusions and future directions
The development of viral-mediated gene delivery tools, transgenic mice approaches, and the increased accessibility of RNA sequencing (RNA-seq) in single cells have created exciting avenues for in depth analysis of the mesolimbic circuitry including the NAc, VP, and VTA. Even with several decades of research on reward circuitry, the molecular diversity of these brain circuitry has not been fully elucidated. As we have seen, discrete cell types (i.e. dopaminergic neurons) within a single brain region might display considerable functional heterogeneity depending on its efferent target. Thus, uncovering separate molecular markers that can discriminate between these two populations will make development of precise therapeutics that can pharmacologically target them more tractable. Single cell molecular profiling techniques such as Drop-seq, which utilizes cell-specific DNA barcodes to sequence individual cells, allow for unbiased screening and identification of potentially novel subpopulations of neurons not previously identified that could have functional relevance in depression-related behaviors (Macosko et al., 2015). Further refinement of other existing techniques utilizing fluorescence-activated cell sorting (FACS) combined with single cell profiling techniques would allow for researchers to virally label cell-type and projection-specific populations and obtain sequencing data for these separate populations as well (Tirosh et al., 2016). Combining the available anatomical circuit information with the precise molecular identity of neurons will be beneficial to provide a fundamental framework to understand the neural basis of MDD as well as reward-related behaviors and their disorders.
Given these recent advances, why then is there such a disconnect between bench discoveries to bedside treatments? Clearly, no obvious answer exists except for that MDD is a multi-faceted, complex condition; but we can offer a supplementary explanation. Provided that most manipulations either directly or indirectly target activity within the reward and/or motivational circuitry, it is fair to wonder if these experiments are simply temporary alleviations of depression-related behaviors by virtue of acutely increasing reward, but ultimately don’t address the underlying etiology. Acute optogenetic-induced reversals of depression-related behavior may simply be epiphenomena. How do we separate temporary induced aversion from exacerbation of the core cause of depression, or is it simply a debate of semantics? It is telling that the vast majority of studies using optogenetic manipulations or overexpression of certain genes after CMS or CSDS look predominantly at a single time-point tested right after the CMS or CSDS protocol, even though certain CSDS-induced symptoms are reported to persist 40 days after stress cessation (Krishnan et al., 2007). Most manipulations report a reduction in social avoidance and anhedonic behavior during manipulation; however, no long-term follow up is provided. Do depression-related behaviors that are temporarily attenuated during manipulation re-emerge one day, a week, or even immediately after cessation? While manipulating reward or motivation-related brain areas may be necessary or sufficient to induce acute reward that can temporarily ‘override’ depressive-like behaviors in animals, the ultimate goal towards the development of better treatments or a cure, so to speak, for MDD based on animal research is not just a temporary fix but understanding and reversing the core etiology. Thus, adjusting the standard for studies to use long-term follow up as a critical study endpoint is imperative for the future.
In sum, the wide patient to patient variability of behavioral symptoms of depression compel researchers to understand how separate circuits or cell-types in the brain can underlie specific depressive-like phenotypes. Future studies that take a systematic approach to integrate separate pathways and how they differentially regulate behavioral symptoms of depression will be critical to uncover the etiology of MDD and drive the discovery of more effective therapeutics.
References
- Adachi M, Autry AE, Mahgoub M, Suzuki K, Monteggia LM, 2017. TrkB signaling in dorsal raphe nucleus is essential for antidepressant efficacy and normal aggression behavior. Neuropsychopharmacology 42, 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park S Il, Marcinkiewcz CM, Crowley NA, et al. , 2015. Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 87, 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, Dragomirecka E, Kohn R, Keller M, Kessler RC, Kawakami N, Kilic C, Offord D, Ustun TB, Wittchen HU, 2003. The epidemiology of major depressive episodes: results from the international consortium of psychiatric epidemiology (ICPE) surveys. Int. J. Methods Psychiatr. Res 12, 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, Persaud B, Cachope R, Bolanos-Guzman CA, Cheer JF, Deisseroth K, Han MH, Nestler EJ, 2015. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun 6, 7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Cates HM, Purushothaman I, Lorsch ZS, Walker DM, Wang J, Huang X, Schluter OM, Maze I, Pena CJ, Heller EA, Issler O, Wang M, Song WM, Stein JL, Liu X, Doyle MA, Scobie KN, Sun HS, Neve RL, Geschwind D, Dong Y, Shen L, Zhang B, Nestler EJ, 2016. Circuit-wide transcriptional profiling reveals brain region-specific gene networks regulating depression susceptibility. Neuron 90, 969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, LaBonte B, Pena CJ, Shen L, Wittenberg GM, Nestler EJ, 2017. Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol. Psychiatry 81, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L, 2015. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Nestler EJ, 2006. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci 7, 137–151. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ, 2006. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311, 864–868. [DOI] [PubMed] [Google Scholar]
- Brancato A, Bregman D, Ahn HF, Pfau ML, Menard C, Cannizzaro C, Russo SJ, Hodes GE, 2017. Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience 350, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC, 2014. Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology 77, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Covington III HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, Nestler EJ, Han MH, 2010. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci 30, 16453–16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I, 2010. Antidepressant-Like Effects of κ-Opioid Receptor Antagonists in Wistar Kyoto Rats. Neuropsychopharmacology 35, 752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R, Francis TC, Nam H, Riggs LM, Engeln M, Rudzinskas S, Konkalmatt P, Russo SJ, Turecki G, Iniguez SD, Lobo MK, 2017. Reduced Slc6a15 in nucleus accumbens D2-neurons underlies stress susceptibility. J. Neurosci 37, 6527–6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Grace AA, 2014. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol. Psychiatry 76, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH, 2013. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Liu H, Han MH, 2015. Neuronal correlates of depression. Cell. Mol. Life Sci 72, 4825–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, Dey A, Smith M, Rebusi N, Pfau M, Ables JL, Aleyasin H, Khibnik LA, Hodes GE, Ben-Dor GA, Deisseroth K, Shapiro ML, Malenka RC, IbanezTallon I, Han MH, Russo SJ, 2015. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat. Neurosci 18, 962–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL, 2007. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology 194, 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE III, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, Neve RL, Deisseroth K, Nestler EJ, 2010. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci 30, 16082–16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE III, Maze I, Vialou V, Nestler EJ, 2015. Antidepressant action of HDAC inhibition in the prefrontal cortex. Neuroscience 298, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravchik A, Goldman D, 2000. Neurochemical individuality: genetic diversity among human dopamine and serotonin receptors and transporters. Arch. Gen. Psychiatry 57, 1105–1114. [DOI] [PubMed] [Google Scholar]
- Creed M, Ntamati NR, Chandra R, Lobo MK, Luscher C, 2016. Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron 92, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A, 1988. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J. Pharmacol. Exp. Ther 244, 1067–1080. [PubMed] [Google Scholar]
- Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ, 2012. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J. Neurosci 32, 6957–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Mayberg HS, 2014. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin. Neurosci 16, 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB, 2007. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 64, 327–337. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH, 2010. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology 209, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ, 2003. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol. Psychiatry 54, 994–1005. [DOI] [PubMed] [Google Scholar]
- Faget L, Osakada F, Duan J, Ressler R, Johnson AB, Proudfoot JA, Yoo JH, Callaway EM, Hnasko TS, 2016. Afferent inputs to neurotransmitter-defined cell types in the ventral tegmental area. Cell Rep. 15, 2796–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, et al. , 2014. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Flaxman AD, Patten SB, Vos T, Whiteford HA, 2013. The epidemiological modelling of major depressive disorder: application for the global burden of disease study 2010. PLoS One 8, e69637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Lobo MK, 2017. Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol. Psychiatry 81, 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, Brooks JM, Iniguez SD, O’Donnell P, Kravitz A, Lobo MK, 2015. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol. Psychiatry 77, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han MH, 2014. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 344, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ, 2007. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30, 220–227. [DOI] [PubMed] [Google Scholar]
- Greenberg GD, Steinman MQ, Doig IE, Hao R, Trainor BC, 2015. Effects of social defeat on dopamine neurons in the ventral tegmental area in male and female California mice. Eur. J. Neurosci 42 (12), 3081–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE 3rd, Berton O, Russo SJ, 2011. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc 6 (8), 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ, Nobrega JN, 2010. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol. Psychiatry 67, 117–124. [DOI] [PubMed] [Google Scholar]
- Hamilton PJ, Burek DJ, Lombroso SI, Neve RL, Robison AJ, Nestler EJ, Heller EA, 2018. Cell-type-specific epigenetic editing at the Fosb gene controls susceptibility to social defeat stress. Neuropsychopharmacology 43 (2), 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heninger GR, Delgado PL, Charney DS, 1996. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 29, 2–11. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, et al. , 2015. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J. Neurosci 35, 16362–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M, 2010. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology 211, 69–77. [DOI] [PubMed] [Google Scholar]
- Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A, Martin G, Robinson J, Moquin L, Marti F, et al. , 2016. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat. Neurosci 19 (4), 560. [DOI] [PubMed] [Google Scholar]
- Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, Sanchez DO, Lobo MK, Serrano PA, Braren SH, Castillo SA, 2018. Vicarious Social Defeat Stress Induces Depression-Related Outcomes in Female Mice. Biol. Psychiatry 83 (1), 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun TY, Pae CU, Chae JH, Bahk WM, Kim KS, 2001. Polymorphism of CTLA-4 gene for major depression in the Korean population. Psychiatry Clin. Neurosci 55, 533–537. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB, 1993. Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity and recurrence. J. Affect. Disord 29, 85–96. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE, 2005. Prevalence, Severity, and Comorbidity of. Arch. Gen. Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khibnik LA, Beaumont M, Doyle M, Heshmati M, Slesinger PA, Nestler EJ, Russo SJ, 2016. Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol. Psychiatry 79, 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiening K, Sartorius A, 2013. A new translational target for deep brain stimulation to treat depression. EMBO Mol. Med 5, 1151–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA, 2010. Dynorphin, stress, and depression. Brain Res. 1314, 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowland D, Lilascharoen V, Pacia CP, Shin S, Wang EH, Lim BK, 2017. Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell 170 (284–297), e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ, 2008. The molecular neurobiology of depression. Nature 455, 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ, 2011. Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci 7, 121–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ, 2007. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hultman R, Hughes D, Michel N, Katz BM, Dzirasa K, 2014. Prefrontal cortex reactivity underlies trait vulnerability to chronic social defeat stress. Nat. Commun 5, 4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW, 2015. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci 18, 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh Y-HE, Cahill M, et al. , 2017. Sex-specific transcriptional signatures in human depression. Nat. Med 23, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman-Maharg AR, Copeland T, Sanchez EO, Campi KL, Trainor BC, 2017. The long-term effects of stress and kappa opioid receptor activation on conditioned place aversion in male and female California mice. Behav. Brain Res 332, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J, 2008. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57, 760–773. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC, 2011. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70, 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC, 2014. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76 (Pt B), 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R, 2011. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470, 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, Malinow R, Yates JR, Hu H, 2013. βCaMKII in lateral habenula mediates core symptoms of depression. Science 341, 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC, 2012. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li J, Li T, Wang T, Li Y, Zeng Z, Li Z, Chen P, Hu Z, Zheng L, Ji J, Lin H, Feng G, Shi Y, 2011. CTLA-4 confers a risk of recurrent schizophrenia, major depressive disorder and bipolar disorder in the Chinese Han population. Brain Behav. Immun 25, 429–433. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ, 2013. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J. Neurosci 33, 18381–18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Leon S, Croes EA, Sayed-Tabatabaei FA, Claes S, Van Broeckhoven C, van Duijn CM, 2005. The dopamine D4 receptor gene 48-base-pair-repeat polymorphism and mood disorders: a meta-analysis. Biol. Psychiatry 57, 999–1003. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID, 2011. The Neuropeptide Oxytocin Facilitates Pro-Social Behavior and Prevents Social Avoidance in Rats and Mice. Neuropsychopharmacology 36, 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Little JP, Cassel JM, Carter AG, 2012. Subcellular connectivity underlies pathway-specific signaling in the nucleus accumbens. Nat. Neurosci 15, 1624–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Cassel JM, Carter AG, 2014. Cocaine exposure reorganizes cell type-and input-specific connectivity in the nucleus accumbens. Nat. Neurosci 17, 1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA, 2015. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jones RM, Portoghese PS, Carlezon WA, 2003. Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther 305 (1), 323–330. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH, 2005. Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C, 2003. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci 23, 5674–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJ, Daan S, Van Den Hoofdakker RH, Koolhaas JM, 1996. Changes in behaviour and body weight following a single or double social defeat in rats. Stress 1, 21–32. [DOI] [PubMed] [Google Scholar]
- Mendels J, Weinstein N, Cochrane C, 1972. The relationship between depression and anxiety. Arch. Gen. Psychiatry 27, 649–653. [DOI] [PubMed] [Google Scholar]
- Meye FJ, Soiza-Reilly M, Smit T, Diana MA, Schwarz MK, Mameli M, 2016. Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nat. Neurosci 19, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Moreines JL, Owrutsky ZL, Grace AA, 2017. Involvement of Infralimbic Prefrontal Cortex but not Lateral Habenula in Dopamine Attenuation After Chronic Mild Stress. Neuropsychopharmacology 42, 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A, 1985. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology 86, 274–280. [DOI] [PubMed] [Google Scholar]
- Muir J, Lorsch ZS, Ramakrishnan C, Deisseroth K, Nestler EJ, Calipari ES, Bagot RC, 2018. In vivo fiber photometry reveals signature of future stress susceptibility in nucleus accumbens. Neuropsychopharmacology 43 (2), 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, Neumeister A, 2011. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology 213, 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, 1998. Antidepressant treatments in the 21st century. Biol. Psychiatry 44, 526–533. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE, 2010. Animal models of neuropsychiatric disorders. Nat. Neurosci 13, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, Root DC, Hamblin MW, 1996. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology 15, 515–522. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS, 2002. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J. Neurosci 22, 10883–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP Jr., 2008. Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of mu-opioid receptor mRNA and FosB/DeltaFosB immunoreactivity. Eur. J. Neurosci 27, 2272–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ED, Hale MW, Lukkes JL, Valentine MJ, Sarchet DM, Lowry CA, 2011. Repeated social defeat increases reactive emotional coping behavior and alters functional responses in serotonergic neurons in the rat dorsal raphe nucleus. Physiol. Behav 104, 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, Issler O, Loh YE, Leong T, Kiraly DD, Goodman E, Neve RL, Shen L, Nestler EJ, 2017. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356, 1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau ML, Purushothaman I, Feng J, Golden SA, Aleyasin H, Lorsch ZS, Cates HM, Flanigan ME, Menard C, Heshmati M, Wang Z, 2016. Integrative Analysis of Sex-Specific microRNA Networks Following Stress in Mouse Nucleus Accumbens. Front. Mol. Neurosci 9, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Knepel W, Braun S, Meyer HD, Lohmann H, Brantl V, 1986. Effects of a kappa-opioid agonist on adrenocorticotropic and diuretic function in man. Horm. Metab. Res 18, 842–848. [DOI] [PubMed] [Google Scholar]
- Proulx CD, Hikosaka O, Malinow R, 2014. Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci 17, 1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Andreoli M, Michielin F, Quarta D, Sokal DM, 2011. Increased phasic activity of VTA dopamine neurons in mice 3 weeks after repeated social defeat. Behav. Brain Res 218, 253–257. [DOI] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC, 2015. The ventral pallidum: subregion-specific functional anatomy and roles in motivated behaviors. Prog. Neurobiol 130, 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, De Jong J, Linnoila M, 1989. Cerebrospinal fluid monoamine metabolites and suicidal behavior in depressed patients. A 5-year follow-up study. Arch. Gen. Psychiatry 46, 609–612. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Charney DS, 2013. Next generation antidepressants. Proc. Natl. Acad. Sci. U. S. A 110, 4441–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E, 2006a. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav. Brain Res 174, 188–192. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Hiemke C, Fuchs E, Ruther E, Havemann-Reinecke U, 2006b. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav. Pharmacol 17, 19–29. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Ni JR, Caron MG, 2015. Brain 5-HT deficiency increases stress vulnerability and impairs antidepressant responses following psychosocial stress. Proc. Natl. Acad. Sci. U. S. A 112, 2557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A, 2010. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol. Psychiatry 67, e9–e11. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V, 2008. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33, 368–377. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R, 2012. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron 74, 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Piriz J, Malinow R, 2014. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science 345, 1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Rea W, 1997. Sensitization to the behavioral effects of cocaine: modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol. Biochem. Behav 57, 449–455. [DOI] [PubMed] [Google Scholar]
- Szegedi A, Rujescu D, Tadic A, Muller MJ, Kohnen R, Stassen HH, Dahmen N, 2005. The catechol-O-methyltransferase Val108/158Met polymorphism affects short-term treatment response to mirtazapine, but not to paroxetine in major depression. Pharmacogenomics J. 5, 49–53. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C, 2012. GABA neurons of the VTA drive conditioned place aversion. Neuron 73, 1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, et al. , 2016. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352 (6282), 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA, 1993. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol. Behav 53, 983–993. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Landeros RV, Knoblauch NW, Takahashi EY, Silva AL, Crean KK, 2011. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PLoS One 6 (2), e17405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ, 2006. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci 9, 519–525. [DOI] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K, 2013. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD, 2012. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerakumar A, Challis C, Gupta P, Da J, Upadhyay A, Beck SG, Berton O, 2014. Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems. Biol. Psychiatry 76, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Thibault M, Kaska S, Cooper S, Gajewski P, Eagle A, Mazei-Robison M, Nestler EJ, Robison AJ, 2015. Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology 99, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, Burnham VL, Mazei-Robison MS, Ferguson D, Golden SA, Koo JW, Chaudhury D, Christoffel DJ, Pomeranz L, Friedman JM, Russo SJ, Nestler EJ, Han MH, 2014. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat. Neurosci 17 (1), 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RM, Shen EY, Bagot RC, Anselmo A, Jiang Y, Javidfar B, Wojtkiewicz GJ, Cloutier J, Chen JW, Sadreyev R, Nestler EJ, Akbarian S, Hochedlinger K, 2017. Phf8 loss confers resistance to depression-like and anxiety-like behaviors in mice. Nat. Commun 8, 15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K, 2012. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N, 2012. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873. [DOI] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L, 2014. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 83, 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, 2005. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52, 90–110. [DOI] [PubMed] [Google Scholar]
- Winter C, Vollmayr B, Djodari-Irani A, Klein J, Sartorius A, 2011. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav. Brain Res 216, 463–465. [DOI] [PubMed] [Google Scholar]
- Wook Koo J, Labonte B, Engmann O, Calipari ES, Juarez B, Lorsch Z, Walsh JJ, Friedman AK, Yorgason JT, Han MH, Nestler EJ, 2016. Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol. Psychiatry 80, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Yokoyama C, Mizuma H, Kurai S, Finnema SJ, Halldin C, Doi H, Onoe H, 2014. A possible mechanism of the nucleus accumbens and ventral pallidum 5-HT1B receptors underlying the antidepressant action of ketamine: a PET study with macaques. Transl. Psychiatry 4, e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD, 2016. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wienecke CF, Nachtrab G, Chen X, 2016. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]