ABSTRACT
The demand for novel antimicrobial therapies due to the threat posed by antimicrobial resistance has resulted in a growing interest in the protective role of our skin bacteria and the importance of competition among bacteria on the skin. A survey of the cultivable bacteria on human skin was undertaken to identify the capacity of the skin microbiota to produce bacteriocins with activity against skin pathogens. Twenty-one bacteriocins produced by bacteria isolated from seven sites on the human body of each subject exhibited inhibition spectra ranging from broad to narrow range, inhibiting many Gram-positive bacteria, including opportunistic skin pathogens such as Propionibacterium acnes (recently renamed Cutibacterium acnes), Staphylococcus epidermidis and methicillin-resistant Staphylococcus aureus (MRSA). Sequencing indicated that the antimicrobial-producing isolates were predominately species/strains of the Staphylococcus genus. Colony mass spectrometry revealed peptide masses that do not correspond to known bacteriocins. In an era where antibiotic resistance is of major concern, the inhibitory effect of novel bacteriocins from the bacteria of skin origin demonstrates the antimicrobial potential that could be harnessed from within the human skin microbiota.
Keywords: skin pathogens, staphylococci, antimicrobial potential, bacteriocin, skin microbiota, skin microbiome
In the search for novel topical skin therapies, we have examined skin-derived bacteriocins, produced by microbes which have evolved in such a way as to outcompete pathogens, and successfully colonise the skin environment.
INTRODUCTION
The human skin microbiome is home to hundreds of diverse bacterial species from the four phyla Actinobacteria, Proteobacteria, Firmicutes and Bacteriodetes, which act as a part of the body's first line of defence against the external environment (Natsuga 2014). The commensal microbiota of the skin contributes to host health and is thought to play a role in protecting the host against a wide range of infections. One such defence mechanism is the production of bacteriocins. Bacteriocins are ribsosomally synthesised, heat-stable antimicrobial peptides that are produced by bacteria that can exhibit both broad and narrow range inhibition spectra. Lantibiotics belong to class I bacteriocins, which are heavily post-translationally modified—characterised by the presence of lanthionine/B-methyllanthionine residues (McAuliffe, Ross and Hill 2001). Bacteriocins may exert a diverse array of functions from colonising peptides, which help the producer to become established in a niche, to killing peptides for eliminating competitors or as signalling peptides through interactions with other bacteria and the immune system (Schauber and Gallo 2008; Bastos et al. 2009; Dobson et al. 2012). The importance of these traits can be seen where imbalances in the ratio or composition of skin microbiota have been linked to skin diseases/infections such as psoriasis, atopic dermatitis, acne and impetigo (Yamasaki and Gallo 2008; Otto 2009; Zeeuwen et al. 2013; Grice 2014). It has been hypothesised that restoration of this natural microbial balance could potentially alleviate or prevent such skin infections and would reduce the use of antibiotics to treat these conditions (Sanford and Gallo 2013).
Staphylococcus species are the dominant bacterial colonisers of the skin and are divided into two main groups on the basis of coagulase activity. Coagulase triggers the coagulation of soluble fibrinogen-forming insoluble fibrin, resulting in the formation of a clot. Thus, coagulase-positive staphylococci, for example Staphylococcus aureus, which can reside on the skin surface as a commensal organism, are potentially pathogenic and are capable of producing haemolysins and a range of heat-stable toxins. Conversely, the skin is also colonised by a diverse array of coagulase-negative Staphylococcus species, which form a part of the normal commensal bacteria of the skin. The production of lantibiotics is abundant within commensal coagulase-negative staphylococci; for example S. gallinarum, S. epidermidis and S. hominis produce the lantibiotics gallidermin, epidermin and hominicin, respectively (Götz et al. 2014). Studies have characterised the human skin microbiome in healthy and diseased states (Grice and Segre 2011), and while little has been done in screening the human skin specifically for novel bacteriocins, there is increasing interest in the human skin microbiome as a source of competitive strains and novel antimicrobials. This has led to the discovery of a novel antibiotic, lugdunin, isolated from a nasal strain of Staphylococcus lugdunensis (Zipperer et al. 2016). Furthermore, the application of human skin commensals as sources of protection against S. aureus and skin neoplasms (Nakatsuji et al., 2017, 2018) highlight the potential of human skin commensals as possible alternative solutions to antibiotics.
Here, we describe one approach targeted specifically at the isolation of coagulase-negative staphylococcal skin isolates and investigate their inhibitory activity against a range of skin pathogens associated with skin infections such as MRSA, atopic dermatitis and Acne vulgaricus, as well as the causative agents of mastitis.
MATERIALS AND METHODS
Isolation of antimicrobial-producing skin isolates
Twenty healthy volunteers, 18–65 years of age, 13 females and 7 males, were recruited for this study, which was approved by the Cork Clinical Research Ethics Committee under Protocol number APC071. Seven different areas of the body were swabbed, specifically the retro auricular crease (behind ear), the axillary vault (underarm), the inguinal crease (groin), the umbilicus (belly button), the gluteal crease (between lower back and buttocks), the alar crease (side of nose) and the toe web space (between toes). Each swab (Amies, PS, Viscose swabs; Sarstedt, Sinnottstown Lane, Drinagh, Wexford, Ireland) was dipped in sterile saline before swabbing the body area (Landers, Hoet and Wittum 2010) and swabs were stored for no more than 12 hr at 4°C before being homogenised in 2 mL of Maximum Recovery Diluent (MRD; Oxoid Ltd., Basingstoke, Hampshire, UK). Tenfold serial dilutions were performed in MRD and 100 µL aliquots were spread-plated onto Mannitol Salt Agar (MSA; Oxoid Ltd.) and Brain Heart Infusion (BHI) agar (Merck, Darmstadt, Germany). All plates were incubated aerobically at 37°C for 48 hr. After incubation, colonies were counted and colonies of different morphologies and colours were streaked for purity and grown aerobically in 10 mL of BHI broth (Merck) at 37°C in a shaking incubator (311DS Labnet) overnight.
Detection of antimicrobial activity
Spot bioassays for antimicrobial production were then carried out on the isolates by spotting 10 µL of the overnight culture onto BHI agar; the plates were incubated overnight at 37°C and then overlaid with MRS sloppy Lactobacillus MRS broth (BD™ Difco™ Trafalgar Scientific Ltd, Leicester, United Kingdom) containing 0.75% agar seeded with 0.25% of an overnight culture of Lactobacillus delbrueckii ssp. bulgaricus LMG 6901 and grown anaerobically overnight (see Table 1 for optimal growth conditions of bacterial strains used in this study). The remaining colonies on the BHI plates were also overlaid with L. delbrueckii ssp. bulgaricus LMG 6901. The MSA plates were overlaid with sloppy (0.75%) BHI agar seeded with an overnight inoculum of either 0.25% Listeria innocua DPC 3572 or 0.25% MRSA DPC 5645. Colonies from spot assays and plate overlays that exhibited zones of inhibition were then inoculated into BHI broth, grown overnight at 37°C and stocked in 20% glycerol at −80°C for further characterisation (Fig. 1).
Table 1.
Growth conditions of the indicator strains used in this study.
| Growth conditions | ||||
|---|---|---|---|---|
| Species | Strain | Temp (°C) | Atmosphere | Growth media |
| Bifidobacterium longum subsp. infantis | ATCC 15697 | 37 | Anaerobic* | mMRS |
| Bifidobacterium longum subsp. longum | DSM 20097 | 37 | Anaerobic | mMRS |
| Corynebacterium xerosis | DPC 5629 | 37 | Aerobic | BHI |
| Corynebacterium variablis | NCIMB 702097 | 30 | Aerobic | BHI |
| Cutibacterium acnes | LMG 16711 | 37 | Anaerobic | mRCM & RCA |
| Enterococcus faecalis | ATCC 19433 | 37 | Anaerobic | MRS |
| Fusobacterium varium | DSM 19868 | 37 | Anaerobic | BHI |
| Lactobacillus delbrueckii subsp. bulgaricus | LMG 6901 | 37 | Anaerobic | MRS |
| Lactobacillus fermentum | APC 2582 | 37 | Anaerobic | MRS |
| Lactobacillus rhamnosus | APC 3483 | 37 | Anaerobic | MRS |
| Listeria innocua | DPC 3572 | 37 | Aerobic | BHI |
| Listeria monocytogenes | DPC 5788 | 37 | Aerobic | BHI |
| Listeria monocytogenes | DPC 6893 | 37 | Aerobic | BHI |
| Listeria monocytogenes | NCTC 5348 | 37 | Aerobic | BHI |
| Pseudomonas aeruginosa | NCIMB 8295 | 37 | Aerobic | BHI |
| Pseudomonas aeruginosa | APC 2064 | 37 | Aerobic | BHI |
| Pseudomonas fluorescens | DPC 6056 | 30 | Aerobic | BHI |
| Pseudomonas putita | ATCC 17522 | 37 | Aerobic | BHI |
| MRSA | DPC 5645 | 37 | Aerobic | BHI |
| Staphylococcus aureus | ATCC 25923 | 37 | Aerobic | BHI |
| Staphylococcus aureus | DPC 7016 | 37 | Aerobic | BHI |
| Staphylococcus capitis | APC 2923 | 37 | Aerobic | BHI |
| Staphylococcus epidermidis | DPC 5990 | 37 | Aerobic | BHI |
| Staphylococcus simulans | APC 3482 | 37 | Aerobic | BHI |
| Streptococcus agalactiae | ATCC 13813, APC 1055 | 37 | Aerobic | BHI |
| Streptococcus bovis | DPC 6491 | 37 | Aerobic | GM17 |
| Streptococcus dysgalactiae | APC 3484 | 37 | Aerobic | BHI |
| Streptococcus uberis | DPC 5344 | 37 | Aerobic | BHI |
mMRS = modified MRS, mRCM = modified Reinforced Clostridial Media (made following ATCC Medium:2107 Modified Reinforced Clostridial agar/broth (pre-reduced) protocol), RCA = Reinforced Clostridium agar.
= Anaerobic conditions, where required, were achieved through the use of anaerobic jars and Anaerocult A gas packs (Merck, Darmstadt, Germany).
ATCC = American Type Culture Collection, APC = APC Microbiome Ireland Culture Collection, DPC = Teagasc Culture Collection, WSLC = Weihenstephan Listeria Collection, LMG = Laboratorium voor Microbiologie, Universteit Gent, Belgium.
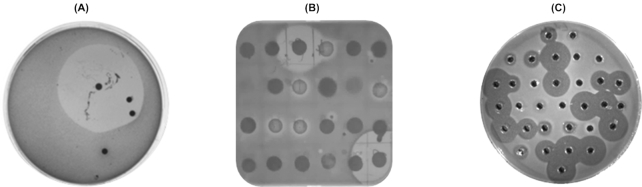
Figure 1.
(A) Overlay of an original plate containing dilution of a toe swab with L. delbrueckii ssp. bulgaricus LMG 6901. (B) Spot assay of 10 µL of inoculum from colonies of varying morphologies isolated from swab dilution plates, overlaid with L. delbrueckii ssp. bulgaricus LMG 6901. (C) Well diffusion assay of antimicrobial-producing skin isolates in agar seeded with L. delbrueckii ssp. bulgaricus LMG 6901 to confirm that the positive result in spot assay was due to antimicrobial substance secreted into CFS.
Characterisation of antimicrobial activity of skin isolates
Well diffusion assays (WDA)
Cell-free supernatants (CFS) were prepared from 10 mL overnight cultures of putative antimicrobial-producing strains (isolated from BHI and MSA plates) by centrifugation (Sorvall Legend RT) at 4000 × g for 20 min following overnight growth in BHI broth in a shaking incubator at 37°C (Ryan et al. 1996). To eliminate inhibition due to acids, the CFS were neutralised to pH 6.9–7.2 using 1 M NaOH. Wells were bored in MRS agar plates, previously seeded with 0.25% inoculum of an overnight culture of L. delbrueckii ssp. bulgaricus LMG 6901, and 50 µL of each neutralised supernatant was added to the wells. The plates were incubated anaerobically at 37°C overnight and observed for zones of inhibition around the well (Ryan et al. 1996). Strains showing zones of inhibition were selected for further study.
16S rRNA sequencing
Genomic DNA was extracted from the antimicrobial-producing strains (grown overnight in 10 mL BHI broth at 37°C) using a Sigma-Aldrich DNA purification kit as described by the manufacturer (Sigma-Aldrich Ireland Limited, Vale Road, Arklow, Co. Wicklow, Ireland). For 16S rRNA sequencing, universal primers Uni F 5′-AGAGTTTGATCCTGGCTCAGG-3′ and Uni R 5′-ACGGCAACCTTGTTACGAGT-3′ were used for PCR reactions (run conditions: initial denaturation: 94°C × 5 min, cycling conditions: 30 cycles of 94°C × 40 s, 55°C × 30 s, 72°C × 1 min, final extension: 72°C × 10 min) to initially identify the antimicrobial-producing strains. To confirm species, degenerate primers targeting the dnaJ gene (Shah et al. 2007) forward primer, 5′-GCCAAAAGAGACTATTATGA-3′, and reverse primer, 5′-ATTGYTTACCYGTTTGTGTACC-3′, were used for the PCR reactions, following conditions described by Shah et al. (2007). Quantification of the extracted DNA and PCR products was conducted using a Qubit 2.0 fluorometer (Thermo Fisher Scientific, 168 Third Avenue Waltham, MA USA 02451). Sequencing was conducted by Genewiz (Hope End, Takeley, Essex, CM22 6TA, United Kingdom) and analyses of the sequencing data were performed utilising Lasergene 8 software (DNAStar Inc., Madison, WI). Basic local alignment search tool (BLAST) on the National Centre for Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov) was used to compare the sequencing data of the isolates to existing genomic data.
Heat stability and protease sensitivity
To establish the heat stability of bacteriocins produced, CFS were incubated for 10 min at a range of temperatures, 37, 60, 70, 80, 90, 100°C, and for 15 min at 121°C before performing a WDA in 1.5% MRS agar seeded with 0.25% L. delbrueckii ssp. bulgaricus LMG 6901 as previously described; see Fig. S2A (Supporting Information).
To investigate whether the antimicrobials produced were proteinaceous in nature, the CFS of all antimicrobial-producing isolates were incubated with Proteinase K (Sigma) to a final concentration of 20 mg/mL at 37°C for 1 hr and tested for antimicrobial activity by a WDA using L. delbrueckii ssp. bulgaricus LMG 6901 as the target organism as described above. The corresponding CFS, mixed with an equivalent volume of water, was used as a control; see Fig. S2B and S2C (Supporting Information).
Cross-immunity
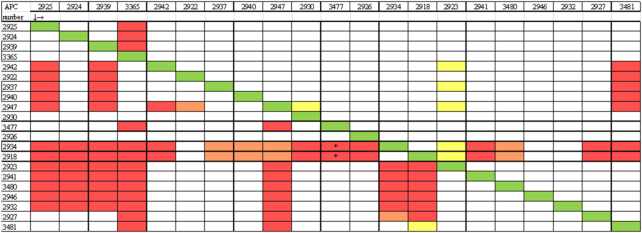
To determine the relatedness of the bacteriocins produced by the strains, cross-immunity assays were performed as follows: CFS of all producing strains were tested against each other by conducting WDA assays as previously described using each producing strain as a producer and also as a target strain. Each producing strain was inoculated individually (at 0.25%) into BHI agar, which was allowed to solidify and 50 µL of the pH-neutralised CFS of each strain was added to wells of all plates; see Fig. 2.
Figure 2.
Cross-immunity assays using CFS from all the antimicrobial-producing strains tested against themselves and each other, with the list of antimicrobial-producing isolates down the left-hand side and the indicator strains across the top of the table. The diagonal green line  reveals that the antimicrobial producer is immune to its own antimicrobial, as expected. The red box
reveals that the antimicrobial producer is immune to its own antimicrobial, as expected. The red box  represents inhibition of the indicator strain indicating that these strains might be different or sensitive to each other.
represents inhibition of the indicator strain indicating that these strains might be different or sensitive to each other.  (orange) = small/ medium hazy zones.
(orange) = small/ medium hazy zones.  (yellow) = very small zones but sensitive. White boxes represent no inhibition, revealing target strains not inhibited by the producing strain, indicating they are immune (related) to the bacteriocins produced by other producers. * = initially sensitive but bactostatic activity observed.
(yellow) = very small zones but sensitive. White boxes represent no inhibition, revealing target strains not inhibited by the producing strain, indicating they are immune (related) to the bacteriocins produced by other producers. * = initially sensitive but bactostatic activity observed.
Spectrum of inhibition
A spectrum of inhibition was completed on pH-adjusted CFS of all antimicrobial-producing isolates using 28 indicator strains by a WDA as described previously. All indicator strains were diluted to the same optical density, (OD600 nm 0.8), using a Biochrom Libra S2 Colorimeter (Biochrom Ltd., Cambourne Business Park, Cambridge, United Kingdom) before seeding the agar. Indicator strains employed to detect antimicrobial activity and their optimal growth conditions are listed in Table 1. Zone size was measured as follows: area of zone πr2 − area of well πr2 in millimetres.
Pulsed Field Gel Electrophoresis
All isolates identified as Staphylococcus species were fingerprinted using Pulsed Field Gel Electrophoresis (PFGE) as described by Bannerman et al. (1995) with minor modifications. Cultures were inoculated at 0.02% and grown in a shaking incubator overnight in 5 mL BHI broth at 37°C. The overnight culture (0.7 mL) was centrifuged for 2 min at 4500 × g in an Eppendorf centrifuge. The supernatant was discarded, and the cell pellets were washed in 1 mL of sterile TE buffer (0.1 M Tris Cl, 0.15 M NaCl, 0.1 M EDTA, pH7.5) and recentrifuged. Washed cells were resuspended in 0.3 mL sterile EC buffer (6 mM Tris-HCl, 1 M NaCl, 0.1 M EDTA, 1% Sarkosyl). A solution of lysostaphin (1 mg/mL) was prepared in 20 mM sodium acetate (pH 4.5) and 2 µL was added to the cell suspension and then vortexed. A 2% Sea Plaque agarose (Bio-Rad Laboratories Ltd., Watford, Hertfordshire, United Kingdom) solution was prepared in EC buffer and 300 µL added to 300 µL lysostaphin-cell suspension was mixed gently to avoid the formation of bubbles and then quickly pipetted into PFGE plug molds. The agar plugs were allowed to solidify at room temperature for 10 min. When solidified, the plugs were incubated in 3 mL EC buffer overnight at 37°C. After overnight incubation, the EC buffer was removed and replaced with 3 mL TE buffer (10 mM Tris Cl, 1 mM EDTA) and incubated for 1 hr at 55°C without shaking. Prior to electrophoresis, the plugs were cut into small slices of 2 × 5 mm and transferred into Eppendorf tubes containing 125 µL of 20U Sma1 restriction enzyme (New England Biolabs, 75–77 Knowl Piece, Wilbury Way, Hitchin, Herts, United Kingdom) in buffer. Plugs were incubated overnight with shaking (300 rpm) at 25°C. Following digestion, trimmed slices of plug were loaded onto the PFGE comb, fixed with 1% PFGE-grade agarose (Bio-Rad Laboratories) in 0.5X Tris-borate EDTA (TBE) buffer. Lambda PFGE marker (New England Biolabs) was loaded onto the gel as described above and used as a reference ladder. The gel was run in 0.5X TBE buffer using a CHEF-DR II®PFGE apparatus using the following gel running parameters: initial pulse 5 s, final pulse 40 s, voltage 200 V or 6 V/cm for 20 hr at 12–14°C. The gel was stained for 2 hr with ethidium bromide (0.5 µg/mL), destained for 1 hr in distilled water and photographed using a GelDoc-It Imager (Ultra-Violet Products Ltd., Cambridge, United Kingdom).
Activity units
For the 13 different antimicrobial-producing strains identified from PFGE, bacteriocin activity units (AU) were calculated as described by Nilsen, Nes and Holo (2002) based on the WDA. Briefly, 50 µL of CFS was diluted twofold and dilutions were dispensed into wells of plates seeded with indicators listed in Table 4. AU were calculated by multiplying the reciprocal of the lowest dilution of the CFS, which produced zones of inhibition by 20 in order to obtain AU/mL; see Table 4. This assay was performed in duplicate.
Table 4.
Activity units per mL (AU/mL) from well diffusion assays of the 13 different bacteriocin-producing skin isolates against indicator strains. (S. hominis APC 2925, 2924, 3365; S. warneri APC 2922, 2937, 2947, 2930; S. epidermidis APC 3477; S. simulans APC 2926; S. capitis APC 2934, 2918, 2923, 2927).
| Bacteriocin producing strains | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indicator strains | 2925 | 2924 | 3365 | 2922 | 2937 | 2947 | 2930 | 3477 | 2926 | 2934 | 2918 | 2923 | 2927 | |
| Corynebacterium xerosis | DPC 5629 | 40 | 40 | – | 80 | 80 | 80 | – | – | – | – | – | – | – |
| Cutibacterium acnes | LMG 16711 | – | – | 40 | – | – | – | – | – | – | 40 | 40 | 40 | – |
| Lactobacillus bulgaricus | LMG 6901 | 80 | 80 | 320 | 640 | 640 | 640 | 160 | 320 | 40 | 80 | 80 | 1280 | 640 |
| Lactobacillus fermentum | APC 2582 | 40 | 40 | 20 | 80 | 80 | 80 | – | – | – | 20 | 20 | 40 | 20 |
| Listeria monocytogenes | DPC 6893 | 40 | 40 | – | – | – | – | – | – | – | – | – | – | – |
| MRSA | DPC 5645 | – | – | – | – | – | – | – | – | – | 80 | 40 | – | – |
| Staphylococcus aureus | ATCC 25923 | – | – | – | – | – | – | – | – | – | 40 | 40 | – | – |
| Staphylococcus aureus | DPC 7016 | – | – | – | 80 | 20 | 40 | – | – | – | 40 | 40 | – | – |
| Staphylococcus epidermidis | DPC 5990 | – | – | – | – | – | – | – | – | – | 160 | 160 | – | – |
| Staphylococcus simulans | APC 3482 | – | – | – | – | – | – | – | – | – | – | – | 40 | 40 |
| Streptococcus agalactiae | APC 1055 | 20 | 20 | 20 | 320 | 80 | 80 | – | – | – | – | – | 20 | – |
| Streptococcus agalactiae | ATCC 13813 | 20 | 20 | – | 80 | 80 | 80 | – | – | – | – | – | – | – |
| Streptococcus bovis | DPC 6491 | 40 | 40 | 20 | 40 | 40 | 80 | – | – | – | – | – | 320 | – |
| Streptococcus dysgalactiae | APC 3484 | 40 | 40 | – | 80 | 40 | 40 | – | – | – | – | – | – | – |
| Streptococcus uberis | DPC 5344 | – | – | – | 80 | 40 | 40 | – | – | – | – | – | 40 | 20 |
MALDI TOF mass spectrometry
Colonies from the 13 antimicrobial-producing skin isolates were mixed with 50 µL propan-2-ol 0.1% TFA, vortexed three times and centrifuged at 16 000 × g for 30 s. MALDI TOF mass spectrometry was performed on the CFS using an Axima TOF2 MALDI TOF mass spectrometer (Shimadzu Biotech, Manchester, UK). An aliquot (0.5 µL) of matrix solution (α-cyano 4-hydroxy cinnamic acid, 10 mg mL−1 in acetonitrile-0.1% (v/v) trifluoroacetic acid) was deposited onto the target and left for 10 s before being removed. The residual solution was allowed to air-dry and 0.5 µL of the sample solution was deposited onto the pre-coated sample spot; 0.5 µL of matrix solution was added to the deposited sample and allowed to air-dry. The sample was then analysed in the positive-ion linear mode and peptide masses were compared to the bacteriocin database ‘Bactibase’ to identify putative bacteriocins.
RESULTS AND DISCUSSION
The human skin microbiome is home to trillions of bacteria but is predominately colonised by members of the genus Staphylococcus (Otto 2010). The skin is the defensive barrier that separates our internal organs from the external world. It acts as a first line of defence against pathogens, and the layers of skin help prevent harmful substances from accessing the body (Sanford and Gallo 2013; Zeeuwen et al. 2013). Significant therapeutic potential has been identified for the staphylococcal genus, including the production of class 1a lantibiotics such as epidermin, nukacin, gallidermin and aureocin (Schnell et al., 1989, 1992; Sashihara et al. 2000; Netz et al. 2002), as well as a novel antibiotic lugdenin (Zipperer et al. 2016). Consequently, a culture-based approach was adopted in this study to isolate antimicrobial-producing bacteria with a particular focus on those that exhibit inhibitory activity against bacteria involved in the pathogenesis of skin infections. These include MRSA, S. epidermidis, Cutibacterium acnes and a number of mastitic pathogens, such as Streptococcus uberis, Streptococcusbovis, Streptococcusagalactiae, Streptococcusdysgalactiae and S. aureus.
Detection and isolation of antimicrobial-producing skin isolates
Twenty volunteers of mixed age, gender and race were recruited to provide swabs from seven body locations. The body sites were chosen based on the varied bacterial compositions of different skin areas (Grice and Segre 2011). Superficial swabs were employed in this study because the swabbing method was less invasive and was reported to yield a largely similar microbiota profile to that associated with skin scraping of epidermis or punch biopsy of the full thickness of epidermis and dermis when next-generation sequencing methods were used (Grice et al. 2008). Over 90,000 colonies were initially screened for antimicrobial activity; these were isolated from 7 sites from the 20 individuals (140 sites) using the agar overlay technique on the original isolation plates. Samples were serially diluted and spread-plated onto the surface of BHI and MSA agar plates and all plates that contained visible growth were counted as potential antimicrobial producers and overlaid with L. delbrueckii ssp. bulgaricus or MRSA/L. innocua, respectively. At this stage of the study, the genetic relationship between the colonies was not determined. Spot bioassays on selected individual colonies and original plate overlays identified 101 possible antimicrobial-producing isolates (see the supplementary information for more details). To confirm that an antimicrobial substance was secreted into the growth medium, neutralised CFS was tested against L. delbrueckii ssp. bulgaricus LMG 6901 by WDA and this reduced the number of possible bacteriocin producers to 25, giving an observed frequency of 0.03% from the 90 000 colonies screened in this study. Due to small zone sizes, four of the antimicrobial producers were discarded. Lactobacillus delbrueckii ssp. bulgaricus LMG 6901 was selected as the primary indicator for all assays because of its insensitivity to acids, thus reducing the frequency of false positives (Casey et al. 2004). While it is now accepted that many bacteria in natural ecosystems have the genetic potential to produce bacteriocin-like inhibitors, their detection in vitro is largely dependent on the screening methods undertaken. The low frequency observed in this study may contribute to the fact that only L. delbrueckii ssp. bulgaricus was used as the primary indicator bacterium.
Identification of antimicrobial-producing skin isolates
16S rRNA sequencing of the 25 antimicrobial-producing isolates revealed four Bacillus species—Bacillus licheniformis (2), Bacillus endophyticus and Bacillus safensis—but the remainder were coagulase-negative Staphylococcus species. However, additional analysis by amplification of the dnaJ gene was required to further differentiate the Staphylococcus species. Five Staphylococcus species were identified, including S. capitis (9), S. hominis (4), S. epidermidis (1), S. simulans (1) and S. warneri (6) (Table 2). Staphylococcus capitis, a frequent coloniser of human skin, was the most prevalent species isolated (43%) followed by S. warneri (29%), which has been reported to be a less-frequent skin coloniser (Otto 2010) ). Twelve strains (∼50%) of antimicrobial-producing staphylococci were isolated from the toe web-space body area. The four antimicrobials which were omitted from the study, as they were considered poor producers, were Bacillus species.
Table 2.
Identification of antimicrobial-producing strains isolated from skin.
| Strain Identity | Strain number | Subject no. | Body area | PFGE lane no. |
|---|---|---|---|---|
| Staphylococcus hominis | APC 2925 | SM019 | toe web-space | 1$ |
| Staphylococcus hominis | APC 2924 | SM012 | toe web-space | 2 |
| Staphylococcus hominis | APC 2939 | SM019 | gluteal crease | 3$ |
| Staphylococcus hominis | APC 3365 | SM020 | gluteal crease | 4 |
| Staphylococcus warneri | APC 2942 | SM009 | axillary vault | 5* |
| Staphylococcus warneri | APC 2922 | SM001 | toe web-space | 6 |
| Staphylococcus warneri | APC 2937 | SM006 | gluteal crease | 7* |
| Staphylococcus warneri | APC 2947 | SM007 | toe web-space | 8 |
| Staphylococcus warneri | APC 2940 | SM007 | gluteal crease | 9* |
| Staphylococcus warneri | APC 2930 | SM015 | toe web-space | 10 |
| Staphylococcus epidermidis | APC 3477 | SM015 | toe web-space | 11 |
| Staphylococcus simulans | APC 2926 | SM002 | toe web-space | 12 |
| Staphylococcus capitis | APC 2934 | SM003 | inguinal crease | 13 |
| Staphylococcus capitis | APC 2918 | SM001 | alar crease | 14 |
| Staphylococcus capitis | APC 2923 | SM007 | toe web-space | 15¤ |
| Staphylococcus capitis | APC 2941 | SM010 | umbilicus | 16¤ |
| Staphylococcus capitis | APC 3480 | SM009 | umbilicus | 17¤ |
| Staphylococcus capitis | APC 2946 | SM008 | toe web-space | 18¤ |
| Staphylococcus capitis | APC 2932 | SM008 | toe web-space | 19¤ |
| Staphylococcus capitis | APC 2927 | SM015 | toe web-space | 20π |
| Staphylococcus capitis | APC 3481 | SM015 | toe web-space | 21π |
These symbols reflect identical PFGE patterns from Figure 3.
Characterisation of antimicrobial-producing skin isolates
A characteristic of bacteriocins is that they are relatively heat stable and are susceptible to degradation by proteolytic enzymes (Jack, Tagg and Ray 1995). Antimicrobial activity in the CFS from all strains was eliminated following Proteinase K treatment, indicating that the antimicrobials were proteinaceous in nature (Fig. S2, Supporting Information). In addition, all antimicrobials were stable up to 100°C for 10 min and showed only a 50% reduction in activity after autoclaving at 121°C for 15 min (Fig. S2, Supporting Information).
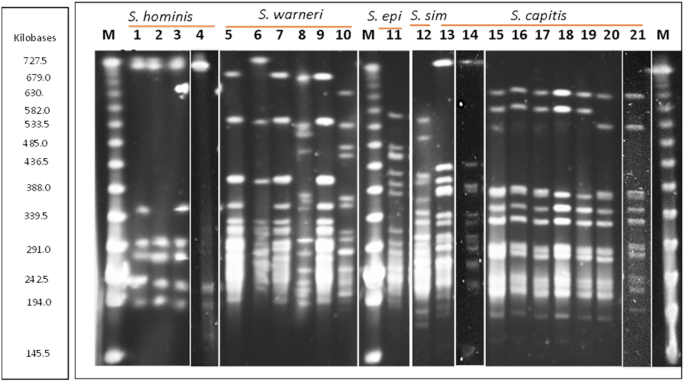
One of the features of bacteriocin production is that the genome of producers must harbour immunity gene(s), which protect producers from the antimicrobial effects of the bacteriocin they produce (Cotter, Hill and Ross 2005; Yang et al. 2014). Cross-immunity assays were performed to determine whether the producing strains were sensitive to the antimicrobial activity of the other producers (Fig. 2). To determine the genetic relatedness of the 21 staphylococci isolated, the strains were fingerprinted using PFGE and similarities were categorised on the basis of the procedure described by Tenover et al. (1995). Strains of the same species are closely related, in some cases only differing from each other by a single DNA band, for example S. hominis strains APC 2925, APC 2924 and S. capitis strains APC 2934 and APC 2918 (Fig. 3). However, the PFGE analysis revealed 13 genetically different antimicrobial-producing staphylococci—three S. hominis, four S. warneri, one S. epidermidis, one S. simulans and four S. capitis.
Figure 3.
PFGE macrodigestion patterns of staphylococcal skin isolates following genomic digestion with Sma1. Lanes 1–4 = Staphylococcus hominis: APC 2925, 2924, 2939 and 3365; lanes 5–10 = Staphylococcus warneri: APC 2942, 2922, 2937, 2947, 2940 and 2930; lane 11 = Staphylococcus epidermidis: APC 3477; lane 12 = Staphylococcus simulans APC 2926; lanes 13–21 = Staphylococcus capitis APC 2934, 2918, 2923, 2941, 3480, 2946, 2932, 2927 and 3481. Lanes 4, 14 and 21 were taken from other PFGE gels with the same lambda marker (M) and input into this gel. epi = epidermidis; sim = simulans. Artefact in lane 3 below the first band is not a Sma1 DNA band.
Combining data from cross-immunity assays and PFGE analysis revealed that antimicrobials produced by skin isolates of the same species and of the same or similar pulsotype were immune to each other, suggesting they are producing the same bacteriocin and are possibly the same strain. For example, two S. hominis strains APC 2925 and APC 2939, isolated from different body areas (toe web-space and gluteal crease) of the same subject, yielded the same pulsotype (lanes 1 and 3 on PFGE gel) and had the same immunity pattern as another S. hominis strain, APC 2924, from a different subject whose pulsotype differed by just one Sma1 digestion DNA band (lane 2 on PFGE gel). This was also the case for two S. capitis strains from two different subjects (APC 2934 and APC 2918, lanes 13 and 14 of PFGE gel). This shows that some different strains that are closely related can produce the same bacteriocin.
PFGE profiles demonstrated that the same pulsotype can be shared across a number of individuals, with the same S. capitis pulsotype isolated from four different subjects (Fig. 3, lanes 15–19) and three subjects sharing the same S. warneri pulsotype: APC 2942, APC 2937 and APC 2940 (Fig. 3, lanes 5, 7 and 9). Interestingly, we also showed that the same subject can carry more than one strain of the same species; S. warneri APC 2947 and APC 2940 were isolated from the subject 7. These strains were isolated from different locations on the body and exhibited different pulsotypes—(Fig. 3, lanes 8 and 9). S.warneri APC 2947 had a very different pulsotype to other S. warneri strains isolated in this study, and while it was immune to the bacteriocin produced by others of the same species, it had a different immunity pattern in that it was inhibited by S. capitis skin isolates unlike other S. warneri (Figs 2 and 3, lane 8).
Bacteriocins such as thuricin CD and nisin have either a narrow or broad spectrum of activity, respectively (Rea et al. 2010; Shin et al. 2016), and both of these characteristics may be beneficial to the producing strain depending on the niche inhabited by the bacterium. In a crowded environment like the skin, bacteriocin production might help producing strains establish themselves in a particular skin environment. For example, the production of narrow spectrum bacteriocins may confer a competitive advantage to strains, allowing them to inhibit closely related species that would be competing for similar nutrients on the skin (Dobson et al. 2012). Table 3 shows the spectrum of inhibition of the bacteriocins produced against a panel of bacteria including skin pathogens such as C. acnes, S. epidermidis, Corynebacterium xerosis (C. xerosis) and MRSA. The majority of S. warneri exhibited the broadest spectrum of activity, inhibiting nine of the indicator strains, while the inhibitory activity of three strains (APC 2930, APC 3477 and APC 2926) was shown to exhibit a narrow inhibition spectrum—inhibiting only the primary indicator L. delbrueckii ssp. bulgaricus LMG 6901.
Table 3.
Spectrum of inhibition of bacteriocin-producing skin isolates against indicator strains subjected to well diffusion assays. +<50 mm2; ++50–150mm2; +++150–249mm2; ++++ 250–349mm2; 350–449mm2; ++++++>450 (calculated as area of zone πr2 – area of well πr2in millimetres).
| S. hominis | S. warneri | S. epidermidis | S. simulans | S. capitis | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Producer strains | ||||||||||||||||||||||
| 2925 | 2924 | 2939 | 3365 | 2942 | 2922 | 2937 | 2947 | 2940 | 2930 | 3477 | 2926 | 2934 | 2918 | 2923 | 2941 | 3480 | 2946 | 2932 | 2927 | 3481 | ||
| Corynebacteria xerosis | DPC5629 | ++ | ++ | ++ | − | +++ | +++ | +++ | +++ | +++ | − | − | − | − | − | − | − | − | − | − | − | − |
| Cutibacterium acnes | LMG 16711 | − | − | − | ++ | − | − | − | − | − | − | − | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | − |
| Lactobacillus bulgaricus | LMG6901 | ++ | ++ | ++ | ++++ | ++++++ | ++++++ | +++++ | ++++++ | ++++++ | +++ | +++++ | + | ++ | ++ | +++++ | ++++++ | ++++++ | ++++++ | ++++++ | +++++ | +++++ |
| Lactobacillus fementum | APC 2582 | ++ | ++ | ++ | + | +++ | ++ | ++ | ++ | ++ | − | − | − | ++ | ++ | + | + | + | + | + | + | + |
| Listeria monocytogenes | DPC 6893 | ++ | ++ | ++ | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| MRSA | DPC5645 | − | − | − | − | − | − | − | − | − | − | − | − | ++ | ++ | − | − | − | − | − | − | − |
| Staphylococcus aureus | ATCC 25923 | − | − | − | − | − | − | − | − | − | − | − | − | ++ | ++ | − | − | − | − | − | − | − |
| Staphylococcus aureus | DPC 7016 | − | − | − | ++ | ++ | ++ | ++ | ++ | − | − | − | − | ++ | ++ | − | − | − | − | − | − | − |
| Staphylococcus epidemidis | DPC 5990 | − | − | − | − | − | − | − | − | − | − | − | − | +++++ | +++++ | − | − | − | − | − | − | − |
| Staphylococcus simulans | APC 3482 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Streptococcusagalactiae | APC 1055 | ++ | ++ | ++ | − | +++ | +++ | +++ | +++ | +++ | − | − | − | − | + | + | + | + | + | − | − | |
| Streptococcus agalactiae | ATCC 13813 | ++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ | − | − | − | − | − | − | − | − | − | − | − | − |
| Streptococcus bovis | DPC 6491 | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | − | − | − | − | − | +++ | +++ | +++ | +++ | +++ | − | − |
| Streptococcus dysgalactiae | APC 3484 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | − | − | − | − | − | − | − | − | − | − | − |
| Streptococcus tiberis | DPC 5344 | − | − | − | − | +++ | +++ | +++ | +++ | ++ | − | − | − | − | +++ | +++ | +++ | +++ | +++ | ++ | ++ | |
Other indicator strains that the skin antimicrobial producers were tested against can be seen on Table 1.
While the majority of our commensal or resident skin microbiota are beneficial to skin health, occasionally the microbes that inhabit our skin can develop into opportunistic pathogens, for example S. lugdunensis and S. capitis (Böcher et al. 2009; Cameron et al. 2015). Even the most abundant species of Staphylococcus on human skin, S. epidermidis, can become pathogenic if the conditions are suitable (Cogen, Nizet and Gallo 2008). Disruptions to the microbial composition of the skin are responsible for skin conditions such as psoriasis, atopic dermatitis, impetigo and acne, and sometimes these imbalances can also prevent healing of chronic wounds (Grice 2014). Two S. capitis strains (APC 2934 and APC 2918) isolated in this study exhibited inhibitory activity of 160 AU/mL against S. epidermidis (DPC 5990) (Tables 3 and 4).
S. aureus in particular is known for developing antibiotic resistance (Chambers and Deleo 2010). MRSA was first identified in 1960 and since then has become one of the most prevalent antibiotic resistant pathogens worldwide and is characterised by swollen red lesions containing pus following infection of skin wounds; it also causes many other diseases, including pneumatic diseases that can result in mortality in the elderly. A study investigating incidence of MRSA in S. aureus isolates in the Prince of Songkhla Hospital, Thailand found that 60.9% of the S. aureus isolates were methicillin resistant (Indrawattana et al. 2013), while a study on skin and soft tissue infections carried out in medical centres in Europe revealed that the most prevalent pathogen was MRSA (22.5%) (Johnson 2011). MRSA is first treated with glycopeptide antibiotics such as vancomycin, (Santajit and Indrawattana 2016). However, the development of novel antimicrobial therapies for methicillin-resistant and vancomycin-resistant S. aureus are included in the second highest priority group in a list of priority pathogens published by the WHO in a recent report describing antimicrobial resistance as a ‘global emergency’ (http://www.who.int/antimicrobial-resistance/en/). Alternative interventions to alleviate the problems posed by MRSA and other antimicrobial resistant pathogens are urgently required. S. capitis strains APC 2934 and APC 2918 with very similar pulsotypes (Fig. 3, lanes 13 and 14) were isolated in this study from two different subjects, from different body areas, namely the inguinal crease and alar crease, and were immune to each other's antimicrobial activity (Fig. 2). These strains exhibited inhibitory activities, 80 AU/mL and 40 AU/mL respectively, against a strain of the skin pathogen MRSA (DPC 5645) (Tables 3 and 4).
Many adolescents and adults will experience the effects of acne vulgaricus at some point in their lives (Webster 2002). Patients suffering from mild acne are often prescribed topical antibiotics or antiseptics at the beginning of treatment, such as clindamycin, erythromycin, benzoyl peroxide, topical retinoid or a combination of these products. However, if these are ineffective, or if the acne is classified as moderate to severe, then patients are often prescribed systemic treatments including oral antibiotics including tetracyclines, erythromycin, trimethoprim–sulfamethoxazole hormone therapy and retinoid-isotreretinoin, often for long periods (Rathi 2011). According to TIME Health, acne patients stay on antibiotics for four times the recommended time period. Eight skin isolates in this study displayed inhibitory activity of 40 AU/mL against C. acnes, which is a leading cause of acne vulgaricus (see Tables 3 and 4). Seven of these isolates were S. capitis; however, there were three distinct pulsotypes. S.hominis APC 3365 was also active against C. acnes.
C. xerosis is a Gram-positive aerobic bacterium, and a commensal of the human skin. C. xerosis can cause bacteremia, skin infections, endocarditis and pneumonia in immune-compromised patients (Pessanha et al. 2003). Three S. hominis and five S. warneri demonstrated inhibitory activities of 40 AU/mL and 80 AU/mL against C. xerosis (Tables 3 and 4), and against other Corynebacterium species (results not shown). PFGE analysis revealed five distinct pulsotypes (two S. hominis and three S. warneri strains (Fig. 3)). In addition to highlighting the therapeutic potential of coagulase-negative staphylococci on human skin by inhibiting skin pathogens, we also observed the ability of skin commensals to inhibit other pathogens such as Listeria monocytogenes (Table 3, APC 2924, APC 2925, APC 2939- 2 distinct strains from PFGE, Fig. 3) and Streptococcus species involved in bovine mastitis, such as St. uberis, St. agalactiae and St. dysgalactiae (Table 3). While traditionally S. aureus was the most common cause of mastitis in dairy cows, more recently St. uberis has been shown to be increasingly associated with infection (Kromker, et al. 2014). In this study, three Staphylococcus species, S. hominis, S. warneri and S. capitis inhibited a range of Streptococcus species (St. uberis, St. bovis, St. agalactiae and St. dysgalactiae), which have been implicated in bovine mastitis (Table 3) with AU ranging from 20 to 320 AU/mL (Table 4). Streptococcus uberis is of particular interest as it has been increasingly shown to be one of the causative agents of bovine mastitis in the UK. This shows that the antimicrobial-producing human skin isolates may also have applications in veterinary medicine for the treatment of mastitis, given their ability to inhibit both Staphylococcus and Streptococcus species, and could aid in the search for non-antibiotic solutions for mastitis treatment to reduce antibiotics in the food chain. Mastitis in humans is also a major health issue (Michie, Lockie and Lynn 2003). Staphylococcus epidermidis and streptococci are among the most prevalent causative agents of this infection, found in 87.6% and 68.6% of mastitic human breast milk samples, respectively (Marín et al. 2017). Bacteriocin producers from human skin, identified in this study, exhibited inhibitory activity against Staphylococcal and Streptococcal species, thus highlighting their potential as live bio-therapeutics for the treatment of human mastitis.
Colony mass spectrometry can be used to identify antimicrobial peptides if they can be matched to known peptide masses in the Bactibase database. However, in this case the peptide masses detected did not match any known bacteriocins, suggesting these isolates are potentially producing novel bacteriocins. The Maldi-TOF MS profiles of the 13 antimicrobial-producing staphylococci are shown in Fig. S3, Supporting Information.
CONCLUSION
There is a need to identify new antimicrobial strains with the potential to outcompete pathogens in the skin environment. The skin is a protective defence, shielding us from the external environment (Yamasaki and Gallo 2008). This study has highlighted that the skin microbiota is home to many bacteriocin-producing strains, which may indicate that the skin microbiota is an important tool in our fight against antimicrobial resistance. Indeed, this screening has resulted in the isolation of a set of 13 novel bacteriocin-producing human skin isolates with potential to restore imbalances of skin microbiota as well as to inhibit skin pathogens such as MRSA and C. acnes. More importantly, these strains may prove useful as probiotics for topical skin applications to provide colonization resistance by replacement of skin pathogens and particularly MRSA. Further characterisation studies will be carried out on these bacteriocin-producing skin isolates.
Supplementary Material
ACKNOWLEDGEMENTS
JO'S was supported by a studentship from Science Foundation Ireland (SFI; www.sfi.ie) under Grant Number SFI/12/RC/2273.
FUNDING
This work was supported by a grant from Science Foundation Ireland (SFI; www.sfi.ie) (grant Number SFI/12/RC/2273).
Conflicts of interest. None declared.
REFERENCES
- Bannerman TL, Hancock GA, Tenover FC et al. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos MCF, Ceotto H, Coelho MLV et al. Staphylococcal antimicrobial peptides: relevant properties and potential biotechnological applications. Curr Pharm Biotechnol. 2009;10:38–61. [DOI] [PubMed] [Google Scholar]
- Böcher S, Tønning B, Skov RL et al. Staphylococcus lugdunensis, a common cause of skin and soft tissue infections in the community. J Clin Microbiol. 2009;47:946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DR, Jiang JH, Hassan KA et al. Insights on virulence from the complete genome of Staphylococcus capitis. Front Microbiol. 2015;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey PG, Casey GD, Gardiner GE et al. Isolation and characterization of anti-Salmonella lactic acid bacteria from the porcine gastrointestinal tract. Lett Appl Microbiol. 2004;39:431–8. [DOI] [PubMed] [Google Scholar]
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2010;7:629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence?. Br J Dermatol. 2008;158:442–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PD, Hill C, Ross PR. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777–88. [DOI] [PubMed] [Google Scholar]
- Dobson A, Cotter PD, Ross PR et al. Bacteriocin production: a probiotic trait?. Appl Environ Microbiol. 2012;78:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice E. The skin microbiome: potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin Cutan Med Surg. 2014;33:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Renaud G et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz F, Perconti S, Popella P et al. ‘Epidermin and gallidermin: Staphylococcal lantibiotics’. Int J Med Microbiol. 2014;304:63–71. [DOI] [PubMed] [Google Scholar]
- Indrawattana N, Sungkhachat O, Sookrung N et al. Staphylococcus aureus clinical isolates: antibiotic susceptibility, molecular characteristics, and ability to form biofilm. BioMed Res Int. 2013;2013:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack RW, Tagg JR, Ray B. Bacteriocins of gram-positive bacteria. Am Soc Microbiol. 1995;59:171–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AP. Methicillin-resistantStaphylococcus aureus: the European landscape. J Antimicrob Chemother. 2011;66:43–48. [DOI] [PubMed] [Google Scholar]
- Kromker V, Friederike Reinecke F, Paduch JH et al. Bovine S treptococcus uberis intramammary infections and mastitis. Clin Microbial. 2014;3:4 [Google Scholar]
- Landers TF, Hoet A, Wittum TE. Swab type, moistening, and preenrichment for Staphylococcus aureus on environmental surfaces. J Clin Microbiol. 2010;48:2235–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín M, Arroyo R, Espinosa-Martos I et al. Identification of emerging human mastitis pathogens by MALDI-TOF and assessment of their antibiotic resistance patterns. Front Microbiol. 2017;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe O, Ross RP, Hill C. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol Rev. 2001;25:285–308. [DOI] [PubMed] [Google Scholar]
- Michie C, Lockie F, Lynn W. The challenge of mastitis. Arch Dis Child. 2003;88:818–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Butcher AM et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci Adv. 2018;4:eaao4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Saisindhu N et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsuga K. Epidermal barriers. Cold Spring Harbor Perspect Med. 2014;4:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz DJ, Pohl R, Beck-Sickinger AG et al. Biochemical characterisation and genetic analysis of aureocin A53, a new, atypical bacteriocin from Staphylococcus aureus. J Mol Biol. 2002;319:745–56. [DOI] [PubMed] [Google Scholar]
- Nilsen T, Nes IF, Holo H. Enterolysin A, a novel cell wall degrading bacteriocin secreted from Enterococcus faecalis LMG 2333. Appl Environ Microbiol. 2002;69:2975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Staphylococcus epidermidis—the 'accidental' pathogen. Nat Rev Microbiol. 2009;7:555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol. 2010;5:183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessanha B, Farb A, Lwin T et al. Infectious endocarditis due to Corynebacterium xerosis. Cardiovasc Pathol. 2003;12:98–101. [DOI] [PubMed] [Google Scholar]
- Rathi SK. Acne vulgaricus treatment : the current scenario. Indian J Dermatol. 2011;56:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MC, Sit CS, Clayton E et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci. 2010;107:9352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MP, Rea MC, Hill C et al. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl Environ Microbiol. 1996;62:612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JA, Gallo RL. Functions of the skin microbiota in health and disease. Semin Immunol. 2013;25:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res Int. 2016;2016:2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashihara T, Kimura H, Higuchi T et al. Cloning of the gene encoding a novel lantibiotic, nukacin ISK-1, of Staphylococcus warneri. J Facult Agricult, Kyushu Univ. 2000;45:149–61. [Google Scholar]
- Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell N, Engelke G, Augustin J et al. Analysis of genes involved in the biosynthesis of lantibiotic epidermin. European J Biochem/FEBS. 1992;204:57–68. [DOI] [PubMed] [Google Scholar]
- Schnell N, Entian KD, Götz F et al. Structural gene isolation and prepeptide sequence of gallidermin, a new lanthionine containing antibiotic. FEMS Microbiol Lett. 1989;49:263–7. [DOI] [PubMed] [Google Scholar]
- Shah MM, Iihara H, Noda M et al. dnaJ gene sequence-based assay for species identification and phylogenetic grouping in the genus Staphylococcus. Int J Syst Evol Microbiol. 2007;57:25–30. [DOI] [PubMed] [Google Scholar]
- Shin JM, Gwak JW, Kamarajan P et al. Biomedical applications of nisin. J Appl Microbiol. 2016;120:1449–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC, Arbeit RD, Goering RV et al. Interpreting chromosomal DNA restriction patterns produced by pulsed- field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GF. Clinical review Acne vulgaris. BMJ (Clin Res Ed). 2002;325:475–9. [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Gallo R. Antimicrobial peptides in human skin disease. Eur J Dermatol. 2008;18:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Lin C, Sung CT et al. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol. 2014;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeuwen PL, Kleerebezem M, Timmerman HM et al. Microbiome and skin diseases. Curr Opin Allergy Clin Immunol. 2013;13:514–20. [DOI] [PubMed] [Google Scholar]
- Zipperer A, Konnerth MC, Laux C et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature. 2016;535:511–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.