Abstract
Objectives
Long-term effects of gastric bypass (GBP) surgery have been presented in observational and randomised studies, but there are only limited data for persons with obesity and type 2 diabetes mellitus (T2DM) regarding postoperative complications.
Design
This is a nationwide observational study based on two quality registers in Sweden (National Diabetes Register, NDR and Scandinavian Obesity Surgery Register, SOReg) and other national databases.
Setting
After merging the data, we matched individuals with T2DM who had undergone GBP with those not surgically treated for obesity on propensity score, based on sex, age, body mass index (BMI) and calendar time. The risks of postoperative outcomes (rehospitalisations) were assessed using Cox regression models.
Participants
We identified 5321 patients with T2DM in the SOReg and 5321 matched controls in the NDR, aged 18–65 years, with BMI >27.5 kg/m² and followed for up to 9 years.
Primary and secondary outcome measures
We assessed risks for all-cause mortality and hospitalisations for cardiovascular disease, severe kidney disease, along with surgical and other medical conditions.
Results
The results agree with the previously suggested lower risks of all-cause mortality (49%) and cardiovascular disease (34%), and we also found positive effects for severe kidney disease but significantly increased risks (twofold to ninefold) of several short-term complications after GBP, such as abdominal pain and gastrointestinal conditions, frequently requiring surgical procedures, apart from reconstructive plastic surgery. Long-term, the risk of anaemia was 92% higher, malnutrition developed approximately three times as often, psychiatric diagnoses were 33% more frequent and alcohol abuse was three times as great as in the control group.
Conclusions
This nationwide study confirms the benefits and describes the panorama of adverse events after bariatric surgery in persons with obesity and T2DM. Long-term postoperative monitoring and support, as better selection of patients by appropriate specialists in interdisciplinary settings, should be provided to optimise the outcomes.
Keywords: diabetes mellitus, obesity, bariatric surgery, postoperative complications, adverse effects
Strengths and limitations of this study.
The major strength of our study is the unique and nationwide character of our population with type 2 diabetes that received gastric bypass operation.
The high data reliability as well the external validity allow the generalising of our results to similar developed countries using the same criteria and contraindications for bariatric surgery and quality of care.
Our non-randomised observational study may be limited by some minor differences between the matched groups on the propensity score.
We tried to eliminate major confounders by careful matching between the two groups as well with an adjusted Cox regression model, however, we cannot exclude underlying residual confounders.
We studied effects and postoperative events after gastric bypass in inpatients (rehospitalisations) leaving unassessed a large proportion of outpatients visiting the primary care.
Introduction
The most effective method for ensuring long-term weight reduction in individuals with obesity as well as beneficial effects on mortality, cardiovascular disease (CVD) and cardiovascular (CV) risk factors is bariatric surgery, Roux-en-Y gastric bypass (GBP) in particular.1 2 These effects of GBP have also been shown in patients with type 2 diabetes mellitus (T2DM) in both observational3–5 and randomised control trials6–8 under different follow-up periods. However, it has also been demonstrated in cohorts with a low proportion of individuals with diabetes that GBP is associated with postoperative complications and readmission rates from 0.6% to 11.3%,9–12 as well as long-term adverse outcomes such as hypoglycaemia,6 anaemia, nutritional deficiencies,13 gallstones,14 depression,15 suicide and non-fatal self-harm16 and alcohol problems.17
Only few reports have addressed the long-term incidence of complications in patients with obesity and T2DM who have undergone bariatric surgery. The Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently study reported adverse events of GBP and sleeve gastrectomy compared with conventional medical therapy, but only in 142 individuals with T2DM randomised at a single centre with follow-up period up to 5 years.6 Similarly, the Diabetes Surgery Study recently reported clinical effects and adverse events after GBP or lifestyle–medical management in 120 individuals after 5 years.18 Larger prospective studies such as Swedish Obese Subjects study1 and large American observational studies with broad samples10 19 have addressed postoperative outcomes and readmission rates of GBP or other types of bariatric surgery, but with only a small proportion of patients who have T2DM.
We recently conducted a nationwide observational study of individuals with T2DM who underwent GBP compared with matched individuals and reported beneficial effects on overall mortality and CV events,3 but we did not address short-term or long-term adverse effects. The objective of this observational cohort study is therefore to identify clinical benefits as well as a wide spectrum of early postoperative, as well as long-term adverse effects of GBP for up to 9 years in individuals with T2DM compared with individuals with obesity who have not received surgical treatment.
Research design and methods
This study is based on two nationwide quality registers in Sweden: the National Diabetes Register (NDR) and the Scandinavian Obesity Surgery Register (SOReg), as well as linked data from the Swedish Inpatient Register, the Cause of Death Register and the Statistics Sweden. All these databases have previously been described and validated.20 21 The NDR is a quality register tool that provides nearly full coverage (90% for T2DM and 95% for T1DM) of Swedes with diabetes since 1996. SOReg started in 2007 as a quality and research register. Since 2010, it has covered virtually all bariatric procedures in Sweden. All bariatric centres report to the register (surgical complications, postoperative reports and longitudinal effects).
After merging the data of SOReg and NDR, we identified individuals with diabetes and obesity who had undergone primary GBP between 1 January 2007 and 31 December 2015 (see online supplementary material). We subsequently matched them with control patients in the NDR who had not undergone bariatric surgery. Propensity score matching (1:1) was performed on the basis of sex, age (18–75 years), body mass index (BMI) (>27.5 kg/m²) and calendar time.
bmjopen-2018-023882supp001.pdf (272.6KB, pdf)
We based our definition of T2DM on classical epidemiological criteria, that is, treatment with diet, oral antihyperglycaemic agents, insulin or different combinations, as well as patients who were ≥40 years of age at the time of diagnosis.
All clinical characteristics at baseline were obtained from the NDR and SOReg, socioeconomic status was taken from Statistics Sweden, and presurgical and postsurgical diagnoses were taken from the Swedish Inpatient Register, International Classification of Diseases (ICD-10) (table S1, online supplementary material), which are held by the National Board of Health and Welfare. The Inpatient Registry records all inpatient admissions since 1987. We studied admissions to the hospitals by including specific diagnoses for coronary heart disease, acute myocardial infarction, stroke, atrial fibrillation, heart failure and valvular heart disease, as well as acute and chronic diseases that were related to diabetes mellitus (hyperglycaemia, hypoglycaemia with coma, amputation, kidney, liver and pulmonary diseases, cancer, anaemia, malnutrition, dementia, psychiatric disorders and alcohol abuse). We also report surgical history, such as hospitalisation due to bleeding, gastrointestinal (GI) surgery and leakage, wound complications, GI ulcers and reflux disease, bowel obstruction, hernia, gall bladder disease and pancreatitis, as well previous plastic surgery.
Patients were followed up to 9 years or until the first admission to the hospital for specific diagnoses or group of diagnoses or death. Controls who were treated with GBP were censored on the date of such treatment.
Statistical analysis
One matched control was selected for each GBP patient using propensity scores for longitudinal exposure.22 The outcome of the propensity score matching was assessed only through descriptive statistics comparing the matched groups. Thus, controls were matched to GBP patients based on the estimated risk score from a Cox regression model with time-updated data, where exposure for GBP was the endpoint. The model contained covariates for sex, age and BMI. Controls were selected in chronological order.
Descriptive statistics are presented using means with SD for age and BMI, median with quartiles for income and counts with percentages for all other variables. Incidence rates for each outcome were estimated using counts and person-years. Comparisons between GBP patients and controls used Cox regression, adjusted for sex, age, BMI and socioeconomic factors (income, marital status, education level and country of origin). No adjustments were made for multiple inferences. Thus, while p values below 5% were considered statistically significant, the outcome of individual hypothesis tests should be interpreted with caution.
Patient and public involvement statement
The authors developed the research question and outcome measures. The patients and public were not involved in the design or conduct of the study. The results will be disseminated to study participants via media and health centres.
Results
We identified 5321 patients in the SOReg who had T2DM and had undergone GBP (96.0% laparoscopic, 1.7% initially laparoscopic and converted to open surgery and 2.3% primary open surgery), as well as 5321 matched controls in the NDR (online supplementary material). Both groups were followed for up to 9 years (mean, 4.5 years). Table 1 shows the baseline characteristics of both groups. There were some minor differences between the groups (standardised differences of more than 0.1): the GBP persons had a slightly higher mean age and BMI and were less likely to be single (marital status), with a greater mean income and higher educational level. The groups were well matched with respect to previous CV, GI, psychiatric and surgical diseases (standardised differences less than 0.1).
Table 1.
Baseline characteristics
| GBP (n=5321) | Control (n=5321) | Standardised difference* | |
| Sex | |||
| Men | 2098 (39.4%) | 1926 (36.2%) | 0.0471 |
| Women | 3223 (60.5%) | 3395 (63.8%) | 0.0471 |
| Age | 49.0 (9.5) | 47.1 (11.5) | 0.122 |
| BMI (kg/m2) | 42.0 (5.7) | 40.9 (7.3) | 0.117 |
| Income (Kr) | 199.638 (139 136; 261 558) | 168.380 (121 840; 239 368) | 0.156 |
| Marital status | |||
| Single | 1602 (30.1%) | 2064 (38.8%) | 0.130 |
| Married | 2518 (47.4%) | 2227 (41.9%) | 0.0781 |
| Separated | 1092 (20.5%) | 881 (16.6%) | 0.0723 |
| Widowed | 106 (2.0%) | 147 (2.8%) | 0.0358 |
| Education level | |||
| Compulsory school | 1069 (20.1%) | 1431 (26.9%) | 0.114 |
| University | 3192 (60.0%) | 2847 (53.5%) | 0.0926 |
| Upper secondary school | 1037 (19.5%) | 930 (17.5%) | 0.0366 |
| Missing data | 23 (0.4%) | 113 (2.1%) | 0.107 |
| Country of origin | |||
| Sweden | 4261 (80.1%) | 4027 (75.7%) | 0.075 |
| Rest of Europe | 514 (9.7%) | 602 (11.3%) | 0.0382 |
| Rest of the world | 546 (10.3%) | 692 (13.0%) | 0.0607 |
| Cardiovascular | |||
| Cardiovascular disease | 273 (5.1%) | 261 (4.9%) | 0.00730 |
| Acute myocardial infarction | 173 (3.2%) | 169 (3.2%) | 0.00301 |
| Coronary heart disease | 395 (7.4%) | 313 (5.9%) | 0.0437 |
| Congestive heart failure | 140 (2.6%) | 168 (3.2%) | 0.0222 |
| Atrial fibrillation | 148 (2.8%) | 149 (2.8%) | 0.000807 |
| Valvular heart disease | 24 (0.4%) | 27 (0.5%) | 0.00577 |
| Stroke | 109 (2.0%) | 103 (1.9%) | 0.00571 |
| Deep vein thrombosis/pulmonary embolism | 71 (1.3%) | 65 (1.2%) | 0.00710 |
| Diabetes related | |||
| Hyperglycaemia | 80 (1.5%) | 130 (2.4%) | 0.0478 |
| Hypoglycaemia (with or without coma) | 57 (1.1%) | 61 (1.2%) | 0.00508 |
| Gastrointestinal | |||
| Gastrointestinal surgery (not GBP) | 549 (10.3%) | 644 (12.1%) | 0.0400 |
| Abdominal pain | 386 (7.2%) | 334 (6.3%) | 0.0275 |
| Gallstone, gallbladder disease and pancreatitis | 419 (7.9%) | 366 (6.9%) | 0.0270 |
| Gastrointestinal ulcer and reflux | 86 (1.6%) | 72 (1.4%) | 0.0154 |
| Hernia | 204 (3.8%) | 160 (3.0%) | 0.0322 |
| Bowel obstruction | 18 (0.3%) | 29 (0.6%) | 0.0220 |
| Gastrointestinal leakage | 7 (0.1%) | 17 (0.3%) | 0.0280 |
| Liver disease | 16 (0.3%) | 26 (0.5%) | 0.0212 |
| Surgical | |||
| Plastic surgery | 54 (1.0%) | 33 (0.6%) | 0.0310 |
| Wound complications | 192 (3.6%) | 156 (2.9%) | 0.0269 |
| Bleeding | 50 (0.9%) | 32 (0.6%) | 0.0273 |
| Other | |||
| Psychiatric disorders | 318 (6.0%) | 346 (6.5%) | 0.0154 |
| Alcohol abuse | 94 (1.8%) | 122 (2.3%) | 0.0264 |
| Cancer | 111 (2.1%) | 158 (3.0%) | 0.0398 |
| Malnutrition | 21 (0.4%) | 41 (0.8%) | 0.0349 |
| Kidney disease | 56 (1.0%) | 83 (1.6%) | 0.0316 |
| Pulmonary disease | 128 (2.4%) | 131 (2.5%) | 0.00259 |
| Anaemia | 55 (1.0%) | 60 (1.1%) | 0.00643 |
| Amputation | 10 (0.2%) | 12 (0.2%) | 0.00585 |
| Dementia | 1 (0.02%) | 4 (0.08%) | 0.0184 |
Numbers and proportions.
*Difference between sample means divided by SD. Acceptable significance when standardised difference <0.1.
GBP, gastric bypass.
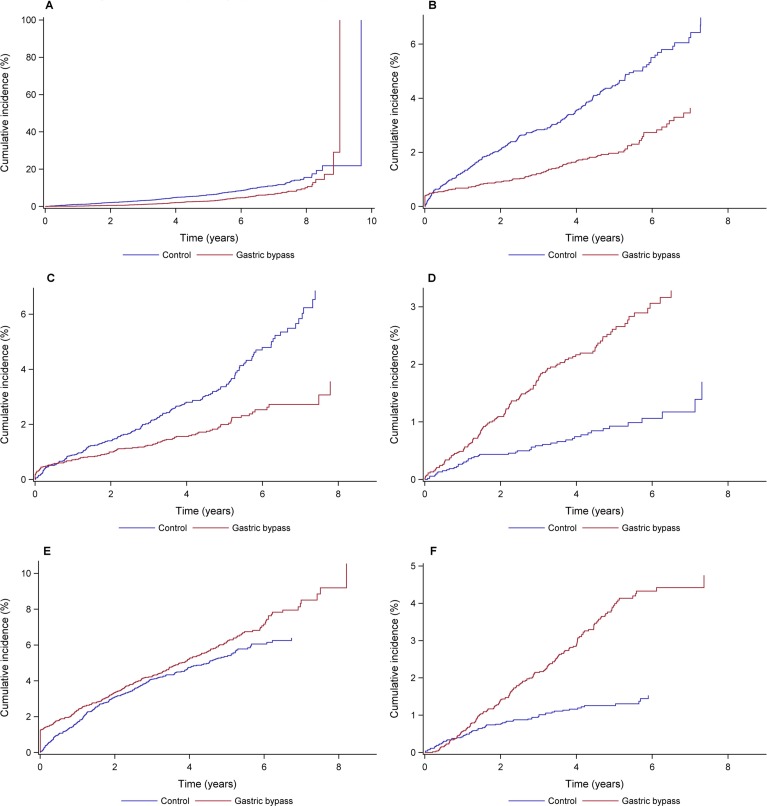
Table 2 shows the number of events and incidence rates during the follow-up period. Event rates for all-cause mortality were 72.9 and 142.1 per 10 000 person-years in GBP and the control group, respectively (HR 0.51, 95% CI 0.43 to 0.62; figure 1A). Risks for CVD or coronary heart disease, acute myocardial infarction and congestive heart failure (figure 1B) were also lower after GBP.
Table 2.
Number of events and event rates during follow-up
| Outcome | GBP (n=5321) | Control (n=5321) | HR (95% CI) | P value |
| All-cause mortality | 183 (72.90) | 351 (142.06) | 0.51 (0.43 to 0.62) | <0.0001 |
| Cardiovascular | ||||
| Cardiovascular disease | 108 (43.54) | 150 (61.54) | 0.66 (0.51 to 0.85) | 0.0014 |
| Fatal cardiovascular disease | 21 (8.38) | 64 (25.94) | 0.34 (0.20 to 0.56) | <0.0001 |
| Acute myocardial infarction | 51 (20.43) | 85 (34.69) | 0.55 (0.39 to 0.79) | 0.0010 |
| Coronary heart disease | 309 (128.66) | 274 (114.28) | 1.13 (0.95 to 1.34) | 0.156 |
| Fatal coronary heart disease | 28 (11.17) | 77 (31.20) | 0.35 (0.22 to 0.54) | <0.0001 |
| Congestive heart failure | 109 (43.94) | 225 (93.05) | 0.49 (0.39 to 0.62) | <0.0001 |
| Atrial fibrillation | 204 (83.64) | 213 (88.16) | 0.93 (0.76 to 1.14) | 0.486 |
| Valvular heart disease | 21 (8.39) | 32 (13.00) | 0.64 (0.36 to 1.14) | 0.131 |
| Stroke | 59 (23.69) | 71 (28.94) | 0.77 (0.54 to 1.10) | 0.158 |
| Deep vein thrombosis/pulmonary embolism | 56 (22.48) | 59 (24.07) | 1.01 (0.69 to 1.48) | 0.952 |
| Diabetes related | ||||
| Hypoglycaemia (with or without coma) | 43 (17.24) | 46 (18.72) | 1.04 (0.68 to 1.60) | 0.844 |
| Hyperglycaemia | 23 (9.20) | 89 (36.37) | 0.33 (0.21 to 0.53) | <0.0001 |
| Gastrointestinal | ||||
| Gastrointestinal surgery (not GBP) | 936 (422.59) | 301 (125.76) | 3.33 (2.91 to 3.80) | <0.0001 |
| Abdominal pain | 558 (239.25) | 124 (50.94) | 5.52 (4.51 to 6.75) | <0.0001 |
| Gallstone, gallbladder disease and pancreatitis | 312 (129.31) | 125 (51.30) | 2.49 (2.02 to 3.08) | <0.0001 |
| Gastrointestinal ulcer and reflux | 239 (98.58) | 46 (18.73) | 5.42 (3.91 to 7.51) | <0.0001 |
| Hernia | 235 (97.00) | 86 (35.17) | 2.75 (2.14 to 3.54) | <0.0001 |
| Bowel obstruction | 232 (95.29) | 27 (10.97) | 9.47 (6.31 to 14.20) | <0.0001 |
| Gastrointestinal leakage | 40 (16.05) | 7 (2.84) | 5.54 (2.46 to 12.45) | <0.0001 |
| Liver disease | 30 (12.00) | 40 (16.26) | 0.73 (0.45 to 1.19) | 0.205 |
| Surgical | ||||
| Plastic surgery | 380 (158.08) | 22 (8.94) | 19.85 (12.86 to 30.67) | <0.0001 |
| Wound complications | 290 (120.87) | 87 (35.55) | 3.45 (2.70 to 4.42) | <0.0001 |
| Bleeding | 172 (70.50) | 26 (10.57) | 6.87 (4.49 to 10.52) | <0.0001 |
| Other | ||||
| Psychiatric disorder | 317 (131.64) | 268 (111.93) | 1.33 (1.13 to 1.58) | 0.0008 |
| Alcohol abuse | 180 (73.10) | 65 (26.52) | 2.90 (2.16 to 3.88) | <0.0001 |
| Cancer | 153 (61.80) | 188 (77.41) | 0.78 (0.63 to 0.97) | 0.0257 |
| Malnutrition | 128 (51.69) | 46 (18.72) | 2.81 (1.98 to 3.97) | <0.0001 |
| Kidney disease | 105 (42.38) | 187 (76.87) | 0.58 (0.45 to 0.75) | <0.0001 |
| Pulmonary complications | 86 (34.66) | 114 (46.64) | 0.84 (0.63 to 1.13) | 0.249 |
| Anaemia | 84 (33.78) | 46 (18.71) | 1.92 (1.33 to 2.76) | 0.0005 |
| Amputation | 15 (5.99) | 23 (9.33) | 0.51 (0.26 to 0.98) | 0.0432 |
| Dementia | 4 (1.60) | 12 (4.87) | 0.46 (0.14 to 1.57) | 0.214 |
Event rates (%) per 10 000 person-years.
GBP, gastric bypass.
Figure 1.
A–F Cumulative incidence of postoperative outcomes during the 9 years follow-up. All-cause mortality; congestive heart failure; kidney disease; malnutrition; psychiatric disorder; alcohol abuse.
Other benefits were observed after GBP. Hospitalisation for hyperglycaemia was less frequent, and the risks of kidney disease (figure 1C), leg amputation and cancer were lower (table 2). GBP individuals were, however, at greater risk for anaemia (HR 1.92, 95% CI 1.33 to 2.76) and malnutrition (HR 2.81, 95% CI 1.98 to 3.97) (figure 1D). The risks of hospitalisation due to psychiatric disorders or alcohol abuse (figure 1E,F) increased after GBP (73.1 and 26.5 per 10 000 person-years in GBP and the control group, respectively, HR 1.33, 95% CI 1.13 to 1.58 and HR 2.90, 95% CI 2.16 to 3.88).
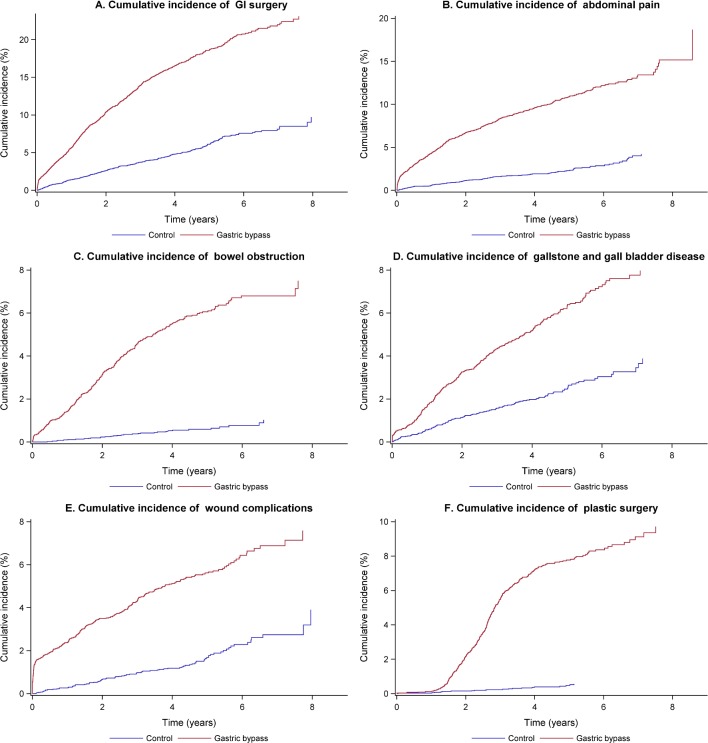
A number of adverse conditions, frequently necessitating additional GI surgery, were also observed more often in the GBP group: abdominal pain, bowel obstruction, gallstones, gallbladder disease, pancreatitis, GI ulcers, reflux, hernia, GI leakage, wound complications and bleeding (figure 2A–E). Subsequent reconstructive plastic surgery (figure 2F) was also required frequently, while the risk for pulmonary complications, embolism, deep vein thrombosis or liver disease was slightly lower.
Figure 2.
A–F Cumulative incidence of postoperative adverse events during the 9 years follow-up. Gastrointestinal surgery; abdominal pain; bowel obstruction; gallstone and gallbladder disease; wound complications; plastic surgery.
We analysed results of GBP treatment in men and women using a Cox regression model adjusted for sex, age, BMI and socioeconomic factors (table S2, online supplementary material). The significant interactions we noted were risks for fatal CVD, atrial fibrillation, congestive heart failure and GI surgery (higher in men after GBP, p<0.05), while women were at a higher risk (1.51, 95% CI 1.23 to 1.85) of being hospitalised due to a psychiatric disorder after GBP.
Discussion
This observational study compares outcomes after GBP (rehospitalisations) in individuals with obesity and T2DM with a matched group of those who have not been surgically treated. We confirm the previously shown beneficial effects on all-cause mortality and CV morbidity in individuals with or without T2DM,1 3 as well as presenting a panorama of short-term and long-term complications after GBP on a nationwide scale. Common reasons for postoperative hospital admissions were GI conditions such as abdominal pain, gallstone/gallbladder disease, pancreatitis, GI ulcer, leakage, reflux, hernia, bowel obstruction, psychiatric disorders and alcohol abuse.
Additional GI surgery was performed in 17.6% of the GBP group, more than three times as much as in the control group. GI leakage, bleeding, abdominal pain and bowel obstruction are likely causes for these surgical interventions, as well as gallstone disease and cholecystitis, which are frequently observed after GBP and rapid weight loss.14 23–25 Wanjura et al recently showed that the incidence of cholecystectomy was substantially elevated before GBP and increased 6–36 months after surgery compared with the general population.24 Previous GBP doubled the risk of complications after cholecystectomy, almost quadrupled the risk of reoperation24 and the simultaneous cholecystectomy increased the risk by increasing of the operation time.25 It has been suggested that defective gallbladder emptying in conjunction with the production of crystallisation-promoting compounds (mucin) can contribute to the development of cholesterol crystals and gallstones in subjects with obesity during weight reduction.23
Some postoperative complications were common shortly after GBP (leakage, wound complications and ulcer/reflux), while others (hernia, bowel obstruction and gallstone) generally increased after 1–2 years. These findings were expected, although the incidence of ulcers and reflux disease soon after GBP may be exaggerated due to the endoscopies for dyspepsia and dysphoric symptoms. Hernias may well be undiagnosed preoperatively but detected during surgery and become symptomatic after weight loss when the associated fat disappears. The incidence of wound complications and GI leakage shortly after GBP was comparable to other studies with short follow-up periods and a small percentage of patients with diabetes.26–28 There were no major differences between men and women in the risk for specific postoperative complications, apart from a slightly higher incidence of additional surgical procedures and CV risk (fatal CVD) in men, as previously suggested.11 29
There was a 42% lower relative risk of hospitalisation due to severe kidney disease after GBP. A systematic review has previously suggested that weight loss is associated with reductions in proteinuria and microalbuminuria. A retrospective cohort study showed a higher mean estimated glomerular filtration rate (eGFR) in patients up to 3 years after bariatric surgery than those with moderately impaired renal function (Chronic Kidney Disease stages 3 and 4) who were referred for, but did not receive, surgery.30 31 There has been no prospective study in patients with severe renal disease. Retrospective data are limited by study design and estimations of renal function. eGFR calculations depend on muscle mass and serum creatinine levels, both of which change after weight loss independent of kidney function. Although the selection of patients eligible for bariatric surgery can contribute to the apparent beneficial effects on risk of severe kidney disease, these results should prompt new studies concerning the effects on renal function, as well as optimal patients for surgery to treat weight loss. Improved glycaemic and blood pressure control after GBP32 33 could also contribute to the apparent effects of including changes in dose of antihypertensives, which are known to affect serum creatinine. We did not evaluate glycaemic control in this study, but pronounced effects after bariatric surgery have been demonstrated repeatedly.6 34 35
The anatomical and physiological consequences of GBP result in a higher risk of long-term deficiencies of several vitamins and minerals.36 The present study had no access to data from primary care, where follow-up should start 2 years after GBP, but malnutrition and anaemia were twice as common. Poor compliance with vitamin and mineral supplements, as well as irregular follow-up, may very likely explain these results. A recent meta-analysis pointed to this potential problem in individuals without diabetes, suggesting that diabetes is not a risk factor per se.13 Adequate supplementation is paramount,37 since deficiencies after GBP tend to increase over time.13 38
A history of psychiatric disorders requiring hospitalisation was not uncommon in either group of individuals with obesity in this study, and was 33% higher after GBP. Previous studies have shown that depression, which may improve in the first year following bariatric surgery, tends to progress39 along with suicide and self-harm, particularly if they are pre-existing conditions.15 16 Thus, greater awareness is needed in order to identify vulnerable patients with a history of self-harm or depression who may need psychiatric services after GBP. Perhaps specific multidisciplinary teams should identify such patients and through treatment algorithms could enhance the safety and efficacy pre and postoperatively.40 In agreement with previous studies,17 41 we confirmed a higher event rate of alcohol-related problems that lead to hospitalisation after GBP, which points to the importance of careful selection of patients who are offered surgery, as well as better follow-up of those with a history of alcohol-related risk behaviour. The mechanisms of this well-known phenomenon are still unknown.
The indications for surgical treatment of obesity were presented by the National Institute of Health in 199142 and have been repeatedly revised and expanded over the years. Severe and untreated psychopathology as well as active alcohol or substance abuse, or eating disorders are contraindications to bariatric surgery, although the decision to offer this treatment should always be individualised based on the stability of conditions and the assessment of multidisciplinary treatment teams.43 The need for more robust criteria and the possible application of scoring systems or algorithms that could facilitate the assessment of patients beyond BMI has been discussed.44
A major strength of this study is its nationwide coverage of patients with obesity and T2DM, all of whom received recent GBP surgery. The results are likely to be generalisable to similar developed countries using the same criteria and contraindications for bariatric surgery and quality of care. All linked databases are characterised by high participation rates and validation of medical data.21 45
Our study was non-randomised and observational, but with carefully matched groups to maximise the size of the cohort as well as to reduce the influence of confounding factors. Minor differences in clinical characteristics may still influence our results, and we also did not include some variables (eg, duration of diabetes, glycated haemoglobin A1c, use of antidiabetic drugs) that potentially also could affect the results. Similarly, we did not exclude patients with multiple comorbidities before the intervention, because we would have lost substantial data and they had all qualified for GBP. We also used Cox proportional hazards regression modelling, including baseline characteristics, to minimise the effects of confounding. Certainly, we cannot rule out residual confounding, unobserved factors that may be related to both exposure and outcome. However, the external validity is most likely high as our study includes virtually all GBP patients with T2DM in Sweden during the time period.
Another limitation is that we captured diagnoses during hospitalisation, not outpatient care. Comorbidities and incidence of postoperative outcomes may be underestimates as a result, but the systematic flaw could not be avoided. Nevertheless, measurement errors may potentially arise because the patients who had received surgery were followed up more frequently than the control group. GBP was the only surgical procedure we studied (96% laparoscopic), given that sleeve gastrectomy and duodenal switch were not performed very often and follow-up data were too limited during the study period. We also did not address the importance of more specific surgical techniques.
Individuals with obesity and T2DM who have undergone GBP are generally at a reduced risk of all-cause mortality and CV morbidity, as well as severe kidney disease and cancer to a lesser extent. They also have, however, significantly higher risks of postoperative complications and adverse events both short term and long term, mostly abdominal pain and GI conditions that frequently require additional surgical procedures, apart from reconstructive plastic surgery. Long-term consequences observed more often are anaemia, malnutrition, psychiatric disorders and alcohol abuse. In order to maximise the benefit and minimise the risk of problems, long-term postoperative monitoring and support should be provided. Better selection of patients for such treatment, performed by appropriate specialists in interdisciplinary settings, could probably also optimise outcomes.
Supplementary Material
Footnotes
Patient consent for publication: Not required.
Contributors: VL, SF, A-MS, MM, JO, IN, SG and BE contributed to the conception and design of the study. SF, MM, A-MS, JO and IN contributed to the acquisition of data and SF performed the statistical analyses. All authors contributed to the interpretation of data. VL and BE drafted the article, and VL, SF, A-MS, MM, JO, IN, SG and BE contributed to critical revision. BE is the guarantor of this work, had full access to the data and assumes responsibility for their integrity and analysis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: The regional ethical review board at the University of Gothenburg, Sweden approved the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: This is a registry study and therefore the data generated are not suitable for sharing beyond that contained within the report. Further information can be obtained from the corresponding author.
References
- 1. Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52. 10.1056/NEJMoa066254 [DOI] [PubMed] [Google Scholar]
- 2. Kwok CS, Pradhan A, Khan MA, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol 2014;173:20–8. 10.1016/j.ijcard.2014.02.026 [DOI] [PubMed] [Google Scholar]
- 3. Eliasson B, Liakopoulos V, Franzén S, et al. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol 2015;3:847–54. 10.1016/S2213-8587(15)00334-4 [DOI] [PubMed] [Google Scholar]
- 4. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–56. 10.1016/j.amjmed.2008.09.041 [DOI] [PubMed] [Google Scholar]
- 5. Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–304. 10.1001/jama.2014.5988 [DOI] [PubMed] [Google Scholar]
- 6. Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376:641–51. 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia 2016;59:945–53. 10.1007/s00125-016-3903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol 2015;3:413–22. 10.1016/S2213-8587(15)00089-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saunders JK, Ballantyne GH, Belsley S, et al. 30-day readmission rates at a high volume bariatric surgery center: laparoscopic adjustable gastric banding, laparoscopic gastric bypass, and vertical banded gastroplasty-Roux-en-Y gastric bypass. Obes Surg 2007;17:1171–7. 10.1007/s11695-007-9210-3 [DOI] [PubMed] [Google Scholar]
- 10. Berger ER, Huffman KM, Fraker T, et al. Prevalence and risk factors for bariatric surgery readmissions: findings from 130,007 admissions in the metabolic and bariatric surgery accreditation and quality improvement program. Ann Surg 2018;267:122–31. 10.1097/SLA.0000000000002079 [DOI] [PubMed] [Google Scholar]
- 11. Dayer-Jankechova A, Fournier P, Allemann P, et al. Complications after laparoscopic roux-en-y gastric bypass in 1573 consecutive patients: are there predictors? Obes Surg 2016;26:12–20. 10.1007/s11695-015-1752-1 [DOI] [PubMed] [Google Scholar]
- 12. Bruze G, Ottosson J, Neovius M, et al. Hospital admission after gastric bypass: a nationwide cohort study with up to 6 years follow-up. Surg Obes Relat Dis 2017;13:962–9. 10.1016/j.soard.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weng TC, Chang CH, Dong YH, et al. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. BMJ Open 2015;5:e006964 10.1136/bmjopen-2014-006964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melmer A, Sturm W, Kuhnert B, et al. Incidence of Gallstone Formation and Cholecystectomy 10 Years After Bariatric Surgery. Obes Surg 2015;25:1171–6. 10.1007/s11695-014-1529-y [DOI] [PubMed] [Google Scholar]
- 15. Lagerros YT, Brandt L, Hedberg J, et al. Suicide, self-harm, and depression after gastric bypass surgery: a nationwide cohort study. Ann Surg 2017;265:235–43. 10.1097/SLA.0000000000001884 [DOI] [PubMed] [Google Scholar]
- 16. Neovius M, Bruze G, Jacobson P, et al. Risk of suicide and non-fatal self-harm after bariatric surgery: results from two matched cohort studies. Lancet Diabetes Endocrinol 2018;6:197–207. 10.1016/S2213-8587(17)30437-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Svensson PA, Anveden Å, Romeo S, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity 2013;21:2444–51. 10.1002/oby.20397 [DOI] [PubMed] [Google Scholar]
- 18. Ikramuddin S, Korner J, Lee WJ, et al. Lifestyle Intervention and Medical Management With vs Without Roux-en-Y Gastric Bypass and Control of Hemoglobin A1c, LDL Cholesterol, and Systolic Blood Pressure at 5 Years in the Diabetes Surgery Study. JAMA 2018;319:266–78. 10.1001/jama.2017.20813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shin JH, Worni M, Castleberry AW, et al. The application of comorbidity indices to predict early postoperative outcomes after laparoscopic Roux-en-Y gastric bypass: a nationwide comparative analysis of over 70,000 cases. Obes Surg 2013;23:638–49. 10.1007/s11695-012-0853-3 [DOI] [PubMed] [Google Scholar]
- 20. Eliasson B, Gudbjörnsdottir S. Diabetes care-improvement through measurement. Diabetes Res Clin Pract 2014;106 Suppl 2:S291–4. 10.1016/S0168-8227(14)70732-6 [DOI] [PubMed] [Google Scholar]
- 21. Hedenbro JL, Näslund E, Boman L, et al. Formation of the scandinavian obesity surgery Registry, SOReg. Obes Surg 2015;25:1893–900. 10.1007/s11695-015-1619-5 [DOI] [PubMed] [Google Scholar]
- 22. Lu B. Propensity score matching with time-dependent covariates. Biometrics 2005;61:721–8. 10.1111/j.1541-0420.2005.00356.x [DOI] [PubMed] [Google Scholar]
- 23. Gustafsson U, Benthin L, Granström L, et al. Changes in gallbladder bile composition and crystal detection time in morbidly obese subjects after bariatric surgery. Hepatology 2005;41:1322–8. 10.1002/hep.20686 [DOI] [PubMed] [Google Scholar]
- 24. Wanjura V, Sandblom G, Österberg J, et al. Cholecystectomy after gastric bypass-incidence and complications. Surg Obes Relat Dis 2017;13:979–87. 10.1016/j.soard.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 25. Wanjura V, Szabo E, Österberg J, et al. Morbidity of cholecystectomy and gastric bypass in a national database. Br J Surg 2018;105:121–7. 10.1002/bjs.10666 [DOI] [PubMed] [Google Scholar]
- 26. Stenberg E, Szabo E, Agren G, et al. Early complications after laparoscopic gastric bypass surgery: results from the Scandinavian Obesity Surgery Registry. Ann Surg 2014;260:1040–7. 10.1097/SLA.0000000000000431 [DOI] [PubMed] [Google Scholar]
- 27. Maciejewski ML, Winegar DA, Farley JF, et al. Risk stratification of serious adverse events after gastric bypass in the bariatric outcomes longitudinal database. Surg Obes Relat Dis 2012;8:671–7. 10.1016/j.soard.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 28. Yong PH, Weinberg L, Torkamani N, et al. The Presence of Diabetes and Higher HbA1c Are Independently Associated With Adverse Outcomes After Surgery. Diabetes Care 2018;41:1172–9. 10.2337/dc17-2304 [DOI] [PubMed] [Google Scholar]
- 29. Livingston EH, Huerta S, Arthur D, et al. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg 2002;236:576–82. 10.1097/00000658-200211000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Afshinnia F, Wilt TJ, Duval S, et al. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant 2010;25:1173–83. 10.1093/ndt/gfp640 [DOI] [PubMed] [Google Scholar]
- 31. Imam TH, Fischer H, Jing B, et al. Estimated GFR Before and After Bariatric Surgery in CKD. Am J Kidney Dis 2017;69:380–8. 10.1053/j.ajkd.2016.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93. 10.1056/NEJMoa035622 [DOI] [PubMed] [Google Scholar]
- 33. Courcoulas AP, King WC, Belle SH, et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg 2018;153:427–34. 10.1001/jamasurg.2017.5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liakopoulos V, Franzén S, Svensson A-M, et al. Changes in risk factors and their contribution to reduction of mortality risk following gastric bypass surgery among obese individuals with type 2 diabetes: a nationwide, matched, observational cohort study. BMJ Open Diab Res Care 2017;5:e000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964–73. 10.1016/S0140-6736(15)00075-6 [DOI] [PubMed] [Google Scholar]
- 36. Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab 2006;91:4223–31. 10.1210/jc.2006-0557 [DOI] [PubMed] [Google Scholar]
- 37. Ziegler O, Sirveaux MA, Brunaud L, et al. Medical follow up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab 2009;35:544–57. 10.1016/S1262-3636(09)73464-0 [DOI] [PubMed] [Google Scholar]
- 38. Olbers T, Beamish AJ, Gronowitz E, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol 2017;5:174–83. 10.1016/S2213-8587(16)30424-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitchell JE, King WC, Chen JY, et al. Course of depressive symptoms and treatment in the longitudinal assessment of bariatric surgery (LABS-2) study. Obesity 2014;22:1799–806. 10.1002/oby.20738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spittal MJ, Frühbeck G. Bariatric surgery: many benefits, but emerging risks. Lancet Diabetes Endocrinol 2018;6:161–3. 10.1016/S2213-8587(17)30435-7 [DOI] [PubMed] [Google Scholar]
- 41. King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. Jama 2012;307:2516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. NIH conference. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956–61. [PubMed] [Google Scholar]
- 43. De Luca M, Angrisani L, Himpens J, et al. Indications for Surgery for Obesity and Weight-Related Diseases: Position Statements from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg 2016;26:1659–96. 10.1007/s11695-016-2271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fruhbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol 2015;11:465–77. [DOI] [PubMed] [Google Scholar]
- 45. Emilsson L, Lindahl B, Köster M, et al. Review of 103 swedish healthcare quality registries. J Intern Med 2015;277:94–136. 10.1111/joim.12303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023882supp001.pdf (272.6KB, pdf)