Abstract
Introduction
Although diabetes distress is found to be associated with decreased glycaemic control among adults with type 1 diabetes, the psychological and emotional impact of living with the condition is often not recognised and often under-reported in diabetes care. Therefore, regular assessment of diabetes distress is recommended. Assessment of diabetes distress using patient-reported outcome measures (PROMs) in clinical practice has the potential to enhance care for people with diabetes by identifying problems and improving patient–clinician communication. In this study protocol, we describe a pilot randomised controlled trial (RCT) aiming to test the feasibility of all components of an empowerment-based intervention using PROMs as dialogue support in clinical diabetes consultations, and to address the uncertainties associated with running a fully powered evaluation study.
Methods and analysis
We will undertake a two-arm pilot RCT of an intervention using the Problem Areas In Diabetes (PAID) scale in clinical diabetes consultations in order to conclude whether a fully powered trial is appropriate and/or feasible. The study will also include qualitative indepth interviews with participants and healthcare providers. Our objectives are to (1) evaluate the recruitment procedures and attrition rates; (2) evaluate the performance of the randomisation procedure; (3) evaluate the participants’ mean scores on the outcome measures before and after the intervention; (4) evaluate if the intervention consultations are acceptable and feasible; and (5) explore patients’ and healthcare providers’ experiences with the use of PAID as dialogue support and empowerment-based communication skills in clinical diabetes consultations. The quantitative data analysis includes descriptive statistics (frequencies, percentages, means, SD and CI). For the qualitative data, we will perform thematic analysis.
Ethics and dissemination
Ethical approval has been obtained from the Western Norway Regional Committee for Medical and Health Research Ethics (2017/1506/REC west). We will present the findings from the study phases at national and international conferences and submit manuscripts to peer-reviewed journals and popular science journals.
Trial registration number
NCT03471104; Pre-results.
Keywords: general diabetes, quality in health care
Strengths and limitations of this study.
This is a study with the potential to provide new knowledge about the use of patient-reported outcome measures (PROMs) as dialogue support in clinical diabetes consultations among patients with type 1 diabetes.
The use of the Medical Research Council’s framework as a guide for the development of study intervention initiatives like this is a strength because the feasibility and uncertainties related to a fully powered randomised controlled trial (RCT) can be illuminated before a resource-intensive fully powered RCT is conducted.
A key challenge includes possible contamination of the control group, although the completed PROMs will not be available in the electronic patient records of the participants in the control group.
Introduction
The management of type 1 diabetes (T1D) is complex, and people living with the condition need to make numerous daily choices related to their medical treatment.1 2 They need to monitor their blood glucose and administer insulin several times each day. The burden of living with T1D remains a challenge despite new insulin types and advances in insulin delivery and glucose monitoring technologies.3 Many Norwegian adults with T1D do not achieve the recommended treatment goals for glycaemic control.4 5 This poor goal attainment might be due to inappropriate choice of insulin regimen for the individual, but research has also shown psychological and emotional aspects as important barriers for satisfactory diabetes self-management.6
The psychological and emotional impact of living with diabetes is often unrecognised and/or under-reported in diabetes care.7 8 Diabetes distress, which reflects the emotional response to the burden, worries, anxieties, frustrations and stressors associated with managing diabetes in everyday life,9 10 is found to be associated with decreased glycaemic control.11 12 Therefore, regular assessment of diabetes distress is recommended.13 Such assessment is considered feasible and beneficial to promote the recognition of psychological and emotional issues that affect diabetes self-management.9 14
Collecting patient-reported outcome measures (PROMs) involves asking people to complete questionnaires concerning the impact of their condition and its treatment on their health.15 The integration of PROMs in clinical practice has the potential to improve care for people with diabetes and other chronic conditions by screening for and identifying problems, monitoring progress over time, improving patient–clinician communication and enabling people to become more involved in managing their own health.16 17 However, using PROMs in itself may not affect health outcomes. The collection of PROMs should be accompanied by a discussion of results to elaborate on any problems identified by the assessment.14 17 Previous research has shown that the use of PROMs to monitor diabetes psychological distress and general well-being followed by a discussion of outcomes improves psychological well-being in both adults and youth with diabetes.14 18 19 In the Cross-National Diabetes Attitudes, Wishes, and Needs (DAWN) Monitoring of Individual Needs in Diabetes (MIND) study,14 the skills used in discussions of PROMs data regarding diabetes distress and well-being were based on empowerment theory and patient-centred communication. Empowerment in nursing and healthcare is defined as a motivational approach and process using specific counselling and communication techniques to assist patients in making health-promoting behaviour changes.20 The approach is patient-centred, with the healthcare providers facilitating and providing information and knowledge to assist the patients in taking informed decisions. The desired outcomes in the empowerment process are control and self-determination. A systematic review by Chen et al 21 states that interventions aiming to empower people with chronic illnesses are able to improve health status, improve outcome indicators of psychological and social aspects, and improve self-management. The authors of the DAWN MIND study suggest further research on process evaluations to explore the role of empowerment-centred and patient-centred skills such as active listening, use of open-ended questions and promoting active patient participation in the decision-making process.14
The overarching aim of the Diabetes Patient-Reported Outcome Measures trial (DiaPROM trial) is to develop, test and evaluate a structured empowerment-based intervention using PROMs regarding diabetes distress as dialogue support in clinical diabetes consultations among adults with T1D. Our proposition is that the DiaPROM intervention initiative will reduce diabetes distress and further improve overall well-being, improve perceived competence for diabetes management and improve glycaemic control. Based on experiences and research,14 18 19 we also believe that improved focus on the psychological and emotional burden of the disease will improve satisfaction with diabetes follow-up. This paper describes the protocol for a pilot randomised controlled trial (RCT) to test the feasibility of and uncertainties associated with a fully powered evaluation study.
The development of the DiaPROM trial
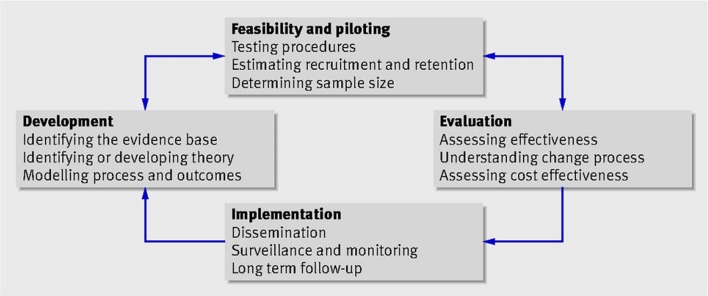
The DiaPROM trial is part of the implementation of PROMs in the Norwegian Diabetes Register for Adults. We wanted to design a study to test a method for using the PROMs data in clinical diabetes practice. The study is multidisciplinary and consists of several interacting components and a number of behaviours required by those receiving and delivering the intervention. Thus, we consider the study as a complex intervention with a need to develop and test the various components gradually before conducting a fully powered RCT. As guidance in this process, we used the Medical Research Council’s framework (MRC framework) for the evaluation of complex interventions.22 23 The framework describes four important phases in the development, evaluation and implementation of a new intervention initiative: (1) the development phase, (2) the feasibility and piloting phase, (3) the evaluation phase and (4) the implementation phase (figure 1).
Figure 1.
Key elements in the Medical Research Council’s guidance for developing, evaluating and implementing complex interventions (MRC framework). Reproduced from Craig et al22 with permission.
The development of the DiaPROM trial took place during 2016 and 2017. Initially, the essential tasks were to determine which PROMs to include and how patients should complete the PROMs.
PROMs to include
We reviewed the literature to identify published articles on the use of PROMs as dialogue support in clinical diabetes practice. We wanted to identify the most commonly used PROMs to measure diabetes distress. We recognised that studies have primarily used PROMs to evaluate interventions’ effects; relatively few publications have reported on the use of PROMs in clinical diabetes care. We did identify, however, the DAWN MIND study which tested the feasibility and impact of the computer-assisted ‘Monitoring of Individual Needs in Diabetes’ procedure aimed to improve recognition and management of the psychological needs of patients with diabetes by implementing PROMs in routine diabetes care.14 24 Regular assessment of psychological needs was implemented as part of the annual review in diabetes clinics across eight countries. The assessment included, among others, diabetes distress measured by the Problem Areas in Diabetes (PAID) scale. Accordingly, Schmitt et al 25 emphasise the necessity of a justified choice of measurement and recommend the use of the PAID when the clinical purpose is to bear in mind a variety of emotional concerns related to living with diabetes. Some other studies have reported PAID as an appropriate instrument for use in clinical diabetes consultations, as well.26–30 The scale may contribute to improved communication by making the dialogue between healthcare providers and patients more therapeutic and goal-oriented.
Patient and public involvement
Involving health service users throughout all phases of a study is important to provide insight into patients’ perspectives and ensure that the research focuses on issues relevant for the health service users and the public.31 32 Patient and public involvement (PPI) is also useful in terms of shaping the research processes.31 In this study, we used the Guidance for Reporting Involvement of Patients and the Public 2 (GRIPP2) short form as guidance for including and reporting PPI.32 To include the voice of the health service users throughout the study, two people with diabetes have been included in the DiaPROM project group, both experienced with PPI and research. They will contribute to all phases of the study. Furthermore, we have included additional people with diabetes to share their views on the various phases of the study, recruited mainly from national and local diabetes associations.
A crucial question when we considered which PROMs to include in the study was what adult people with T1D perceived as the most important and relevant aspects to emphasise in diabetes follow-up. Thus, in parallel with the literature review, we consulted the health service users. In addition to the health service users in the project group, we met the leader of the Norwegian Diabetes Association and a group of four representatives from the local diabetes association (two with T1D and two parents of children with T1D where one had type 2 diabetes herself). First, we used open question to the health service users to determine which topics they perceived as important and relevant to include in a set of PROMs. After an open discussion, we asked them to review several generic instruments (eg, WHO’s 5-Item Well-Being Index [WHO-5], RAND-12 Health Status Inventory, Patient Activation Measure) and diabetes-specific instruments (eg, PAID, Diabetes Distress Scale [DDS], Perceived Competence for Diabetes Scale [PCDS]). The user representatives considered the advantages and shortcomings of using the 20 statements in PAID as dialogue support in the intervention. They found the instrument relevant and suitable to be used in the intervention.
The PAID
Based on the literature review and in accordance with the input from the health service users, we chose the PAID scale for use in the study intervention. The participants’ PAID scores will constitute the basis for the dialogue in the clinical consultations. The scale was developed to gain insight into the breadth of emotional responses to living with diabetes and consists of 20 statements regarding diabetes distress (eg, ‘feeling constantly concerned about food and eating’, ‘worrying about low blood sugar reactions’).33–35 The scores are on a 5-point Likert scale from 0 (not a problem) to 4 (serious problem). An item score of 3 (somewhat serious problem) or 4 (serious problem) indicates moderate to serious diabetes distress related to the specific item. Scale scores are transformed to a 0–100 scale, with higher scores indicating greater distress, and a PAID total score >40 suggests serious diabetes-related distress. To identify both moderate and serious distress, we defined scores of concern as PAID total scores ≥30 or single item scores of 3 or 4. The scale has been translated into several languages, including Norwegian.36
Method for completing PROMs
The literature describes various methods for administration of PROMs, such as paper-based self-administration at home or in the clinic, interviews by telephone or personal meetings, computer-assisted self-administration in the clinic, or mail-based or web-based administration from patients’ homes.16 17 Electronic PROMs collection is preferred since the patients’ responses can be transferred to the electronic patient records (EPRs) without scanning paper forms or punching data.17 In our study, we decided on computer-assisted administration on a touchscreen computer in the outpatient clinic. Using this method has advantages, such as efficient and simultaneous data entry and minor privacy challenges.
Feasibility study
We conducted a feasibility study in 2017 to examine the technical and practical feasibility of collecting PROMs on a touchscreen computer in the outpatient clinic, and evaluate the participants’ perceived understanding and relevance of the items on the PAID and the included outcome measures. We also evaluated the acceptability of completing PROMs annually. Field observations and comments from the participants provided data on the technical and practical procedures. Sixty-nine individuals with T1D ≥40 years participated in the study and 83% of them reported that, to a high or a very high degree, they would be positive about an annual completion of PROMs. However, almost 20% of 137 invited patients did not show up at the clinic (change of appointments, sick, no reason given), and most of the invited ones did not go directly to the computer on arrival at the clinic as instructed in the information sheet. Thus, we developed clearer information and procedures for the pilot study to avoid loss of potential participants among those invited. Further analyses of the results from the feasibility study are ongoing, and we plan to publish these in a separate article.
Aims
The purpose of the pilot RCT reported here is to test the feasibility of the proposed DiaPROM trial components and address the uncertainties associated with running a fully powered RCT in order to conclude whether such a trial is appropriate and/or feasible. The following are our objectives:
Evaluate the recruitment procedures and attrition rates.
Evaluate the performance of the randomisation procedure.
Evaluate the participants’ mean scores on the outcome measures before and after the intervention.
Evaluate if the intervention consultations are acceptable and feasible.
Explore patients’ and healthcare providers’ experiences with the use of PAID as dialogue support and empowerment-based communication skills in clinical diabetes consultations.
Methods and analysis
We will undertake a two-arm pilot RCT with embedded qualitative study on participants’ and healthcare providers’ views of the DiaPROM intervention initiative. We report our protocol here using the Standard Protocol Items: Recommendations for Interventional Trials checklist (http://www.spirit-statement.org/wp-content/uploads/2013/01/SPIRIT-Checklist-download-8Jan13.pdf).
Participants and eligibility criteria
As recommended for pilot RCTs,37 we will include 80 participants: 40 in the intervention group and 40 controls. Participants will have T1D for at least 1 year and be aged ≥18 to <40 years. We will exclude people who are unable to read or complete the PROMs on the touchscreen computer. Furthermore, we will exclude pregnant women, patients with known and recorded cognitive deficiency (eg, Down’s syndrome, Alzheimer), severe somatic comorbidity (eg, end-stage renal disease, severe heart failure, severe cancer), and/or a major psychiatric diagnosis (eg, severe depression or bipolar disorder, schizophrenia) as diabetes distress is often neither ethical nor possible to discuss with these groups of patients. Eligible participants will receive information and consent forms by regular mail before their annual diabetes consultation at the clinic. The information form will include information about the possibility to withdraw from the study at any time point without consequences.
Randomisation procedure and allocation concealment
We will randomise eligible and consenting participants, using computer-generated block randomisation at the patient level, stratified for gender, immediately after the participants have completed both the PAID and the self-reported outcome measures. When participants complete the measures on the touchscreen computer in the outpatient clinic, they will receive an individual four-character code. When the physician downloads the PROMs data using the code, a concealed computerised allocation will take place. Information about which group the person is allocated to will appear on the computer screen and the physician will inform the participant immediately. It is not possible to blind either participants or healthcare providers.
Trial intervention
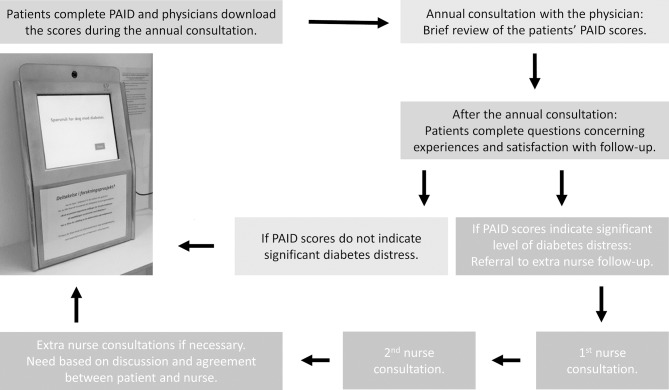
After participants have completed the PAID scale, physicians download the scores into the participants’ EPR as part of the annual consultation (figure 2). Physicians then review and discuss the PAID scores briefly with the participants. Participants with one or more single PAID item(s) score of 3 or 4 (somewhat serious or serious problem), or PAID total score ≥30, will be referred to additional diabetes nurse consultations. Participants with lower scores will receive regular follow-up according to usual clinical protocols.
Figure 2.
The study intervention in the Diabetes Patient-Reported Outcome Measures trial (DiaPROM). PAID, Problem Areas In Diabetes scale.
Additional nurse follow-up will consist of at least two consultations. The first will take place within 4 weeks after randomisation, and the second within a further 3 months. After the second nurse consultation, the nurse and the participant will agree any further follow-up until the next annual consultation with the physician. Diabetes nurses will review the PAID scores and discuss the reported problem areas and distress with participants by following a communication manual based on key elements from empowerment theory and self-determination theory, such as empathetic communication and autonomy support.38–40 These communication skills involve ‘active listening’, ‘asking open questions’, ‘responding’, ‘summing up’ and ‘agreeing on goals and actions to take’. Nurses will record their work on participants’ problem areas, goals and actions. The intervention will last for a maximum of 1 year, until the next annual consultation.
Control procedure
The control group will receive ‘care as usual’, which does not include a structured focus on psychological and emotional diabetes distress. For most patients the annual consultation normally constitutes ‘care as usual’. Although all participants will complete the PAID before randomisation, for control participants the scores will not be accessible to clinicians in the EPR until the study is completed. For ethical reasons, we will not prevent physicians discussing psychological or emotional issues with participants in the control group if participants specifically raise such an issue. Unlike participants in the intervention group, such discussions will not be structured with reference to the PAID data. We will identify to what extent such discussions have taken place by reviewing participants’ EPR.
Training of healthcare providers
Before the study commences, we will have a 1-hour meeting with the participating physicians, and they will be trained in how to download the PAID scores into the EPR and how to briefly discuss the scores in the annual consultations. Further, they will get both oral information and written instructions regarding the interpretation of the PAID scores including instructions on the criteria for referral of participants to extra follow-up by the diabetes nurses. Nurses will get both oral and written information and a 2×1 hour training in how to interpret scores and discuss the reported problem areas, how to follow the communication manual in the consultations, and how to agree on goals and actions to take with the participants.
Data collection and outcome measures
All participants (both intervention and control groups) will complete the outcome measures electronically before the annual consultation at baseline and after 12 months. After the annual consultation, the participants will complete a paper-based questionnaire about their experience and satisfaction with the diabetes follow-up. We will evaluate the recruitment procedures and attrition rates by observing and monitoring the number of eligible participants invited, number of invited people declining participation, number of people who attended the clinic, number of intervention participants who attended the nurse consultations and number of consultations conducted. We will also observe and document the technical performance of the randomisation procedure. Finally, we will document all types of contacts between participants and the diabetes outpatient clinic for all participants throughout the study period.
To describe the study sample and evaluate the technical procedure of data retrieval from EPR, we will perform a computerised retrieval of the following variables from the participants’ EPR: sex, age, ethnicity, body mass index, diabetes duration, haemoglobin A1c (HbA1c) (secondary outcome), insulin regimen, insulin doses, severe hypoglycaemic episodes needing assistance in the past year, hospitalisations, comorbidities and diabetes late complications.
The outcome measures to evaluate the effect of the intervention in the evaluation phase of the study (phase III) were chosen based on a literature review and considerations among the researchers and the health service users. We decided on the DDS as primary outcome. DDS measures diabetes distress and contains 17 items and 4 subscales: emotional burden (five items), physician-related distress (four items), regimen distress (five items) and diabetes-related interpersonal distress (three items).41 The scores are on a 6-point Likert scale from 1 (not a problem) to 6 (serious problem), with a mean total or subscale score from 1 to 6.42 The total or subscale scores >3 are defined as high levels of distress. The DDS has previously shown satisfactory psychometric properties to map diabetes distress and might have advantages for use as outcome measure in clinical trials because it contributes to identification of subdomains of distress.11 25 36 To measure the secondary outcomes, overall well-being and perceived diabetes competence, we have included the WHO-543–45 and the PCDS.46–48 We will use HbA1c as the target for glycaemic control.
We will invite all participants from the intervention group and all healthcare providers (physicians and diabetes nurses) participating in the intervention group to individual indepth interviews to collect qualitative data on their experiences with the intervention, including the use of PAID as dialogue support in clinical consultations. This will provide a sample of about 15–20 participants and 10–15 healthcare providers. All interviews will be conducted at the outpatient clinic and will be audio-recorded after obtaining consent from participants.
Data analysis
We will use Stata SE V.15 for Windows for all statistical analyses,49 and for data entry range checks for data values will be performed. We will report the recruitment of participants and the number of trial dropouts descriptively (frequencies and percentages). Further, we will report the means, SD and CI of the DDS and the other outcome measurements before and after the intervention period for both the intervention and control groups. As the study is a pilot and the sample size is small, we will not perform inferential statistics and analyse between-group calculations. The participants’ PAID scores will be analysed descriptively (mean, SD), as well.
We will transcribe verbatim and analyse participants’ and healthcare providers’ experiences with the intervention by using thematic analysis.50 Thematic analysis is a flexible qualitative method without any specific theoretical foundation and consists of six steps: (1) transcribing, reading and rereading, (2) generating initial codes, (3) searching for themes, (4) reviewing themes, (5) defining and naming the themes, and (6) producing the report.
Ethics and dissemination
Haukeland University Hospital, Bergen, Norway, is the responsible research institution (trial sponsor) where the study data will be stored on a secure research server. In order to protect confidentiality, names of the potential and enrolled participants will be stored separate from the other study data. Only the principal investigator and other clearly identified members of the project group have access to the study data. If important protocol modifications happen, this will be communicated to the ethics committee and ClinicalTrials.gov. Further information can be obtained from ClinicalTrials.gov, trial registration number NCT03471104.
Completing the PROMs may activate latent psychological or psychosocial problems and negative feelings. To care for any participants in the control group reporting worryingly high levels of distress (eg, above cut points for severe levels of distress measured by PAID and/or DDS), the research team will continuously review the reported distress levels. We will discuss potential needs for more intensive care or referral to psychological or psychiatric follow-up for those reporting worryingly levels of distress with the physicians and diabetes nurses.
We will present the findings of the study phases at national and international conferences and submit manuscripts to peer-reviewed journals and popular science journals. Further, we will also publish the findings in popular science journals, public newspapers and journals for relevant health service user groups. One of the health service users included in the project group will participate in the writing and publication process.
Discussion
In the pilot RCT study described in this protocol, we aim to test the feasibility of and address the clinical and methodological uncertainties associated with running a fully powered RCT testing the effect of an intervention incorporating the use of PAID to decrease diabetes distress among people with T1D. The study will provide knowledge on the use of PAID in clinical diabetes practice, although the purpose primarily is to prepare the ground for the design and conduct of a fully powered RCT. In addition, the qualitative evaluation will provide important knowledge on the specific empowerment-based communication skills used to discuss PAID scores of concern in the clinical consultations. In an upcoming fully powered evaluation study (phase III), we plan to test the effect of the entire intervention package including both the use of PAID and the empowerment-based follow-up. A major limitation of such an effect study is the lack of information on how specific parts of the intervention may affect the results.
Diabetes distress has been shown to be a barrier to satisfactory glycaemic control,11 12 and a more structured focus on diabetes distress may have the potential to improve long-term health for people with T1D by reducing distress and improving glycaemic control. A previous literature review by Carlsen et al 51 found that the use of PAID could benefit patients but emphasised the need for follow-up studies to evaluate whether the PAID should be implemented in routine diabetes care to enhance a more structured focus on diabetes distress.
The choice of using PAID as dialogue support in the intervention and the DDS as the primary outcome measure is in accordance with previous research. Both instruments have previously shown satisfactory psychometric properties to map individual levels of diabetes distress, but it has been claimed that the PAID has advantages for use in clinical practice and that the DDS has advantages for use in clinical trials, because it also contributes to identifying subdomains of distress.11 25 36 However, there will be an overlap between the intervention measure (PAID) and the primary outcome measure (DDS) in this study. Using PAID in the intervention may prime the participants’ responses to the DDS, but the inclusion of WHO-5 and the PCDS as additional outcomes may compensate for the overlap between PAID and DDS. Previous research has shown links between diabetes distress measured by PAID, and well-being and perceived competence. Snoek et al 14 indicated an overlap between predictors for diabetes distress and general well-being measured by WHO-5, and Mohn et al 48 showed an association between greater diabetes distress and lower perceived competence for diabetes self-management measured by PCDS.
Strengths and limitations
The use of the MRC framework is a strength in the development of this study because it includes several complex and interacting components that need to be considered and tested with the purpose to reveal uncertainties before conducting a fully powered RCT. In addition, the use of the GRIPP2 short form to guide the PPI throughout all phases of the development of the intervention initiative is considered a strength. The health service users included in the project have influenced, among others, the choice of PROMs, the choice of the theoretical foundation for the intervention and the discussions related to the qualitative component of the study.
We have included primarily disease-specific outcome measures, but also one generic PROM (WHO-5). Disease-specific PROMs are used to capture information that is most pertinent to particular patient groups, but they might miss domains affecting the patient that are unrelated to their disease.16 52 Generic instruments may capture broad dimensions of health and allow for comparisons between populations but might not be sensitive to changes in disease-specific health domains over time or in relation to interventions.15
The fact that the control group in the study will also complete the PAID and the evaluation PROMs before the annual diabetes consultation, and that the same physicians meet participants from both the intervention and the control groups, might lead to intervention contamination challenges. This might be a challenge, although the scores will not be accessible in the EPRs of participants in the control group.
Supplementary Material
Acknowledgments
Thanks to the Norwegian Nurses Association, the Norwegian Diabetes Association and the Western Norway University of Applied Sciences for funding the study. Thanks also to the Norwegian Diabetes Register for Adults and DIPS AS for fruitful collaboration. Further, we thank all the healthcare providers and the healthcare service user representatives involved in the study. Thanks also to senior research fellow Peter Craig for giving us the permission to reproduce the figure describing the study phases in the development of a complex intervention.
Footnotes
Contributors: All authors have contributed in accordance with the criteria for authorship. AH and MG applied for funding of the trial. AH, IH, MG and RBS designed the study with involvement of GST, DAR and RMN. AH wrote the first draft of the study protocol. All other authors have edited and critically reviewed the manuscript, and all authors read and approved the final version.
Funding: The study is funded by the Norwegian Nurses Association, the Western Norway University of Applied Sciences, the Norwegian Diabetes Association and the Norwegian Diabetes Register for Adults. The first two funded a postdoctoral position and a PhD position, respectively. The last two contributed with running funds.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Western Norway Regional Committee for Medical and Health Research Ethics has approved the study (2017/1506/REK west).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. American Association of Diabetes Educators (ADE). AADE7 self-care behaviors. Diabetes Educ 2008;34:445–9. 10.1177/0145721708316625 [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association (ADA). Introduction: standards of medical care in diabetes. Diabetes Care 2018;4:2. [Google Scholar]
- 3. Jones A, Vallis M, Pouwer F. If it does not significantly change HbA1c levels why should we waste time on it? A plea for the prioritization of psychological well-being in people with diabetes. Diabet Med 2015;32:155–63. 10.1111/dme.12620 [DOI] [PubMed] [Google Scholar]
- 4. Cooper JG, Claudi T, Thordarson HB, et al. Treatment of type 1 diabetes in the specialist health service--data from the Norwegian Diabetes Register for Adults. Tidsskr Nor Laegeforen 2013;133:2257 10.4045/tidsskr.13.0153 [DOI] [PubMed] [Google Scholar]
- 5. Carlsen S, Skrivarhaug T, Thue G, et al. Glycemic control and complications in patients with type 1 diabetes - a registry-based longitudinal study of adolescents and young adults. Pediatr Diabetes 2017;18:188–95. 10.1111/pedi.12372 [DOI] [PubMed] [Google Scholar]
- 6. Pouwer F, Nefs G, Nouwen A. Adverse effects of depression on glycemic control and health outcomes in people with diabetes: a review. Endocrinol Metab Clin North Am 2013;42:529–44. 10.1016/j.ecl.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 7. Pouwer F, Beekman AT, Lubach C, et al. Nurses’ recognition and registration of depression, anxiety and diabetes-specific emotional problems in outpatients with diabetes mellitus. Patient Educ Couns 2006;60:235–40. 10.1016/j.pec.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 8. Peyrot M, Rubin RR, Lauritzen T, et al. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005;22:1379–85. 10.1111/j.1464-5491.2005.01644.x [DOI] [PubMed] [Google Scholar]
- 9. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabet Med 2014;31:764–72. 10.1111/dme.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol 2015;3:450–60. 10.1016/S2213-8587(15)00135-7 [DOI] [PubMed] [Google Scholar]
- 11. Strandberg RB, Graue M, Wentzel-Larsen T, et al. Longitudinal relationship between diabetes-specific emotional distress and follow-up HbA1c in adults with Type 1 diabetes mellitus. Diabet Med 2015;32:1304–10. 10.1111/dme.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fisher L, Hessler D, Polonsky W, et al. Diabetes distress in adults with type 1 diabetes: prevalence, incidence and change over time. J Diabetes Complications 2016;30:1123–8. 10.1016/j.jdiacomp.2016.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. International Diabetes Federation. International diabetes federation diabetes atlas. 8th edn, 2017. [Google Scholar]
- 14. Snoek FJ, Kersch NY, Eldrup E, et al. Monitoring of Individual Needs in Diabetes (MIND)-2: follow-up data from the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) MIND study. Diabetes Care 2012;35:2128–32. 10.2337/dc11-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenhalgh J, Dalkin S, Gooding K, et al. Functionality and feedback: a realist synthesis of the collation, interpretation and utilisation of patient-reported outcome measures data to improve patient care. Health Serv and Res 2017;5:1–280. 10.3310/hsdr05020 [DOI] [PubMed] [Google Scholar]
- 16. Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res 2012;21:1305–14. 10.1007/s11136-011-0054-x [DOI] [PubMed] [Google Scholar]
- 17. Eton DT, Beebe TJ, Hagen PT, et al. Harmonizing and consolidating the measurement of patient-reported information at health care institutions: a position statement of the Mayo Clinic. Patient Relat Outcome Meas 2014;5:7–15. 10.2147/PROM.S55069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pouwer F, Snoek FJ, van der Ploeg HM, et al. Monitoring of psychological well-being in outpatients with diabetes: effects on mood, HbA(1c), and the patient’s evaluation of the quality of diabetes care: a randomized controlled trial. Diabetes Care 2001;24:1929–35. 10.2337/diacare.24.11.1929 [DOI] [PubMed] [Google Scholar]
- 19. de Wit M, Delemarre-van de Waal HA, Bokma JA, et al. Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being: a randomized controlled trial. Diabetes Care 2008;31:1521–6. 10.2337/dc08-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellis-Stoll CC, Popkess-Vawter S. A concept analysis on the process of empowerment. ANS Adv Nurs Sci 1998;21:62–8. 10.1097/00012272-199812000-00007 [DOI] [PubMed] [Google Scholar]
- 21. Chen YC, Li IC, Ic L. Effectiveness of interventions using empowerment concept for patients with chronic disease: a systematic review. JBI Libr Syst Rev 2009;7:1179–233. 10.11124/01938924-200907270-00001 [DOI] [PubMed] [Google Scholar]
- 22. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655–83. 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Craig P, Petticrew M. Developing and evaluating complex interventions: reflections on the 2008 MRC guidance. Int J Nurs Stud 2013;50:585–7. 10.1016/j.ijnurstu.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 24. Snoek FJ, Kersch NY, Eldrup E, et al. Monitoring of Individual Needs in Diabetes (MIND): baseline data from the Cross-National Diabetes Attitudes, Wishes, and Needs (DAWN) MIND study. Diabetes Care 2011;34:601–3. 10.2337/dc10-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmitt A, Reimer A, Kulzer B, et al. How to assess diabetes distress: comparison of the Problem Areas in Diabetes Scale (PAID) and the Diabetes Distress Scale (DDS). Diabet Med 2016;33:835–43. 10.1111/dme.12887 [DOI] [PubMed] [Google Scholar]
- 26. Chawla A, Saha C, Marrero DG. A novel application of the Problem Areas in Diabetes (PAID) instrument to improve glycemic control and patient satisfaction. Diabetes Educ 2010;36:337–44. 10.1177/0145721709354607 [DOI] [PubMed] [Google Scholar]
- 27. van Bastelaar KM, Pouwer F, Geelhoed-Duijvestijn PH, et al. Diabetes-specific emotional distress mediates the association between depressive symptoms and glycaemic control in Type 1 and Type 2 diabetes. Diabet Med 2010;27:798–803. 10.1111/j.1464-5491.2010.03025.x [DOI] [PubMed] [Google Scholar]
- 28. Meeuwissen JAC, Holleman GJM, de Jong FJ, et al. Screening and guided self-help intervention for anxiety and depression in patients with type 2 diabetes. European Diabetes Nursing 2011;8:47–52. 10.1002/edn.177 [DOI] [Google Scholar]
- 29. Fleer J, Tovote KA, Keers JC, et al. Screening for depression and diabetes-related distress in a diabetes outpatient clinic. Diabet Med 2013;30:88–94. 10.1111/dme.12001 [DOI] [PubMed] [Google Scholar]
- 30. Reddy J, Wilhelm K, Campbell L. Putting PAID to diabetes-related distress: the potential utility of the problem areas in diabetes (PAID) scale in patients with diabetes. Psychosomatics 2013;54:44–51. 10.1016/j.psym.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 31. Staley K. ‘Is it worth doing?’ Measuring the impact of patient and public involvement in research. Res Involv Engagem 2015;1:6 10.1186/s40900-015-0008-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358:j3453 10.1136/bmj.j3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–60. 10.2337/diacare.18.6.754 [DOI] [PubMed] [Google Scholar]
- 34. Welch GW, Jacobson AM, Polonsky WH. The problem areas in diabetes scale. An evaluation of its clinical utility. Diabetes Care 1997;20:760–6. 10.2337/diacare.20.5.760 [DOI] [PubMed] [Google Scholar]
- 35. Welch G, Weinger K, Anderson B, et al. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med 2003;20:69–72. 10.1046/j.1464-5491.2003.00832.x [DOI] [PubMed] [Google Scholar]
- 36. Graue M, Haugstvedt A, Wentzel-Larsen T, et al. Diabetes-related emotional distress in adults: reliability and validity of the Norwegian versions of the Problem Areas in Diabetes Scale (PAID) and the Diabetes Distress Scale (DDS). Int J Nurs Stud 2012;49:174–82. 10.1016/j.ijnurstu.2011.08.007 [DOI] [PubMed] [Google Scholar]
- 37. Richards DA, Hallberg IR. Section 2. Investigating feasibility and undertaking pilot testing of complex interventions. Complex interventions in health. An overview of research methods: Routledge, 2015:121–82. [Google Scholar]
- 38. Anderson RM, Funnell M. The art of empowerment: stories and strategies for diabetes educators. Alexandria, Virginia: American Diabetes Association, 2000. [Google Scholar]
- 39. Deci EL, Ryan RM. Handbook of self-determination research. Rochester, NY: University of Rochester Press, 2002. [Google Scholar]
- 40. Zoffmann V, Hörnsten Å, Storbækken S, et al. Translating person-centered care into practice: A comparative analysis of motivational interviewing, illness-integration support, and guided self-determination. Patient Educ Couns 2016;99:400–7. 10.1016/j.pec.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 41. Polonsky WH, Fisher L, Earles J, Psyd JE, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626–31. 10.2337/diacare.28.3.626 [DOI] [PubMed] [Google Scholar]
- 42. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care 2013;36:2551–8. 10.2337/dc12-2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Wit M, Pouwer F, Gemke RJ, et al. Validation of the WHO-5 Well-Being Index in adolescents with type 1 diabetes. Diabetes Care 2007;30:2003–6. 10.2337/dc07-0447 [DOI] [PubMed] [Google Scholar]
- 44. Hajos TR, Pouwer F, Skovlund SE, et al. Psychometric and screening properties of the WHO-5 well-being index in adult outpatients with Type 1 or Type 2 diabetes mellitus. Diabet Med 2013;30:e63–e69. 10.1111/dme.12040 [DOI] [PubMed] [Google Scholar]
- 45. Topp CW, Østergaard SD, Søndergaard S, et al. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom 2015;84:167–76. 10.1159/000376585 [DOI] [PubMed] [Google Scholar]
- 46. Williams GC, McGregor HA, Zeldman A, et al. Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychol 2004;23:58–66. 10.1037/0278-6133.23.1.58 [DOI] [PubMed] [Google Scholar]
- 47. Williams GC, McGregor HA, King D, et al. Variation in perceived competence, glycemic control, and patient satisfaction: relationship to autonomy support from physicians. Patient Educ Couns 2005;57:39–45. 10.1016/j.pec.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 48. Mohn J, Graue M, Assmus J, et al. Self-reported diabetes self-management competence and support from healthcare providers in achieving autonomy are negatively associated with diabetes distress in adults with Type 1 diabetes. Diabet Med 2015;32:1513–9. 10.1111/dme.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. StataCorp LLC. STATA data analysis and statistical software. Stata SE 15 ed. College Station, Texas, USA: StataCorp LLC, 2018. [Google Scholar]
- 50. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 51. Carlsen K, Haugstvedt A, Graue M. PAID as screening and working tool in clinical consultations]. Norwegian J Clin Nurs 2015;10:228–37. [Google Scholar]
- 52. Black N, Burke L, Forrest CB, et al. Patient-reported outcomes: pathways to better health, better services, and better societies. Qual Life Res 2016;25:1103–12. 10.1007/s11136-015-1168-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.