Abstract
Background and Objective
Platelets play a pivotal role in atherothrombosis and are potentially involved in the pathogenesis of atherosclerosis. We investigated whether mean platelet volume (MPV) predicts clinical outcome and progression of atherosclerosis in patients with asymptomatic carotid artery disease.
Methods
We studied 1006 of 1268 prospectively collected consecutive patients with asymptomatic carotid atherosclerosis who were evaluated by duplex sonography. Patients were followed up clinically for the occurrence of a major adverse cardiovascular event (MACE), a composite of myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft, stroke and death.
Results
During a median follow-up of 3·1 years (interquartile range, 2·5–3·5), a total of 316 (31·5%) MACEs were recorded. Increased levels of MPV were significantly associated with increased risk of the occurrence of MACEs (adjusted hazard ratio [HR] for an increase in one standard deviation [SD] of MPV 1·22, confidence interval [CI] 1·05–1·35, P < 0·01). Patients with MPV levels above 11·8 femtolitre (= fifth quintile) had a significantly higher event rate (41·3% vs. 29·3%, P < 0·001) with an adjusted HR for MACEs of 1·65 (95% CI 1·26–2·16, P < 0·001) compared with patients with MPV levels in the first to fourth quintile. No significant association was found between baseline MPV levels with either baseline degree or progression during a 6-month follow-up of carotid stenosis.
Conclusion
Mean platelet volume was independently and significantly associated with adverse cardiovascular outcome in patients with asymptomatic carotid atherosclerosis.
Keywords: Atherosclerosis, carotid artery disease, mean platelet volume, platelet reactivity
Introduction
Activated platelets play a crucial role in the pathogenesis of atherothrombosis. There is an increasing evidence suggesting an important contribution of platelets in atherogenesis not only as mediators of thrombus formation, but also as inducers of inflammation [1]. Platelets are anucleate cells with an average size of 2–5 μm in diameter and 0·5 μm thickness and a lifespan of 7–8 days. In some individuals, the platelet volume exceeds average levels observed in the respective population. In addition to acquired factors, human and animal studies have shown that characteristics of blood cells in general and platelets in particular have high heritability. According to recent data, the platelet volume is also genetically determined [2].
Platelets participate in the surveillance and maintenance of the integrity of the endothelium through the release of various cytokines and growth factors. Any exaggerated response on damaged or altered endothelial cells appears to be crucial for the initiation and development of atherosclerosis [1]. Recent studies suggest that platelet activation might be a trigger for increased leucocyte recruitment to the subendothelial space [3]. Enhanced platelet reactivity is associated with thrombogenic activation and an increased risk of cardiovascular disease. It has been shown that large platelets have increased reactivity, aggregate more rapidly with collagen, produce higher levels of thromboxane A2 and express more glycoprotein Ib and IIb/IIIa receptors compared with normal-size platelets [4–6]. Furthermore, large platelets contain more granules and aggregate more rapidly with adenosine diphosphate [7]. Additionally, large platelets are less sensitive to inhibitory effects of prostacyclin on aggregation and secretion than smaller platelets [8]. It is speculated that several biologically active substances for which the concentrations change during ageing, with increasing body fat, in the presence of diabetes, with high blood pressure, by nicotine exposure and following (athero-) thrombotic events stimulate the bone marrow to produce larger platelets [9].
In vitro measurements of platelet function provide an index of the functional capacity of platelets. Several markers, such as P-selectin, activated glycoprotein IIb/IIIa, platelet factor 4 and beta-thromboglobulin, are indicators of platelet activity and activation [10–12]. However, they are not determined routinely in the clinical setting, because the necessary methods are not available everywhere, they are time consuming, require specialized technique and equipment, and are relatively costly. The analysis of mean platelet volume (MPV), on the other hand, is simple, automated and cheap. The MPV is the most commonly utilized methodology to measure platelet size. Although there is controversy about the most reliable methods to determine MPV [13], it is routinely available at relatively low cost. MPV correlates well with platelet function and activation, and elevated MPV values are associated with a shortened bleeding time and increased thromboxane B2 plasma levels [14]. Thus, higher MPV can be considered a reliable indicator of increased platelet activity. Compared with other markers of platelet activity, which often require special laboratories and expertise, MPV seems to be a simple and cost-effective marker for platelet activity.
Currently, the relationship between platelet parameters and clinical outcome in patients with carotid artery disease is poorly understood. Recent publications suggest a critical role of enhanced platelet reactivity in patients with carotid stenosis [15,16]. The purpose of this study was therefore to investigate the relationship between MPV and the occurrence of major adverse cardiovascular events (MACEs) in a prospectively collected cohort of neurologically asymptomatic patients with carotid artery disease.
Materials and methods
In this single-centre study, we prospectively enrolled 1268 consecutive patients who underwent duplex ultrasound investigations of the extracranial carotid arteries between March 2002 and March 2003. Study design, inclusion and exclusion criteria have been published previously [17]. Briefly, patients with prevalent atherosclerotic carotid artery disease, as defined by the presence of nonstenotic plaques or carotid stenosis of any degree, who were neurologically asymptomatic at the time of screening, were enrolled. Patients underwent baseline carotid ultrasound (US) investigation and a second US examination after 6–9 months. The main indications for performing carotid ultrasound investigation were carotid bruits, prevalence of risk factors and known atherosclerotic diseases in other vessel areas. Patients with a myocardial infarction (MI), stroke, coronary revascularisation or peripheral vascular surgery during the preceding 6 months, were excluded from the study. The rationale behind this was the assumption that acute cardiovascular events may affect laboratory measures and rather reflect the severity of an acute situation than chronic atherosclerotic disease of the carotid artery. The study complied with the Declaration of Helsinki and was approved by the review board and the institutional ethics committee of the Medical University of Vienna. All patients gave their written informed consent. The reporting of the study confirms to the STROBE statement and EQUATOR guidelines [18].
Clinical and laboratory data
After enrolment, the medical history and data from physical examination were recorded. All demographic and vital parameters were ascertained by two independent observers. Antecubital venous blood samples were drawn and analysed directly without freezing according to local laboratory standard procedure. Blood anticoagulated with ethylenediaminetetraacetic acid was used for the performance of an automated blood picture. Measurements were taken within 1 h of sampling. MPV and total platelet count (PTC) were determined at admission in a Sysmex XE-2100 automated cell counter (Sysmex Corporation, Norderstedt, Germany) from blood samples. Treating physicians and ultrasonographers were blinded for all laboratory values.
Colour-coded duplex sonography and grading of internal carotid artery stenosis
Duplex examinations at baseline were performed on an Acuson 128 XP10 with a 7·5-MHZ linear array probe (Acuson, Malvern, PA, USA). Duplex grading of the carotid narrowing was performed as described previously [19]. We used four categories to quantify the degree of carotid stenosis at baseline and follow-up: 0–29% (carotid plaques), 30–49% (advanced plaques), 50–69% (moderate stenosis) and 70% up to 100% (high-grade stenosis to occlusion).
Definitions
Definitions of traditional cardiovascular risk factors were published previously [17]. For stroke, computed tomography or magnetic resonance imaging was used for confirmation.
Study end point and surveillance protocol
The primary study end point was defined as the occurrence of a first MACE, a composite of MI, percutaneous coronary intervention, coronary artery bypass graft, stroke and death by all-cause during the follow-up period. Patients were seen at follow-up visits to the outpatient ward in 6- to 9-month intervals after the initial presentation for clinical re-examination. A follow-up questionnaire was sent to each patient to re-evaluate the occurrence of MACEs. If the questionnaire was not returned, personal telephone contact to the patients or to the treating physicians was established. The performance of percutaneous coronary interventions or coronary bypass graft was validated by review of the procedure protocols. If information about death was not available, contact with the municipality was established. Outcome was assessed by two independent observers who were blinded with respect to patient’s baseline clinical and laboratory data.
Statistical methods
Continuous data are presented as median and interquartile range (IQR, range from the 25th to the 75th percentile). Discrete data are given as counts and percentages. MPV and PTC were categorized in quintiles, to obtain clinically useful measures for the effect sizes. Analysis of variance and the chi-squared test were used for comparisons between quintiles, as appropriate. MPV quintiles were compared by means of the log-rank. Event-free survival probabilities were estimated with the Kaplan–Meier method. Univariable and multivariable Cox proportional hazards models were applied to assess the association between MPV levels and the occurrence of a first MACE. The following variables that showed an association (P < 0·05) with the study end point, and those that were significantly associated with the highest MPV quintile were entered in the final multivariable model: age (years), sex (male/female), hypertension (binary), diabetes (binary), statin treatment (binary), treatment with clopidogrel (binary), BMI (kg/m2), history of MI (binary), history of coronary heart disease (binary), peripheral artery disease (binary), haemoglobin (g/dL), serum creatinine (mg/dL) and high-sensitivity C-reactive protein (hs-CRP; mg/L). Results of the Cox models are presented as hazard ratios (HRs) and the 95% CI. We assessed the overall model fit using Cox–Snell residuals. We also tested the proportional hazard assumption for all covariates using Schoenfeld residuals (overall test) and the scaled Schoenfeld residuals (variable-by-variable testing). A two-sided P-value of < 0·05 was considered significant. All calculations were performed with spss for Windows (version 20.0; SPSS Inc., Chicago, IL, USA).
Results
A total of 1268 patients met the inclusion criteria and were enrolled in the study. Of 59 patients, samples for determination of MPV were not available, and of 203 patients (17%), only the inclusion but no clinical follow-up data were available. These patients were excluded leaving 1006 patients for final analysis. The cohort comprised 633 male patients (63%), the median age was 69 years (IQR 61–76). Demographic data and baseline characteristics are given in Table 1. The 262 patients who had to be excluded from the analysis were not significantly different in regard to baseline clinical characteristics (age, sex, frequency of atherothrombotic risk factors and cardiovascular comorbidities) compared with the study population of 1006 patients (data not shown).
Table 1.
Baseline characteristics and risk factors for 1006 patients
| MPV in femtolitre | |||||||
|---|---|---|---|---|---|---|---|
| 1st quintile | 2nd quintile | 3rd quintile | 4th quintile | 5th quintile | |||
| Variable | All patients | < 10·3 (9·9–10·1) | 10·3–10·7 (10·4–10·6) | 10·8–11·3 (10·9–11·2) | 11·4–11·8 (11·5–11·7) | ≥ 11·9 (12–12·7) | |
| No. of patients* | 1006 | 221 | 193 | 230 | 178 | 184 | P-value |
| Age, years | 69·1 (61–76) | 69·9 (66–79) | 69·1 (66–77) | 70·5 (71–79) | 68·9 (67–79) | 67·3 (67–78) | 0·22 |
| Male gender | 633 (62·9) | 133 (60·2) | 121 (62·7) | 143 (62·2) | 118 (66·3) | 118 (64·1) | 0·78 |
| Medical history | |||||||
| Previous PAD | 424 (42·1) | 93 (42·1) | 78 (40·4) | 107 (46·5) | 84 (47·2) | 62 (33·7) | 0·05 |
| Previous CHD | 542 (53·9) | 116 (52·5) | 104 (53·9) | 124 (53·9) | 93 (52·2) | 105 (57·1) | 0·89 |
| Previous MI | 241 (24) | 42 (19) | 42 (21·8) | 55 (23·9) | 48 (27) | 54 (29·3) | 0·12 |
| Previous stroke | 165 (16·4) | 37 (16·7) | 26 (13·5) | 34 (14·8) | 36 (20·2) | 32 (17·4) | 0·45 |
| Risk factors | |||||||
| Diabetes | 222 (22·1) | 43 (19·5) | 36 (18·7) | 49 (21·3) | 51 (28·7) | 43 (23·4) | 0·03 |
| Hypertension | 693 (68·9) | 147 (66·5) | 127 (65·8) | 169 (73·5) | 126 (70·8) | 124 (67·4) | 0·38 |
| Current smokers | 272 (27) | 68 (30·8) | 49 (25·4) | 53 (23·0) | 52 (29·2) | 50 (27·2) | 0·39 |
| F. hist. of atheroscl. | 554 (55·1) | 119 (53·8) | 100 (51·8) | 143 (62·2) | 93 (52·2) | 99 (53·8) | 0·18 |
| Clinical characteristics | |||||||
| BMI | 26·3 (21·2–27·3) | 25·9 (22·3–28·2) | 25·9 (22·1–29·5) | 26·2 (23·2–27·1) | 26·8 (24–28·8) | 26·8 (23·1–29·2) | 0·02 |
| Statins | 586 (58·3) | 111 (50·2) | 131 (67·9) | 136 (59·1) | 109 (61·2) | 99 (53·8) | 0·001 |
| Acetylsalicylic acid | 563 (56·0) | 110 (49·8) | 112 (58·9) | 123 (53·5) | 106 (59·6) | 112 (60·9) | 0·13 |
| Clopidogrel | 237 (23·6) | 62 (28·1) | 44 (22·8) | 60 (26·1) | 45 (25·3) | 26 (14·1) | 0·01 |
| Laboratory findings | |||||||
| Platelet count/mm3 | 224 (187–260) | 253 (202–268) | 235 (227–322) | 224 (191–248) | 213 (179–248) | 193 (164–229) | < 0·001 |
| Haemoglobin, g/dL | 14 (12·9–15·1) | 13·9 (12·2–14·3) | 13·7 (12·4–15·4) | 14 (11·9–14·8) | 14 (13·1–15·4) | 14·3 (12·7–15·1) | 0·001 |
| HbA1c, % | 6 (5·6–6·6) | 6 (5·6–7) | 5·9 (5·7–6·5) | 6 (5·9–7·2) | 6 (5·8–7·3) | 6·1 (5·6–7·2) | 0·65 |
| Triglycerides, mg/dL | 149 (107–216) | 151 (99–227) | 150 (113–235) | 147 (102–205) | 151 (101–201) | 147 (98–159) | 0·68 |
| HDL cholesterol, mg/dL | 50 (42–60) | 50 (38–61) | 50 (42–61) | 49 (43–63) | 51 (39–55) | 49 (43–69) | 0·97 |
| LDL cholesterol, mg/dL | 118 (94–146) | 120 (98–134) | 122 (100–143) | 117 (108–151) | 113 (92–154) | 119 (96–152) | 0·64 |
| hs-CRP, mg/dL | 0·29 (0·14–0·64) | 0·32 (0·14–0·77) | 0·3 (0·22–0·8) | 0·24 (0·15–0·83) | 0·3 (0·22–0·92) | 0·33 (0·18–0·72) | 0·16 |
| Serum creatinine, mg/dL | 1·06 (0·93–1·23) | 1·03 (0·91–1·38) | 1·08 (0·97–1·32) | 1·05 (0·96–1·37) | 1·09 (0·94–1·37) | 1·06 (1·04–1·34) | 0·49 |
| APTT, s | 35 (32·6–38·7) | 35·0 (32·5–38·5) | 34·4 (32·3–38·1) | 34·6 (32·5–38·2) | 35·5 (32·8–39·1) | 35·7 (33·0–40·5) | 0·34 |
| NT, % | 108 (93–123) | 112 (94–125) | 107 (93–123) | 108 (94–126) | 107 (93–122) | 104 (90–120) | 0·16 |
Continuous data are presented as the median and the interquartile range. Discrete data are given as counts and percentages.
APTT, activated partial thromboplastin time; CHD, coronary heart disease; F. hist. of atheroscl, family history of atherosclerosis; HbA1c, glycated haemoglobin A1; hs-CRP, high-sensitivity C-reactive protein; MI, myocardial infarction; MPV, mean platelet volume; NT, Normotest®; PAD, peripheral arterial disease.
*Because MPV was estimated with one decimal, numbers of individuals were slightly unequal among quintiles due to ties in values.
Progression of carotid atherosclerosis
At baseline, 57% of the patients had carotid plaques, 33% had moderate stenosis and 10% had a narrowing of more than 70% of the internal carotid artery. Progression of carotid lesions was found in 92 of 1006 patients (9%). No significant association between baseline MPV values and degree of baseline stenosis (P = 0·86) or progression (P = 0·27) of carotid stenosis was found.
Cardiovascular outcome
During the median follow-up of 3·1 years (IQR 2·5–3·5), corresponding to 2887 overall person-years, a total of 362 MACEs were observed in 317 (31·5%) patients. A total of 21 patients suffered from two MACEs and 12 patients from three MACEs. A total of 151 (15·4%) patients died, 103 (12·2%) of them by all-cause cardiovascular death. No significant association between death caused by other diseases and MPV was found (P = 0·5). A total of 41 patients (3·6%) suffered from MI, 54 (5·4%) experienced an ischaemic stroke, 76 (6·5%) had a percutaneous coronary intervention and 40 (2·7%) had a coronary artery bypass graft.
Mean platelet volume and major adverse cardiovascular events
The mean MPV level (fl) ± SD was 11 ± 0·96 and correlated negatively with PTC (r = −0·36, P < 0·001). Univariate and independent predictors of a first MACE are presented in Table 2.
Table 2.
Results of univariate and multivariate Cox regression analysis of independent variables, which showed a significant association with major adverse cardiovascular events (MACEs). In addition, statin treatment (binary), treatment with clopidogrel (binary), BMI (kg/m2) and haemoglobin (g/dL) were included in the multivariate Cox model. MPV > 11·8 fl refers to the fifth quintile
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | HR | CI | P-value | HR | CI | P-value |
| MPV > 11·8 fl | 1·71 | 1·26–2·16 | < 0·001 | 1·65 | 1·21–2·10 | < 0·001 |
| Age* | 1·01 | 1·00–1·02 | 0·03 | 1·01 | 0·99–1·02 | 0·14 |
| Female | 0·75 | 0·59–0·96 | 0·02 | 0·82 | 0·62–1·08 | 0·04 |
| Previous CHD | 1·7 | 1·34–2·16 | < 0·001 | 1·17 | 0·86–1·56 | 0·23 |
| Previous MI | 1·79 | 1·41–2·27 | < 0·001 | 1·45 | 1·08–1·94 | 0·02 |
| Diabetes | 1·65 | 1·30–2·11 | < 0·001 | 1·38 | 1·05–1·77 | 0·05 |
| RR | 1·38 | 1·06–1·79 | 0·02 | 1·17 | 0·89–1·54 | 0·15 |
| Serum creatinine* | 1·08 | 1·00–1·15 | 0·04 | 1·04 | 0·95–1·59 | 0·38 |
| hs-CRP* | 1·32 | 1·16–1·49 | < 0·001 | 1·21 | 1·04–1·40 | 0·005 |
CI, 95% confidence interval; HR, hazard ratio; MI, myocardial infarction; MPV, mean platelet volume; RR, arterial hypertension.
*HRs refer to a 1-SD increase.
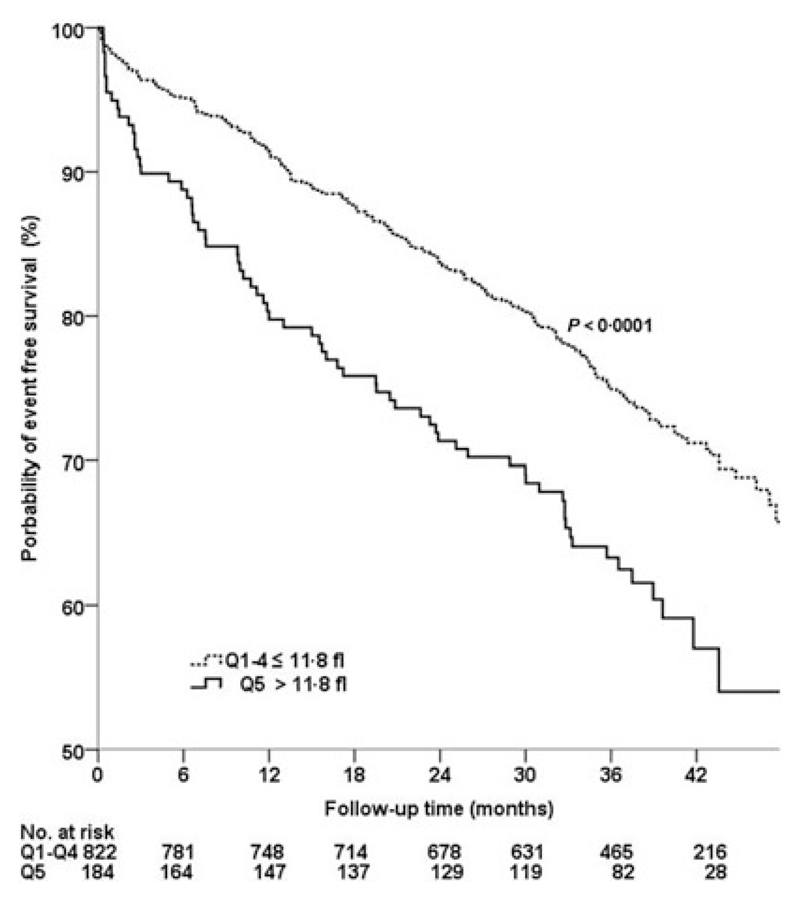
In the Cox proportional hazard regression analysis, increased levels of MPV were significantly associated with adverse cardiovascular outcome (HR for an increase of 1 SD of MPV 1·24, CI 1·10–1·42, P < 0·01; adjusted HR for an increase of 1 SD of MPV 1·22, CI 1·06–1·36, P = 0·003). In addition, Kaplan–Meier analysis showed a significantly higher event rate in patients with MPV levels above 11·9 fl (corresponding to the 5th quintile) compared with patients with lower levels of MPV (< 11·9 fl; 41·3% vs. 29·3%, log-rank: P < 0·001; Fig. 1). In the Cox proportional hazard regression analysis, patients with MPV levels ≥ 11·9 fl were at increased risk for MACEs (HR 1·71, 95% CI 1·26–2·16, P < 0·001; adjusted HR 1·65, 95% CI 1·21–2·10, P < 0·001) compared with patients with lower MPV levels (first to fourth quintile). No statistically significant association between baseline PTC and the occurrence of MACEs was found (P = 0·1).
Figure 1.
Kaplan–Meier estimates for major adverse cardiovascular events (composite of myocardial infarction, percutaneous coronary interventions, coronary bypass graft, stroke, and death) according to the fifth vs. the first to fourth quintile (Q) of MPV. MPV, mean platelet volume.
Discussion
We present data of a significant association of high MPV levels with increased risk of adverse outcome in patients with asymptomatic carotid artery disease.
Increased production of large platelets could contribute to the pathogenesis of atherothrombosis. In patients with atherosclerotic diseases such as ischaemic cerebrovascular disease [20], MI [21], coronary artery disease [22,23] and recently also peripheral artery disease [24], high values of MPV have been shown to contribute to the identification of ‘high-risk’ patients who are more likely to have a poorer clinical outcome. Taglieri et al. [22] observed that increased MPV values predict remyocardial infarctions within 1 year. Moreover, Bath et al. [20] found that increased MPV levels are independent predictors for the risk of stroke among patients with a history of stroke or transient ischaemic attack. However, our study is the first to investigate the prognostic impact of MPV on clinical outcome in patients with carotid artery disease.
We found that patients with MPV levels above 11·8 fl had a 1·65-fold higher risk of a MACE compared with patients with lower MPV values during a median observation period of 3·1 years (P < 0·001). There was no significant difference in outcome between patients within the first to the fourth quintile of MPV levels. As expected [25], we found a significant negative correlation between MPV and PTC, yet we did not observe a significant association between PTC and the occurrence of MACEs (P = 0·1). Our data are in good agreement with the results of other studies in which also no association between the incidence of cardiovascular events and PTC was found [21,26]. The presence of an increased number of large platelets usually parallels a decreased PTC [21,25]. Possibly, this represents a compensatory mechanism to maintain a constant platelet function in the presence of small platelet numbers [27]. However, the relationship between MPV and PTC is not fully understood and needs further investigation.
Recently published data show controversial results concerning associations between degree of carotid stenosis and MPV. Arévalo-Lorido et al. [28] found a significant correlation of severity of carotid stenosis with MPV values in patients with ischaemic atherothrombotic stroke. In contrast, Giuseppe De Luca et al. [29] reported no significant association between carotid intima–media thickness and MPV levels in a large prospective study with patients undergoing coronary angiography. In our patient cohort, no statistically significant association was found between MPV and carotid stenosis.
Increased MPV has been described in subjects with established cardiovascular risk factors, such as metabolic syndrome [30], smoking [31], diabetes [32], obesity [33], hypertension [34] and hyperlipidaemia [35]. We did not detect any associations between MPV and conventional cardiovascular risk factors. MPV was only independently associated with diabetes. However, we found an independent association with statin therapy, which has also been reported in previous studies [36,37]. We detected a significant association between chronic treatment with clopidogrel and baseline levels of MPV, which, to the best of our knowledge, has yet not been described in the literature. However, the study design is not appropriate to draw conclusions regarding the potential influence of clopidogrel on the MPV.
Up to now, literature does not provide insights into whether elevated MPV causes cardiovascular events or is simply associated with them. Martin et al. [38] found that platelet size was still elevated 6 weeks after hospital discharge, indicating that increases in MPV are persistent and do not only reflect the acute event. Interestingly, in our study, patients with a previous MI also showed significantly higher MPV values (P < 0·01). However, we cannot demonstrate that the MPV in these patients was already increased before the event.
Our data show an association between platelet size and MACE in patients with asymptomatic carotid artery disease. These findings support our hypothesis that large MPV represents a risk factor for development of cardiovascular events. The detailed mechanisms responsible for the development of atherothrombosis have to be elucidated in future studies. Currently, there is no evidence available that antiplatelet therapies influence levels of MPV [39]. Drugs, such as losartan [40] and lipid lowering therapies [36,37], may affect MPV, but no study to date has shown that lowering levels of MPV also lowers cardiovascular risk.
Limitations
Several limitations of our study should be noted: additional confounding that was not considered in the statistical calculations cannot be totally excluded as various comorbidities and drugs may influence MPV. However, no major difference was found in the association between MPV and risk of MACEs after adjustment for antiplatelet therapy, established cardiovascular risk factors and previous ischaemic events. Moreover, categorization into quintiles resulted in a relatively low number of patients per group. However, outliers were rare and the IQR of the first and fifth was comparable with the second, third and fourth quintile.
Conclusion
In this study, we show that in patients with asymptomatic carotid artery disease, levels of MPV are independent predictors of future cardiovascular events. The measurement of MPV could provide prognostic information in patients at risk for a cardiovascular event. Additional studies will be necessary to demonstrate that MPV represents a useful marker in clinical practice.
Acknowledgments
Conflict of interest
None of the authors have any personal or financial relationships that have any potential to inappropriately influence his or her actions or manuscript, and no financial or other potential conflict of interests exist (includes involvement with any organization with a direct financial or other interest) regarding the manuscript.
References
- 1.Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program. 2011;2011:51–61. doi: 10.1182/asheducation-2011.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Gieger C, Radhakrishnan A, Cvejic A, Tang W, Porcu E, Pistis G, et al. New gene functions in megakaryopoiesis and platelet formation. Nature. 2011;480:201–8. doi: 10.1038/nature10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix IC, et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116:4317–27. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157–61. [PubMed] [Google Scholar]
- 5.Park Y, Schoene N, Harris W. Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets. 2002;13:301–6. doi: 10.1080/095371002220148332. [DOI] [PubMed] [Google Scholar]
- 6.Giles H, Smith RE, Martin JF. Platelet glycoprotein IIb-IIIa and size are increased in acute myocardial infarction. Eur J Clin Invest. 1994;24:69–72. doi: 10.1111/j.1365-2362.1994.tb02062.x. [DOI] [PubMed] [Google Scholar]
- 7.Jakubowski JA, Thompson CB, Vaillancourt R, Valeri CR, Deykin D. Arachidonic acid metabolism by platelets of differing size. Br J Haematol. 1983;53:503–11. doi: 10.1111/j.1365-2141.1983.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 8.Jakubowski JA, Adler B, Thompson CB, Valeri CR, Deykin D. Influence of platelet volume on the ability of prostacyclin to inhibit platelet aggregation and the release reaction. J Lab Clin Med. 1985;105:271–6. [PubMed] [Google Scholar]
- 9.Boos CJ, Lip GY. Assessment of mean platelet volume in coronary artery disease – what does it mean? Thromb Res. 2007;120:11–3. doi: 10.1016/j.thromres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Michelson AD. Methods for the measurement of platelet function. Am J Cardiol. 2009;103:20A–6A. doi: 10.1016/j.amjcard.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Capitanio AM, Niewiarowski S, Rucinski B, Tuszynski GP, Cierniewski CS, Hershock D, et al. Interaction of platelet factor 4 with human platelets. Biochim Biophys Acta. 1985;839:161–73. doi: 10.1016/0304-4165(85)90033-9. [DOI] [PubMed] [Google Scholar]
- 12.Rinder HM, Snyder EL, Bonan JL, Napychank PA, Malkus H, Smith BR. Activation in stored platelet concentrates: correlation between membrane expression of P-selectin, glycoprotein IIb/IIIa, and beta-thromboglobulin release. Transfusion. 1993;33:25–9. doi: 10.1046/j.1537-2995.1993.33193142305.x. [DOI] [PubMed] [Google Scholar]
- 13.Latger-Cannard V, Hoarau M, Salignac S, Baumgart D, Nurden P, Lecompte T. Mean platelet volume: comparison of three analysers towards standardization of platelet morphological phenotype. Int J Lab Hematol. 2012;34:300–10. doi: 10.1111/j.1751-553X.2011.01396.x. [DOI] [PubMed] [Google Scholar]
- 14.Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 2009;63:1509–15. doi: 10.1111/j.1742-1241.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 15.Csongradi E, Nagy B Jr, Fulop T, Varga Z, Karanyi Z, Magyar MT, et al. Increased levels of platelet activation markers are positively associated with carotid wall thickness and other atherosclerotic risk factors in obese patients. Thromb Haemost. 2011;106:683–92. doi: 10.1160/TH11-01-0030. [DOI] [PubMed] [Google Scholar]
- 16.Lukasik M, Rozalski M, Luzak B, Michalak M, Ambrosius W, Watala C, et al. Enhanced platelet-derived microparticle formation is associated with carotid atherosclerosis in convalescent stroke patients. Platelets. 2013;24:63–70. doi: 10.3109/09537104.2011.654292. [DOI] [PubMed] [Google Scholar]
- 17.Schillinger M, Exner M, Mlekusch W, Sabeti S, Amighi J, Nikowitsch R, et al. Inflammation and carotid artery–risk for atherosclerosis study (ICARAS) Circulation. 2005;111:2203–9. doi: 10.1161/01.CIR.0000163569.97918.C0. [DOI] [PubMed] [Google Scholar]
- 18.Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35–53. doi: 10.1111/j.1365-2362.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 19.Sabeti S, Exner M, Mlekusch W, Amighi J, Quehenberger P, Rumpold H, et al. Prognostic impact of fibrinogen in carotid atherosclerosis: nonspecific indicator of inflammation or independent predictor of disease progression? Stroke. 2005;36:1400–4. doi: 10.1161/01.STR.0000169931.96670.fc. [DOI] [PubMed] [Google Scholar]
- 20.Bath P, Algert C, Chapman N, Neal B. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35:622–6. doi: 10.1161/01.STR.0000116105.26237.EC. [DOI] [PubMed] [Google Scholar]
- 21.Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46:284–90. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 22.Taglieri N, Saia F, Rapezzi C, Marrozzini C, Bacchi Reggiani ML, Palmerini T, et al. Prognostic significance of mean platelet volume on admission in an unselected cohort of patients with non ST-segment elevation acute coronary syndrome. Thromb Haemost. 2011;106:132–40. doi: 10.1160/TH10-12-0821. [DOI] [PubMed] [Google Scholar]
- 23.Varol E, Icli A, Ozaydin M, Erdogan D, Arslan A. Mean platelet volume is elevated in patients with myocardial infarction with normal coronary arteries, as in patients with myocardial infarction with obstructive coronary artery disease. Scand J Clin Lab Invest. 2009;69:570–4. doi: 10.1080/00365510902829354. [DOI] [PubMed] [Google Scholar]
- 24.Berger JS, Eraso LH, Xie D, Sha D, Mohler ER., 3rd Mean platelet volume and prevalence of peripheral artery disease, the National Health and Nutrition Examination Survey, 1999–2004. Atherosclerosis. 2010;213:586–91. doi: 10.1016/j.atherosclerosis.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles C. The platelet count and mean platelet volume. Br J Haematol. 1981;48:31–7. doi: 10.1111/j.1365-2141.1981.00031.x. [DOI] [PubMed] [Google Scholar]
- 26.D’Erasmo E, Aliberti G, Celi FS, Vecci E, Mazzuoli G. Sequential evaluation of platelet count and mean platelet volume during myocardial infarction. Medicina (Firenze) 1988;8:58–60. [PubMed] [Google Scholar]
- 27.Thompson CB, Jakubowski JA. The pathophysiology and clinical relevance of platelet heterogeneity. Blood. 1988;72:1–8. [PubMed] [Google Scholar]
- 28.Arevalo-Lorido JC, Carretero-Gomez J, Villar-Vaca P. Mean platelet volume predicting carotid atherosclerosis in atherothrombotic ischemic stroke. Ir J Med Sci. 2012;181:179–83. doi: 10.1007/s11845-011-0755-8. [DOI] [PubMed] [Google Scholar]
- 29.De Luca G, Santagostino M, Secco GG, Cassetti E, Giuliani L, Franchi E, et al. Mean platelet volume and the extent of coronary artery disease: results from a large prospective study. Atherosclerosis. 2009;206:292–7. doi: 10.1016/j.atherosclerosis.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Kutlucan A, Bulur S, Kr S, Onder E, Aslantas Y, Ekinozu I, et al. The relationship between mean platelet volume with metabolic syndrome in obese individuals. Blood Coagul Fibrinolysis. 2012;23:388–90. doi: 10.1097/MBC.0b013e328352e8fa. [DOI] [PubMed] [Google Scholar]
- 31.Kario K, Matsuo T, Nakao K. Cigarette smoking increases the mean platelet volume in elderly patients with risk factors for atherosclerosis. Clin Lab Haematol. 1992;14:281–7. doi: 10.1111/j.1365-2257.1992.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 32.Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T, et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475–8. doi: 10.1080/0953710042000267707. [DOI] [PubMed] [Google Scholar]
- 33.Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981–2. doi: 10.1111/j.1742-1241.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 34.Nadar S, Blann AD, Lip GY. Platelet morphology and plasma indices of platelet activation in essential hypertension: effects of amlodipine-based antihypertensive therapy. Ann Med. 2004;36:552–7. doi: 10.1080/07853890410017386. [DOI] [PubMed] [Google Scholar]
- 35.Pathansali R, Smith N, Bath P. Altered megakaryocyteplatelet haemostatic axis in hypercholesterolaemia. Platelets. 2001;12:292–7. doi: 10.1080/09537100120058810. [DOI] [PubMed] [Google Scholar]
- 36.Coban E, Afacan B. The effect of rosuvastatin treatment on the mean platelet volume in patients with uncontrolled primary dyslipidemia with hypolipidemic diet treatment. Platelets. 2008;19:111–4. doi: 10.1080/09537100701230444. [DOI] [PubMed] [Google Scholar]
- 37.Broijersen A, Eriksson M, Larsson PT, Beck O, Berglund L, Angelin B, et al. Effects of selective LDL-apheresis and pravastatin therapy on platelet function in familial hypercholesterolaemia. Eur J Clin Invest. 1994;24:488–98. doi: 10.1111/j.1365-2362.1994.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 38.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338:1409–11. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 39.Colkesen Y, Coskun I, Muderrisoglu H. The effect of aspirin on mean platelet volume in patients with paroxysmal atrial fibrillation. Platelets. 2012;24:263–6. doi: 10.3109/09537104.2012.682106. [DOI] [PubMed] [Google Scholar]
- 40.Jagroop IA, Mikhailidis DP. Angiotensin II can induce and potentiate shape change in human platelets: effect of losartan. J Hum Hypertens. 2000;14:581–5. doi: 10.1038/sj.jhh.1001102. [DOI] [PubMed] [Google Scholar]