Abstract
Objective
To calculate the incidence of hospitalisation due to acute respiratory failure, pneumonia, acute respiratory distress syndrome (ARDS), febrile seizures and encephalitis/encephalopathy among influenza-positive patients in Japan, where point-of-care tests are routinely used to diagnose influenza.
Design
A cross-sectional study using routinely collected data.
Setting
Japanese clinics and hospitals between 2012 and 2016.
Participants
Japanese patients aged 0–74 years diagnosed with influenza by a rapid test in employment-related health insurance records.
Primary outcome measures
Incidence of hospitalisation per 100 000 influenza-positive episodes.
Results
We included over 16 million influenza-positive episodes, 1.0% of whom were hospitalised. Of these, 3361 were acute respiratory failure, 27 253 pneumonia, 18 ARDS, 2603 febrile seizure and 159 encephalitis/encephalopathy. The percentage of hospitalisations by age was 2.96% of patients aged 0–1 years, 0.77% aged 2–5, 0.51% aged 6–12, 0.78% aged 13–18, 1.36% aged 19–44, 1.19% aged 45–64, and 2.21% aged 65–74. The incidence of hospitalisations from these five complications combined was highest in influenza-positive patients aged 0–1 years (943 per 100 000) compared with 307 in those aged 2–5 years and 271 in those aged 65–74 years. For pneumonia, the incidence was highest for influenza-positive patients aged 0–5 years and 65 years or more. There were statistically significant decreasing trends over the years in the incidence of all-cause hospitalisations, pneumonia and febrile seizures.
Conclusions
Japanese administrative data revealed that 1.0% of influenza-positive patients aged under 75 years were hospitalised. Male patients had a higher incidence of pulmonary complications and febrile seizures. Children aged 0–5 years and adults aged 65–74 years were at high risk of being admitted to hospital for pneumonia.
Keywords: influenza, hospitalisation, pneumonia, influenza encephalopathy, influenza encephalitis, febrile seizure
Strengths and limitations of this study.
This study uses Japanese routinely collected data where uniquely diagnostic tests are used to identify influenza infections in the population.
Point-of-care testing for influenza has limited sensitivity, but its high specificity means that nearly all the participants in this study were infected with influenza.
Limitations of the data set prevent analysis of mortality and patients over the age of 74 years.
Introduction
Influenza is a major burden on health systems worldwide. Every year, an estimated one billion people,1 including 90 million children younger than 5 years of age, are infected with influenza globally, and 1 million people have influenza-associated acute lower respiratory tract infection,2 which causes 290 000–600 000 deaths.3
Complications of influenza which cause hospitalisations are a serious public health concern. In both Western and Asian countries, majority of influenza-related hospital admissions are due to respiratory or neurological complications: pneumonia, febrile seizure, acute respiratory failure, acute respiratory distress syndrome (ARDS) and encephalitis/encephalopathy.4–8 We refer to these as ‘severe complications’ here. Hospitalisation rates from influenza infection have been investigated,4 9 but most studies were conducted in Western countries, where testing for influenza is not routine. This means that studies have used either limited sample sizes of positively identified individual hospitalised patients, or extrapolated from influenza surveillance data.9–11 Hospital-based studies may have underestimated the risk and the number of infections and complications in the community.12 Previous studies have used estimates of the general population as denominators, rather than assessing the risk of admission among the infected population, combining the risk of infection and the risk of complications. This is problematic because programmes targeting high-risk groups, such as vaccination or prophylaxis, may reduce the number of infections in high-risk groups, biasing estimates of the risk of complications if infected.12 Also, many studies pre-date the option of administering new neuraminidase inhibitors.13
Although it is also seen internationally,14–18 influenza encephalitis is a particular concern among Japanese physicians owing to a high incidence and mortality rate in Japan.7 19–23 The prognosis for patients with influenza encephalitis/encephalopathy is very poor; approximately 30% of affected patients die and 20%–30% have neurological sequelae.24 To understand the aetiology and prevalence of this severe outcome, surveillance has been conducted.25 26 In Japan, influenza-associated encephalopathy is a notifiable disease.23 Japanese physicians are required to report influenza infection cases with (1) death after coma or hospitalisation with coma for 24 hours or more; and (2) a fever of 38°C or higher, central nervous system manifestation or prior influenza infection symptoms. This surveillance system has detected 60–100 influenza encephalitis cases annually27 and 331 cases during the 2009–2010 pandemic26; however, under-reporting of cases has been acknowledged.27 Another survey of paediatric departments in 265 hospitals reported 263 influenza-associated encephalopathy cases over 3 years.25 The authors estimate that there are 200–300 influenza encephalopathy cases per annum in Japan28; therefore, the incidence of influenza encephalitis/encephalopathy is not accurately known.
To understand the incidence of severe complications in patients with influenza, an analysis of large-scale, real-world data is needed, encompassing hospital and community sites. Previous studies using large data sets of routinely collected medical records have had to rely on clinical diagnoses of influenza-like illness or modelling of influenza and other respiratory virus infections using incomplete laboratory data.11 In Japan, diagnostic testing for influenza is routine, which presents a unique opportunity to combine the benefits of large data sets with positive diagnoses.6 We therefore sought to estimate the incidence of hospitalisation with the above five severe complications per influenza infection, using Japanese health insurance claim data.
Methods
Patients and data
We analysed administrative data provided by Japan Medical Data Center (renamed to JMDC), Tokyo, Japan.29 The data source was the monthly health insurance claim records between January 2012 and December 2016 of approximately three million employees and their dependents, representing 2.4% of the Japanese population. Within health insurance coverage in Japan, people can consult physicians in any type of hospital and department, and medical doctors in any specialty can diagnose influenza and prescribe anti-influenza medications. The age of patients in the data set ranged from 0 to 74 years because all Japanese people aged 75 or more (except for individuals who are on public assistance) are covered by another health insurance programme with lower out-of-pocket expenses.
From the database, we extracted the data of individuals who consulted physicians with influenza-like illness episodes. We then included only patients with a diagnosis of influenza virus infection. In Japan, the use of immunochromatogenic assay point-of-care tests (POCT) in clinical practice has been covered by public health insurance from 1999.22 As recommended in Japanese guidelines,30 a test-and-treat strategy is routine.22 31 Even if physicians only slightly suspect influenza infection, they use a POCT to diagnose influenza and administer antivirals to the positive patients.30 32 33 Testing would be indicated in fever, sore throat, malaise, non-productive cough or a history of infection in the family, for example.22 34 During the 2009–2010 pandemic influenza A(H1N1) season, physicians performed this test in majority of cases (>90%), and we believe this was likely to be the case during the period of this study because in Japan paediatric patients with an influenza-like illness are required to obtain a medical certificate showing they do not have influenza before returning to school.35
Outcomes
Hospitalisation was recorded in the health insurance claims of inpatients. In patients with a diagnosis of influenza infection, we identified those who were hospitalised with a diagnosis of acute respiratory failure, pneumonia, ARDS, febrile seizure and encephalitis/encephalopathy on their records, according to the International Classification of Diseases (ICD-10) codes. The primary outcomes were the incidence of each of the five severe complications per 100 000 influenza infections. Acute respiratory failure was coded as J960, J988, R060, R068 or R092, pneumonia was coded as J10–J18 or J20–J22, ARDS as J80, and febrile seizures as R560. We defined influenza encephalitis/encephalopathy as patients who were diagnosed using ICD-10 codes for influenza infection and encephalitis/encephalopathy (G00–G09 or G41) and had been administered steroid pulse or immunoglobulin therapy.36
Statistical analysis
We examined the number of diagnosed influenza infections and severe complications by sex, age, outpatient/inpatient status, number of beds in the facility and clinical specialty. In Japan, clinical facilities with fewer than 20 beds are denoted a ‘clinic’ by law. Clinics are usually run by a single medical doctor and function as a primary care department. Most clinics have no beds, but a very small subset of clinics have 1–19 beds to accommodate inpatients. In contrast, facilities with 20 beds or more are legally termed a ‘hospital’. Hospitals have primary care, specialised outpatient, and general and specialised inpatient departments. In this study, hospitalised influenza-positive patients were inpatients in both ‘clinic’ and ‘hospital’ settings. We plotted histograms of the ages of outpatients and inpatients infected with influenza. We determined the incidence of inpatients with the five severe complications by dividing the number of complications by the number of infections. We stratified this by sex, influenza season and age. We also examined the numbers of the influenza-infected patients and the proportions of inpatients over calendar time at the request of a reviewer. Influenza seasons were defined as lasting from September through to the following August. We calculated p values for secular trends of incidence over influenza seasons. Statistical analyses were performed using SAS V.9.4 statistical software. All reported p values were two-sided and we considered p<0.05 to indicate a statistical significance.
Patient and public involvement
Patients were not actively involved in developing the research question and protocol, including outcome measures. The participants will be provided the final study results by clinical research information services and home page of the University of Yamanashi.
Results
Characteristics of patients diagnosed with influenza
Table 1 summarises the number of patients with diagnoses of influenza infection in the study population. Among 16 636 913 infections, 53.4% of the patients were men and 1.0% were hospitalised. Approximately a quarter (25.7%) of infections were in children aged 6–12 years. Overall 32% of diagnoses were made in the internal medicine department and 23% in paediatrics. Most infections were diagnosed in clinics (n=13 572 391; 83%). Figures 1 and 2 illustrate the number of outpatients and inpatients with influenza infection, respectively, by age. Influenza was most often diagnosed in outpatients aged 0–12 years, with a second small peak in middle-aged patients. In contrast, inpatient cases were the most common among patients aged less than 1 year. Table 2 shows the number of complicated cases by department, hospital size and type of hospital management. A total of 3361 patients (0.02%) were admitted to hospital with acute respiratory failure, 27 253 (0.16%) with pneumonia, 18 (0.0001%) with ARDS, 2603 (0.02%) with febrile seizures and 159 (0.001%) with encephalitis/encephalopathy. Most complicated cases were admitted to paediatric departments, with 19 012 pneumonia admissions (70% of the total), 1794 with acute respiratory failure (53% of the total), 2461 with febrile seizures (95% of the total) and 63 encephalitis (40% of all cases). The number of inpatients with acute respiratory failure, pneumonia and febrile seizure tended to increase with the number of hospital beds.
Table 1.
Population characteristics: total number (%) of 1 663 6913 Japanese patients with a physician’s diagnosis of influenza infection between 2012 and 2016, in health insurance administrative data
| Sex, n (%) | Men | Women | |||||||
| 8 885 699 (53.4) | 7 751 214 (46.6) | ||||||||
| Patient status, n (%) | Outpatient | Inpatient | |||||||
| 16 488 970 (99.0) | 164 394 (1.0) | ||||||||
| Age, years | 0–1 | 2–5 | 6–12 | 13–18 | 19–44 | 45–64 | 65–74 | ||
| n (%) | 823 875 (5.1) | 2 886 462 (17.7) | 4 193 137 (25.7) | 1 480 030 (9.1) | 3 815 970 (23.4) | 2 872 125 (17.6) | 231 120 (1.4) | ||
| Hospital beds, n | 0–19 | 20–99 | 100–199 | 200–299 | 300–499 | 500+ | |||
| Patients, n (%) | 13 572 391 (83.3) | 392 179 (2.4) | 450 850 (2.8) | 324 418 (2.0) | 616 989 (3.8) | 945 892 (5.8) | |||
| Clinical department of diagnosis | Internal medicine | Paediatrics | Otorhinolaryngology | Orthopaedics | Dermatology | Surgery | Ophthalmology | Obstetrics and gynaecology | Psychiatry |
| Patients, n (%) | 1 187 638 (32.4) | 827 942 (22.6) | 310 514 (8.5) | 308 146 (8.4) | 250 737 (6.8) | 206 763 (5.6) | 165 915 (4.5) | 156 615 (4.3) | 99 624 (2.7) |
Figure 1.
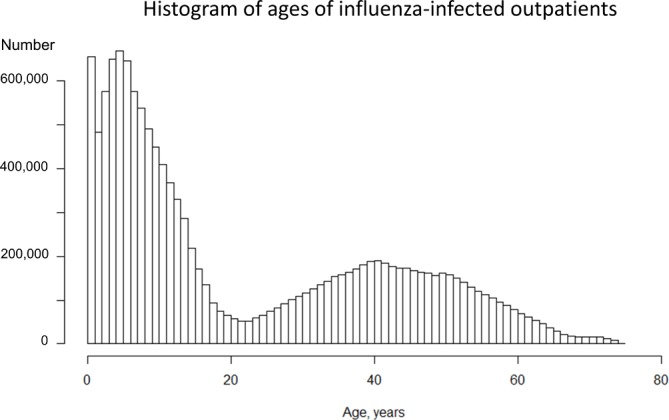
Histogram of the ages of influenza-infected outpatients in the 2012/2013, 2013/2014, 2014/2015 and 2015/2016 seasons, according to health insurance administrative data.
Figure 2.
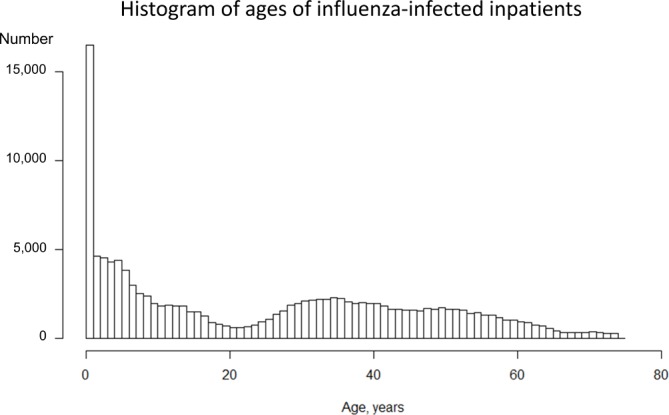
Histogram of the ages of influenza-infected inpatients in the 2012/2013, 2013/2014, 2014/2015 and 2015/2016 seasons, according to health insurance administrative data.
Table 2.
Total number of inpatients with severe influenza complications by department and hospital type among 16 636 913 Japanese influenza cases between 2012 and 2016
| Category | Inpatients | Acute respiratory failure | Pneumonia | ARDS | Febrile seizure | Encephalitis/encephalopathy |
| n | 164 394 | 3361 | 27 253 | 18 | 2603 | 159 |
| Clinical department, n (%) | ||||||
| Internal medicine | 23 722 (14.4) | 682 (20.3) | 3633 (13.3) | 6 | 23 (0.9) | 57 (35.8) |
| Paediatrics | 47 138 (28.7) | 1794 (53.4) | 19 012 (69.8) | 2 | 2461 (94.5) | 63 (39.6) |
| Otorhinolaryngology | 12 825 (7.8) | 43 (1.3) | 217 (0.8) | 0 | 2 (0.1) | 0 |
| Orthopaedics | 7158 (4.4) | 43 (1.3) | 338 (1.2) | 0 | 6 (0.2) | 0 |
| Dermatology | 1100 (0.7) | 3 (0.1) | 45 (0.2) | 0 | 0 | 0 |
| Surgery | 17 138 (10.4) | 189 (5.6) | 720 (2.6) | 3 | 18 (0.7) | 0 |
| Ophthalmology | 2302 (1.4) | 0 | 23 (0.1) | 0 | 1 (0.04) | 0 |
| Obstetrics and gynaecology | 15 155 (9.2) | 88 (2.6) | 330 (1.2) | 0 | 0 | 0 |
| Psychiatry | 2486 (1.5) | 28 (0.8) | 197 (0.7) | 0 | 8 (0.3) | 37 (23.3) |
| Others or not specified | 35 370 (21.5) | 486 (14.5) | 2738 (10.0) | 0 | 84 (3.2) | 2 (1.3) |
| Hospital beds, n (%) | ||||||
| 0–19 | 16 843 (10.2) | 167 (5.0) | 805 (3.0) | 0 | 18 (0.7) | 0 |
| 20–99 | 10 202 (6.2) | 106 (3.2) | 913 (3.4) | 3 | 36 (1.4) | 0 |
| 100–199 | 12 661 (7.7) | 308 (9.2) | 2394 (8.8) | 0 | 147 (5.6) | 0 |
| 200–299 | 15 701 (9.6) | 358 (10.7) | 2933 (10.8) | 7 | 220 (8.5) | 26 (16.4) |
| 300–499 | 40 753 (24.8) | 922 (27.5) | 9179 (33.7) | 0 | 1003 (38.5) | 57 (35.8) |
| 500+ | 68 234 (41.5) | 1500 (44.7) | 11 029 (40.5) | 8 | 1179 (45.3) | 76 (47.8) |
| Hospital type, n (%) | ||||||
| Clinic | 16 817 (10.2) | 167 (5.0) | 805 (3.0) | 0 | 18 (0.7) | 0 |
| National or municipal hospital | 48 243 (29.3) | 985 (29.4) | 10 995 (40.3) | 10 | 1314 (50.5) | 82 (51.6) |
| University hospital | 21 898 (13.3) | 285 (8.5) | 2049 (7.5) | 0 | 162 (6.2) | 34 (21.4) |
| Other hospital | 77 185 (47.0) | 1919 (57.2) | 13 404 (49.2) | 8 | 1109 (42.6) | 43 (27.0) |
| Not specified | 251 (0.2) | 5 (0.1) | 0 | 0 | 0 | 0 |
ARDS, acute respiratory distress syndrome.
Hospitalisation rates from severe complications
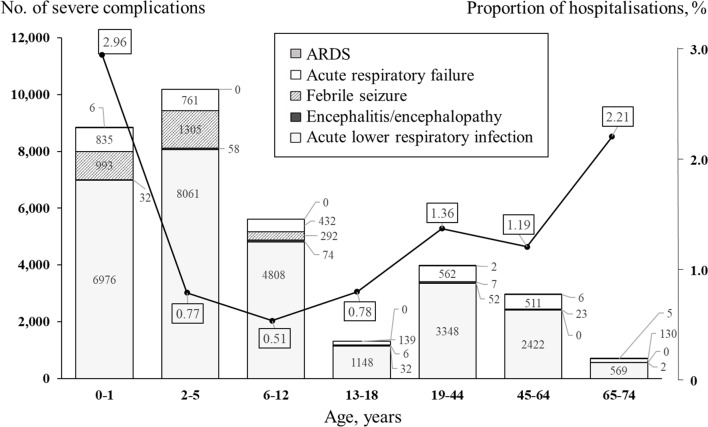
The combined incidence of the five complications was 189 per 100 000 diagnosed infections. Pneumonia was the most common complication, with 164 per 100 000 diagnosed infections, followed by acute respiratory failure (20.2), febrile seizures (15.7), encephalitis/encephalopathy (0.9) and ARDS (0.10). Table 3 shows the incidence of five severe complications by age, sex and influenza season. Whereas the incidence of acute respiratory failure, pneumonia, ARDS and febrile seizures was higher in men, encephalitis/encephalopathy was higher in women. There were decreasing trends over the years in the incidence of hospital admissions from pneumonia (p for trend <0.0001), febrile seizure (p for trend <0.0001) and the five severe complications combined (p for trend <0.0001), but not acute respiratory failure (p for trend=0.07), encephalitis/encephalopathy (p for trend=0.19) or ARDS (p for trend=0.98). In each age group, pneumonia was the most common complication. The incidence of acute respiratory failure, pneumonia and febrile seizure was highest in patients aged 0–1 years, ARDS was highest in those 65–74 years and encephalitis/encephalopathy was highest in patients aged 13–18 years.
Table 3.
Incidence of hospitalisation with severe complications per 100 000 confirmed influenza infections
| Inpatients per 100 000 influenza infections, n | Hospitalisation | Any of five complications | Acute respiratory failure | Pneumonia | ARDS | Febrile seizure | Encephalitis/encephalopathy |
| Sex* | p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 | p=0.08 | p<0.0001 | p=0.08 |
| Male (n=8 885 794) | 970 | 191 | 22.2 | 171 | 0.15 | 17.8 | 0.8 |
| Female (n=7 751 279) | 1011 | 171 | 17.8 | 156 | 0.06 | 13.2 | 1.1 |
| Year† | p<0.0001 | p<0.0001 | p=0.07 | p<0.0001 | p=0.98 | p<0.0001 | p=0.19 |
| January 2012–August 2012 (n=1 611 699) | 1114 | 249 | 23 | 229 | 0.25 | 23 | 0.4 |
| September 2012–August 2013 (n=2 912 806) | 1079 | 199 | 22 | 180 | 0.07 | 17 | 0.8 |
| September 2013–August 2014 (n=3 532 559) | 1023 | 180 | 20 | 160 | 0.06 | 17 | 1.3 |
| September 2014–August 2015 (n=3 628 976) | 965 | 169 | 19 | 150 | 0.17 | 14 | 1.1 |
| September 2015–August 2016 (n=3 530 057) | 951 | 172 | 21 | 157 | 0.06 | 14 | 0.7 |
| September 2016–December 2016 (n=1 103 073) | 946 | 166 | 20 | 152 | 0.18 | 11 | 1.4 |
| Age, years | |||||||
| 0–1 (n=823 875) | 2551 | 943 | 101 | 847 | 0.73 | 121 | 1.3 |
| 2–5 (n=2 886 462) | 776 | 307 | 26 | 279 | 0 | 45 | 0.9 |
| 6–12 (n=4 193 137) | 526 | 124 | 10 | 115 | 0 | 7 | 1.3 |
| 13–18 (n=1 480 030) | 734 | 87 | 9.4 | 78 | 0 | 0.41 | 1.4 |
| 19–44 (n=3 815 970) | 1337 | 100 | 15 | 88 | 0.05 | 0.18 | 0.9 |
| 45–64 (n=2 872 125) | 1141 | 95 | 18 | 82 | 0.21 | 0 | 0.5 |
| 65–74 (n=231 120) | 1919 | 271 | 56 | 245 | 1.7 | 0 | 0.4 |
*p for difference of incidence.
p for trend.
ARDS, acute respiratory distress syndrome.
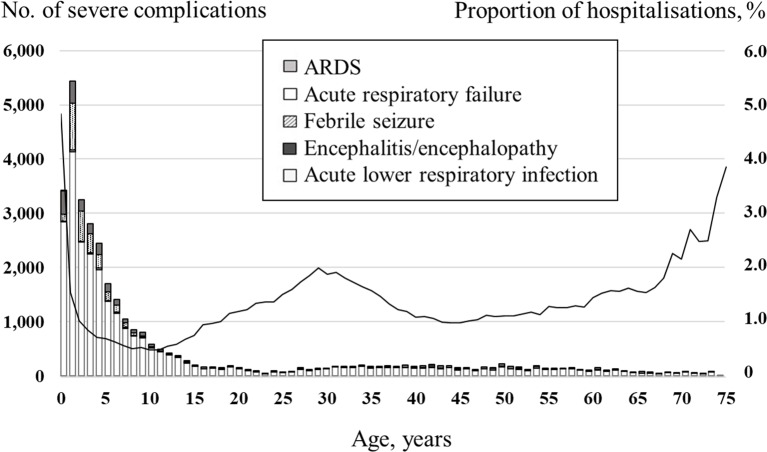
Figures 3 and 4 show the number and percentage of inpatients with complications by age group and age over the study period. Young children had most of the severe complications. Across all age groups, pneumonia was by far the most common of the five complications, and the age group with the largest number of cases was children aged 2–5 years. In contrast, the proportion of infections hospitalised was highest in patients aged 0–1 years (2.96%) and second highest in those aged 65–74 years (2.21%).
Figure 3.
Number of influenza-infected inpatients with severe complications and proportion of hospitalisation in a health insurance claim database, by age group, between 2012 and 2016. Bars represent the number of each severe complication; the line represents the proportion of infections resulting in hospitalisation in each age group. ARDS, acute respiratory distress syndrome.
Figure 4.
Number of influenza-infected inpatients with severe complications and proportion of hospitalisation in a health insurance claim database, by age, between 2012 and 2016. Bars represent the number of each severe complication; the line represents the proportion of infections with hospitalisation. ARDS, acute respiratory distress syndrome.
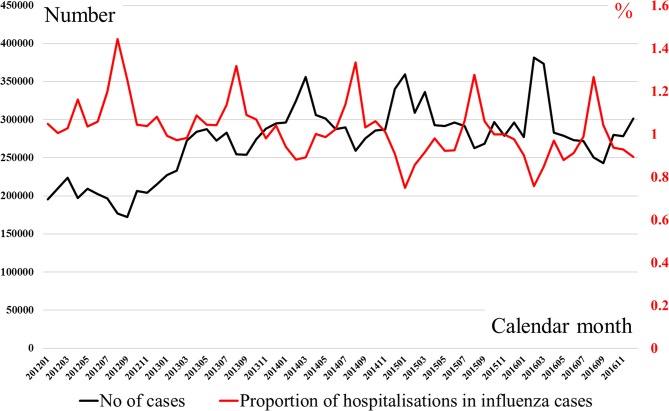
Figure 5 shows the number of influenza-infected patients and the proportion who were inpatients by calendar month between 2012 and 2016. Every year the number of infections increased from winter to spring, while the proportion admitted peaked in summer. The number of infections was similar between 2014 and 2016. The proportion hospitalised gradually decreased between 2012 and 2016.
Figure 5.
Number of influenza-infected patients and proportion of hospitalisation in health insurance claim database between 2012 and 2016. The black line represents the number of patients, while the red line represents the proportion of infections hospitalised.
Discussion
Principal findings
We described the epidemiology of hospital admissions and five key severe complications in large cross-sectional data of influenza-positive patient visits. Influenza diagnoses were the most common in young children and middle-aged patients (table 1 and figures 1–2). The incidence and absolute number of hospital admissions with complications of influenza were highest in young children (figure 2 and table 3). The most common complication was pneumonia (table 1). The incidence and absolute number of admissions for pulmonary complications were highest in children (figures 3–4 and table 3). Patients aged 65–74 years were also at high risk for admission from complications, but the absolute number of both influenza infections and serious complications was lower than for children in our data set (figures 3–4 and table 3). The incidence of admissions for encephalitis/encephalopathy was relatively high in children aged 0–18 years (table 3). There was a decreasing trend in the proportions of infections hospitalised for any reason and with any of the five complications, pneumonia or febrile seizures between 2012 and 2016 (table 3 and figure 5).
Comparison with previous research
Male patients suffered more complications than female ones, especially for acute respiratory failure, pneumonia and febrile seizures (table 3). This is consistent with previous studies reporting that during the 2009–2010 pandemic influenza A(H1N1) season, the incidence of hospitalisation in male children was greater than in female children in the USA (56%),37 Canada (60%)38 and Japan (64.3%).6 Asthma is a risk factor for pneumonia in children with influenza.39 Our finding of an increased risk of pneumonia in males may be because asthma is a more common disease in boys than girls.40 It is also known that boys get febrile seizures more often than girls.41 This was also observed in our data for febrile seizures with influenza infection. In contrast, our data suggest the possibility of a higher risk of encephalitis/encephalopathy in women with influenza infection (table 3). However, in Japanese surveillance reports from 2007 to 2010, 153 of 263 (58.2%) paediatric patients with encephalopathy were male.25 Because the means of data collection in previous studies were different, we are unable to conclude in which sex complications are more common. Further study with another large data set is needed to investigate risk factors, including sex, for hospitalisation and incidence of encephalitis/encephalopathy in Asian people.
Our results by age are consistent with those of previous studies and add to understanding the risk of specific complications among those with a diagnosis of influenza. Previous studies, being unable to identify a large number of influenza-positive patients, have used small numbers of influenza-positive admissions as cases and general population estimates as the denominators. This combines the risk of infection and the risk of complications. We found that the risk of hospitalisation was highest in infected infants aged 0–1 years (figures 2–4 and table 3). This is consistent with a US study which found that the highest hospitalisation rate was among infants aged under 1 year (11.9 per 100 000 population) during the 2009–2010 influenza A(H1N1) pandemic9; in addition, we are able to show that the risk of admission is as high as 943 per 100 000 infections. A study from the UK also reported that children aged 6 months to 4 years had the high influenza-related hospitalisation rates between 2001 and 2007 (3360 per 100 000 population).8
A systematic review found large gaps in the evidence base (describing the evidence for risk factors for admissions as ‘limited to absent’).12 They found contrasting results with children under the age of 2 years at higher risk of admission to hospital than older children with pandemic H1N1 influenza but the reverse with seasonal influenza.12 They also found evidence of spectrum bias; studies from hospitals and intensive care units gave lower estimates of risk of death than community-based studies for both the elderly and children compared with young adults. As well as including positively diagnosed patients, our study covers both the community and hospital settings and is of sufficient size to allow estimation for specific causes of admission. In addition, because vaccination reduces the hospitalisation rate,42–44 vaccination programmes in other countries that target high-risk groups may bias hospitalisation rate estimates for these patients. In Japan all individuals have had to pay a fee to receive influenza vaccine irrespective of their risk profile since 1994, when free vaccination for primary and secondary school students was stopped.45 This means that high-risk groups in Japan are less resistant to severe disease than in other countries, reducing this bias in our hospitalisation rates.
Implications for clinicians and policymakers
During the 2009–2010 pandemic, the WHO46 and the Centers for Disease Control and Prevention47 issued guidelines for early neuraminidase inhibitor treatment. There are suggestions that administration of this medication within 48 hours reduces mortality and severe outcomes.48 A Cochrane review, while critical of the quality of trial evidence, found a reduction in secondary infections among children who were prescribed oseltamivir.49 During the 2000s, four neuraminidase inhibitors became available in Japan: zanamivir in 2000, oseltamivir in 2001, and laninamivir and peramivir in 2010.22 The Japanese Association for Infectious Diseases also recommended postexposure prophylaxis of zanamivir and oseltamivir in hospitals and geriatric facilities in 2012. Reportedly, seven to eight million patients per annum were prescribed with neuraminidase inhibitors from 2011 to 2015; more than half of all patients infected with influenza received these medicines.22 The decreasing trend with time in the hospitalisation and the composite incidence of the five severe complications (table 3; p<0.0001) might be attributed to the increasingly widespread use of13 32 and more options for neuraminidase inhibitors.6 22 This decreasing trend was not altered in the 2014/2015 season when influenza A(H3N2) spread internationally, including in Japan.50 A recent meta-analysis of randomised controlled trials reported that in patients with pathogen-ascertained influenza, as is practice in Japan, treatment with oseltamivir reduced hospital admissions by 63%.51
In our study, the incidence of hospitalisations, acute pneumonia and febrile seizures decreased with time (table 3, all p values <0.0001). This was observed in parallel with increased administration of neuraminidase inhibitors. However, there appears to be no trend in the risk of encephalitis/encephalopathy. Because influenza encephalitis appears to be mediated by an acute process during infection,14 it is important to prevent influenza infection to reduce the incidence of encephalitis. The primary countermeasure to protect individuals from infection is vaccination. National vaccine policies may have impacted on variation in the hospitalisation incidence between countries. In Japan, schoolchildren were vaccinated routinely from 1976 to 1993, when influenza was removed from the list of free routine vaccinations, and vaccination of high-risk groups was only recommended from 2001, although for a fee which has limited uptake.52 Studies of this natural experiment suggest that the former routine vaccination programme for schoolchildren indirectly reduced excess mortality among the elderly.45 53 The previous results suggest indirect effect of the vaccines on reducing severe complication risk among children; however, the effect on the incidence of encephalitis is unknown. Because current encephalitis treatments are of limited effectiveness, a vaccination programme covering a broad population may be the best way to reduce the morbidity associated with influenza encephalitis.
Strengths and weaknesses
A main strength of this study is that the included patients were diagnosed with influenza by testing. The majority of influenza-like illness is usually caused by infections other than influenza.54 Diagnoses were based on rapid antigen detection with immunochromatogenic assay. Because POCTs for influenza are less invasive and require less time than laboratory tests, they are an essential tool for physicians to evaluate influenza in outpatient and inpatient clinical practice in Japan.6 Although low sensitivity (59%–93%)55 is a weak point for POCTs, the specificity is 98%–100%.33 56 This means that nearly all individuals with influenza-like illness who have POCT-positive results (the participants in this study) were infected with influenza. This makes routinely collected Japanese data unique. Additionally, all Japanese people who are hospitalised with severe complications of influenza infection should be present in universal health insurance data. Therefore, we did not greatly overestimate the number of infected patients or underestimate severe complications, and thus did not underestimate the risk of complications and hospitalisations.
There are limitations inherent to the data set used in this study. First, POCTs for influenza are known to have variable sensitivity. In the 2010s, 20 or more POCT kits were available in Japan.57 Sensitivity would have been influenced by the following factors:
Time from the onset of illness. Reportedly, the sensitivity is lower 0–24 hours from symptom onset and higher in days 2–4.58 59 Parents tend to bring children to paediatricians at an earlier stage of the infection, while infected employed adults tend to consult physicians in the mid or later stages. This would bias sensitivity towards comparatively low in children compared with adults.
Patient age. In contrast children are known to have higher viral load and longer shedding, and consequently POCTs have higher sensitivity in children.60 61
Influenza type A/B/C. POCT sensitivity is higher in influenza A than in B.60 In Japan, influenza type A spreads early in winter, type B late in winter and type C in all seasons. Therefore, the sensitivity might have been relatively low between January 2012 and August 2012 and higher between September 2016 and December 2016 (table 3).
Operator technique.59 In almost all Japanese medical care facilities, physicians conduct POCT for individuals with influenza-like illness.30 31 Because physicians are trained to appropriately sample specimen material, operator bias within our Japanese data would be small.
Number of times patients were tested. In Japan, physicians are permitted to conduct POCTs up to twice per patient in a calendar month within health insurance coverage. Even if the first POCT had failed to detect influenza-positive patients, the second POCT might identify the infection. Thus the sensitivity in Japanese clinical practice would be higher than the nominal sensitivity. Overall, the sensitivity of POCTs can vary unpredictably according to the circumstances. Our denominator (influenza-positive episodes) may be underestimated in low-sensitivity situations and to a smaller degree in high-sensitivity situations. In contrast, we would expect almost all of the numerator population (hospitalised patients with severe symptoms) would have been positively diagnosed. Thus, the estimated incidence of hospitalisation among influenza-positive patients may have been overestimated.
Second, the administrative data from employees and their families used here do not permit analysis of patients aged 75 years or more. Also, due to employment patterns in Japan, the number of male patients was slightly higher than female patients (table 1). The sex ratio varies across generations in Japan, with more males in younger populations and females predominating in older populations.61 However, the incidence of hospitalisation among infected patients in both sexes and the studied age groups should not be biased by this imbalance. Third, we were unable to estimate influenza-related mortality in this data set, but the data are sufficient to allow examination of serious complications that are major public health concerns. Although we were unable to define encephalitis/encephalopathy cases virologically, we added the requirement of receiving specific therapy to the definition of encephalitis/encephalopathy cases.36 This more stringent definition reduces the likelihood that we have overestimated this outcome. Further analyses of influenza-related mortality in Japan are needed, and this should encompass older adults. The effect of neuraminidase inhibitors should be examined using observational data, as clinical trials are likely to be underpowered for rare but important complications such as encephalopathy.
Conclusions
Using Japanese administrative data, 1.0% of patients who tested positive for influenza infection were hospitalised. Male patients had a higher incidence of pulmonary complications and febrile seizures. Children aged 0–5 years and adults aged 65–74 years were at high risk of being admitted to hospital for pneumonia, with the highest absolute numbers of hospitalised patients among young children. Further efforts are needed, such as active prescription of neuraminidase inhibitors and vaccination programmes, to prevent hospitalisations from severe complications in these age groups.
Supplementary Material
Acknowledgments
The authors are immensely grateful to the Japan Medical Data Center for providing the administrative data.
We thank Ms Analisa Avila, ELS, of Edanz Group for editing a draft of this manuscript.
Footnotes
Patient consent for publication: Not required.
Contributors: All of the authors agreed with the manuscript’s results and conclusion and approved the final version of the manuscript. HY conceived the study. HY, MM, JJL, RK, TY and ZY contributed to the design of the study and interpretation of the data analyses. HY analysed the data. HY, JJL, MM and RK wrote the first draft of the manuscript. JJL, RK, MM, ZY and HY contributed to revision of the manuscript. ZY and HY were responsible for data integrity. HY obtained funding.
Funding: This work was supported by funding from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (KAKENHI grant numbers JP15K08730 and JP18K17376).
Competing interests: None declared.
Ethics approval: The ethics committee of the School of Medicine, University of Yamanashi approved this study (approval number: H29-1709), in accordance with the ethical guidelines and regulations of the Declaration of Helsinki. The data were properly anonymised by the JMDC in the manner permitted by Japanese guideline of Personal Information Protection Commission, Cabinet Office, Government of Japan for the use of data from medical examinations in medical research without individual participants' consent (Act on the Protection of Personal Information, Act No 57 of 30 May 2003; last version amendment of Act No 65 of 2015).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The original administrative data are available through a formal request to the JMDC, subject to fees.
References
- 1. Ghebrehewet S, MacPherson P, Ho A. Influenza. BMJ 2016;355:i6258 10.1136/bmj.i6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaiser L, Wat C, Mills T, et al. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med 2003;163:1667–72. 10.1001/archinte.163.14.1667 [DOI] [PubMed] [Google Scholar]
- 3. Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018;391:1285–300. 10.1016/S0140-6736(17)33293-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333–40. 10.1001/jama.292.11.1333 [DOI] [PubMed] [Google Scholar]
- 5. Newland JG, Laurich VM, Rosenquist AW, et al. Neurologic complications in children hospitalized with influenza: characteristics, incidence, and risk factors. J Pediatr 2007;150:306–10. 10.1016/j.jpeds.2006.11.054 [DOI] [PubMed] [Google Scholar]
- 6. Sugaya N, Shinjoh M, Mitamura K, et al. Very low pandemic influenza A (H1N1) 2009 mortality associated with early neuraminidase inhibitor treatment in Japan: analysis of 1000 hospitalized children. J Infect 2011;63:288–94. 10.1016/j.jinf.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 7. Influenza Working Group of the Japan Pediatric Society. Secondary treatment guideline of 2013/2014 influenza infection. Japanese: Influenza Working Group of the Japan Pediatric Society, 2014. [Google Scholar]
- 8. Cromer D, van Hoek AJ, Jit M, et al. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect 2014;68:363–71. 10.1016/j.jinf.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 9. Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 2009;302:1896–902. 10.1001/jama.2009.1583 [DOI] [PubMed] [Google Scholar]
- 10. Nicholson KG, McNally T, Silverman M, et al. Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine 2006;24:102–8. 10.1016/j.vaccine.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 11. Pitman RJ, Melegaro A, Gelb D, et al. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect 2007;54:530–8. 10.1016/j.jinf.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 12. Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ 2013;347:f5061 10.1136/bmj.f5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugaya N. Widespread use of neuraminidase inhibitors in Japan. J Infect Chemother 2011;17:595–601. 10.1007/s10156-011-0288-0 [DOI] [PubMed] [Google Scholar]
- 14. Amin R, Ford-Jones E, Richardson SE, et al. Acute childhood encephalitis and encephalopathy associated with influenza: a prospective 11-year review. Pediatr Infect Dis J 2008;27:390–5. 10.1097/INF.0b013e31816507b2 [DOI] [PubMed] [Google Scholar]
- 15. Ekstrand JJ, Herbener A, Rawlings J, et al. Heightened neurologic complications in children with pandemic H1N1 influenza. Ann Neurol 2010;68:762–6. 10.1002/ana.22184 [DOI] [PubMed] [Google Scholar]
- 16. Evans A, Agadi S, Siegel J, et al. Neurologic complications associated with novel influenza A (H1N1) virus infection in children - Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep 2009;58:773–8. [PubMed] [Google Scholar]
- 17. Rellosa N, Bloch KC, Shane AL, et al. Neurologic manifestations of pediatric novel h1n1 influenza infection. Pediatr Infect Dis J 2011;30:165–7. 10.1097/INF.0b013e3181f2de6f [DOI] [PubMed] [Google Scholar]
- 18. Baltagi SA, Shoykhet M, Felmet K, et al. Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Pediatr Crit Care Med 2010;11:179–84. 10.1097/PCC.0b013e3181cf4652 [DOI] [PubMed] [Google Scholar]
- 19. Kasai T, Togashi T, Morishima T. Encephalopathy associated with influenza epidemics. Lancet 2000;355:1558–9. 10.1016/S0140-6736(05)74614-6 [DOI] [PubMed] [Google Scholar]
- 20. Morishima T, Togashi T, Yokota S, et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis 2002;35:512–7. 10.1086/341407 [DOI] [PubMed] [Google Scholar]
- 21. Sugaya N. Influenza-associated encephalopathy in Japan. Semin Pediatr Infect Dis 2002;13:79–84. 10.1053/spid.2002.122993 [DOI] [PubMed] [Google Scholar]
- 22. Zaraket H, Saito R. Japanese surveillance systems and treatment for influenza. Curr Treat Options Infect Dis 2016;8:311–28. 10.1007/s40506-016-0085-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okumura A, Nakagawa S, Kawashima H, et al. Deaths associated with pandemic (H1N1) 2009 among children, Japan, 2009-2010. Emerg Infect Dis 2011;17:1993–2000. 10.3201/eid1711.110649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang GF, Li W, Li K. Acute encephalopathy and encephalitis caused by influenza virus infection. Curr Opin Neurol 2010;23:305–11. 10.1097/WCO.0b013e328338f6c9 [DOI] [PubMed] [Google Scholar]
- 25. Hoshino A, Saitoh M, Oka A, et al. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev 2012;34:337–43. 10.1016/j.braindev.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 26. Gu Y, Shimada T, Yasui Y, et al. National surveillance of influenza-associated encephalopathy in Japan over six years, before and during the 2009-2010 influenza pandemic. PLoS One 2013;8:e54786 10.1371/journal.pone.0054786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Institute of Infectious Diseases and Mnistry of Health, Labout and Welfare. Infectious agents surveillance report. 36 Japanese: National Institute of Infectious Diseases and Mnistry of Health, Labout and Welfare, 2015. [Google Scholar]
- 28. Mizuguchi M. Influenza encephalopathy and related neuropsychiatric syndromes. Influenza Other Respir Viruses 2013;7:67–71. 10.1111/irv.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanaka S, Seto K, Kawakami K. Pharmacoepidemiology in Japan: medical databases and research achievements. J Pharm Health Care Sci 2015;1:16 10.1186/s40780-015-0016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Uehara S, Sunakawa K, Eguchi H, et al. Japanese guidelines for the management of respiratory infectious diseases in children 2007 with focus on pneumonia. Pediatr Int 2011;53:264–76. 10.1111/j.1442-200X.2010.03316.x [DOI] [PubMed] [Google Scholar]
- 31. Ito M, Watanabe M, Nakagawa N, et al. Rapid detection and typing of influenza A and B by loop-mediated isothermal amplification: comparison with immunochromatography and virus isolation. J Virol Methods 2006;135:272–5. 10.1016/j.jviromet.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 32. Sugaya N, Mitamura K, Yamazaki M, et al. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis 2007;44:197–202. 10.1086/509925 [DOI] [PubMed] [Google Scholar]
- 33. Suzuki M, Yoshimine H, Harada Y, et al. Estimating the influenza vaccine effectiveness against medically attended influenza in clinical settings: a hospital-based case-control study with a rapid diagnostic test in Japan. PLoS One 2013;8:e52103 10.1371/journal.pone.0052103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watanabe M, Nakagawa N, Ito M, et al. Sensitivity of rapid immunoassay for influenza A and B in the early phase of the disease. Pediatr Int 2009;51:211–5. 10.1111/j.1442-200X.2008.02696.x [DOI] [PubMed] [Google Scholar]
- 35. Komiya N, Gu Y, Kamiya H, et al. Clinical features of cases of influenza A (H1N1)v in Osaka prefecture, Japan, May 2009. Euro Surveill 2009;14:19272. [DOI] [PubMed] [Google Scholar]
- 36. Study Group of Influenza Encephalitis. Guideline of treatmant for influenza encephalitis. revised edition. Japanese: Ministry of Health, Labour and Welfare, 2011. [Google Scholar]
- 37. Kumar S, Havens PL, Chusid MJ, et al. Clinical and epidemiologic characteristics of children hospitalized with 2009 pandemic H1N1 influenza A infection. Pediatr Infect Dis J 2010;29:591–4. 10.1097/INF.0b013e3181d73e32 [DOI] [PubMed] [Google Scholar]
- 38. O’Riordan S, Barton M, Yau Y, et al. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. Can Med Assoc J 2010;182:39–44. 10.1503/cmaj.091724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee JJ, Bankhead C, Smith M, et al. Risk factors for influenza-related complications in children during the 2009/10 pandemic: a UK primary care cohort study using linked routinely collected data. Epidemiol Infect 2018;146:817–23. 10.1017/S0950268818000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Strachan DP, Butland BK, Anderson HR. Incidence and prognosis of asthma and wheezing illness from early childhood to age 33 in a national British cohort. BMJ 1996;312:1195–9. 10.1136/bmj.312.7040.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsuboi T. Epidemiology of febrile and afebrile convulsions in children in Japan. Neurology 1984;34:175–75. 10.1212/WNL.34.2.175 [DOI] [PubMed] [Google Scholar]
- 42. Talbot HK, Zhu Y, Chen Q, et al. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011-2012 influenza season. Clin Infect Dis 2013;56:1774–7. 10.1093/cid/cit124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yokomichi H, Kurihara S, Yokoyama T, et al. The pandemic influenza A (H1N1) 2009 vaccine does not increase the mortality rate of idiopathic interstitial pneumonia: a matched case-control study. PLoS One 2014;9:e88927 10.1371/journal.pone.0088927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yokomichi, H, et al. Safety of the Influenza A (H1N1)2009 vaccine in chronic obstructive pulmonary disease: a matched case-control study. J Vaccines Vaccin 2012;03:1000148 10.4172/2157-7560.1000148 [DOI] [Google Scholar]
- 45. Reichert TA, Sugaya N, Fedson DS, et al. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001;344:889–96. 10.1056/NEJM200103223441204 [DOI] [PubMed] [Google Scholar]
- 46. World Health Organization. Rapid advice guidelines for pharmacological management of pandemic influenza (H1N1) 2009 and other influenza viruses. 2010. http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf (Accessed 1 Dec 2018). [PubMed]
- 47. Centers for Disease Control and Prevention. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season. https://www.cdc.gov/h1n1flu/recommendations.htm (Accessed 1 Dec 2018).
- 48. Muthuri SG, Myles PR, Venkatesan S, et al. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009-2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis 2013;207:553–63. 10.1093/infdis/jis726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2012;1:CD008965 10.1002/14651858.CD008965.pub3 [DOI] [PubMed] [Google Scholar]
- 50. Pebody R, Warburton F, Andrews N, et al. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Euro Surveill 2015;20:30013 10.2807/1560-7917.ES.2015.20.36.30013 [DOI] [PubMed] [Google Scholar]
- 51. Dobson J, Whitley RJ, Pocock S, et al. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015;385:1729–37. 10.1016/S0140-6736(14)62449-1 [DOI] [PubMed] [Google Scholar]
- 52. Hirota Y, Kaji M. History of influenza vaccination programs in Japan. Vaccine 2008;26:6451–4. 10.1016/j.vaccine.2008.06.042 [DOI] [PubMed] [Google Scholar]
- 53. Charu V, Viboud C, Simonsen L, et al. Influenza-related mortality trends in Japanese and American seniors: evidence for the indirect mortality benefits of vaccinating schoolchildren. PLoS One 2011;6:e26282 10.1371/journal.pone.0026282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hayward AC, Fragaszy EB, Bermingham A, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med 2014;2:445–54. 10.1016/S2213-2600(14)70034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paules C, Subbarao K. Influenza. The Lancet 2017;390:697–708. 10.1016/S0140-6736(17)30129-0 [DOI] [PubMed] [Google Scholar]
- 56. Poehling KA, Zhu Y, Tang YW, et al. Accuracy and impact of a point-of-care rapid influenza test in young children with respiratory illnesses. Arch Pediatr Adolesc Med 2006;160:713–8. 10.1001/archpedi.160.7.713 [DOI] [PubMed] [Google Scholar]
- 57. Sakai-Tagawa Y, Ozawa M, Tamura D, et al. Sensitivity of influenza rapid diagnostic tests to H5N1 and 2009 pandemic H1N1 viruses. J Clin Microbiol 2010;48:2872–7. 10.1128/JCM.00439-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hata A, Asada J, Mizumoto H, et al. [Appropriate use of rapid diagnostic testing for influenza]. Kansenshogaku Zasshi 2004;78:846–52. 10.11150/kansenshogakuzasshi1970.78.846 [DOI] [PubMed] [Google Scholar]
- 59. Landry ML. Diagnostic tests for influenza infection. Curr Opin Pediatr 2011;23:91–7. 10.1097/MOP.0b013e328341ebd9 [DOI] [PubMed] [Google Scholar]
- 60. Chartrand C, Leeflang MM, Minion J, et al. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med 2012;156:500–11. 10.7326/0003-4819-156-7-201204030-00403 [DOI] [PubMed] [Google Scholar]
- 61. Central Intelligence Agency. The world factbook: sex ratio (male/female). 2018. https://www.cia.gov/library/publications/the-world-factbook/fields/2018.html (Accessed 1 Dec 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.