Abstract
Background
Leptomeningeal carcinomatosis (LC) is a severe complication of metastatic tumor spread to the central nervous system. Prognosis is dismal with a median overall survival (OS) of ~10–15 weeks. Treatment options include radiotherapy (RT) to involved sites, systemic chemo- or targeted therapy, intrathecal chemotherapy and best supportive care with dexamethasone. Craniospinal irradiation (CSI) is a more aggressive radiotherapeutic approach, for which very limited data exists. Here, we report on our 10-year experience with palliative CSI of selected patients with LC.
Patients and methods
Twenty-five patients received CSI for the treatment of LC at our institution between 2008 and 2018. Patients were selected individually for CSI based on clinical performance, presenting symptoms and estimated benefit. Median patient age was 53 years (IQR: 45–59), and breast cancer was the most common primary. Additional brain metastases were found in 18 patients (72.0%). RT was delivered at a TomoTherapy machine, using helical intensity-modulated radiotherapy (IMRT). The most commonly prescribed dose was 36 Gy in 20 fractions, corresponding to a median biologically equivalent dose of 40.8 Gy (IQR: 39.0–2.5). Clinical performance and neurologic function were assessed before and in response to therapy, and deficits were retrospectively quantified on the 5-point neurologic function scale (NFS). A Cox proportional hazards model with univariate and multivariate analyses was fitted for survival.
Results
Twenty-one patients died and four were alive at the time of analysis. Median OS from LC diagnosis was 19.3 weeks (IQR: 9.3–34.0, 95% CI: 11.0–32.0). In univariate analysis, a Karnofsky performance scale index (KPI) ≥70% (P=0.001), age ≤55 years at LC diagnosis (P=0.022), cerebrospinal fluid (CSF) protein <100 mg/dL (P=0.018) and no more than mild or moderate neurologic deficits (NFS ≤2; P=0.007) were predictive of longer OS. So were the neurologic response to treatment (P=0.018) and the application of systemic therapy after RT completion (P=0.029). The presence of CSF flow obstruction was predictive of shorter OS (P=0.026). In multivariate analysis, age at LC diagnosis (P=0.018), KPI (P<0.001) and neurologic response (P=0.037) remained as independent prognostic factors for longer OS. Treatment-associated toxicity was manageable and mostly grades I and II according to the Common Terminology Criteria for Adverse Events v4.0. Eight patients (32%) developed grade III myelosuppression. Neurologic symptom stabilization could be achieved in 40.0% and a sizeable improvement in 28.0% of all patients.
Conclusion
CSI for the treatment of LC is feasible and may have therapeutic value in carefully selected patients, alleviating symptoms or delaying neurologic deterioration. OS after CSI was comparable to the rates described in current literature for patients with LC. The use of modern irradiation techniques such as helical IMRT is warranted to limit toxicity. Patient selection should take into account prognostic factors such as age, clinical performance, neurologic function and the availability of systemic treatment options.
Keywords: leptomeningeal metastases, radiotherapy, TomoTherapy, carcinomatous meningitis, neuroaxis, neurologic function
Background
Approximately 5% of patients presenting with metastatic cancer develop leptomeningeal carcinomatosis (LC) as an advanced-stage complication of tumor spread to the central nervous system (CNS).1,2 LC, as defined by the current European Association of Neuro-Oncology–European Society for Medical Oncology guidelines, is a state in which tumor cells have multifocally affected the leptomeninges (pia or arachnoidea) and are frequently observed floating freely within the cerebrospinal fluid (CSF).3 Additional parenchymal brain metastases can be identified in 50%–80% of the cases.4–7 The incidence of LC varies depending on the primary histology, and it is diagnosed most frequently in patients with breast or lung cancer (10%–35%), as well as melanoma (5%–25%). However, LC diagnosis has become more frequent in gastrointestinal cancers (4%–14%), as well as in cancers of unknown primary (1%–7%).2,4,8,9 It is assumed that advances regarding the precision of diagnostic imaging have contributed to the rising overall incidence of LC. The same holds true for the prolonged survival attributable to newly available and efficacious systemic therapies.4,8,10,11
Due to the dismal prognosis of LC, treatment indications and modality are frequently discussed; median overall survival is reported to be in the vicinity of 10–15 weeks.6,12–14 There have, however, been reports identifying patient subgroups with a favorable outcome and even long-term survival in selected cases.6,12–14 Among the most decisive factors reported in the literature to influence survival are initial clinical performance, the availability of efficacious systemic treatment options and an aggressive treatment of LC.6,13–16 The benefit of intrathecal chemotherapy (ITC) has been established in several studies with different regimens based on methotrexate or liposomal cytarabine, among others.17–19 An emerging role can be observed for newer small-molecule and targeted drugs such as epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), potentially guided by molecularly informed decisions.20
Radiotherapy (RT) is frequently employed in the form of whole-brain irradiation (WBRT) with a potential survival benefit, especially if additional brain metastases have been diagnosed.14,15 To achieve symptom palliation and preserve neurologic function, this is often combined with focal RT of symptomatic spinal lesions.14
Craniospinal irradiation (CSI) as a more aggressive and extensive form of RT implies a total or subtotal irradiation of all CNS compartments, including the whole brain and typically complete spine.21 It is frequently performed as part of multimodal and curatively intended treatment approaches for pediatric tumors, such as medulloblastomas, or primary CNS tumors with cerebrospinal spread, such as ependymomas.22,23 For the palliative treatment of metastatic LC, there exists very sparse data supporting CSI, and clinical experience is limited.24 Critical consideration has to be given to several clinical as well as technical aspects that make CSI a challenging approach. On the clinical side, dose-limiting toxicity, primarily myelosuppression, can jeopardize the potential benefit and deplete the hematologic reserves needed for subsequent systemic therapy.25 On the technical side, field junctions have been necessary to achieve the required vertical radiation field extension, posing dosimetric challenges. However, the use of modern irradiation techniques, such as intensity-modulated radiotherapy (IMRT), helical treatment delivery and proton therapy, has helped overcome some of the obstacles.21,26–29
In the sparse body of literature available, it has been reported that in rare cases amounting to 10%–15% of the patients, comparably long-term survival after LC diagnosis could be achieved with the help of decisive treatment.24 This report aims to present our institution’s experience with the CSI of 25 metastatic patients with LC over a period of 10 years, evaluating the outcome, toxicity and predictive clinical factors to facilitate patient selection.
Patients and methods
We identified 25 patients who received CSI for the treatment of LC at our institution between 2008 and 2018. Patient data were extracted from an oncologic research database maintained at our institution, as well as from the patients’ detailed medical records. All reviews were performed following institutional guidelines and the Declaration of Helsinki of 1975 in its most recent version.
Patient characteristics
Median patient age at LC diagnosis was 53 years (IQR: 45–59; min.–max.: 17–74), and median interval from primary diagnosis was 25 months (IQR: 15–85; min.–max.: 0–279). Detailed patient characteristics are illustrated in Table 1. Primary histology varied: breast cancer was the most frequent (n=15, 60%), followed by lung cancer (n=6, 24%).
Table 1.
Patient characteristics (N=25)
| Age (years) | |
| Median | 53 |
| Q1–Q3 | 45–59 |
| Min.–max. | 17.0–74.0 |
| Gender | |
| Female | 16 (64.0%) |
| Male | 9 (36.0%) |
| Primary histology | |
| Breast cancer | 15 (60.0%) |
| Lung cancer | 6 (24.0%) |
| Gastrointestinal cancers | 2 (8.0%) |
| Myeloid leukemia | 1 (4.0%) |
| Sarcoma | 1 (4.0%) |
| Interval from primary diagnosis to LC diagnosis (months) | |
| Median | 25 |
| Q1–Q3 | 15–85 |
| Min.–max. | 0.0–279.0 |
| KPI at LC diagnosis (%) | |
| Median | 70 |
| Q1–Q3 | 60–70 |
| Min.–max. | 30–90 |
| Metastases outside CNS | |
| Yes | 16 (64.0%) |
| No | 9 (36.0%) |
| Parenchymal brain metastases | |
| Yes | 18 (72.0%) |
| No | 7 (28.0%) |
| Last therapy before CSI | |
| None | 11 (44.0%) |
| Intrathecal chemotherapy | 4 (16.0%) |
| Targeted therapy | 3 (12.0%) |
| Antihormonal therapy | 3 (12.0%) |
| Systemic chemotherapy | 3 (12.0%) |
| Combination therapy | 1 (4.0%) |
| Systemic therapy after CSI | |
| None | 16 (64.0%) |
| Systemic chemotherapy | 5 (20.0%) |
| Targeted therapy | 3 (12.0%) |
| Antihormonal therapy | 1 (4.0%) |
| Diagnostics | |
| MRI | 25 (100.0%) |
| Lumbar puncture | 20 (80.0%) |
| Both | 20 (80.0%) |
| LC spread | |
| Spine | 25 (100.0%) |
| Brain | 11 (44.0%) |
| Both | 11 (44.0%) |
| CSF protein level | |
| Mean | 384.7 |
| SD | 684.39 |
| Median | 194 |
| Q1–Q3 | 99.3–338 |
| Min.–max. | 59.3–3,200 |
| Neurologic function (NFS) before treatment | |
| 0 | 0 (0.0%) |
| 1 | 7 (28.0%) |
| 2 | 12 (48.0%) |
| 3 | 6 (24.0%) |
Notes: NFS 0, asymptomatic; NFS 1–2, mild or moderate neurologic symptoms; NFS 3–4, severe neurologic symptoms, requiring hospitalization. Data shown as n (%) unless indicated otherwise..
Abbreviations: CNS, central nervous system; CSI, craniospinal irradiation; KPI, Karnofsky performance scale index; LC, leptomeningeal carcinomatosis; MRI, magnetic resonance imaging; NFS, neurologic function scale.
For LC diagnosis, all patients received an MRI of the neuroaxis, identifying radiographic evidence of contrast enhancement of the spinal or cerebral meninges.3 Histologic confirmation by means of lumbar puncture revealed floating tumor cells in the CSF in 20 cases (80%). The spinal meninges were affected in all presented cases, whereas only eleven patients (44%) showed additional involvement of the intracranial meninges. Additional parenchymal brain metastases were identified in 18 cases (72%). The presence of nodular/bulky disease and CSF flow obstruction were assessed radiologically, as no CSF flow studies were performed.
Symptoms and overall clinical performance (Karnofsky performance scale index [KPI]) at the beginning of RT and during follow-up were extracted from the patients’ medical records and quantified regarding symptom control, improvement or worsening after therapy. Neurologic function was assessed by an experienced radiation oncologist based on the documented symptoms, and a neurologic function scale (NFS) was used to assess the palliative effect achieved by RT.30 Neurologic function was classified as follows: asymptomatic (0), minor neurologic symptoms (1), moderate neurologic symptoms (2), neurologically seriously limited, requiring hospitalization (3) and requiring hospitalization and constant nursing care (4). The outcome of NFS was assessed as either stable, improved or worsened, according to documented symptoms. Symptom control was defined as a constant value of the NFS at therapy completion or first follow-up if available, whereas improvement was defined as a reduction of the NFS by at least 1 point from baseline. Date of death was obtained from medical and official records. Treatment-related toxicity was rated according to the Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0.31 Detailed patient characteristics are illustrated in Table 1.
Treatment planning and delivery
Treatment indication was discussed interdisciplinarily in the context of our institution’s comprehensive cancer center. Patients were selected individually for CSI according to clinically estimated benefit. Selection criteria qualifying for CSI were mild neurologic symptoms, young age, good clinical performance, favorable histology and limited or controlled systemic tumor burden in addition to systemic treatment availability. Patients with poorer clinical performance could receive CSI if they showed multifocally symptomatic spread and symptom improvement by CSI was deemed feasible. Total CSI was performed in 19 patients (76.0%) and subtotal CSI with a maximum total sparing of eight spinal segments in 6 patients (24.0%). Twenty patients (80.0%) received simultaneous CSI, while six patients had previously completed WBRT (sequential CSI). Nine patients had previously received dosimetrically relevant irradiation to parts of the CNS. In those cases, during CSI, the previously irradiated areas were spared or received a lower dose according to the remaining radiotolerance.
Helical IMRT at a TomoTherapy® (Accuray Inc., Sunny-vale, CA, USA) machine was used for CSI. An individual head fixation mask was fitted for each patient. Treatment planning was performed using a 5 mm computed tomography and gadolinium-enhanced magnetic resonance imaging when needed. Dose optimization aimed at a homogeneous dose distribution to all parts of the CNS, including the brain, simultaneously treating additional brain metastases, if existent. No dedicated sparing of intracranial organs at risk (eg, the hippocampus) was performed. All aspects of TomoTherapy planning for CSI have been described earlier.22 Five patients received treatment in part at a conventional linear accelerator using laterally opposing fields for cranial irradiation and multi-field three-dimensional conformal radiotherapy for spinal irradiation, as has been previously described.32,33 Reasons were emergency start of treatment with subsequent TomoTherapy replanning, sequential CSI or subtotal CSI. The most commonly prescribed dose was 36 Gy in 20 fractions. Details on treatment parameters are presented in Table 2.
Table 2.
Prior radiotherapy and detailed treatment parameters for CSI
| Prior radiotherapy outside CNS, n (%) | |
| No | 21 (84.0%) |
| Yes | 4 (16.0%) |
| Prior radiotherapy to parts of the CNS, n (%) | |
| None | 16 (64.0%) |
| WBRT | 5 (20.0%) |
| SRS | 2 (8.0%) |
| WBRT and SRS | 1 (4.0%) |
| Other | 1 (4.0%) |
| Cumulative physical dose for CSI | |
| Mean | 32.4 |
| SD | 5.96 |
| Median | 35.2 |
| Q1–Q3 | 30.6–36 |
| Min.–max. | 14.4–36.0 |
| Physical dose per fraction for CSI | |
| Mean | 2.0 |
| SD | 0.43 |
| Median | 1.8 |
| Q1–Q3 | 1.8–1.8 |
| Min.–max. | 1.6–3.0 |
| Number of fractions | |
| Median | 20 |
| Q1–Q3 | 14–20 |
| Min.–max. | 8.0–22.0 |
| Biologically equivalent cumulative dose for CSI | |
| Mean | 38.2 |
| SD | 7.36 |
| Median | 40.8 |
| Q1–Q3 | 39–42.5 |
| Min.–max. | 17.0–43.8 |
Note: BED is calculated with an underlying assumed α/β=10 for malignant cells.
Abbreviations: BED, biologically equivalent dose; CNS, central nervous system; CSI, craniospinal irradiation; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Statistical analyses
For baseline analyses, descriptive statistics were used. Continuous variables are given as means (SD) and/or median (IQR and range, as appropriate) and categorical variables as absolute and relative frequencies. The median followup time was calculated using the reverse Kaplan–Meier method.34 Overall survival (OS) was calculated from the date of LC diagnosis to the last follow-up or death. OS was investigated using the method of Kaplan–Meier. Survival curves for prognostic factors were compared using a two-sided log-rank test. To identify the prognostic factors for OS, univariate and multivariate Cox regression were used. To preserve the validity of multivariate modeling in light of the limited number of patients, a maximum of three covariates that were significant in univariate analysis were chosen for multivariate Cox regression. The covariates thus chosen were the ones most frequently and strongly prognostic in comparable literature, while at the same time being easily and universally assessable in a clinical context. A univariate logistic regression was performed to screen for clinical factors with predictive value regarding symptom response to treatment (NFS stabilization or improvement, according to aforementioned definition). Since this was a retrospective exploratory data analysis, P-values are of descriptive nature. A descriptive P-value of <0.05 was considered as statistically significant. Statistical analyses were performed with the software R Version 3.4.3.
Results
Survival and prognostic factors
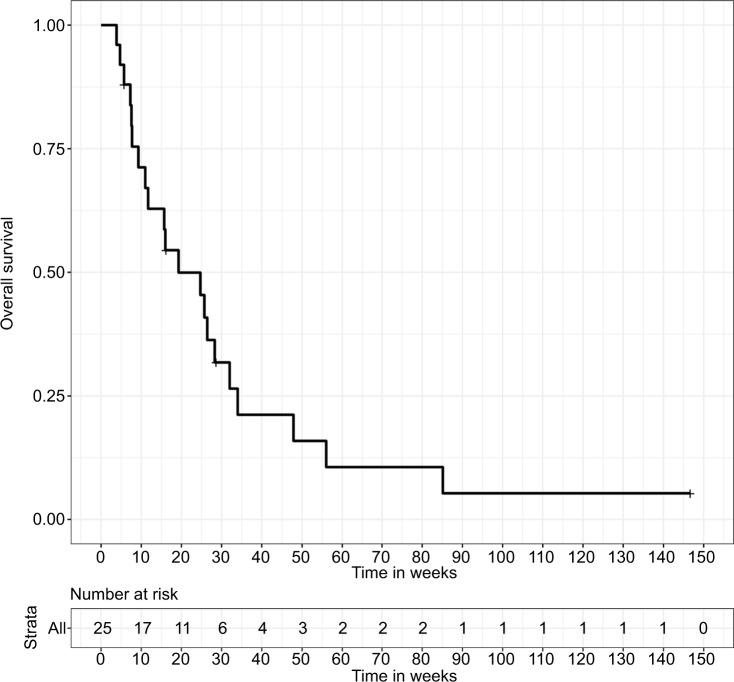
Median follow-up, as calculated by the reverse Kaplan–Meier method, was not reached. At the time of analysis, 21 patients had died and 4 patients were still alive. Median OS in all treated patients was 19.3 weeks (IQR: 9.3–34.0). Actuarial follow-up at the time of median OS was 44% (Figure 1).
Figure 1.
Overall survival for 25 patients receiving CSI, calculated from the date of LC diagnosis until death or the last follow-up.
Abbreviations: CSI, craniospinal irradiation; LC, leptomeningeal carcinomatosis.
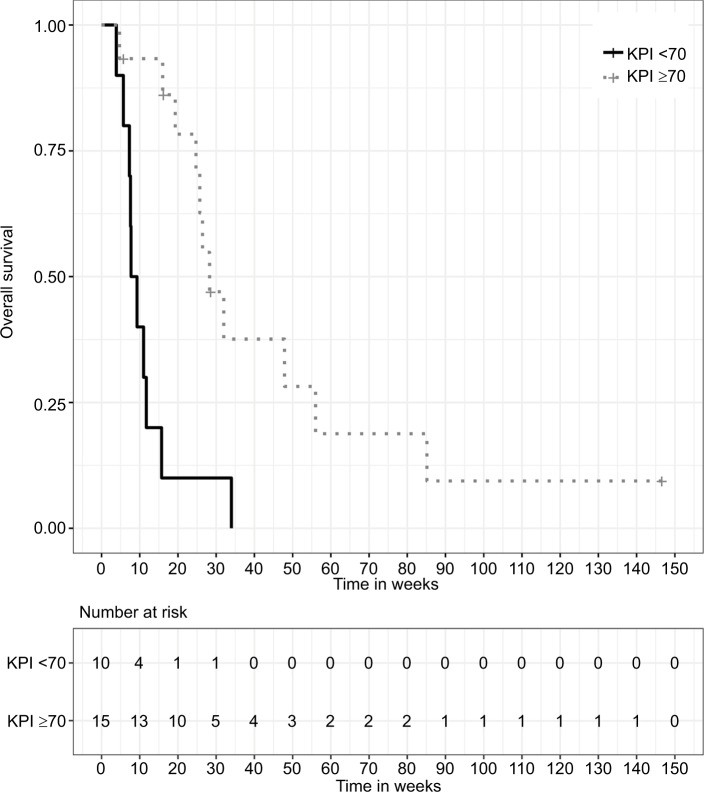
A KPI ≥70% at the beginning of RT was a significant prognostic factor for improved OS with a median OS of 28.3 weeks (IQR: 24.7–56.0) in patients with a good clinical performance (n=15, 60%), whereas patients with poor performance (KPI <70%, n=10, 40%) showed a median OS of 9.3 weeks (IQR: 7.3–11.7). This prognostic impact was shown in both univariate (HR 0.19, 95% CI: [0.07, 0.52], P=0.001) and multivariate (HR 0.13, 95% CI: [0.04, 0.40], P<0.001) analyses. Similar prognostic value was found for the patients’ age at LC diagnosis, with patients ≤55 years of age showing an improved median OS of 28.3 weeks (IQR: 15.7–47.9) in contrast to a median OS of 7.6 weeks (IQR: 5.7–25.7) for older patients. Again, the prognostic value of age confirmed in both univariate (HR 0.30, 95% CI: [0.11, 0.84], P=0.022) and multivariate (HR 0.21, 95% CI: [0.06, 0.77], P=0.018) analyses.
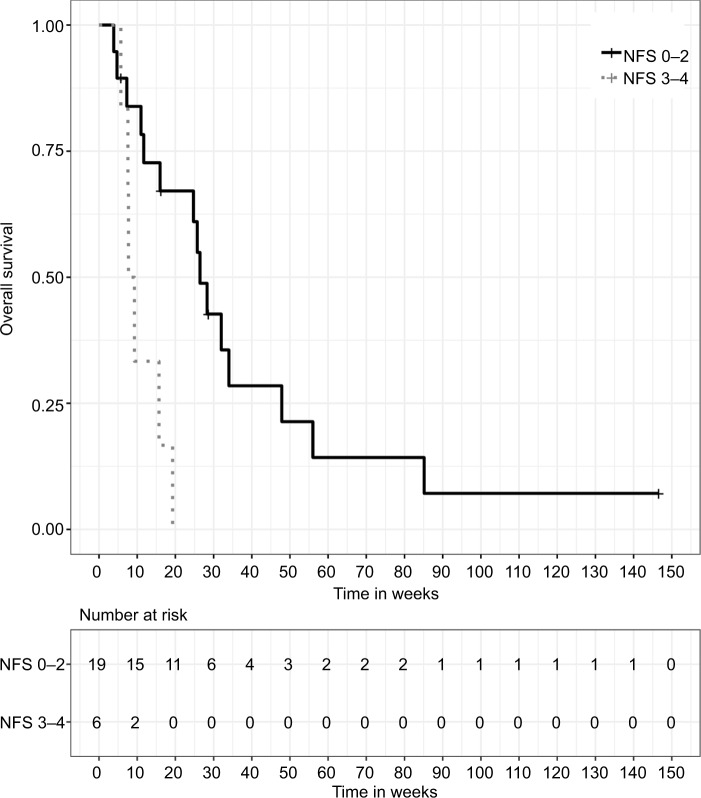
Patients initially presenting with mild or moderate symptoms (NFS ≤2) showed an improved OS in univariate analysis (HR 0.20, 95% CI: [0.06, 0.65], P=0.007). Median OS for these patients was 26.4 weeks (IQR: 11.7–47.9), compared to 9.3 weeks (IQR: 7.6–15.7) for patients presenting with severe neurologic symptoms. Symptom control or improvement in response to CSI was associated with a beneficial effect on OS (median 26.4 weeks, IQR: 15.7–47.9), whereas further neurologic deterioration during and after RT was prognostic for inferior OS (median 7.3 weeks, IQR: 5.7–16.0). The prognostic value of clinical response for improved OS was evident in univariate (HR 0.28, 95% CI: [0.10, 0.81], P=0.018) and multivariate (HR 0.24, 95% CI: [0.06, 0.92], P=0.037) analyses. Systemic treatment after CSI completion was administered in a total of nine cases (36.0%), and consisted of chemotherapy in five cases, targeted therapy in three cases and antihormonal therapy in one case. The application of systemic therapy after RT completion was prognostic for improved OS in univariate analysis (HR 0.34, 95% CI: [0.13, 0.90], P=0.029). The presence of CSF flow obstruction was prognostic of inferior OS (HR 3.02, 95% CI: [1.14, 7.98], P=0.026), whereas CSF protein levels below 100 mg/dL were prognostic of superior OS in univariate analysis (HR 0.16, 95% CI: [0.03, 0.73], P=0.018).
Clinical parameters without statistically significant impact on OS in our current analysis were the overall applied biologically equivalent dose and the extent of meningeal spread, involving only the spinal or additionally intracranial meninges. Furthermore, the presence or absence of parenchymal brain metastases, extracerebral metastases, nodular/bulky disease as well as the interval between primary and LC diagnoses showed no significant impact on survival. The clinical factors considered for univariate and multivariate analyses, the corresponding P-values and HRs are listed in Table 3. Age <55 years at LC diagnosis, KPI ≥70% at LC diagnosis and neurologic response to CSI were considered for multivariate analysis, according to the aforementioned rationale. Kaplan–Meier survival curves stratified by prognostic factors significant in the multivariate analysis are shown in Figures 2–4.
Table 3.
Factors analyzed in univariate and multivariate Cox regression with the corresponding HRs and P-values
| Factor analyzed | HR | 95% CI | P-value |
|---|---|---|---|
|
| |||
| Univariate Cox regression | |||
| Age ≤55 years at LC diagnosis | 0.298 | 0.106–0.837 | 0.022 |
| Male gender | 0.861 | 0.340–2.179 | 0.752 |
| NFS ≤2 at LC diagnosis | 0.022 | 0.063–0.649 | 0.007 |
| KPI ≥70% at LC diagnosis | 0.192 | 0.071–0.516 | 0.001 |
| Breast cancer histology | 1.052 | 0.426–2.599 | 0.912 |
| Systemic therapy after CSI completion | 0.337 | 0.127–0.897 | 0.029 |
| Involvement of cranial meninges | 0.821 | 0.338–1.998 | 0.664 |
| Tumor cells in CSF | 0.986 | 0.322–3.017 | 0.980 |
| Parenchymal brain metastases present | 0.957 | 0.365–2.509 | 0.929 |
| Interval between primary diagnosis and LC diagnosis | 1.002 | 0.996–1.008 | 0.473 |
| High-grade myelosuppression after CSI | 1.185 | 0.479–2.934 | 0.713 |
| Neurologic response to CSI | 0.284 | 0.100–0.806 | 0.018 |
| Metastases outside CNS present | 1.420 | 0.555–3.630 | 0.464 |
| Applied BED | 0.933 | 0.865–1.008 | 0.077 |
| CSF flow obstruction | 3.0201 | 1.143–7.982 | 0.026 |
| Nodular/bulky disease | 1.9299 | 0.809–4.602 | 0.138 |
| CSF protein level <100 mg/dL | 0.1581 | 0.034–0.727 | 0.018 |
| Multivariate Cox regression | |||
| Age ≤55 years at LC diagnosis | 0.213 | 0.059–0.768 | 0.018 |
| KPI ≥70% at LC diagnosis | 0.128 | 0.041–0.400 | |
| Neurologic response to CSI | 0.235 | 0.060–0.919 | 0.037 |
Note: Significant P-values indicated in bold type.
Abbreviations: BED, biologically equivalent dose; CNS, central nervous system; CSF, cerebrospinal fluid; CSI, craniospinal irradiation; KPI, Karnofsky performance scale index; LC, leptomeningeal carcinomatosis; NFS, neurologic function scale.
Figure 2.
Kaplan–Meier survival curves stratified by age at LC diagnosis, P=0.015 (two-sided log-rank test).
Abbreviation: LC, leptomeningeal carcinomatosis.
Figure 3.
Kaplan–Meier survival curves stratified by KPI, P<0.001 (two-sided log-rank test).
Abbreviation: KPI, Karnofsky performance scale index.
Figure 4.
Kaplan–Meier survival curves stratified by neurologic symptom response, P=0.012 (two-sided log-rank test).
Note: NFS 0, asymptomatic; NFS 1–2, mild or moderate neurologic symptoms; NFS 3–4, severe neurologic symptoms, requiring hospitalization.
Abbreviation: NFS, neurologic function scale.
Toxicity and symptom response
Acute treatment-related toxicity consisted mainly of nausea, fatigue and skin erythema. Those symptoms were mild and became manifest during treatment or within the first 4 weeks from treatment completion. Fatigue and nausea were most common among these symptoms, affecting 21 (84.0%) and 9 (36.0%) patients, respectively. None of these treatment-associated symptoms were considered severe or unexpected, and they were rated grade I or II according to CTCAE v 4.0. Of 25 patients, 20 (80.0%) patients completed the treatment and in 5 cases, treatment was discontinued due to tumor-associated general clinical deterioration. Prophylactic or therapeutic corticosteroids were administered in all patients, and all patients developed complete alopecia in the context of WBRT. Regular blood samples were taken for the early detection of high-grade myelosuppression which was defined as follows: anemia with hemoglobin levels <8 g/dL, neutropenia with leukocyte levels <1.0/nL or thrombopenia with thrombocyte levels <50/nL. Such hematologic toxicity, rated grade III according to CTCAE, was developed by eight patients (32.0%), who required medical intervention under which it was controlled. Six of these patients did not receive any systemic therapy after CSI. As far as can be determined in the context of this retrospective analysis, reasons in all these cases were either general performance related or due to the lack of efficacious medicamentous options. None was directly related to hematologic toxicity. No treatment-associated toxicities of grade IV or V were observed. Details on treatment-related toxicity are given in Table 4.
Table 4.
Acute treatment-associated toxicity
| Toxicity | n | % |
|---|---|---|
| Nausea | 9 | 36.0 |
| Fatigue | 21 | 84.0 |
| Skin erythema | 7 | 28.0 |
| Myelosuppression | 8 | 32.0 |
According to NFS, the majority of patients (n=19, 76.0%) initially presented with mild to moderate neurologic impairments (NFS 1–2). Severe neurologic impairment (NFS 3–4) was observed in six patients (24.0%). No patient was asymptomatic at the initial presentation. Most common among the documented symptoms were motor and/or sensory deficits (80.0% and 68.0%, respectively), often having led up to LC diagnosis. Headaches and vomiting as a sign of cerebral edema and elevated intracranial pressure (ICP) were frequent in patients presenting with additional brain metastases and were observed in 24.0% (n=6) and 16.0% (n=4) of the patients, respectively. Visual deficits and seizures affected 36.0% (n=9) and 12.0% (n=3) of the patients, respectively.
Regarding the clinical outcome and response to CSI, a stabilization of individual symptoms could be achieved in 44.0%–80.0% of the patients, depending on symptom. ICP-related symptoms were most commonly controlled (76.0%–80.0%) or even improved (16.0%–16.0%) by WBRT. Motor deficits were improved in 36.0% and sensory deficits in 20.0% of the patients. Those symptoms were stabilized in 44.0% and 68.0% of the patients, respectively. However, a fraction of the patients (20.0% for motor deficits and 12.0% for sensory deficits) suffered further symptom deterioration, irrespective of the treatment. Considering overall NFS, symptom palliation in the form of stabilization could be achieved in 40.0% and a sizeable improvement in 28.0% of all patients. Details on symptom response are given in Table 5.
Table 5.
Response of individual symptoms and NFS to treatment
| Before CSI | Clinical outcome after CSI | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinical symptoms and NFS | Improved | Stable | Worsened | |||||
| n | % | n | % | n | % | n | % | |
| Headache | 19 | 76.0 | 4 | 16.0 | 19 | 76.0 | 2 | 8.0 |
| Vomiting | 14 | 56.0 | 4 | 16.0 | 20 | 80.0 | 1 | 4.0 |
| Visual impairment | 4 | 16.0 | 4 | 16.0 | 18 | 72.0 | 3 | 12.0 |
| Seizures | 3 | 12.0 | 4 | 16.0 | 20 | 80.0 | 1 | 4.0 |
| Motor deficits | 20 | 80.0 | 9 | 36.0 | 11 | 44.0 | 5 | 20.0 |
| Sensory deficits | 17 | 68.0 | 5 | 20.0 | 17 | 68.0 | 3 | 12.0 |
| NFS 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| NFS 1–2 | 19 | 76.0 | 4 | 16.0 | 9 | 36.0 | 6 | 24.0 |
| NFS 3–4 | 7 | 28.0 | 3 | 12.0 | 1 | 4.0 | 2 | 8.0 |
Note: NFS 0, asymptomatic, NFS 1–2, mild or moderate neurologic symptoms; NFS 3–4, severe neurologic symptoms, requiring hospitalization.
Abbreviations: CSI, craniospinal irradiation; NFS, neurologic function scale.
Positive predictive relevance for symptom response, as determined by the univariate logistic regression, was observed for age ≤55 years (OR 14.00, 95% CI: [2.13, 138.42], P=0.011), whereas the presence of CSF flow obstruction was a significant negative predictor (OR 0.13, 95% CI: [0.02, 0.79], P=0.034). A trend toward negative predictive impact was detected for the presence of metastases outside the CNS, but significance was not reached (OR 0.19, 95% CI: [0.03, 1.07], P=0.069). ORs and the corresponding P-values of univariate logistic regression for the endpoint of symptom response are listed in Table 6.
Table 6.
Factors analyzed in univariate logistic regression for the endpoint of symptom response with the corresponding ORs and P-values
| Factor analyzed | OR | 95% CI | P-value |
|---|---|---|---|
|
| |||
| Age ≤55 years at LC diagnosis | 14.000 | 2.130–138.417 | 0.011 |
| Male gender | 0.417 | 0.069–2.387 | 0.323 |
| NFS ≤2 at LC diagnosis | 1.083 | 0.125–7.359 | 0.936 |
| KPI ≥70% at LC diagnosis | 1.833 | 0.326–10.622 | 0.486 |
| Breast cancer histology | 0.250 | 0.038–1.405 | 0.125 |
| Systemic therapy after CSI completion | 6.222 | 0.837–129.893 | 0.120 |
| Involvement of cranial meninges | 0.327 | 0.051–1.803 | 0.209 |
| Tumor cells in CSF | 0.464 | 0.022–3.960 | 0.527 |
| Parenchymal brain metastases present | 1.250 | 0.197–10.564 | 0.819 |
| Interval primary diagnosis to LC diagnosis | 0.994 | 0.983–1.005 | 0.271 |
| High-grade myelosuppression after CSI | 0.694 | 0.117–4.429 | 0.687 |
| Metastases outside CNS present | 0.185 | 0.026–1.073 | 0.069 |
| Applied BED | 1.213 | 1.036–1.700 | 0.082 |
| CSF flow obstruction | 0.129 | 0.016–0.792 | 0.034 |
| Nodular/bulky disease | 0.420 | 0.067–2.291 | 0.325 |
| CSF protein level <100 mg/dL | 1.500 | 0.212–13.563 | 0.691 |
Note: Significant P-values are indicated in bold type.
Abbreviations: BED, biologically equivalent dose; CNS, central nervous system; CSF, cerebrospinal fluid; CSI, craniospinal irradiation; KPI, Karnofsky performance scale index; LC, leptomeningeal carcinomatosis; NFS, neurologic function scale.
Discussion
We retrospectively assessed our experience with 25 patients who received CSI as a palliative treatment for LC over a period of 10 years. Overall, from a technical, as well as clinical point of view, treatment was feasible and resulted in OS rates that compare favorably to those described in the literature for this patient collective.13,14 Acute treatment-related toxicity was moderate and palliative symptom control or improvement was achieved in the majority of patients. Despite the limited number of subjects, we were able to identify age at LC diagnosis, clinical performance and neurologic treatment response as independent prognostic factors for survival, potentially helping in terms of future patient selection.
Regarding the published literature, very little data is available on the subject of CSI for the palliative treatment of LC. To the best of our knowledge, only one other report has specifically evaluated this approach in the past 20 years: Hermann et al reported on their experience treating a total of 16 patients between 1995 and 2000.24 They achieved a median OS of 12 weeks and symptom palliation in 68% of the patients. The symptoms that most commonly improved were pain, motor impairments and bladder/bowel incontinence. Reported treatment-related side effects were manageable and included mucositis, nausea and dysphagia. One-third of the patients developed high-grade myelosuppression.24
The current National Comprehensive Cancer Network (NCCN) guidelines recommend CSI for LC only for highly select cases (eg, hematologic malignancies, breast cancer) due to this treatment’s toxicity profile.35 In general, if the neurologic status and clinical performance are adequate, ITC is the recommended treatment option for non-adherent LC, as stated in the European Association of Neuro-Oncology–European Society for Medical Oncology guidelines.3,10 Treatment is usually administered via an intraventricular catheter connected to a subcutaneous reservoir (Ommaya reservoir).36 Efficacy has been demonstrated for different agents: several randomized trials have established the role of high-dose methotrexate for LC from breast cancer, lymphoma and leukemia,17,19 as well as the roles of thiotepa and rituximab in hematologic malignancies.17,37 In breast cancer patients, methotrexate, topotecan, etoposide and trastuzumab were shown to be effective.37–39 According to the NCCN guidelines, the administration of RT is recommended mainly to the involved regions in order to achieve symptom palliation, address bulky disease and remove CSF flow obstruction.4,35
One of the main obstacles, which in the past limited the feasibility of CSI and resulted in substantial toxicity, was the use of non-conformal, non-image-guided RT techniques. At a conventional linear accelerator, treatment planning for CSI typically included two opposing fields for cranial irradiation and two dorsal fields for spinal irradiation.24 To achieve vertical RT field extension, including the entire spine, field junctions were necessary with the associated risk of field overlap or underdosage due to positioning errors. To compensate for those risks, craniocaudal field borders were shifted in regular intervals over the course of RT.24 The introduction of TomoTherapy resolved the problem of field junctions, since the continuous movement of the treatment couch during beam administration results in a helical application of the dose, achieving homogeneous dose distributions over a long vertical field extension.40,41 Combined with the use of IMRT, high-dose conformity can be achieved and dosimetric superiority over conventional RT techniques has been conclusively demonstrated for TomoTherapy in several studies.42–44 This allows for the effective sparing of organs at risk, substantially reducing treatment-associated toxicity. Improvements could be achieved regarding the occurrence and severity of mucositis, as well as pulmonary and hematologic toxicity.22,45,46 Regarding hematologic toxicity, however, the wider low-dose distribution of TomoTherapy may, in some cases, result in an increase of red bone marrow exposed to relevant low-dose irradiation (the so-called dose-bath effect).47 Modern linear accelerators can perform IMRT employing alternate techniques such as volumetric modulated arc therapy, achieving a similarly favorable reduction of toxicity.48 Several comparative studies between Tomo-Therapy and other IMRT techniques have been performed and overall, the resulting dose distributions provide adequate sparing of organs at risk for both techniques.48,49 Regarding field junctions, different techniques have been conceived to compensate for the positioning errors and avoid relevant over- or underdosage: exploiting the advantages of IMRT, low-gradient junction techniques, as well as non-coplanar IMRT techniques were proven effective dosimetrically.50,51 Superior sparing of bone marrow in the vertebral bodies, as well as even higher dose conformity can be achieved using proton irradiation.29,52 Favorable results regarding toxicity and tumor control have been described for proton CSI in pediatric tumors in several recent works, although proton therapy generally reintroduces the issue of field junctions.53–55
Median OS at 19.3 weeks in our current analysis compares favorably to that in previously published analyses in patients with LC: for LC from non-small-cell lung cancer, two relatively large analyses, including 125 and 149 patients, have been published by Morris et al6 and Lee et al,13 respectively. Median OS estimates were 12.9 and 15.0 weeks in those cohorts that received heterogeneous treatment regimens, including WBRT and RT to the involved regions and differing combinations with ITC and/or systemic chemotherapy. A small subgroup of patients, for whom the administration of EGFR-TKIs was indicated, showed a favorable median OS of up to 14 months.6 Similar analyses for breast cancer patients have been recently reviewed by Kak et al.56 In several mid-sized cohorts of between 60 and 100 patients, median OS estimates of 10–16 weeks could be achieved.7,57–59 Therapeutic modalities comprised mostly ITC with methotrexate and second-line thiotepa, although Le Rhun et al described a reasonably sized cohort of 103 patients treated with liposomal cytarabine as a first-line therapy, achieving a median OS of 16.3 weeks.12 Overall results for breast cancer are thus comparable to those previously discussed for lung cancer.
A recent publication by Brower et al assessed the outcome of 124 patients with LC from heterogeneous solid tumors, including unfavorable histologies such as small-cell lung-cancer.14 In this unselected patient collective, median OS at 9.2 weeks was slightly inferior to the works mentioned before.14 In our analysis, no statistical significance could be found for the impact of primary histology and in view of the recently discussed works, this is possibly due to the limited number of patients.
In the published literature, as well as our current analysis, survival seems to be highly dependent on patient selection. This is demonstrated most clearly in clinical performance manifesting itself as a central and consistent prognostic factor throughout the great majority of publications available on the subject of LC.13–16,60–62 Our analysis is in agreement with that body of literature in confirming the prognostic value of the KPI. For a more comprehensive assessment of the patients’ neurologic function, the NFS scale has proven a useful tool with independent prognostic significance. Its key value lies in providing functional quantification to a reasonable extent and summarizing the neurologic symptoms for better comparability. Several recent publications have confirmed the role and applicability of the NFS for the clinical assessment of baseline status and treatment outcome in patients with CNS metastases.30,63 Age at LC diagnosis was identified as another prognostic factor in our analysis and in this, consistency can be found with the widely established recursive partitioning analysis (RPA) and graded prognostic assessment (GPA) indices for the prognostic evaluation of CNS metastases.64–66 The statistically significant identification of these factors despite the limited number of patients in the current analysis emphasizes their decisiveness and suggests that it is, in fact, a very select subgroup of patients that may benefit from CSI as a palliative measure. Furthermore, this implies that rigorous patient selection, based on thorough clinical assessment, and interdisciplinary decision making are necessary to choose the best treatment approach after LC diagnosis.
Limitations of the current study include its retrospective nature and the small number of patients, as well as a possible selection bias, owing to the fact that CSI for the palliative treatment of LC is not an approach generally recommended by the current guidelines. Consequently, it was decided upon on an individual basis for each patient in this group and for differing reasons, including mostly young age, good performance and clinically estimated benefit. Furthermore, the very small number of patients receiving ITC in our cohort permits no assessment of the effect ITC may have had on survival. Analogously, in the era of targeted therapies with proven efficacy inside the CNS,67,68 comparative data with this form of treatment would be warranted.
Conclusion
To the best of our knowledge, this is the larger of only two cohorts in the current literature, for which the specific effect of CSI in the palliative treatment of LC has been evaluated. We could demonstrate treatment feasibility and potential therapeutic value in carefully selected patients, alleviating preexisting symptoms or delaying neurologic deterioration. OS after CSI was comparable to rates described in the current literature for patients with LC. To achieve a reasonable toxicity profile, the use of modern irradiation techniques, such as helical IMRT, is warranted. Patient selection should be done on an individual basis, taking into account prognostic factors such as age, clinical performance, neurologic function and the availability of systemic treatment options.
Ethics approval
Ethics approval for the study and a waiver of written informed consent, as applicable to the retrospective in-house research, were granted by the Heidelberg University ethics committee on April 12, 2018 (#S-172/2018). Patient confidentiality was maintained by anonymizing patient data to remove any identifying information.
Acknowledgments
This work received indirect financial support by Heidelberg University young investigator grants to RAES, DB and JHR.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin. 2003;21(1):25–66. doi: 10.1016/s0733-8619(02)00032-4. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan JG, Desouza TG, Farkash A, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol. 1990;9(3):225–229. doi: 10.1007/BF02341153. [DOI] [PubMed] [Google Scholar]
- 3.Le Rhun E, Weller M, Brandsma D, et al. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28(Suppl 4):iv84–iv99. doi: 10.1093/annonc/mdx221. [DOI] [PubMed] [Google Scholar]
- 4.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74(18):1449–1454. doi: 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umemura S, Tsubouchi K, Yoshioka H, et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer. 2012;77(1):134–139. doi: 10.1016/j.lungcan.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7(2):382–385. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 7.Lara-Medina F, Crismatt A, Villarreal-Garza C, et al. Clinical features and prognostic factors in patients with carcinomatous meningitis secondary to breast cancer. Breast J. 2012;18(3):233–241. doi: 10.1111/j.1524-4741.2012.01228.x. [DOI] [PubMed] [Google Scholar]
- 8.Nugent JL, Bunn PA, Matthews MJ, et al. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer. 1979;44(5):1885–1893. doi: 10.1002/1097-0142(197911)44:5<1885::aid-cncr2820440550>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Yap HY, Yap BS, Tashima CK, Distefano A, Blumenschein GR. Meningeal carcinomatosis in breast cancer. Cancer. 1978;42(1):283–286. doi: 10.1002/1097-0142(197807)42:1<283::aid-cncr2820420142>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25(2):103–119. doi: 10.1053/ctrv.1999.0119. [DOI] [PubMed] [Google Scholar]
- 12.Le Rhun E, Taillibert S, Zairi F, et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol. 2013;113(1):83–92. doi: 10.1007/s11060-013-1092-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Lee JI, Nam DH, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol. 2013;8(2):185–191. doi: 10.1097/JTO.0b013e3182773f21. [DOI] [PubMed] [Google Scholar]
- 14.Brower JV, Saha S, Rosenberg SA, Hullett CR, Ian Robins H. Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci. 2016;27:130–137. doi: 10.1016/j.jocn.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Ozdemir Y, Yildirim BA, Topkan E. Whole brain radiotherapy in management of non-small-cell lung carcinoma associated leptomen-ingeal carcinomatosis: evaluation of prognostic factors. J Neurooncol. 2016;129(2):329–335. doi: 10.1007/s11060-016-2179-9. [DOI] [PubMed] [Google Scholar]
- 16.Gwak HS, Joo J, Kim S, et al. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol. 2013;8(5):599–605. doi: 10.1097/JTO.0b013e318287c943. [DOI] [PubMed] [Google Scholar]
- 17.Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11(3):561–569. doi: 10.1200/JCO.1993.11.3.561. [DOI] [PubMed] [Google Scholar]
- 18.Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. 1987;5(10):1655–1662. doi: 10.1200/JCO.1987.5.10.1655. [DOI] [PubMed] [Google Scholar]
- 19.Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (Depo-Cyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- 20.Kwon J, Chie EK, Kim K, et al. Impact of multimodality approach for patients with leptomeningeal metastases from solid tumors. J Korean Med Sci. 2014;29(8):1094. doi: 10.3346/jkms.2014.29.8.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernier V. Technical aspects in cerebrospinal irradiation. Pediatr Blood Cancer. 2004;42(5):447–451. doi: 10.1002/pbc.10468. [DOI] [PubMed] [Google Scholar]
- 22.Schiopu SR, Habl G, Häfner M, et al. Craniospinal irradiation using helical tomotherapy for central nervous system tumors. J Radiat Res. 2017;58(2):238–246. doi: 10.1093/jrr/rrw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang DS, Murphy ES, Ezell SE, Lucas JT, Tinkle C, Merchant TE. Craniospinal irradiation for treatment of metastatic pediatric low-grade glioma. J Neurooncol. 2017;134(2):317–324. doi: 10.1007/s11060-017-2525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermann B, Hültenschmidt B, Sautter-Bihl ML. Radiotherapy of the neuroaxis for palliative treatment of leptomeningeal carcinomatosis. Strahlenther Onkol. 2001;177(4):195–199. doi: 10.1007/pl00002398. [DOI] [PubMed] [Google Scholar]
- 25.Kumar N, Miriyala R, Thakur P, et al. Impact of acute hematological toxicity on treatment interruptions during cranio-spinal irradiation in medulloblastoma: a tertiary care institute experience. J Neurooncol. 2017;134(2):309–315. doi: 10.1007/s11060-017-2524-7. [DOI] [PubMed] [Google Scholar]
- 26.Zong-Wen S, Shuang-Yan Y, Feng-Lei D, et al. Radiotherapy for adult medulloblastoma: evaluation of helical tomotherapy, volumetric intensity modulated arc therapy, and three-dimensional conformal radiotherapy and the results of helical tomotherapy therapy. Biomed Res Int. 2018;2018:1–8. doi: 10.1155/2018/9153496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang K, Meng H, Chen J, Zhang W, Feng Y. Plan quality and robustness in field junction region for craniospinal irradiation with VMAT. Phys Med. 2018;48:21–26. doi: 10.1016/j.ejmp.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Seravalli E, Bosman M, Lassen-Ramshad Y, et al. Dosimetric comparison of five different techniques for craniospinal irradiation across 15 European centers: analysis on behalf of the SIOP-E-BTG (radiotherapy working group) Acta Oncol. 2018;57(9):1240–1249. doi: 10.1080/0284186X.2018.1465588. [DOI] [PubMed] [Google Scholar]
- 29.Farace P, Bizzocchi N, Righetto R, et al. Supine craniospinal irradiation in pediatric patients by proton pencil beam scanning. Radiother Oncol. 2017;123(1):112–118. doi: 10.1016/j.radonc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Bezjak A, Adam J, Barton R, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer. 2002;38(4):487–496. doi: 10.1016/s0959-8049(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 31.National Institutes of Health NCI Common Terminology Criteria for Adverse Events v4.0 (CTCAE) [Accessed December 19, 2016]. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Published 2010.
- 32.Rief H, Bischof M, Bruckner T, et al. The stability of osseous metastases of the spine in lung cancer – a retrospective analysis of 338 cases. Radiat Oncol. 2013;8(1):200. doi: 10.1186/1748-717X-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scharp M, Hauswald H, Bischof M, Debus J, Combs SE. Re-irradiation in the treatment of patients with cerebral metastases of solid tumors: retrospective analysis. Radiat Oncol. 2014;9(1):4. doi: 10.1186/1748-717X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 35.Bower M, Waxman J. Lecture Notes Oncology. PA, USA: National Comprehensive Cancer Network; 2018. Central nervous system cancers; pp. 96–97. V2. [Google Scholar]
- 36.Sandberg DI, Bilsky MH, Souweidane MM, Bzdil J, Gutin PH. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery. 2000;47(1):49–54. doi: 10.1097/00006123-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Groves MD, Glantz MJ, Chamberlain MC, et al. A multicenter Phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. 2008;10(2):208–215. doi: 10.1215/15228517-2007-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chamberlain MC, Tsao-Wei DD, Groshen S. Phase II trial of intracerebrospinal fluid etoposide in the treatment of neoplastic meningitis. Cancer. 2006;106(9):2021–2027. doi: 10.1002/cncr.21828. [DOI] [PubMed] [Google Scholar]
- 39.Zagouri F, Sergentanis TN, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13–22. doi: 10.1007/s10549-013-2525-y. [DOI] [PubMed] [Google Scholar]
- 40.Bauman G, Yartsev S, Coad T, Fisher B, Kron T. Helical tomotherapy for craniospinal radiation. Br J Radiol. 2005;78(930):548–552. doi: 10.1259/bjr/53491625. [DOI] [PubMed] [Google Scholar]
- 41.Sterzing F, Schubert K, Sroka-Perez G, Kalz J, Debus J, Herfarth K. Helical tomotherapy. Strahlenther Onkol. 2008;184(1):8–14. doi: 10.1007/s00066-008-1778-6. [DOI] [PubMed] [Google Scholar]
- 42.Sharma DS, Gupta T, Jalali R, Master Z, Phurailatpam RD, Sarin R. High-precision radiotherapy for craniospinal irradiation: evaluation of three-dimensional conformal radiotherapy, intensity-modulated radiation therapy and helical TomoTherapy. Br J Radiol. 2009;82(984):1000–1009. doi: 10.1259/bjr/13776022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong JY, Kim GW, Kim CU, et al. Supine linac treatment versus tomotherapy in craniospinal irradiation: planning comparison and dosimetric evaluation. Radiat Prot Dosimetry. 2011;146(1–3):364–366. doi: 10.1093/rpd/ncr190. [DOI] [PubMed] [Google Scholar]
- 44.Kunos CA, Dobbins DC, Kulasekere R, Latimer B, Kinsella TJ. Comparison of helical tomotherapy versus conventional radiation to deliver craniospinal radiation. Technol Cancer Res Treat. 2008;7(3):227–233. doi: 10.1177/153303460800700308. [DOI] [PubMed] [Google Scholar]
- 45.Sugie C, Shibamoto Y, Ayakawa S, et al. Craniospinal irradiation using helical tomotherapy: evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat. 2011;10(2):187–195. doi: 10.7785/tcrt.2012.500194. [DOI] [PubMed] [Google Scholar]
- 46.Peñagarícano J, Moros E, Corry P, Saylors R, Ratanatharathorn V. Pediatric craniospinal axis irradiation with helical tomotherapy: patient outcome and lack of acute pulmonary toxicity. Int J Radiat Oncol Biol Phys. 2009;75(4):1155–1161. doi: 10.1016/j.ijrobp.2008.12.083. [DOI] [PubMed] [Google Scholar]
- 47.Petersson K, Gebre-Medhin M, Ceberg C, et al. Haematological toxicity in adult patients receiving craniospinal irradiation – indication of a dose-bath effect. Radiother Oncol. 2014;111(1):47–51. doi: 10.1016/j.radonc.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Myers PA, Mavroidis P, Papanikolaou N, Stathakis S. Comparing conformal, arc radiotherapy and helical tomotherapy in craniospinal irradiation planning. J Appl Clin Med Phys. 2014;15(5):12–28. doi: 10.1120/jacmp.v15i5.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mascarin M, Giugliano FM, Coassin E, et al. Helical tomotherapy in children and adolescents: dosimetric comparisons, opportunities and issues. Cancers. 2011;3(4):3972–3990. doi: 10.3390/cancers3043972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen AT, Lukacova S, Lassen-Ramshad Y, Petersen JB. Comparison of a new noncoplanar intensity-modulated radiation therapy technique for craniospinal irradiation with 3 coplanar techniques. Med Dosim. 2015;40(4):296–303. doi: 10.1016/j.meddos.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar B, Munshi A, Manikandan A, et al. A low gradient junction technique of craniospinal irradiation using volumetric-modulated arc therapy and its advantages over the conventional therapy. Cancer Radiother. 2018;22(1):62–72. doi: 10.1016/j.canrad.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 52.Macewan I, Chou B, Moretz J, Loredo L, Bush D, Slater JD. Effects of vertebral-body-sparing proton craniospinal irradiation on the spine of young pediatric patients with medulloblastoma. Adv Radiat Oncol. 2017;2(2):220–227. doi: 10.1016/j.adro.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giantsoudi D, Seco J, Eaton BR, et al. Evaluating intensity modulated proton therapy relative to passive scattering proton therapy for increased vertebral column sparing in craniospinal irradiation in growing pediatric patients. Int J Radiat Oncol Biol Phys. 2017;98(1):37–46. doi: 10.1016/j.ijrobp.2017.01.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a Phase 2 single-arm study. Lancet Oncol. 2016;17(3):287–298. doi: 10.1016/S1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]
- 55.Farace P, Vinante L, Ravanelli D, Bizzocchi N, Vennarini S. Planning field-junction in proton cranio-spinal irradiation – the ancillary-beam technique. Acta Oncol. 2015;54(7):1075–1078. doi: 10.3109/0284186X.2014.970667. [DOI] [PubMed] [Google Scholar]
- 56.Kak M, Nanda R, Ramsdale EE, Lukas RV. Treatment of leptomeningeal carcinomatosis: current challenges and future opportunities. J Clin Neurosci. 2015;22(4):632–637. doi: 10.1016/j.jocn.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 57.Gauthier H, Guilhaume MN, Bidard FC, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010;21(11):2183–2187. doi: 10.1093/annonc/mdq232. [DOI] [PubMed] [Google Scholar]
- 58.Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis – the role of multimodality treatment. J Neurooncol. 2007;84(1):57–62. doi: 10.1007/s11060-007-9340-4. [DOI] [PubMed] [Google Scholar]
- 59.Boogerd W, Hart AA, van der Sande JJ, Engelsman E. Meningeal carcinomatosis in breast cancer. Prognostic factors and influence of treatment. Cancer. 1991;67(6):1685–1695. doi: 10.1002/1097-0142(19910315)67:6<1685::aid-cncr2820670635>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 60.Morris Z, Whiteley WN, Longstreth WT, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gani C, Müller AC, Eckert F, et al. Outcome after whole brain radiotherapy alone in intracranial leptomeningeal carcinomatosis from solid tumors. Strahlenther Onkol. 2012;188(2):148–153. doi: 10.1007/s00066-011-0025-8. [DOI] [PubMed] [Google Scholar]
- 62.de Azevedo CR, Cruz MR, Chinen LT, et al. Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. 2011;104(2):565–572. doi: 10.1007/s11060-010-0524-y. [DOI] [PubMed] [Google Scholar]
- 63.Bernhardt D, Bozorgmehr F, Adeberg S, et al. Outcome in patients with small cell lung cancer re-irradiated for brain metastases after prior prophylactic cranial irradiation. Lung Cancer. 2016;101:76–81. doi: 10.1016/j.lungcan.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 65.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70(2):510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 66.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13(12):1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two Phase II trials. Ann Oncol. 2018;29(3):687–693. doi: 10.1093/annonc/mdx820. [DOI] [PubMed] [Google Scholar]