Abstract
Haemophagocytic lymphohistiocytosis (HLH) is a potentially life-threatening syndrome caused by excessive immune activation. Secondary HLH has been described in autoimmune diseases. We detail the case of a 28-year-old African American woman who developed HLH in the setting of systemic lupus erythematosus with collapsing lupus podocytopathy superimposed on mesangial proliferative lupus nephritis class II. Genotyping for APOL1 risk alleles revealed the presence of double (G1/G2) risk alleles. Our patient achieved a complete renal recovery and resolution of HLH within 1 month of treatment with steroids and mycophenolate mofetil, highlighting the importance of prompt, aggressive therapy.
Keywords: renal system, acute renal failure, proteinurea, systemic lupus erythematosus, haematology (INCL blood transfusion)
Background
Haemophagocytic lymphohistiocytosis (HLH) is a heterogeneous disorder characterised by excessive macrophage activation and hypercytokinaemia. Diagnosis of HLH requires the presence of at least five of eight defining features: fever, splenomegaly, bi/pancytopenia, hypertriglyceridaemia and/or hypofibrinogenaemia, hyperferritinaemia, hemophagocytosis, soluble CD25 (soluble IL-2 receptor) >2400 U/mL and low or absent NK cell activity.1 HLH can occur as a primary genetic condition due to mutations in genes governing T-cell and NK-cell cytotoxic inflammatory responses, or secondary to autoimmune diseases, infections, or hematological malignancies. We describe a unique case of HLH secondary to systemic lupus erythematosus (SLE) in an African-American patient with double APOL1 risk allele genotype and acute kidney injury due to collapsing lupus podocytopathy superimposed on class II lupus nephritis.
Case presentation
A 28-year-old African American woman presented with a 3-month history of recurrent high spiking fevers, shortness of breath, unintentional weight loss, myalgias, arthralgias and erythematous skin lesions. Physical examination revealed scarring alopecia and a discoid rash involving the aural conchal bowls. Notably, she was found to have severe neutropaenia, moderate thrombocytopaenia, elevated erythrocyte sedimentation rate (ESR) and C reactive protein (CRP), low complement C3, positive ANA and other positive lupus serologies (table 1). She was commenced on treatment with prednisone 1 mg/kg/daily based on the clinical suspicion of SLE in evolution.
Table 1.
Laboratory parameters
| Urinalysis | Cloudy, large blood, >300 protein, negative for leucocyte esterase, negative nitrite, negative glucose, pH 6.5, specific gravity 1.020 |
| Urine microscopy | Many dysmorphic red blood cells, acanthocytes, few white blood cells, renal tubular epithelial cells, few oval fat bodies and no casts |
| Urine protein/creatinine ratio | 1291.7 mg/g |
| WBC count (cells/mm3) | 800 (3500–11 000 cells/mm3) |
| Haemoglobin (g/dl) | 10.5 (12–16 g/dL) |
| Haematocrit (%) | 30.8 (36–46%) |
| Platelets (cells/mm3) | 101 (150–440 cells/mm3) |
| Sodium (mEq/L) | 134 (135–145 mEq/L) |
| Potassium (mEq/L) | 4.2 (3.5–5 mEq/L) |
| Chloride (mEq/L) | 105 (98–108 mEq/L) |
| Bicarbonate (mEq/L) | 19.6 (24–30 mEq/L) |
| BUN (mg/dL) | 19 (5–26 mg/dL) |
| Creatinine (mg/dL) | 1.3 (0.6–1.2 mg/dL) |
| eGFR* (mL/min/1.73 m2) | 59 (82–137)† |
| PT/INR (sec/ratio) | 9/0.9 (9.5–12.2 sec/ 0.9–1.2 ratio) |
| aPTT (sec) | 23.8 (20.1–31.2 s) |
| Fibrinogen (mg/dL) | 410 (200–400 mg/dL) |
| D-Dimer (mg/L FEU) | 1.25 (0–0.5 mg/L FEU) |
| Ferritin (μg/L) | 2520 (12–150 ug/L) |
| Albumin (g/dL) | 2.9 (3.5–5.5 g/dL) |
| AST (U/L) | 535 (1–40 U/L) |
| ALT (U/L) | 185 (1–40 U/L) |
| Total bilirubin (mg/dL) | 1 (0.1–1.2 mg/dL) |
| Direct bilirubin (mg/dL) | 0.6 (0.1–0.6 mg/dL) |
| LDH (U/L) | 637 (100–210 U/L) |
| Fibrinogen | 410 (200–400 mg/dL) |
| Triglyceride (mg/dL) | 272 (35–135 mg/dL) |
| Lactate (mmol/L) | 1.31 (0.3–1.3 mmol/L) |
| ACE, serum (U/L) | 110 (14–82 U/L) |
| CMV | IgM negative IgG negative |
| Ebstein-Barr virus Ab | IgM negative IgG positive |
| HIV Ab 1/2 | Negative |
| Parvovirus B-19 | IgM negative IgG positive |
| Hepatitis C Ab | Negative |
| Hepatitis B Ab | Reactive ≥11.5 mIU/mL |
| Hepatitis BsAg | Nonreactive |
| Myeloperoxidase Ab (units) | <5 (normal range ≤20.0 units) |
| Proteinase – 3 Ab (units) | 10.6 (normal range ≤20.0 units) |
| C3 complement (mg/dL) | 83 (90–180 mg/dL) |
| C4 complement (mg/dL) | 13.8 (10–40 mg/dL) |
| ESR (mm/h) | 123 (0–30 mm/hr) |
| CRP (mg/L) | 23.2 (0–5 mg/L) |
| ANA titre | Nucleolar, 1:160 |
| SSA/SSA Index (normal range) | Positive/226 (≤99) AU/mL |
| SSB/SSB Index (normal range) | Negative/22 (≤99) AU/mL |
| Smith/Smith Index | Low positive/142 (≤99) AU/mL |
| RNP/RNP Index | Low positive/155 (≤99) AU/mL |
| SCL-70/SCL-70 Index | Negative/21 (≤99) AU/mL |
| Jo 1/Jo 1 Index | Negative/34 (≤99) AU/mL |
| dsDNA/dsDNA Index | Negative/76 (≤99) IU/mL |
| Centromere/Centromere Index | Negative/8 (≤99) AU/mL |
| Histone/Histone Index | Negative/62 (≤99) AU/mL |
| Rheumatoid factor (IU/mL) | 160 (0–20 IU/mL) |
| Soluble IL-2 receptor (U/mL) | 869 (406–1100 U/mL) |
*eGFR calculated by four variable Modification of Diet in Renal Disease MDRD) formula.
†Reference.20
ACE, angiotensin converting enzyme; ANA, antinuclear antibody; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen; CMV, cytomegalovirus; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; Ig, immunoglobulin; IL-2, interleukin 2; LDH, lactate dehydrogenase; PT, prothrombin time; RNP, required navigation performance.
One week later, she was admitted due to worsening fevers. Her physical examination showed: pulse 115/min, BP 97/62 mm Hg, temperature 103 °F, bilateral pulmonary rales and 1+pitting lower extremity oedema. During this hospital admission, the patient developed acute kidney injury with a rise in serum creatinine from baseline value of 0.6 mg/dL to a peak of 1.3 mg/dL over 2 weeks, at which time nephrology consult was called.
Investigations
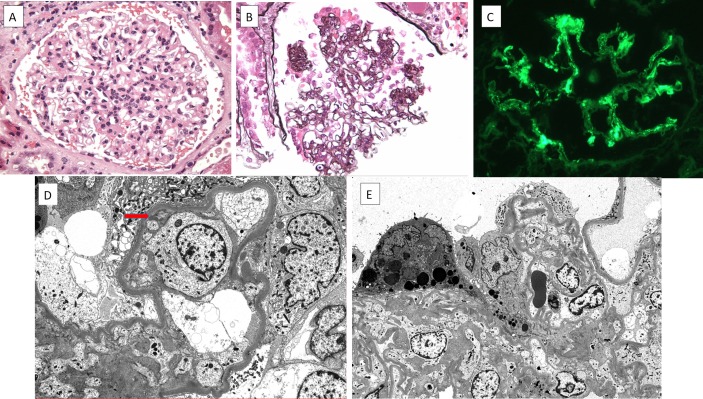
The laboratory values in table 1 are the values obtained when nephrology was consulted after the patient had received prednisone for 3–6 weeks. They were notable for increased levels of transaminases, elevated lactate dehydrogenase (LDH), hyperferritinaemia, and hypertriglyceridemia. Infectious viral aetiologies were excluded based on negative serological tests for hepatitis A, B and C, HIV, Parvovirus B-19 IgM, cytomegalovirus IgM and Epstein-Barr virus IgM. CT scans of the chest and abdomen revealed multiple patchy, ground-glass opacities, enlarged hilar and mediastinal lymph nodes and hepatosplenomegaly. Endobronchial ultrasound biopsy of the right lower lobe of lung revealed interstitial pulmonary fibrosis with a predominantly lymphocytic infiltrate. A bone marrow biopsy revealed normal trilineage haematopoiesis and no evidence of haematological malignancy; however, tissue for flow cytometric analysis was deemed inadequate to detect hemophagocytosis. The serological evaluation was negative for vasculitis. At the time she developed acute kidney injury, urinalysis revealed 3+protein, with a random protein–creatinine ratio of 1.29 g/g. Urine microscopy showed 21–50 red blood cells per high-power field, many of which were dysmorphic. Renal ultrasound showed hyperechoic 11.7 and 11.0 cm kidneys. A diagnostic kidney biopsy was performed and showed diffuse mesangial proliferative glomerulonephritis with exclusively mesangial immune deposits staining for IgG, IgM, IgA, C1q and C3 and many endothelial tubuloreticular inclusions, consistent with lupus nephritis class II (figure 1). There was a superimposed focal segmental glomerulosclerosis (FSGS) with collapsing features involving 4 of 26 glomeruli sampled, with 95% foot process effacement by electron microscopy. Of note, podocyte effacement was diffuse in the absence of peripheral capillary wall immune deposits, consistent with ‘lupus podocytopathy’.
Figure 1.
(A) A representative glomerulus displays global mesangial hypercellularity. The glomerular capillary lumina are patent, without evidence of endocapillary proliferation or leucocyte infiltration (H&E, ×400). (B) There is segmental wrinkling and collapse of the glomerular basement membranes with hypertrophy and hyperplasia of the overlying glomerular epithelial cells, producing a collapsing lesion of sclerosis. These collapsing lesions were present in 4–26 glomeruli sampled (Jones methenamine silver, ×400). (C) Immunofluorescence staining showing a glomerulus with global mesangial staining for IgG (fluorescence micrograph, ×400). (D) By electron microscopy, there is complete foot process effacement with swelling of the podocyte cell bodies. A focal small mesangial electron dense deposit is seen (arrow). No subendothelial or subepithelial electron dense deposits are identified (electron micrograph, ×5000). (E) A low power view showing collapsing sclerosis of the glomerular tuft causing obliteration of the glomerular capillary lumina. The overlying glomerular epithelial cells are swollen with intracytoplasmic protein resorption droplets and complete foot process effacement (electron micrograph, ×3000).
Differential diagnosis
Primary haemophagocytic lymphohistiocytosis.
Secondary haemophagocytic lymphohistiocytosis due to systemic lupus erythematosus.
Lupus nephritis.
Treatment
A diagnosis of HLH was made based on the fulfilment of five of eight criteria stipulated by the original HLH-2004 diagnostic guidelines,1 specifically fever, splenomegaly, bicytopaenia, hypertriglyceridaemia and hyperferritinaemia (table 1). Furthermore, the predictability of secondary HLH in our patient was 92.8% based on the validated H-Score developed by Fardet et al.2 HLH was presumed to be secondary to SLE, with the fulfilment of 8 out of 17 Systemic Lupus International Collaborating Clinics (SLICC) criteria: positive ANA, positive anti-Smith antibody, low serum C3, leucopaenia, thrombocytopaenia, non-scarring alopecia, discoid lupus and renal involvement. As collapsing FSGS is an uncommon form of lupus podocytopathy that is most often associated with APOL1 risk alleles, we performed APOL1 genotyping and found double-risk G1/G2 alleles. The patient was treated with intravenous methylprednisolone (three times per day doses of 1000 mg) followed by oral prednisone 40 mg daily and mycophenolate mofetil (MMF) 500 mg two times per day.
Outcome and follow-up
At the last clinic visit 3 months after initiation of treatment, the patient’s clinical symptoms of fevers, shortness of breath, splenomegaly, myalgias and arthralgias had resolved, while her ears showed a residual violaceous rash. Repeat laboratory studies showed haemoglobin 12.6 g/dL, WBC 3.8/nL, serum creatinine 0.7 mg/dL and normal urinalysis with random urine protein/creatinine ratio 282 mg/g. The patient continued to receive MMF 500 mg two times per day and was started on a tapering regimen of prednisone for a total of 4 months.
Discussion
The entity of collapsing glomerulopathy (CG) is an aggressive, rapidly progressive podocytopathy that usually presents with acute kidney injury and nephrotic-range proteinuria. CG has been described predominantly in patients of African descent and is highly associated with the APOL1 high-risk genotype.3 4 African descendants exhibit a 30%–70% likelihood of harbouring one of the APOL1 (G1 or G2) risk allele variants, which arose under selective evolutionary pressure because they confer protection against the parasite that causes African sleeping sickness, Trypanosoma brucei.5 In a heterozygous model, APOL1 risk alleles provide resistance to trypanosomal infection. However, carrying two risk alleles (G1/G1, G2/G2 or G1/G2) in a homozygous model confers susceptibility to host disease, including forms of FSGS, CG and progressive CKD. Double-risk allele genotypes are found in approximately 13% of the African American population.6
HLH is a clinicopathological entity caused by excessive activation and proliferation of macrophages. CG, the most common renal manifestation of HLH, results from cytokine-mediated effects on podocytes, especially in African patients with susceptible genetic background.7 Podocyte injury in CG leads to cellular de-differentiation and dysregulation, loss of expression of maturity markers such as WT-1, podocyte shedding and the acquisition of proliferative marker Ki-67 by visceral epithelial cells.8 As podocytes are depleted, the denuded glomerular tuft becomes covered by proliferating cells of parietal cell lineage.9 10
SLE is the second most rheumatologic inciting diagnosis for HLH, after systemic juvenile rheumatoid arthritis.11 In both SLE and HLH, activation of an interferon/cytokine-induced molecular programme in the podocyte has been postulated to mediate the development of lupus podocytopathy and CG. Podocytes upregulate APOL1 in response to interferon signalling.12 How APOL1 variant overexpression injures podocytes is thought to involve activation of stress responses and dysregulated endosomal trafficking.13 APOL1 is expressed in podocytes, glomerular endothelium and tubules.14 In African Americans with lupus nephritis, the presence of microcystic tubule dilatation and pseudocrescents resulting from glomerular epithelial cell proliferation has been shown to be associated with two APOL1 risk alleles.4 The combination of double-risk allele genotype with hypercytokinaemia mediated by SLE and HLH fulfils the ‘two-hit’ hypothesis in the pathogenesis of CG.4 15
Our patient had concurrent features of both lupus nephritis class II and CG. While each of these pathological findings has been reported to occur independently in association with SLE, this is the first instance in which they are reported to occur simultaneously in the setting of both SLE and HLH. Most investigations consider CG to represent the most severe morphological expression of lupus podocytopathy, in which there is rapid and widespread podocyte loss.16 17
Recent studies suggest that the possession of two APOL1 risk alleles does not influence treatment response, but does predict faster progression to ESRD in primary FSGS.15 Treatment with a combination of a corticosteroid and other immunosuppressive agents has led to significantly improved renal response rates in primary FSGS, even in those with CG.18 It is generally thought that lupus-induced CG may have a more favourable treatment response than idiopathic CG; however, the presence of homozygous G2 alleles of APOL1, at least in one case report, tends to contradict this belief, as the patient progressed quickly to ESRD despite aggressive treatment.19
In summary, our patient with high-risk APOL1 genotype developed the CG variant of lupus podocytopathy superimposed on class II lupus nephritis in the setting of SLE and HLH. The current treatment guideline for mildly to moderately ill patients with HLH is to address the underlying trigger, with or without the addition of corticosteroids. Given the complex diagnoses of coexistent HLH, SLE and CG, we opted for aggressive therapy with high-dose steroids and MMF, resulting in excellent systemic and renal responses.
Patient’s perspective.
Even though the course of my illness was scary most of the times, I am glad that I am finally better.
Learning points.
Systemic lupus erythematosus (SLE) is one of the most common rheumatological aetiologies of haemophagocytic lymphohistiocytosis (HLH) and can manifest in the kidney as a combination of immune complex-mediated lupus nephritis and lupus podocytopathy with collapsing features.
Patients of African descent carrying double APOL1 risk alleles are at risk to develop collapsing glomerulopathy (CG) in the setting of both SLE and HLH via activation of innate immune pathways in the podocyte.
The combination of APOL1 double-risk allele genotype with hypercytokinaemia mediated by SLE and HLH fulfils the ‘two-hit’ hypothesis in the pathogenesis of CG.
Lupus podocytopathy of the collapsing type usually follows a more aggressive course than minimal change disease and other focal segmental glomerulosclerosis variants and may benefit from steroids and an additional immunosuppressive agent.
APOL1 genetic testing in patients with CG appears to have prognostic value and may help guide treatment decisions in the setting of high interferon states such as SLE and HLH.
Footnotes
Contributors: BC: helped to report the findings and acquired relevant literature for writing and wrote the manuscript. VD’A: helped to interpret the pathology findings and significantly improved the discussion and writing of the manuscript. LB: helped to acquire literature for writing and contributed to the writing of the paper. BM: helped to report the clinical findings, reviewed and approved the final version of the paper. BJ: helped to report the clinical findings, reviewed and approved the final version of the paper, orchestrated the reporting, writing and review of the literature for publication. All the authors have agreed to be accountable for the article and accuracy and integrity of the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. 10.1002/pbc.21039 [DOI] [PubMed] [Google Scholar]
- 2. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 2014;66:2613–20. 10.1002/art.38690 [DOI] [PubMed] [Google Scholar]
- 3. Nicholas Cossey L, Larsen CP, Liapis H. Collapsing glomerulopathy: a 30-year perspective and single, large center experience. Clin Kidney J 2017;10:443–9. 10.1093/ckj/sfx029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larsen CP, Beggs ML, Saeed M, et al. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 2013;24:722–5. 10.1681/ASN.2012121180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010;329:841–5. 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dummer PD, Limou S, Rosenberg AZ, et al. APOL1 Kidney disease risk variants: an evolving landscape. Semin Nephrol 2015;35:222–36. 10.1016/j.semnephrol.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thaunat O, Delahousse M, Fakhouri F, et al. Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int 2006;69:1892–8. 10.1038/sj.ki.5000352 [DOI] [PubMed] [Google Scholar]
- 8. Mubarak M. Collapsing focal segmental glomerulosclerosis: current concepts. World J Nephrol 2012;1:35–42. 10.5527/wjn.v1.i2.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Appel D, Kershaw DB, Smeets B, et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 2009;20:333–43. 10.1681/ASN.2008070795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smeets B, Kuppe C, Sicking EM, et al. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 2011;22:1262–74. 10.1681/ASN.2010090970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atteritano M, David A, Bagnato G, et al. Haemophagocytic syndrome in rheumatic patients. A systematic review. Eur Rev Med Pharmacol Sci 2012;16:1414–24. [PubMed] [Google Scholar]
- 12. Nichols B, Jog P, Lee JH, et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 2015;87:332–42. 10.1038/ki.2014.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beckerman P, Bi-Karchin J, Park AS, et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med 2017;23:429–38. 10.1038/nm.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Madhavan SM, O’Toole JF, Konieczkowski M, et al. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol 2011;22:2119–28. 10.1681/ASN.2011010069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kopp JB, Winkler CA, Zhao X, et al. Clinical Features and Histology of Apolipoprotein L1-Associated Nephropathy in the FSGS Clinical Trial. J Am Soc Nephrol 2015;26:1443–8. 10.1681/ASN.2013111242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salvatore SP, Barisoni LM, Herzenberg AM, et al. Collapsing glomerulopathy in 19 patients with systemic lupus erythematosus or lupus-like disease. Clin J Am Soc Nephrol 2012;7:914–25. 10.2215/CJN.11751111 [DOI] [PubMed] [Google Scholar]
- 17. Haas M. Collapsing glomerulopathy in systemic lupus erythematosus: an extreme form of lupus podocytopathy? Clin J Am Soc Nephrol 2012;7:878–80. 10.2215/CJN.03590412 [DOI] [PubMed] [Google Scholar]
- 18. Laurin LP, Gasim AM, Derebail VK, et al. Renal survival in patients with collapsing compared with not otherwise specified FSGS. Clin J Am Soc Nephrol 2016;11:1752–9. 10.2215/CJN.13091215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kofman T, Narjoz C, Raimbourg Q, et al. Collapsing glomerulopathy associated lupus in a black female with homozygous APOL1 mutation. Lupus 2012;21:1459–62. 10.1177/0961203312460114 [DOI] [PubMed] [Google Scholar]
- 20. Poggio ED, Rule AD, Tanchanco R, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int 2009;75:1079–87. 10.1038/ki.2009.11 [DOI] [PMC free article] [PubMed] [Google Scholar]