Abstract
A 51-year-old woman with known primary antiphospholipid syndrome presented with a 4-day history of chest and abdominal pain, inferior ST-segment elevation on a 12-lead ECG and a subtherapeutic international normalised ratio. In view of a significantly raised high-sensitivity troponin I assay, inferior wall hypokinesis on transthoracic echocardiography and despite unobstructed epicardial vessels on emergency coronary angiography, a diagnosis of myocardial infarction was made. Furthermore, the patient also developed both bilateral adrenal haemorrhages leading to acute adrenal insufficiency and microvascular thrombotic renal disease concurrently. The patient therefore fulfilled the diagnostic criteria for catastrophic antiphospholipid syndrome presenting with cardiac, endocrine and renal involvement. Early diagnosis permitted appropriate treatment with anticoagulation, dual antiplatelet therapy, secondary prevention and corticosteroid replacement therapy and led to a full recovery. This case highlights first the importance of adequate anticoagulation in antiphospholipid syndrome and, second, the potentially fatal, multiorgan complication of failure to do so.
Keywords: ischaemic heart disease, venous thromboembolism, adrenal disorders, haematology (incl blood transfusion), renal medicine
Background
The submitted case describes a rare but both avoidable and treatable exacerbation of antiphospholipid syndrome (APS). This case report highlights both the importance of adequate anticoagulation in APS and the potentially fatal, multiorgan complication of failure to do so. Awareness of this condition is essential to permit prompt diagnosis and treatment and thus avoid a potentially fatal outcome.
Case presentation
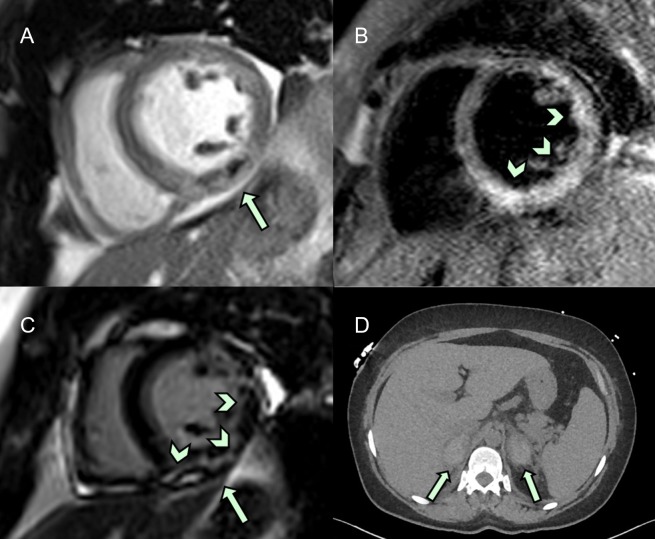
A 51-year-old woman with known primary APS presented with a 4-day history of chest and abdominal pain, inferior ST-segment elevation on 12-lead ECG and a subtherapeutic international normalised ratio. Emergency coronary angiography revealed unobstructed epicardial vessels, however, with slow flow in a dominant right coronary artery. A significantly raised high-sensitivity troponin I assay (more than 1000-fold of the upper limit of normal range) in conjunction with inferior wall hypokinesis on transthoracic echocardiography led to a provisional diagnosis of myocardial infarction with non-obstructive coronary arteries (MINOCA).1 A thromboembolic occlusion with subsequent recanalisation of an otherwise atheroma free right coronary artery was considered to be the most likely underlying cause. MRI with late gadolinium enhancement confirmed subendocardial changes in keeping with microvascular infarction and excluded common alternative MINOCA causes such as the apical structural changes of takotsubo cardiomyopathy or the non-territorial patchy enhancement of myocarditis (figure 1A–C). The patient was therefore fully anticoagulated with treatment dose low molecular weight heparin and warfarin.
Figure 1.
Parasternal short axis view on cardiac MRI demonstrating abnormal inferoposterior left ventricular myocardium in keeping with a vascular occlusive event in the left circumflex territory (A, B) and corresponding patterns of enhancement with gadolinium enhancement (C). Unenhanced CT image demonstrating with bilateral adrenal haemorrhages (D).
Despite prompt treatment the patient deteriorated clinically becoming hypotensive, febrile and confused in the absence of focal neurology. Serum biochemistry revealed a rapid drop in sodium from 136 to 120 mEq/L in less than 24 hours. While hyponatraemia can be common in patients in cardiogenic or septic shock the most likely diagnosis in keeping with the rapidly changing clinical and biochemical picture was felt to be an adrenal crisis. Serum endocrine testing on samples prior to steroid treatment and fluid resuscitation was in keeping with primary adrenal insufficiency revealing hypocortisolaemia and raised adrenocorticotropic hormone levels. In order to avoid treatment delay an adrenocorticotropic hormone stimulation (synacthen) test was not performed, however, the diagnosis was subsequently supported by CT imaging demonstrating bilateral adrenal haemorrhages (figure 1D). The occurrence of acute adrenal haemorrhages in this clinical setting is commonly caused by adrenal vein thromboses and therefore indicative of a thrombotic event in a second organ system. Corticosteroid replacement therapy led to prompt improvement in the patient’s symptoms.
Furthermore, the patient was noted to have microscopic haematuria and proteinuria as well as an elevated protein:creatinine ratio in the absence of any deterioration in glomerular filtration rate. While this represents a clinically less dramatic picture it is consistent with microvascular thrombotic renal disease. Importantly, the observed renal derangements predated iatrogenic renal insults with contrast agent used for angiography and MRI and the adrenal crisis induced hypotension. It is therefore likely to represent a third organ system affected by a thrombotic complication. The patient was discharged home after a period of supportive therapy, full anticoagulation, steroid replacement and secondary prevention therapy for left ventricular systolic dysfunction.
The above patient therefore presented with MINOCA, acute adrenal crisis and subclinical renal dysfunction in the setting of subtherapeutic anticoagulation for APS. These findings are in keeping with widespread development of microvascular thrombotic occlusions, consistent with a diagnosis of catastrophic antiphospholipid syndrome (CAPS).2
Investigations
Histological evidence of intravascular thrombosis was not sought from either cardiac or kidney tissue as the results would have not changed the patient’s management and thus expose the patient to the risks of an invasive procedure unnecessarily. In the absence of a tissue diagnosis the case was classified as probably CAPS.
APS is characterised by a tendency to thrombosis or fetal miscarriage in the presence of persistently elevated antiphospholipid antibodies, defined by the Sydney criteria as the presence of lupus anticoagulants (LA), a heterogeneous group of immunoglobulins, and/or IgG or IgM anticardiolipin (aCL) antibodies, and/or anti-beta-2 glycoprotein antibodies, present on at least two occasions more than 12 weeks apart. This patient’s APS had been diagnosed 8 years previously after she suffered from recurrent below knee deep venous thromboses, testing positive for LA and aCL antibodies at diagnosis. aCL titres from testing at her APS routine follow-up 2 weeks before this presentation were 145 for IgG and 32.7 for IgM, with a dilute Russell Viper Venom Test (dRVVT) ratio of 1.34 (in keeping with the presence of LA). A dRVVT test taken 3 days after presentation was elevated at 2.9. Histologically, the hallmark of CAPS is microvascular thrombosis, however, in the case we present since the diagnosis was made based on other clinicoradiological findings, biopsies were not taken.
Outcome and follow-up
Early diagnosis permitted appropriate treatment with anticoagulation, dual antiplatelet, secondary prevention and corticosteroid replacement therapy and led to a full recovery.
Discussion
CAPS was first described in 1992 and affects fewer than 1% of the antiphospholipid patient population.3 It is characterised by sudden-onset, multiorgan dysfunction in the presence of antiphospholipid antibodies, and carries a mortality of approximately 30%.4 According to an online registry of over 350 cases, a precipitant can be identified in 65% of cases, most commonly infection (22%) or the cessation or alteration of anticoagulation, often for elective surgery (18%).4 Organ involvement is most commonly renal, pulmonary or neurological. Although cardiac involvement is not infrequent it is most often evidenced by valvular involvement while both myocardial infarction and adrenal haemorrhage are less common with incidences of 25% and 13%, respectively.5 This case report describes a rare instance of CAPS involving these organs.
Learning points.
Myocardial infarction can be part of a multisystem disease.
Cardiac MRI with late gadolinium enhancement plays a key role in identifying the underlying cause of myocardial infarction with non-obstructive coronary arteries.
Failure of appropriate anticoagulation in the setting of antiphospholipid syndrome can lead to potentially fatal multiorgan failure.
Footnotes
Contributors: JC, UK, KT and RMD were involved in the clinical care of the patient. JC and RMD diagnosed the patient’s condition. KT provided the clinical images and figure legends. JC, UK and RMD contributed to the writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Agewall S, Beltrame JF, Reynolds HR, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–53. 10.1093/eurheartj/ehw149 [DOI] [PubMed] [Google Scholar]
- 2. Asherson RA, Cervera R, de Groot PG, et al. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus 2003;12:530–4. 10.1191/0961203303lu394oa [DOI] [PubMed] [Google Scholar]
- 3. Asherson RA. The catastrophic antiphospholipid syndrome. J Rheumatol 1992;19:508–12. [PubMed] [Google Scholar]
- 4. Cervera R, Espinosa G. Update on the catastrophic antiphospholipid syndrome and the “CAPS Registry”. Semin Thromb Hemost 2012;38:333–8. 10.1055/s-0032-1304718 [DOI] [PubMed] [Google Scholar]
- 5. Guntz J, Layios N, Damas P. Catastrophic antiphospholipid syndrome: case reports and review of the literature. Acta Anaesthesiol Belg 2014;65:87–94. [PubMed] [Google Scholar]