Abstract
Cystic neoplasms of the liver are rare tumours. According to the recent WHO classification, they are classified into mucinous cystic neoplasm and intraductal papillary neoplasm based on the presence of ovarian-like stroma and biliary communication. We report two rare cases of mucinous cystadenoma of the liver with biliary communication and discuss the shortcomings of current classification.
Keywords: surgical Oncology, hepatic cancer
Background
Cystic neoplasms of the liver are rare tumours and constitute less than 5% of cystic lesions of the liver.1 Initially, they were termed as biliary cystadenoma and cystadenocarcinoma.2 Recently, WHO had classified the cystic neoplasm of the liver into mucinous cystic neoplasm (MCN) and intraductal papillary neoplasm of the bile duct (IPN-B) similar to the classification used in pancreas.3 MCN is characterised by the presence of ovarian stroma and absence of bile duct communication. Whereas, IPN-B lesions lack ovarian- like stroma and have predominant intrabiliary component. We report two rare cases of MCN of the liver with biliary communication and discuss the limitations of current WHO classification of cystic neoplasm of the liver.
Case presentation
Case 1
A 20-year-old woman presented with intermittent upper abdominal pain, jaundice, fever and pruritus for 1 month. She had a firm tender mass arising from the left lobe of the liver extending 6 cm below the xiphoid in midline.
Case 2
A 28-year-old woman presented with jaundice, pruritus and anorexia for 2 months. She was initially evaluated elsewhere, where investigations suggested a simple hepatic cyst with compression of the bile duct. The patient underwent laparotomy and subtotal excision of the cyst elsewhere which on histopathological examination (HPE) was reported as simple hepatic cyst. Postoperatively, the patient had persistent jaundice.-
Investigations
Case 1
Her liver function test was suggestive of obstructive jaundice. Ultrasound abdomen revealed a 6×5 cm multiloculated cystic lesion in segment IV of liver with bilateral intrahepatic biliary radical dilatation (IHBRD). Common Bile Duct (CBD) was normal, and gall bladder was collapsed. On contrast-enhanced CT (CECT) abdomen, there were hypodense lesions predominantly in segment IV with internal septations, however there were no solid components or calcifications (figure 1A). MR cholangiopancreatography (MRCP) revealed a multiloculated 7.0×6.2×5.3 cm heterogeneous predominantly T2 hyperintense lesion in segment IV of the liver. Bilobar IHBRD with probable cystobiliary communication was present (figure 1B).
Figure 1.
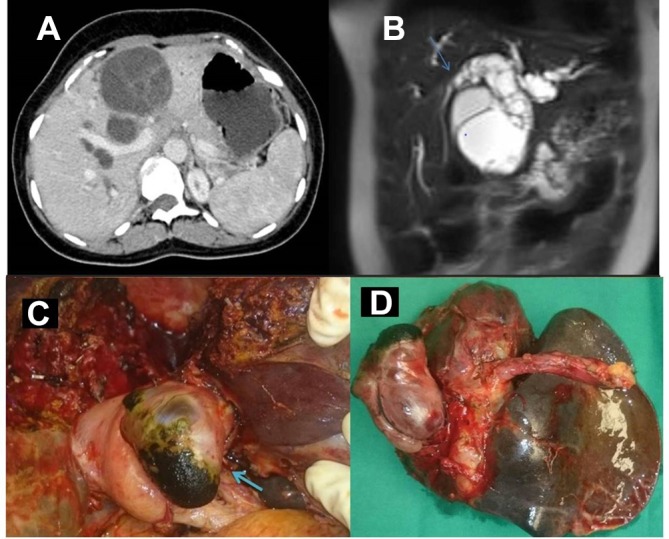
(A) CECT abdomen showing hypodense lesion in segment IV of the liver with few enhancing internal septations. (B) MRCP showing bilobar IHBRD with a heterogeneous T2 hyperintense lesion, extending into the proximal CBD causing abrupt narrowing at the mid-CBD level (arrow). (C) Intraoperative photograph showing portion of the tumour within the CBD milked out (arrow). (D) Resected specimen showing both hepatic and biliary components. CECT, contrast-enhanced CT; IHBRD, intrahepatic biliary radical dilatation; MRCP, MR cholangiopancreatography.
Case 2
She was evaluated with MRI abdomen and MRCP which revealed cystic lesion in segment IV with obstruction at the level of the proximal CBD and bilateral IHBRD. Slide review of previous operative specimen at our institute was reported as mucinous cystadenoma of the liver. On CECT abdomen, there was a hypodense lesion in the segment IV with communication with the left biliary system. Endoscopic ultrasound revealed a 2.4×1.9 cm cystic lesion with soft tissue thickening compressing the common hepatic duct and another 2.8×1.4 cm multiseptated cystic lesion seen along the left ductal system.
Treatment
Case 1
The patient underwent open left hepatectomy. On laparotomy, there was a 6×6 cm solid cystic lesion in segment IVb of the liver with a mobile mass within proximal CBD. Choledochotomy was performed at the level of the dilated portion of CBD, where communication of the cyst with bile duct through left hepatic duct was seen (figure 1C,D). HPE of the resected specimen confirmed the diagnosis of MCN (figure 2).
Figure 2.
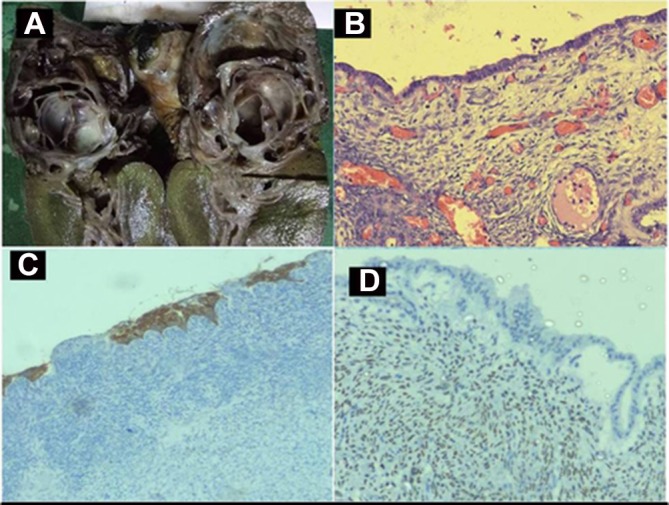
(A) Cut section of specimen showing solid cystic lesion. (B) Photomicrograph showing low columnar epithelial lining cells with underlying ovarian-like stroma (H&E, magnification x400). (C) Immunohistochemistry shows columnar lining epithelium with cytoplasmic staining of cytokeratin and (D) underlying ovarian stroma with nucleus staining positive for oestrogen receptor.
Case 2
On laparotomy, there was a 5×4 cm cystic lesion in segment IVb of the liver, adherent to the left hepatic duct. Intraoperative cholangiography and choledochoscopy confirmed communication of the cyst with bile duct. Complete excision of the hepatic portion and the biliary component along with the sleeve of the left hepatic duct was performed. Choledochotomy was closed primarily over T tube. HPE of the resected specimen confirmed MCN. The T tube was removed 4 weeks later, after T tube cholangiogram did not show any evidence of leak or obstruction.
Outcome and follow-up
Case 1
At the 2-month follow-up, the patient is asymptomatic with a normal liver function test.
Case 2
At the 12-month follow-up, the patient is asymptomatic with a normal liver function test.
Discussion
Cystic neoplasms of the liver are rare tumours and constitute less than 5% of symptomatic cystic lesions of the liver.1 In the WHO classification of liver tumours proposed in 2000, they were termed as biliary cystadenoma (benign) and cystadenocarcinoma (malignant).2 Biliary cystadenoma were further classified into mucinous (common type) and serous types (rare). The term biliary papillomatosis was used for tumours with predominant intrabiliary growth. Recently in 2010, WHO proposed a new classification system for cystic neoplasms of the liver similar to the terminology used for pancreatic cystic tumours and classified them into MCN and IPN-B.3 The diagnostic criteria for MCN were presence of ovarian-like stroma and absence of bile duct communication in addition to the presence of typical mucin-secreting biliary type cuboidal or columnar epithelium. IPN-B lesions were characterised by absence of ovarian-like stroma and predominant intraductal growth pattern. Similarity in clinical pathological features of MCN of the liver, pancreas and ovary suggests an association.4 During embryogenesis, right gonad lies dorsolateral to the liver, and left gonad lies dorsal to the pancreas and spleen until 8 weeks of gestation. Close relation of these structures to gonad could explain the similarity in microscopic features of these tumours.4
MCN commonly affects females in their reproductive period. Small lesions are usually asymptomatic while large cysts present with abdominal pain or mass. Jaundice is rare, and it occurs when there is biliary communication.5 Preoperative diagnosis is difficult, and they are easily mistaken for simple hepatic cysts or hydatid cysts. Hydatid serology can help in differentiating from hydatid cyst. Elevated levels of CA 19–9 (serum and cyst fluid) have been suggested by some authors to help in the diagnosis of MCN.6 On ultrasound, MCNs appear as anechoic lesions with internal septations.7 Intracystic haemorrhage or papillary projections appear as focal hyperechoic areas. Features of MCN on CECT abdomen are hypodense lesions with internal septations which enhance with contrast.8 Presence of irregular wall thickening, mural solid nodules, thick calcification and papillary projections are suggestive of a cystadenocarcinoma. On MRI abdomen, these lesions appear hyperintense in T2-weighted images and hypointense on T1-weighted images. Use of the biliary-specific contrast agent gadobenate dimeglumine can help in preoperative diagnosis of biliary communication.8 On HPE, MCN are lined by biliary type mucus secreting cuboidal or columnar epithelium. Characteristic feature of MCN is presence of dense subepithelial ovarian-like stroma with spindle cells expressing female sex hormone receptors.1 9 Historically, MCN were treated by marsupialisation, internal Roux-en-Y drainage, aspiration, sclerosis or partial resection. Considering their malignant potential and risk of recurrence complete excision is the treatment of choice.10
Peculiar features of the present cases are presentation with jaundice which is a rare clinical manifestation of MCN. Possible mechanisms for jaundice are intraductal extension, intracystic haemorrhage or mucin secretion. Very few case reports have described cystadenoma prolapsing into bile duct.11 In these two cases, it is due to intraductal extension of tumour which is a rare feature of MCN. We also highlight the problem with current classification. Since ovarian-like stroma was present, a diagnosis of MCN was made. However, bile duct communication was also present which is a feature against MCN according to current diagnostic criteria. Hence, we suggest that MCN should be further subclassified into MCN with or without biliary communication.
Learning points.
Mucinous cystic neoplasm of the liver is a rare tumour and should be considered in the differential diagnosis of patients with cystic neoplasm and jaundice.
Current classification needs to be modified to include these rare cases of mucinous cystic neoplasm with biliary communication.
Acknowledgments
We acknowledge Dr B H Srinivas for helping in reporting the histopathology part of the article.
Footnotes
Contributors: SA: planning and reporting. SC, KR and BP: reporting.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol 1994;18:1078–91. [PubMed] [Google Scholar]
- 2. Wittekind C, Fischer HP, Ponchon T. Bile duct cystadenoma and cystadenocarcinoma : Hamilton SR, Aaltonen LA, World Health Organization classification of tumors. pathology and genetics of tumors of the digestive system. Lyon: IARC Press, 2000:182–3. [Google Scholar]
- 3. Tsui WMS, Adsay NV, Crawford JM, et al. Mucinous cystic neoplasms of the liver : Bosman FT, Carneiro F, Hruban RH, Theise ND, World Health Organization classification of tumors. pathology and genetics of tumors of the digestive system. Lyon: IARC Press, 2010:IARC Press–8. [Google Scholar]
- 4. Erdogan D, Kloek J, Lamers WH, et al. Mucinous cystadenomas in liver: management and origin. Dig Surg 2010;27:19–23. 10.1159/000268110 [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez M, Majno P, Terraz S, et al. Biliary cystadenoma revealed by obstructive jaundice. Dig Liver Dis 2009;41:e11–13. 10.1016/j.dld.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 6. Thomas JA, Scriven MW, Puntis MC, et al. Elevated serum CA 19-9 levels in hepatobiliary cystadenoma with mesenchymal stroma. Two case reports with immunohistochemical confirmation. Cancer 1992;70:1841–6. [DOI] [PubMed] [Google Scholar]
- 7. Korobkin M, Stephens DH, Lee JK, et al. Biliary cystadenoma and cystadenocarcinoma: CT and sonographic findings. AJR Am J Roentgenol 1989;153:507–11. 10.2214/ajr.153.3.507 [DOI] [PubMed] [Google Scholar]
- 8. Park MS, Yu JS, Lee DK, et al. Gadobenate dimeglumine-enhanced MRI of intraductal papillary mucinous tumor of the bile ducts. J Magn Reson Imaging 2007;25:625–7. 10.1002/jmri.20791 [DOI] [PubMed] [Google Scholar]
- 9. Zen Y, Pedica F, Patcha VR, et al. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol 2011;24:1079–89. 10.1038/modpathol.2011.71 [DOI] [PubMed] [Google Scholar]
- 10. Delis SG, Touloumis Z, Bakoyiannis A, et al. Intrahepatic biliary cystadenoma: a need for radical resection. Eur J Gastroenterol Hepatol 2008;20:10–14. 10.1097/MEG.0b013e3282f16a76 [DOI] [PubMed] [Google Scholar]
- 11. Takano Y, Nagahama M, Yamamura E, et al. Prolapse into the bile duct and expansive growth is characteristic behavior of mucinous cystic neoplasm of the liver: report of two cases and review of the literature. Clin J Gastroenterol 2015;8:148–55. 10.1007/s12328-015-0569-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


