Abstract
Rheumatoid meningitis (RM) is a rare extra-articular manifestation of rheumatoid arthritis (RA). A 59-year-old man presented with a 10-day history of right-sided frontal headache and a 7-day history of subacute left-sided weakness. He had no history of RA. He was febrile (38.2°C). Left ankle dorsiflexion and plantarflexion were graded at 4+/5. He developed focal onset motor seizures. He was intermittently febrile with minimal improvement despite intravenous antivirals and antimicrobials. Serology revealed elevated rheumatoid factor 88.2 IU/mL and anti-cyclic citrullinated peptide (anti-CCP) IgG >340 AU/mL. Initial cerebrospinal fluid (CSF) was predominantly lymphocytic 96%, with elevated protein 672 mg/L and normal glucose 3.4 mmol/L. Interval CSF revealed newly low glucose 2.6 mmol/L. Extensive CSF microbiology tests were negative. CSF cytology confirmed reactive lymphocytes. MRI brain revealed right frontoparietal leptomeningeal enhancement. Brain and leptomeningeal biopsy demonstrated florid leptomeningeal mixed inflammatory infiltrate without granulomas. The combination of elevated anti-CCP IgG, erosive arthropathy, CSF lymphocytosis, asymmetrical leptomeningeal enhancement and biopsy findings confirmed RM.
Keywords: rheumatoid arthritis, meningitis, headache (including migraines)
Background
Rheumatoid meningitis (RM) is a rare extra-articular manifestation of rheumatoid arthritis (RA). It has rarely been described among individuals with no history of RA and might be overlooked without specific serology and meningeal biopsy.
Case presentation
A 59-year-old coal worker presented to the emergency department with a 10-day history of a new right-sided frontal headache. The pain worsened transiently on coughing or straining. He also reported a 7-day history of gradual onset left-sided weakness and recent fever and chills. His medical history included asthma/chronic obstructive pulmonary disease overlap syndrome, atrial fibrillation, coronary artery disease and hypertension. Over a decade, he had experienced recurrent but infrequent inflammation of the wrists and metatarsophalangeal joints, attributed to osteoarthritis. He lived with his wife. He was an ex-smoker with a 40-pack-year smoking history.
On examination, he was febrile (38.2°C). There was distal left lower limb weakness, with ankle dorsiflexion and plantarflexion graded at 4+/5 on Medical Research Council grading. Sensorium was normal. Reflexes were symmetrical with downgoing plantars. Blood tests showed a neutrophil leucocytosis with white cells count 12.0 (4.4–11.3×109/L), absolute neutrophil count 7.02 (1.4–6.6×109/L) and mildly elevated inflammatory markers C-reactive protein (CRP) 18.1 mg/L (0–10 mg/L). On the day of admission, he had a first focal onset motor seizure with secondary bihemispheric spread. We gave a loading dose of intravenous phenytoin and started on broad-spectrum antivirals and antimicrobials. He underwent extensive investigations as detailed below.
Investigations
Relevant serology revealed markedly elevated rheumatoid factor (RF) 88.2 IU/mL (1–16 IU/mL) and anti-cyclic citrullinated peptide (anti-CCP) IgG >340 AU/mL (0–7 AU/mL). The remainder of the autoimmune serology was negative including antinuclear antibody (ANA), antineutrophil cytoplasmic antibodies and antithyroid peroxidase antibodies. Serum protein electrophoresis and immunoglobulins, including IgG4 subtype, were normal. Antineuronal antibodies were negative. Serum ACE was elevated at 70 U/L (0–45 U/L).
He had six sets of sterile blood cultures. Extensive microbiology serology was negative including HIV, Treponema pallidum, Borrelia burgdorferi, Epstein-Barr virus IgM, Enterovirus PCR, Coxiella burnetti IgM and IgG, Brucella IgM and IgG and serum cryptococcal antigen. He had a positive Quantiferon and Mantoux (purified protein derivative test). Chest X-ray was unremarkable. Three months prior, he had a bronchoscopy and bronchoalveolar lavage that was negative for acid-fast bacilli and tuberculosis (TB) PCR. The findings were suggestive of latent TB only.
Initial cerebrospinal fluid (CSF) was predominantly lymphocytic 96%, with elevated protein 672 mg/L (200–400 mg/L) and normal glucose 3.4 mmol/L (CSF:serum ratio 0.75). Interval CSF on day 14 of admission revealed newly low glucose 2.6 mmol/L (CSF:serum ratio 0.52). CSFs were negative for bacterial, viral, fungal and TB PCR and TB culture. CSF cytology and flow cytometries were negative for malignant cells. Oligoclonal bands were negative. CSF ACE was elevated at 1.88 umol/min/L (0–1.20 umol/min/L); this was attributed to blood-brain barrier disruption.
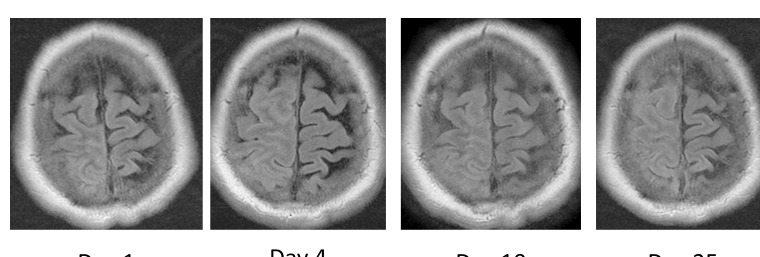
Initial MRI brain revealed leptomeningeal enhancement over the right frontoparietal lobe with subsequent progression on interval imaging over 3 weeks (figure 1). CT of thorax, abdomen and pelvis and positron emission tomography CT of body and bone marrow aspirate and trephine were all negative for systemic illness including malignancy.
Figure 1.
T2 fluid attenuated inversion recovery (FLAIR) axial MRI brain: increased signal with obliteration of sulci due to leptomeningeal thickening over the right frontoparietal lobe, with progression on four interval imaging studies over 3 weeks.
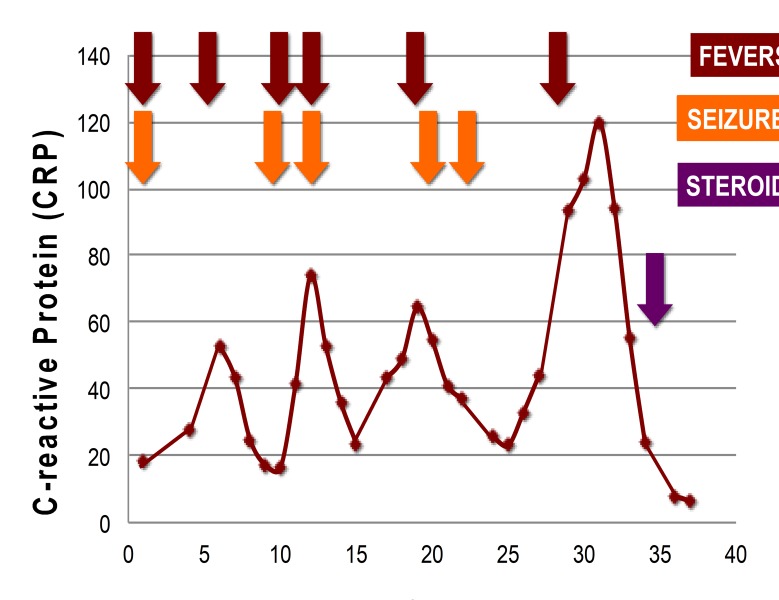
Despite escalation of broad-spectrum antibacterials including empirical antituberculous therapy, antivirals and two antiepileptic agents, he remained clinically unwell over 4 weeks. He had a constant right frontal headache. He was intermittently febrile. He continued to have headache and two times per week focal onset motor seizures with loss of awareness. He was initially treated with phenytoin monotherapy; it was escalated to a combination of increasing doses of phenytoin and adjunctive levetiracetam therapy. He had persistently elevated inflammatory markers, with CRP peaking at 120 mg/L (figure 2).
Figure 2.
Recurrent fevers, focal onset motor seizures and elevated inflammatory markers.
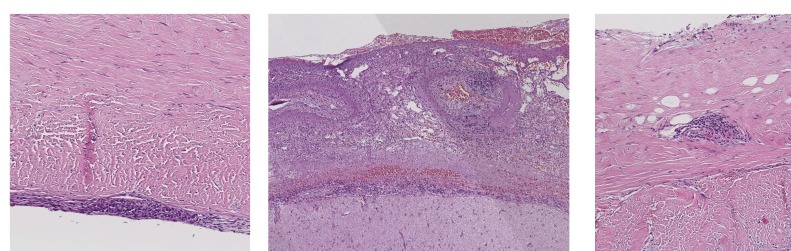
We proceeded to brain and leptomeningeal biopsy. It demonstrated florid leptomeningeal mixed inflammatory infiltrate without granulomas (figure 3). There was involvement of small venules and occasional small arterioles in the leptomeninges without fibrinoid change. Biopsy Gram stain, Ziehl-Neelsen stain and periodic acid-Schiff fungus stain and TB culture were all negative. The biopsy was also stained for IgG4. There were less than 10% plasma cells, no eosinophils, no storiform fibrosis and no obliterative vasculitis, that is, the histological features of IgG4-related disease were not present. These findings are non-specific but suggested an underlying inflammatory aetiology.
Figure 3.
Brain and leptomeningeal biopsy: florid leptomeningeal mixed inflammatory infiltrate without granulomas. There was involvement of small venules and occasional small arterioles in the leptomeninges without fibrinoid change.
Elevated anti-CCP IgG is 95% specific for diagnosis of RA. Taken with the inflammatory CSF, the radiological pattern of asymmetrical leptomeningeal enhancement and the biopsy findings, a diagnosis of RM was reached.
Treatment
We gave 5 days of 1 g intravenous methylprednisolone followed by oral prednisolone at 1 mg/kg body weight per day for 1 month; then followed by a 20% taper every 2 weeks to maintenance 15 mg per day. Within 5 days, there was rapid resolution of headache and all neurological signs along with cessation of seizures.
Outcome and follow-up
In retrospect, this patient fulfils the American College of Rheumatology and European League Against Rheumatism Classification Criteria 2010 for RA with his decade long history of intermittent oligopolyarticular small joint arthropathy affecting his wrists and metatarsophalangeal joints, elevated inflammatory markers and markedly high anti-CCP titres. There was no clinical evidence of rheumatoid nodules. His symptoms had previously been attributed to osteoarthritis. Furthermore, the presence of inactive erosive arthritis later demonstrated on foot X-rays was supportive of a diagnosis of RA. Following 3 months of steroids and antituberculous treatment for latent TB, we additionally started methotrexate as a disease-modifying therapy for RA. Interval MRI at 4 months from steroid commencement demonstrated complete resolution of the meningeal enhancement. One year on, he is well and maintained on methotrexate.
Discussion
RM is a rare extra-articular manifestation of RA and has only rarely been described in individuals without a recognised clinical history of articular RA. A diagnostic challenge, RM requires meningeal biopsy for definitive diagnosis.1 It was first described by Ellman et al in London.2 He reported the case of a 63-year-old man with widespread rheumatoid lesions in the cardiovascular and respiratory systems. Two months prior to death, he developed focal onset seizures that were later explained by dural rheumatoid nodules at autopsy.2 In total, less than 60 biopsy proven cases of RM have been reported in the English literature.1 3–12 However, only six cases did not have a prior history of RA; all of these cases subsequently developed classical symptoms of RA within a few months to 1 year after initial presentation of RM.1 12–15
Pathophysiology remains unknown. There are five case reports of RM temporally linked to anti- tumour necrosis factor (TNF) inhibitor initiation.5 7 Anti-TNF inhibitors rarely result in the paradoxical development of rheumatoid nodules; some suggest that this may include meningeal rheumatoid nodules.5 7
The average age of diagnosis is 63 years (32–89 years).1 It has similar distribution among genders.1 Over 50% of patients have >10 year history of RA.1 It occurs irrespective of disease activity.16 Clinical presentation is diverse including focal neurological signs, altered mental status, seizures, headache and fevers.1 17 Stroke like episodes of abrupt hemiparesis have also been described.9
There are no definitive diagnostic criteria; diagnosis is based on a combination of biochemistry, radiology and histopathology findings.9 CSF is inflammatory with predominantly mononuclear pleocytosis, mildly elevated protein and normal or low glucose.1 4 Elevated CSF interleukin-6 (IL-6) has been described.17 This is a non-specific biomarker of inflammation. One study suggested elevated IL-6 may indicate disease severity, and thus have a role to play as a therapeutic biomarker.17 Elevated CSF RF has also been reported.2
MRI of the brain typically demonstrates meningeal enhancement.1 A literature review of 29 case reports illustrated asymmetric meningeal involvement in 62% of cases.4 Bright diffusion-weighted imaging may reflect inflammation or ischaemic changes secondary to associated vasculitis.16 Apparent diffusion coefficient (ADC) restriction may be attributed to the limited movement of viscous proteinaceous exudates.16 18 Plaques, nodules and dural-based mass lesion mimicking intracranial tumour have also been reported.1
There are three main histopathology findings: meningeal inflammation (83%), rheumatoid nodules (56%) and vasculitis (38%).1 About two-thirds of cases will feature one or more of these characteristic findings.1 Meningeal inflammation is predominantly mononuclear.1 The cortex is typically preserved, but may demonstrate non-specific reactive changes such as gliosis.1 4 Rheumatoid nodules are most commonly found in the dura.1 They are defined by an area of central necrosis surrounded by epithelioid histiocytes, lymphocytes and multinucleated giant cells.1 Vasculitis tends to involve small parenchymal and meningeal vessels.1
There are no treatment guidelines.1 Patients are acutely treated with corticosteroids, with consideration for steroid sparing immunosuppressive agents thereafter, namely methotrexate, cyclophosphamide or rituximab.1 It was historically associated with higher rates of morbidity and mortality.1 Improvements in imaging have helped with earlier identification.1 Diagnosis is now mostly made on biopsy rather than autopsy.1
Clinical presentation, demographics, investigations and histopathology are similar whether there is a history of RA or not.1 12–15 To our knowledge, there have been just six cases of RM published in the English literature without a prior history of RA. In this cohort, the mean age is 61 (37–77) years. There is equal distribution among genders. Half of the cases present with focal neurological deficits, confusion and seizures. One case had a headache. Another case had a fever. RF was unequivocally positive in 83% (5/6). Anti-CCP was positive in 75% (3/4). ANA was positive in 40% (2/5). CSF was mostly inflammatory 80% (4/5). Half of the cases had asymmetric meningeal involvement on imaging. All cases proceeded to brain and leptomeningeal biopsy which demonstrated 83% (5/6) inflammation and 33% (3/6) rheumatoid nodules. The characteristic symptoms of RA manifested a few months to 1 year after initial presentation.1 12–15 This case highlights the importance of including RM in the differential of chronic inflammatory meningitis, irrespective of a negative clinical history of inflammatory arthropathy.
Learning points.
Anti-CCP IgG is >95% specific for diagnosis of rheumatoid arthritis (RA).
To include rheumatoid meningitis in the differential diagnosis of chronic inflammatory meningitis.
The extra-articular manifestations of RA may precede symptoms of arthopathy.
Footnotes
Contributors: All authors reviewed and edited the manuscript. MCMcK wrote the manuscript and was involved in case management. DV was involved in case management. NB reviewed the neuropathology. SC led clinical management of case. He also led conception, supervision and editing of manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Magaki S, Chang E, Hammond RR, et al. Two cases of rheumatoid meningitis. Neuropathology 2016;36:93–102. 10.1111/neup.12238 [DOI] [PubMed] [Google Scholar]
- 2. Ellman P, Cudkowicz L, Elwood JS. Widespread serous membrane involvement by rheumatoid nodules. J Clin Pathol 1954;7:239–44. 10.1136/jcp.7.3.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moeyersoons A, Verschueren P, Tousseyn T, et al. Rheumatoid granulomatous disease and pachymeningitis successfully treated with rituximab. Acta Clin Belg 2018;73:1–6. 10.1080/17843286.2017.1375193 [DOI] [PubMed] [Google Scholar]
- 4. Choi S-J, Ho Park Y, Kim JA, et al. Pearls & Oy-sters: Asymmetric meningeal involvement is a common feature of rheumatoid meningitis. Neurology 2017;88:e108–e110. 10.1212/WNL.0000000000003744 [DOI] [PubMed] [Google Scholar]
- 5. Tsuzaki K, Nakamura T, Okumura H, et al. Rheumatoid meningitis occurring during etanercept treatment. Case Rep Neurol Med 2017;2017:1–5. 10.1155/2017/7638539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nihat A, Chinthapalli K, Bridges L, et al. Rheumatoid meningitis. Pract Neurol 2016;16:312–4. 10.1136/practneurol-2015-001306 [DOI] [PubMed] [Google Scholar]
- 7. Seago S, Stroberg E, Metting A. Rheumatoid meningitis associated with infliximab. Proc 2016;29:204–6. 10.1080/08998280.2016.11929419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stretz C, Song X, Killory BD, et al. Rheumatoid meningitis: Diagnostic and therapeutic observations. Conn Med 2016;80:163–6. [PubMed] [Google Scholar]
- 9. Roy B, Uphoff DF, Silverman IE. Rheumatoid meningitis presenting with multiple strokelike episodes. JAMA Neurol 2015;72:1073–6. 10.1001/jamaneurol.2015.1105 [DOI] [PubMed] [Google Scholar]
- 10. Lu L, Chwalisz B, Pfannl R, et al. Rheumatoid meningitis: a rare complication of rheumatoid arthritis. BMJ Case Rep 2015;2015:bcr2014208745 10.1136/bcr-2014-208745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeaney GA, Denby EL, Jahromi BS, et al. Rheumatoid-associated meningitis and vasculopathy. Neurology 2015;84:1717–8. 10.1212/WNL.0000000000001498 [DOI] [PubMed] [Google Scholar]
- 12. Padjen I, Mayer M, Habek M, et al. Redefining a diagnosis: from meningeal plasma cell granuloma to rheumatoid meningitis. Report of a patient follow-up. Neurol Sci 2015;36:1047–8. 10.1007/s10072-015-2075-7 [DOI] [PubMed] [Google Scholar]
- 13. Jones SE, Belsley NA, McLoud TC, et al. RAdiologic-pathologic conference of the massachusetts general hospital. American Journal of Roentgenology 2006;186:1181–3. 10.2214/AJR.05.0859 [DOI] [PubMed] [Google Scholar]
- 14. Starosta MA, Brandwein SR. Clinical manifestations and treatment of rheumatoid pachymeningitis. Neurology 2007;68:1079–80. 10.1212/01.wnl.0000257824.72457.91 [DOI] [PubMed] [Google Scholar]
- 15. Kim HY, Park JH, Oh HE, He O, et al. A case of rheumatoid meningitis: pathologic and magnetic resonance imaging findings. Neurol Sci 2011;32:1191–4. 10.1007/s10072-011-0727-9 [DOI] [PubMed] [Google Scholar]
- 16. Roques M, Tanchoux F, Calvière L, et al. MRI with DWI helps in depicting rheumatoid meningitis. J Neuroradiol 2014;41:275–7. 10.1016/j.neurad.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 17. Kato T, Hoshi K, Sekijima Y, et al. Rheumatoid meningitis: an autopsy report and review of the literature. Clin Rheumatol 2003;22:475–80. 10.1007/s10067-003-0788-0 [DOI] [PubMed] [Google Scholar]
- 18. Matsuda S, Yoshida S, Takeuchi T, et al. Asymptomatic rheumatoid meningitis revealed by magnetic resonance imaging, followed by systemic rheumatic vasculitis: A case report and a review of the literature. Mod Rheumatol 2016:1–7 (published Online First: 23 Sep 2016). 10.1080/14397595.2016.1232333 [DOI] [PubMed] [Google Scholar]