Abstract
Objectives
The present study aimed to evaluate the association between the concurrence of pre-existing chronic liver diseases (CLD) and worse prognosis in patients with HILI.
Design
A case–control study.
Setting
Tertiary hospital specialising in liver diseases in China.
Participants
145 hospitalised HILI patients were assessed with respect to prognosis by comparing HILI with or without pre-existing CLD from February 2007 to January 2017. Twenty-five HILI cases with pre-existing alcoholic liver disease (ALD) or non-alcoholic fatty liver disease (NAFLD) and 200 ALD or NAFLD controls matched 1:8 for sex, age (±4 years old), body mass index (±2 kg/m2), the type of CLD, alcohol intake (±5 g/d) and the presence or absence of cirrhosis.
Primary outcome measures
Mortality and chronicity in HILI patients with or without pre-existing CLD, and matched CLD patients.
Results
Of the 193 714 hospitalised patients with liver diseases, 5703 patients met the diagnostic criteria for drug-induced liver injury (DILI), which was attributed to Polygonum multiflorum Thunb. (PMT) in 145 patients. Among these HILI patients, 22.8% (33 of 145) had pre-existing CLD, including 17 (51.5%) with ALD, 8 (24.2%) with NAFLD, 5 (15.2%) with chronic viral hepatitis and 3 (9.1%) with autoimmune liver disease. Compared with HILI patients without CLD, HILI patients with pre-existing CLD showed higher mortality (0.9% vs 9.1%, p=0.037) and higher chronicity (12.5% vs 30.3%, p=0.016). Compared with matched ALD (136 patients) or NAFLD (64 patients) patients, HILI patients with pre-existing ALD showed higher chronicity (35.3% vs 11.8%, p=0.019). Multivariate logistic regression analysis found that concurrence of pre-existing CLD was an independent risk factor for both of chronicity and mortality (OR 3.966, 95% CI 1.501 to 10.477, p=0.005), especially the chronicity (OR 3.035, 95% CI 1.115 to 8.259, p=0.030).
Conclusions
Concurrence of pre-existing CLD could be an independent risk factor for worse prognosis, especially chronicity, in PMT-related HILI.
Keywords: herbal medicine, chronic liver disease, prognosis, drug induced Liver injury[mesh terms]
Strengths and limitations of this study.
This was a matched (1:8) case–control study in a large clinical database (n=193 714) from a specialised liver disease centre.
This study focused on patients with one herb Polygonum multiflorum Thunb. induced liver injury in order to avoid the confounding effects of different drugs on prognosis.
We had simultaneously made comparisons among the three groups (HILI with chronic liver disease (CLD) group, HILI without CLD group and matched CLD without HILI group) for distinguishing between HILI and CLD interactions.
The present study was limited by the sample size, single-centre and retrospective nature of the study (ie, a tertiary hospital).
Introduction
Concurrence of pre-existing chronic liver disease (CLD) with drug-induced liver injury (DILI) is a special challenge in clinical settings, which might render the liver sensitive to drug toxicity and cause higher fatality rates.1 For instance, a case report showed that long-term alcohol intake could potentiate the hepatotoxicity of low doses of acetaminophen.2 In addition, non-alcoholic fatty liver disease (NAFLD) and obesity might increase the risk for acute DILI caused by several synthetic agents, such as methotrexate and tamoxifen, resulting in more severe liver injury.3–5 According to published data from the Drug-Induced Liver Injury Network (DILIN), a higher total fatality rate (19.0%) occurred in patients with known pre-existing liver diseases 6 months after the onset of DILI than in those without CLD (8.1%).6 However, these results in DILIN registry could be different from outcomes of DILI patients with pre-existing CLD in China due to different spectra of CLD and medication systems. Furthermore, no studies have tested whether the concurrence of pre-existing CLD is a major risk factor for worse prognosis in DILI.3
In particular, herbal medications are frequently used as alternative or supplementary agents to conventional synthetic drugs to treat chronic diseases in low/middle-income countries (LMICs). In previous population surveys, herbal and dietary supplements (HDS) were used by one-third to one-half of the adult population in developed countries.7 In a previous population survey of LMICs, the widespread use of traditional Chinese medicines (TCMs) was reported among 24.5% of middle-aged and older patients with chronic diseases in China.8 However, the risk of herbal hepatotoxicity has not been fully addressed, especially in patients with pre-existing CLD. It was reported that the herbal formula, Xiao Chai Hu Tang, caused jaundice and abnormal liver function in a middle-aged woman with known pre-existing liver disease.9 10 In addition, there has been also a rising trend in the use of HDS in developed countries, although they are not prescribed by physicians. Therefore, HILI coupled with pre-existing CLD is a critical and expanding issue in most of these countries. However, knowledge about the intersection between herb-induced liver injury (HILI) and pre-existing CLD has been largely limited.
In this study, we analysed the clinical characteristics and prognosis of HILI, especially in patients with pre-existing CLD from a single centre in China, and tested whether the concurrence of pre-existing CLD was an independent risk factor for worse prognosis in patients with HILI.
Methods
Study design
The case–control study included inpatients in Beijing 302 Hospital, a tertiary hospital specialising in liver diseases in the Capital Region of China, from February 2007 to January 2017. Since different drugs might have differential effects on prognosis, we found the HILI cases attributed to the same herb in order to avoid the confounding effects of different drugs. Finally, Polygonum multiflorum Thunb. (PMT) was found to be the most frequent herb attributed to HILI, and this herb has been widely considered to cause hepatotoxicity over the past three decades.11 12 Then, we also divided enrolled patients with PMT-related HILI into patients with pre-existing CLD and those without CLD. To determine the effects of different pre-existing CLD on the prognosis of HILI, we selected patients with PMT-related HILI with pre-existing CLD as the case group, and also identified matched CLD patients without HILI as the control group. For each case, we selected eight controls matched by sex, age (±4 years old), body mass index (BMI) (±2 kg/m2), the type of CLD, the daily amount of alcohol intake (±5 g/d) and the presence or absence of cirrhosis.
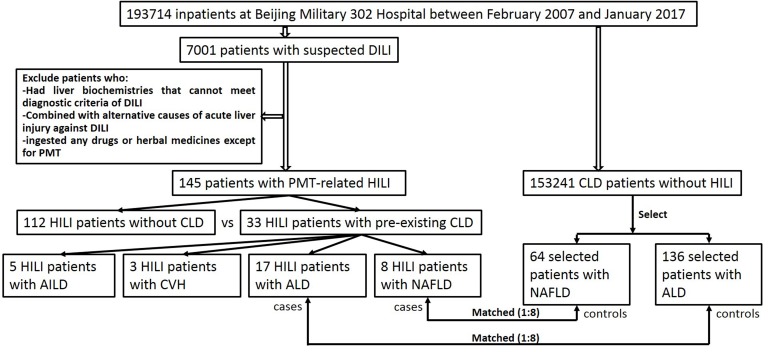
Detailed data about demographics, medical history, clinical features, laboratory tests and histological findings in all eligible patients were extracted from the electronic medical record (EMR). The study protocol was approved, and written informed consent was obtained from each enrolled patient, guardian or next of kin. The study flowchart is depicted in figure 1.
Figure 1.
Flowchart depicting the process for case enrolment. AILD, autoimmune liver disease; ALD, alcoholic liver disease; CLD, chronic liver disease; CVH, chronic viral hepatitis; DILI, drug-induced liver injury; HILI, herb-induced liver injury; NAFLD, non-alcoholic fatty liver disease; PMT, Polygonum multiflorum Thunb.
Diagnostic criteria
DILI or HILI diagnosis was performed according to the American College of Gastroenterology (ACG) clinical guideline for DILI,3 which consists of three parts: (1) any recent abnormal liver biochemistry indices; and (2) chronological use of all drugs and HDS within 6 months prior to the onset of abnormalities in liver testing; and (3) exclusion of recent acute liver injury indicating alternative causes. Abnormal liver biochemistries should meet any of the three following conditions: (1) only a recent rise in alanine or aspartate aminotransferase (ALT or AST) ≥5 times the upper limit of normal (ULN); (2) alkaline phosphatase (AKP) ≥2 times ULN; (3) jaundice (serum total bilirubin (TB) ≥2 mg/dL) and elevations of liver enzymes (ALT ≥3 ULN). For patients with HILI with pre-existing CLD, the ULN was replaced with the previously obtained baseline value prior to exposure to the suspected drugs. When assessing alternative causes of HILI, cases with positive antihepatitis A virus IgM, antihepatitis B core IgM, hepatitis B virus (HBV) DNA, antihepatitis E virus IgM and antihepatitis E virus IgG testing, or with non-hepatotropic virus infection, or with alcoholism within 3 months prior to onset were excluded.3 13–16
In the case and control groups, CLD were defined as persistent liver diseases over 6 months, including alcoholic liver disease (ALD), NAFLD, chronic viral hepatitis (CVH) and autoimmune liver diseases (AILD). ALD was diagnosed in patients with CLD with a history of excessive alcohol consumption over 5 years, ≥40 g/day for men and ≥20 g/day for women, and other causes of CLDs were excluded.17 NAFLD was diagnosed in patients with the radiographic imaging or histological findings compatible with hepatic steatosis in the absence of excessive alcohol intake and other alternative causes such as viral hepatitis, use of agents associated with hepatotoxicity and iron overload.18 CVH was diagnosed based on positive serologic parameters, and in this study, CVH involved chronic HBV infection and chronic hepatitis C virus infection.19 20 AILD consisted of autoimmune hepatitis, primary biliary cholangitis and the overlap syndrome between both of these conditions, and it was diagnosed according to the antibody profiles and liver biopsy findings.21 22
Procedures
In this study, we assessed clinical patterns of liver injury, causality and severity in all eligible patients. By using R value, the ratio of ALT (as a multiple of its ULN) to serum alkaline phosphatase (ALP) (as a multiple of its ULN) at onset after intake of suspected drugs,23 the clinical pattern of DILI was classified into hepatocellular (R≥5), cholestatic (R≤2) and mixed liver injury (2<R<5). Based on the Roussel Uclaf Causality Assessment Method (RUCAM),24 a causal relationship between liver injury and implicated agents among eligible patients was judged as highly probable (≥9), probable (6–8), possible (3–5), unlikely (1–2), or excluded (≤0). According to national and international practice guidelines, the severity assessments of HILI were categorised into five grades, including mild, moderate, severe, liver failure and fatal.25–27 Additionally, the Model for End-Stage Liver Disease (MELD) score was calculated as follows: 9.6*ln [creatinine (mg/dL)]+3.8*ln [bilirubin (mg/dL)]+11.2*ln (international normalised ratio (INR))+6.4.
Liver biopsies were reviewed by two hepatic pathologists, who were blinded to clinical information including patients and suspected agents. And the pathological pattern of liver injury was classified into acute hepatitis and chronic hepatitis, acute and chronic cholestasis and cholestatic hepatitis.28
The discontinuance of the causal agent(s) and alcohol intake was performed in every eligible patient at HILI or CLD recognition. The follow-up visits in eligible cases were scheduled at 6 or 12 months through telephone dictation or uploaded clinical data from EMR. The patient was defined as lost to follow-up if we were unable to contact with him or her at follow-up visit for any reason. Chronicity was considered as the elevations of ALT, AST, TB or ALP >1 ULN or hepatic imaging or histological data in line with chronicity after 6 months from the recognition of HILI or CLD. According to detailed descriptions of the follow-up, all of the eligible patients were categorised with three current outcomes: (1) the recovery group, consisting of cases who had obtained persistent normalisation of liver biochemistry after the withdrawal of implicated agent(s) over the 6-month follow-up; (2) the chronic group, including cases with chronicity beyond 6-month follow-up; and (3) the fatal group, including patients who underwent liver transplantation or died due to liver diseases.
Patient involvement
No patient was involved in setting the research question, the design of the study or their outcome measures. Regular contact with enrolled patients was to improve the implementation of the study. Finally, no patient had advised on dissemination including describing the research and its results.
Statistics
The data are characterised by the means±SDs for normal distribution, the median (Q1, Q3) for abnormal distribution and the frequency distributions for categorical variables. Differences between groups in continuous variables were assessed using Student’s t-test and one-way analysis of variance (ANOVA) or Wilcoxon’s rank-sum test and the Kruskal-Wallis test based on test of normality and homogeneity of variance, respectively. Differences between groups in categorical variables were analysed by the χ2 test or Fisher’s exact test, while results of multiple comparisons were corrected by the Bonferroni’s correction. The identification of factors with p values less than 0.1 in univariate analysis was explored through multivariable logistic regression analysis. OR and 95% CIs were calculated from the model coefficients and SE. P<0.05 was considered statistically significant. All of the statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) software, V.19.0 (SPSS, Chicago, Illinois, USA).
Results
Demographics
Of the 7001 hospitalised patients with temporal association between liver injury and drug exposure among the 193 714 inpatients with liver diseases in the liver unit at Beijing Military 302 Hospital between February 2007 and January 2017, 5703 patients met the diagnostic criteria for DILI, of whom 145 cases were attributed to PMT-related HILI (online supplementary figure S1, tables S1 and S2). Among these cases, 33 (22.76%) with HILI had pre-existing CLD, while 112 cases (77.24%) did not have pre-existing CLD (figure 1). There was no difference in the mean ages between the HILI patients with (45.60 years old, range 21.67–86.74) or without (42.61 years old, range 8.47–70.79) pre-existing CLD. However, male patients accounted for a larger proportion of HILI patients with pre-existing CLD than those without pre-existing CLD (67.7% vs 40.2%, p=0.007) (table 1).
Table 1.
The characteristics among PMT-related HILI patients with or without pre-existing CLD
| Characteristic | Entire cohort of HILI (n=145, 100%) |
HILI with pre-existing CLD (n=33, 22.76%) |
HILI without CLD (n=112, 77.24%) |
P value |
| Males (%) | 67 (46.2%) | 22 (66.7%) | 45 (40.2%) | 0.007 |
| Age (years, mean±SD) | 43.29±13.68 | 45.60±13.04 | 42.61±13.85 | 0.272 |
| BMI (kg/m2, mean±SD) | 23.37±3.40 | 24.64±3.57 | 23.00±3.27 | 0.015 |
| Prior drug allergies (%) | 11 (7.6%) | 1 (3.0%) | 10 (8.9%) | 0.236 |
| Latency (days, median (IQR)) | 50.0 (31.0,91.0) | 45.0 (29.5,105.0) | 51.0 (31.0,88.5) | 0.777 |
| Re-challenge (%) | 7 (4.8%) | 1 (3.0%) | 6 (5.4%) | 0.499 |
| Alcohol use* (%) | 28 (19.3%) | 17 (51.5%) | 11 (9.8%) | <0.001 |
| Laboratory index in DILI recognition | ||||
| WBC (×109/L, median (IQR)) | 5.34 (4.30, 6.56) | 4.89 (4.39,6.50) | 5.37 (4.23,6.56) | 0.899 |
| HGB (g/L, mean±SD) | 135.59±18.20 | 137.82±18.72 | 134.94±18.08 | 0.426 |
| PLT (×109/L, mean±SD) | 213.47±71.00 | 196.79±78.72 | 218.37±68.16 | 0.125 |
| Peripheral eosinophilia (×109/L, median (IQR)) | 0.16 (0.10,0.28) | 0.21 (0.14,0.28) | 0.15 (0.09,0.28) | 0.114 |
| Peak values of laboratory index | ||||
| ALT (U/L, median (IQR)) | 1208.70 (826.05,1537.00) | 1276.00 (806.00,1671.00) | 1173.00 (833.00,1472.05) | 0.551 |
| AST (U/L, median (IQR)) | 739.00 (494.00,1051.00) | 873.00 (445.00,1292.50) | 716.90 (493.50,1041.60) | 0.341 |
| ALP (U/L, median (IQR)) | 179.0 (141.5,215.0) | 177.00 (145.50,230.55) | 180.00 (139.50,213.50) | 0.786 |
| TB (mg/dL, median (IQR)) | 10.76 (6.15,18.96) | 18.75 (7.69,25.18) | 10.38 (5.59,16.77) | 0.008 |
| Albumin (g/L, median (IQR)) | 34(31,38) | 33.00 (27.50,37.00) | 35.00 (32.00,38.00) | 0.036 |
| Cholinesterase (U/L, mean±SD) | 4924.61±1772.28 | 4197.70±1969.99 | 5138.79±1659.09 | 0.007 |
| INR (median (IQR)) | 1.07 (0.98,1.02) | 1.09 (0.97,1.40) | 1.07 (0.99,1.15) | 0.488 |
| Pattern of liver injury | ||||
| HC/Chol/Mixed (%) | 137/4/4 | 30/2/1 | 107/2/3 | 0.399 |
| RUCAM score (median (IQR)) | 8 (7,8) | 7 (6,8) | 8 (7,8) | 0.003 |
| Possible/probable/highly probable | 9/120/16 | 2/30/1 | 7/90/15 | 0.275 |
| Severity of liver injury† (% of column total) | 0.120 | |||
| Mild | 8 (5.5%) | 1 (3.0%) | 7 (6.3%) | |
| Moderate | 18 (12.4%) | 2 (6.1%) | 16 (14.3%) | |
| Severe | 105 (72.4%) | 25 (75.8%) | 80 (71.4%) | |
| Liver failure | 10 (6.9%) | 2 (6.1%) | 8 (7.1%) | |
| Fatal | 4 (2.8%) | 3 (9.1%) | 1 (0.9%) | |
| MELD score (median (IQR)) | 15(12,18) | 17(13,20) | 15(11,17) | 0.038 |
| Prognosis (% of column total) | ||||
| Recovery | 120 (82.8%) | 20 (60.6%) | 97 (86.6%) | 0.001 |
| Chronic | 20 (13.8%) | 10 (30.3%) | 14 (12.5%) | 0.016 |
| Fatal | 5 (3.4%) | 3 (9.1%) | 1 (0.9%) | 0.037 |
*The patients with histories of excessive alcohol use (alcohol intake of ≥40 g/d for men and ≥20 g/d for women) did not drink during 3 months prior to the onset of liver injury.
†The severity assessments of HILI were graded as follows31 32: mild, reversible elevations of serum ALT and/or ALP levels, TB <2.5 mg/dL and international normalised ratio (INR) <1.5; moderate elevations of serum ALT and/or ALP levels with associated TB ≥2.5 mg/dL or INR ≥1.5; severe, elevations of serum ALT and/or ALP levels and TB ≥5 mg/dL, with or without INR ≥1.5; liver failure, elevation of serum ALT and/or ALP level with TB ≥10 mg/dL or a sharp increase of 1 mg/dL per day, INR ≥1.5, with relevant ascites, hepatic encephalopathy or other organ failure related to DILI; death or liver transplantation because of DILI.
ALP, serum alkaline phosphatase; ALT, serum alanine transaminase; AST, serum aspartate aminotransferase; BMI, body mass index; Chol, cholestatic; CLD, chronic liver diseases; DILI, drug-induced liver injury; HC, hepatocellular; HGB, haemoglobin; HILI, herb-induced liver injury; INR, international normalised ratio; MELD, Model for End-Stage Liver Disease; PLT, platelet; PMT, Polygonum multiflorum Thunb.; RUCAM, the Roussel Uclaf Causality Assessment Method; TB, serum total bilirubin; WBC, white blood cell.
bmjopen-2018-023567supp001.pdf (654.4KB, pdf)
Among enrolled patients with PMT-related HILI, 22.76% (33 of 145) had pre-existing CLD, including 17 with ALD, eight with NAFLD, five with CVH and three with AILD (figure 1 and online supplementary table S3). Patients with HILI with pre-existing ALD comprised well over 50% of male patients compared with those without pre-existing CLD (p<0.001), whereas patients with HILI with pre-existing NAFLD implied no sex differences from those without pre-existing CLD (tables 2 and 3). In contrast, BMI values might be significantly higher in HILI cases with pre-existing NAFLD than in HILI cases without pre-existing CLD, but there was no difference in HILI cases with pre-existing ALD and those without CLD (tables 2 and 3).
Table 2.
The characteristics of PMT-related HILI patients with pre-existing ALD compared with those of PMT-related HILI patients without CLD and matched ALD patients
| Characteristic | HILI without CLD group (n=112) |
HILI with pre-existing ALD group (n=17) | Matched ALD group (n=136) |
P value* | P value† | P value‡ | P value§ |
| Males (%) | 45 (40.2%) | 15 (88.2%) | 120 (88.2%) | <0.001 | <0.001 | <0.001 | 1.00 |
| Age (years, median (IQR)) | 43.79 (33.80, 53.41) | 45.15 (38.68, 56.00) | 45.17 (38.34, 51.65) | 0.567 | |||
| BMI (kg/m2, median (IQR)) | 22.48 (20.45, 24.96) | 24.06 (20.67, 26.76) | 23.51 (22.10, 24.73) | 0.133 | |||
| Liver cirrhosis (%) | 8 (7.1%) | 3 (17.6%) | 32 (23.5%) | 0.001 | 0.480 | <0.001 | 1.00 |
| Peak of serum ALT (U/L, median (IQR)) | 1173.00 (833.00,1472.05) | 1389.00 (911.85,1842.50) | 54.50 (30.00, 90.75) | <0.001 | 0.801 | <0.001 | <0.001 |
| Peak of serum AST (U/L, median (IQR)) | 716.90 (493.50,1041.60) | 878.00 (423.50,1359.00) | 46.50 (26.00, 91.00) | <0.001 | 1.00 | <0.001 | <0.001 |
| Peak of serum ALP (U/L, median (IQR)) | 180.00 (139.50,213.50) | 179.00 (149.50,230.55) | 103.00 (80.00, 165.50) | <0.001 | 1.00 | <0.001 | 0.003 |
| Peak of serum TB (mg/dL, median (IQR)) | 10.38 (5.59, 16.77) | 18.75 (8.15,29.51) | 1.11 (0.74, 2.12) | <0.001 | 0.057 | <0.001 | <0.001 |
| Peak of serum GGT (U/L, median (IQR)) | 164.00 (96.25, 260.00) | 160.00 (113.00, 187.00) | 125.00 (46.75, 336.00) | 0.508 | |||
| Peak of serum INR (median (IQR)) | 1.07 (0.99, 1.15) | 1.11 (1.02, 1.40) | 0.99 (0.93, 1.10) | <0.001 | 0.744 | <0.001 | 0.009 |
| Peak of serum TC (mmol/L, median (IQR)) | 3.79 (2.96, 4.42) | 3.78 (2.45, 4.44) | 4.75 (3.90, 5.43) | <0.001 | 0.957 | <0.001 | 0.009 |
| Peak of serum TG (mmol/L, median (IQR)) | 2.32 (1.61, 3.25) | 2.34 (1.77, 3.56) | 1.70 (1.10, 3.01) | 0.004 | 1.00 | 0.009 | 0.156 |
| Laboratory index in the recognition | |||||||
| Serum albumin (g/L, median (IQR)) | 37.00 (35.00, 40.00) | 35.00 (30.50, 39.00) | 39.00 (35.00, 43.00) | 0.001 | 0.303 | 0.003 | 0.021 |
| Serum cholinesterase (U/L, median (IQR)) | 5754.50 (4715.25,6615.75) | 4506.00 (3196.50,5561.00) | 6702.00 (4861.50,8223.75) | <0.001 | 0.018 | <0.001 | <0.001 |
| Recovery (%) | 97 (86.6%) | 10 (58.8%) | 120 (88.2%) | 0.011 | 0.030 | 1.000 | 0.015 |
| Chronic (%) | 14 (12.5%) | 6 (35.3%) | 16 (11.8%) | 0.038 | 0.027 | 0.860 | 0.019 |
| Fatal (%) | 1 (0.9%) | 1 (5.9%) | 0 (0.0%) | 0.058 | |||
*The comparisons were analysed among the three groups.
†The pairwise comparison between the PMT-related HILI group without CLD and the PMT-related HILI with pre-existing ALD group.
‡The pairwise comparison between the PMT-related HILI group without CLD and the matched ALD group.
§The pairwise comparison between the PMT-related HILI with pre-existing ALD group and the matched ALD group.
†‡§Differences between groups in categorical variables were analysed by the chi-squared test or Fisher’s exact test, while results of multiple comparisons were corrected by the Bonferroni’s correction.
ALD, alcoholic liver disease; ALP, serum alkaline phosphatase; ALT, serum alanine transaminase; AST, serum aspartate aminotransferase; BMI, body mass index; CLD, chronic liver disease; GGT, gamma-glutamyl transpeptidase; HILI, herb-induced liver injury; Ig, immunoglobulin; INR, international normalised ratio; PMT, Polygonum multiflorum Thunb.; TB, serum total bilirubin; TC, total cholesterol; TG, total glyceride.
Table 3.
The characteristics of PMT-related HILI patients with pre-existing NAFLD compared with those of PMT-related HILI patients without CLD and matched NAFLD patients
| Characteristic | HILI without CLD group (n=112) |
HILI with pre-existing NAFLD group (n=8) | Matched NAFLD group (n=64) | P value* | P value† | P value‡ | P value§ |
| Males (%) | 45 (40.2%) | 4 (50.0%) | 24 (37.5%) | 0.805 | |||
| Age (years, median (IQR)) | 43.79 (33.80,53.41) | 40.27 (33.81,48.39) | 39.17 (34.08,48.69) | 0.337 | |||
| BMI (kg/m2, median (IQR)) | 22.48 (20.45,24.96) | 27.16 (25.48,28.53) | 26.15 (23.69,28.53) | <0.001 | 0.003 | <0.001 | 1.000 |
| Diabetes mellitus (%) | 5 (4.5%) | 0 (0.0%) | 4 (6.3%) | 0.817 | |||
| Liver cirrhosis (%) | 8 (6.90%) | 0 (0.00%) | 0 (0.00%) | 0.104 | |||
| Complications (%) | 17 (14.3%) | 2 (25.0%) | 0 (0.0%) | 0.001 | 1.000 | <0.001 | 0.009 |
| Peak of serum ALT (U/L, median (IQR)) | 1173.00 (833.00,1472.05) | 1490.50 (861.75,1681.50) | 88.00 (58.50,152.25) | <0.001 | 1.000 | <0.001 | <0.001 |
| Peak of serum AST (U/L, median (IQR)) | 716.90 (493.50,1041.60) | 945.00 (645.18,1377.75) | 50.00 (34.00, 75.25) | <0.001 | 0.468 | <0.001 | <0.001 |
| Peak of serum ALP (U/L, median (IQR)) | 180.00 (139.50,213.50) | 149.00 (105.25,192.75) | 98.50 (77.50,121.75) | <0.001 | 0.864 | <0.001 | 0.045 |
| Peak of serum TB (mg/dL, median (IQR)) | 10.38 (5.59,16.77) | 21.08 (7.65,21.93) | 0.74 (0.57,0.90) | <0.001 | 0.252 | <0.001 | <0.001 |
| Peak of serum GGT (U/L, median (IQR)) | 164.00 (96.25,260.00) | 209.00 (196.25,257.25) | 80.00 (40.50,141.75) | <0.001 | 0.396 | <0.001 | <0.001 |
| Peak of serum INR (median (IQR)) | 1.07 (0.99,1.15) | 1.21 (0.97,1.40) | 0.94 (0.88, 0.96) | <0.001 | 1.000 | <0.001 | 0.003 |
| Peak of serum TC (mmol/L, median (IQR)) | 3.79 (2.96,4.42) | 4.18 (3.76, 4.63) | 5.04 (4.39, 5.58) | <0.001 | 0.780 | <0.001 | 0.084 |
| Peak of serum TG (mmol/L, median (IQR)) | 2.32 (1.61,3.25) | 3.11 (1.71,4.37) | 2.24 (1.58,3.37) | 0.530 | 0.711 | 1.000 | 1.000 |
| Laboratory index in the recognition | |||||||
| Serum albumin (g/L, median (IQR)) | 37.00 (35.00,40.00) | 39.50 (33.25,40.00) | 42.00 (40.00,44.00) | <0.001 | 1.000 | <0.001 | 0.003 |
| Serum cholinesterase (U/L, mean±SD) | 5664.79±1613.11 | 5856.38±1941.11 | 8589.23±1254.07 | <0.001 | 1.000 | <0.001 | <0.001 |
| Recovery (%) | 97 (86.6%) | 5 (62.5%) | 55 (85.9%) | 0.187 | |||
| Chronic (%) | 14 (12.5%) | 3 (37.5%) | 9 (14.1%) | 0.137 | |||
| Fatal (%) | 1 (0.9%) | 0 (0.00%) | 0 (0.00%) | 1.000 | |||
*The comparisons were analysed among the three groups.
†The pairwise comparison between the PMT-related HILI group without CLD and the PMT-related HILI with pre-existing NAFLD group.
‡The pairwise comparison between the PMT-related HILI group without CLD and the matched NAFLD group.
§The pairwise comparison between the PMT-related HILI with pre-existing NAFLD group and the matched NAFLD group.
†‡§Differences between groups in categorical variables were analysed by the chi-squared test or Fisher’s exact test, while results of multiple comparisons were corrected by the Bonferroni’s correction.
ALP, serum alkaline phosphatase; ALT, serum alanine transaminase; AST, serum aspartate aminotransferase; BMI, body mass index; CLD, chronic liver disease; GGT, gamma-glutamyl transpeptidase; HILI, herb-induced liver injury; Ig, immunoglobulin; INR, international normalised ratio; NAFLD, non-alcoholic fatty liver disease; PMT, Polygonum multiflorum Thunb.; TB, serum total bilirubin; TC, total cholesterol; TG, total glyceride.
Clinical characteristics
The clinical features of patients with HILI with or without pre-existing CLD are showed in table 1. By the use of R values, 145 eligible cases were classified into hepatocellular (n=137, 94.6%), cholestatic (n=4, 2.7%) and mixed liver injury (n=4, 2.7%). Of HILI cases with and without pre-existing CLD, based on the RUCAM scale, 11.0% were considered highly probable, 82.8% were probable, 6.2% were possible and no one was unlikely or excluded. The clinical patterns and RUCAM scales in the HILI cases with pre-existing CLD were similar to those in HILI cases without CLD.
The main presenting symptoms, including jaundice (93.9% vs 93.8%), anorexia (72.7% vs 75%), generalised weakness (72.7% vs 68.8%), nausea (51.5% vs 42.9%), abdominal discomfort (27.3% vs 31.3%) and vomiting (9.1% vs 16.1%), were all profiled and showed fewer differences in patients with HILI with or without pre-existing CLD. Further, there were no differences in comorbidities among HILI cases with or without pre-existing CLD, except for cardiovascular disease (12.1% vs 1.8%, p=0.024) (online supplementary table S4).
Nevertheless, there were more differences in clinical and laboratory findings among patients with HILI with pre-existing CLD, those without CLD and matched CLD patients. Compared with the levels in patients with HILI without CLD, higher levels of serum TB (at peak, median, 10.38 vs 18.75 mg/dL, p=0.008) and lower levels of serum albumin (at lowest, median, 35 vs 33 g/L, p=0.036) and cholinesterase (at lowest, 5138.79±1659.09 vs 4197.70±1969.99 U/L, p=0.007) were found in HILI patients with pre-existing CLD. In addition, MELD scores in HILI patients with pre-existing CLD were significantly higher than in those without CLD (median, 15 vs 17, p=0.038). To investigate the impacts of different pre-existing CLD on HILI, we selected and analysed two major types of pre-existing CLDs (ALD and NAFLD) in patients with HILI and matched ALD or NAFLD patients with patients with HILI (1:8) who had corresponding pre-existing CLD (tables 2 and 3). The compared results indicated that patients with HILI with pre-existing ALD or NAFLD had more severe abnormalities in liver biochemistry, including ALT, AST, ALP, TB, INR, serum albumin and cholinesterase, than matched ALD or NAFLD patients (p for all <0.05).
Histological findings
In 145 enrolled PMT-related HILI patients, liver biopsies were performed in 70 cases with and without pre-existing CLD to confirm the diagnosis of HILI. The most common histological patterns were acute (50.8%) hepatitis and acute cholestasis (12.3%), followed by chronic hepatitis (15.4%) and cholestatic hepatitis (21.5%). Lobular inflammation, portal inflammation, interface hepatitis, with typical confluent necrosis, apoptosis and neutrophils, were frequently found in over 50% of HILI cases with histological information. Additionally, hepatocellular and/or canalicular cholestasis in 22 HILI cases, all of whose clinical patterns were hepatocellular liver injury. Histological patterns between patients with HILI with and without pre-existing CLD were similar (p>0.05).
Outcomes
Recorded data on clinical outcomes during follow-up visits are shown in table 1 and online supplementary table S5. All enrolled patients with HILI or CLD were followed up until the end of the study. In 145 patients with PMT-related HILI, four patients with hepatocellular (n=2) or cholestatic liver injury (n=2) died because of haemorrhagic disease, one complication of liver diseases. Four patients progressed to acute and chronic liver failure (ACLF) in 33 patients with HILI with pre-existing CLD, while no one developed ACLF in 112 HILI cases without CLD. Among 33 patients with HILI and CLD, 2 patients died in 4 patients with ACLF, whereas only 1 died in 29 patients without ACLF.
Of patients with HILI patients with fatal outcomes, three patients with HILI with pre-existing CLD and one patient with HILI without a pre-existing CLD died, whereas all of the matched CLD patients survived. Compared with patients with HILI without pre-existing CLD, patients with HILI with pre-existing CLD had a higher mortality rate (0.9% vs 9.1%, p=0.037) and a greater rate of chronicity (12.5% vs 27.3%, p=0.041) (table 1). Moreover, patients with HILI with pre-existing ALD had higher chronicity (11.8% vs 35.3%, p=0.038) and a lower recovery rate (88.2% vs 58.8%, p=0.011) than the matched ALD patients (table 2). In the univariate logistic regression analysis, the concurrence of pre-existing CLD was considered a significant risk factor for worse outcomes (OR 4.203, 95% CI 1.735 to 10.185, p=0.001), including chronicity (OR 3.043, 95% CI 1.201 to 7.713, p=0.019) and mortality (OR 11.100, 95% CI 1.114 to 110.584, p=0.040) (table 4).
Table 4.
Logistic regression for the prognosis of PMT-related HILI with and without pre-existing CLD
| Parameters† | Univariable | Multivariate* | ||||||
| OR | 95% CI | P value | OR | 95% CI | P value | |||
| Chronic | ||||||||
| Age | 1.023 | 0.990 | 1.058 | 0.175 | 1.014 | 0.977 | 1.052 | 0.465 |
| Sex | 1.018 | 0.423 | 2.452 | 0.968 | 0.970 | 0.348 | 2.707 | 0.954 |
| BMI | 1.146 | 1.006 | 1.306 | 0.040 | ||||
| Pre-existing CLD | 3.043 | 1.201 | 7.713 | 0.019 | 3.035 | 1.115 | 8.259 | 0.030 |
| Peak value of ALT | 0.999 | 0.998 | 1.000 | 0.078 | 0.999 | 0.998 | 1.000 | 0.102 |
| Peak value of total bilirubin | 1.025 | 0.976 | 1.075 | 0.323 | ||||
| Peak value of INR | 2.596 | 0.858 | 7.855 | 0.091 | ||||
| Lowest albumin | 0.879 | 0.802 | 0.964 | 0.006 | ||||
| Lowest cholinesterase | 1.000 | 0.999 | 1.000 | 0.010 | ||||
| MELD score | 1.015 | 0.936 | 1.100 | 0.727 | 1.017 | 0.933 | 1.109 | 0.703 |
| Mortality | ||||||||
| Age | 1.042 | 0.965 | 1.124 | 0.293 | 1.028 | 0.960 | 1.101 | 0.433 |
| Sex | 0.277 | 0.028 | 2.729 | 0.271 | 0.512 | 0.085 | 3.076 | 0.465 |
| BMI | 1.071 | 0.804 | 1.426 | 0.640 | ||||
| Pre-existing CLD | 11.100 | 1.114 | 110.584 | 0.040 | 4.385 | 0.846 | 22.714 | 0.078 |
| Peak value of ALT | 0.999 | 0.997 | 1.001 | 0.212 | 0.999 | 0.997 | 1.000 | 0.169 |
| Peak value of total bilirubin | 1.169 | 1.039 | 1.316 | 0.010 | ||||
| Peak value of INR | 12.448 | 2.429 | 63.779 | 0.002 | ||||
| Lowest albumin | 0.545 | 0.328 | 0.904 | 0.019 | ||||
| Lowest cholinesterase | 0.997 | 0.996 | 0.999 | 0.008 | ||||
| MELD score | 1.326 | 1.088 | 1.616 | 0.005 | 1.222 | 1.052 | 1.421 | 0.009 |
| Mortality and chronicity | ||||||||
| Age | 1.028 | 0.996 | 1.061 | 0.089 | 1.021 | 0.985 | 1.058 | 0.265 |
| Sex | 0.828 | 0.363 | 1.890 | 0.654 | 0.819 | 0.300 | 2.236 | 0.696 |
| BMI | 1.143 | 1.010 | 1.294 | 0.034 | ||||
| Pre-existing CLD | 4.203 | 1.735 | 10.185 | 0.001 | 3.966 | 1.501 | 10.477 | 0.005 |
| Peak value of ALT | 0.999 | 0.998 | 1.000 | 0.031 | 0.999 | 0.998 | 1.000 | 0.022 |
| Peak value of total bilirubin | 1.052 | 1.005 | 1.101 | 0.028 | ||||
| Peak value of INR | 7.708 | 1.986 | 29.923 | 0.003 | ||||
| Lowest albumin | 0.804 | 0.726 | 0.890 | <0.001 | ||||
| Lowest cholinesterase | 0.999 | 0.999 | 1.000 | <0.001 | ||||
| MELD score | 1.068 | 0.988 | 1.154 | 0.096 | 1.077 | 0.989 | 1.172 | 0.087 |
*Peak value of total bilirubin, INR and lowest serum albumin and cholinesterase were excluded for multivariate analysis.
†Choosing clinically relevant variables (age and sex) and those with P<0.1 on univariate analysis. For variables with known co-linearity or high correlations, clinical judgement was used to select one predictor for additional modelling.
ALT, serum alanine transaminase; BMI, body mass index; CLD, chronic liver disease; HILI, herb-induced liver injury; INR, international normalised ratio; MELD, Model for End-Stage Liver Disease; PMT, Polygonum multiflorum Thunb.; TCM, traditional Chinese medicine.
In the multivariate logistic regression analysis, clinically relevant variables (age and sex) and those with statistical significance (p<0.1) in the univariate analysis (pre-existing CLD, liver biochemistries and MELD score) were introduced as covariates (table 4). As variables with known colinearity or high correlations, the selection of one predictor for modelling was judged by clinical practice. Multivariate logistic regression analysis showed that the concurrences of pre-existing CLD (OR 3.966, 95% CI 1.501 to 10.477, p=0.005) and peak ALT (OR 0.999, 95% CI 0.998 to 1.000, p=0.022) were independently associated with worse outcomes, including chronicity and mortality. In multivariate logistic regression analysis for different worse outcomes, the concurrence of pre-existing CLD was likely to be an independent risk factor for chronic outcomes of HILI (OR 3.035, 95% CI 1.115 to 8.259, p=0.030) as well as MELD scores for liver-related death (OR 1.222, 95% CI 1.052 to 1.421, p=0.009). In addition, the concurrence of pre-existing CLD might be a potentially relevant factor with a trend close to significance for fatal outcomes of HILI (p=0.078).
Discussion
In this study, PMT-related HILI patients with pre-existing CLD showed a higher mortality rate (0.9% vs 9.1%, p=0.037) and a greater rate of chronicity (12.5% vs 30.3%, p=0.016) than those without CLD. Multivariate logistic regression analysis illustrated that concurrence of pre-existing CLD was an independent risk factor for chronic and fatal outcomes (OR 3.966, 95% CI 1.501 to 10.477, p=0.005), especially the former (OR 3.035, 95% CI 1.115 to 8.259, p=0.030). Thus, the concurrence of pre-existing CLD is likely to be an independent risk factor for worse prognosis, especially chronicity, in PMT-related HILI. These results provide new insights into the clinical study and management of alternative treatment with herbal medications, especially in patients with pre-existing CLD.
In LMICs, herbal medications, rather than synthetic drugs, are frequently used as alternative or supplementary agents to replace conventional synthetic drugs to treat chronic diseases due to the lower cost of TCM and limited access to conventional medicines in remote areas of LMICs.26 29 Thus, this study might partly explain why the proportion of PMT-related HILI patients with pre-existing CLD among all enrolled patients with HILI from China (a LMIC) seemed to be markedly higher than the proportion of patients with DILI with pre-existing CLD among all patients with DILI from the USA (a developed country)13 (22.8% vs 9.9%, respectively). In addition, the use of HDS and the constituent ratio of HILI in DILI cohorts appeared to show increasing trends.7 Self-medication among patients with CLD often accounts for a proportion of herbal medication use.29 Therefore, HILI coupled with pre-existing CLD is a critical and expanding issue in most of these countries.
In this study, we noted that ALD was the primary type of pre-existing CLD involved in PMT-related HILI, followed by NAFLD, CVH and AILD. In contrast, pre-existing hepatitis C or NAFLD often underlie DILI in the DILIN registry.13 The difference between this study and the DILIN registry might be associated with the different spectra of liver diseases, medication systems and socioeconomic backgrounds. According to a retrospective nationwide analysis, the risk of acute DILI could increase with pre-existing ALD (aOR 6.46; 95% CI 4.53 to 9.21) and NAFLD (aOR 7.43; 95% CI 3.30 to 16.7).30 Thus, PMT and its products should be prudently administered to patients with pre-existing ALD or NAFLD.
Interestingly, the laboratory findings of patients with HILI with pre-existing CLD were similar to those of patients with HILI, rather than to those of patients with corresponding CLD. Additionally, histological patterns had no difference between PMT-related HILI patients with and without pre-existing CLD in this study. These results showed that abnormal liver biochemistries and histological findings were dominated by PMT and its products, although these patients with HILI had pre-existing CLD. For instance, patients with HILI with pre-existing CLD and those without CLD could have patterns of sharply increasing levels of ALT, AST, ALP and TB, while patients with CLD could have trends of slightly elevated levels of these factors. Thus, the diagnosis of HILI is likely to depend on the pattern of increasing levels of liver biochemistries and histological findings, especially in patients with pre-existing CLD. However, compared with those in patients with HILI without CLD, the peak value of serum TB and the lowest values of serum albumin and cholinesterase were more severe in patients with HILI with pre-existing CLD, most of whom were diagnosed with hepatocellular liver injury. Previous studies and Zimmerman’s observations have confirmed that increased bilirubin levels and hepatocellular liver injury caused by drugs were associated with 10%–50% mortality and liver transplantation rates from liver failure.31 More severe hypoalbuminaemia and lower choline esterase activity could be explained by underlying impaired liver function due to reduced synthesis.32 33 In a previous study of hepatotoxicity caused by active antiretroviral agents, patients with acute DILI appeared to be more severe in those with CVH.34 Consequently, care should be taken to monitor and manage patients with pre-existing CLD who digest herbal medications by either physician prescription or self-medication.
Notably, this study showed that PMT-related HILI patients with pre-existing CLD had higher mortality and greater chronicity. Furthermore, concurrence of pre-existing CLD could be an independent risk factor for worse prognosis, especially chronicity, in PMT-related HILI. Although PMT-related HILI patients with pre-existing CLD (9.10%) in this study showed similar liver-related mortality rates as patients with DILI with pre-existing CLD (9.12%) in the DILIN registry, PMT-related HILI patients with pre-existing CLD were more likely to develop chronic outcomes (30.3%) than patients with DILI with pre-existing CLD (13.7%) in the DILIN study.13 PMT-related HILI patients with pre-existing ALD were more likely to have CLD than matched ALD patients. In a study of patients with pulmonary tuberculosis treated with various antituberculosis drugs, multivariate analysis revealed prior alcohol consumption to be a risk factor for recurrent DILI.35 Additionally, ACLF might increase the risk for liver-related mortality in patients with HILI with pre-existing CLD. In a retrospective cohort study, hepatic necrosis and hepatic encephalopathy could be significantly associated with liver-related deaths in HILI caused by Ayurvedic and herbal medicines.36 Acute DILI in individuals with pre-existing CLD was hypothesised to result in severe liver injury or slower to recovery due to impaired liver regeneration.3 Therefore, patients with pre-existing CLD following ingestion of herbal TCM should be considered, with a focus on the increased risk of HILI and its worse prognosis.
The present study is noteworthy for several reasons. First, this was a matched (1:8) case–control study on HILI combined with CLD in a large clinical database (n=193 714) from a specialised liver disease centre in China. Second, the HILI cases in the present study attributed to the same herb were found in order to avoid differential effects of confounding variables (different drugs) on prognosis. Third, the comparisons were simultaneously analysed among the three groups (HILI with CLD group, HILI without CLD group and matched CLD without HILI group) for the sake of distinguishing between HILI and CLD interactions. These methods of clinical study was rarely published in previous researches on DILI or HILI.3 13 Additionally, the association between concurrence of pre-existing CLD and worse prognosis of HILI was discovered in this study. In previous studies, knowledge about intersection between HILI and pre-existing CLD has been limited.
However, our study has some limitations. There were potential selection bias and recall bias in this study because of its single-centre and retrospective design. Furthermore, the present study investigated clinical characteristics and prognosis of an herb-PMT-related HILI in patients with CLD, so this affected the sample size of enrolled patients and the power of our study.
In conclusion, patients with HILI with pre-existing CLD should receive heightened attention owing to an increased risk of worse prognosis. Although patients with pre-existing CLD might benefit from the use of complementary and alternative medicines (CAM), especially herbal remedies, they are most likely to experience fatality or chronicity after suffering from HILI caused by herbal TCM. Therefore, providing strict monitoring and supervision of CAM, including herbal TCM, in the treatment of patients with pre-existing CLD is crucial in LMICs. Based on the present research design, further large samples, multicentre and prospective studies are needed to find the distinctive characteristics, risk factors and predictors of prognosis in all-cause DILI patients with pre-existing CLD.
Supplementary Material
Acknowledgments
The authors appreciate the contributions of all the subjects and the staff (the doctors, nurses and administrative staff) at Beijing 302 Hospital for this study.
Footnotes
Patient consent for publication: Obtained.
JJ and R-W contributed equally.
X-X and J-W contributed equally.
Contributors: J-bW, X-hX and JJ designed the study. JJ, R-lW, YZ, MN, L-fW, X-S, T-tH, Y-qS, L-pW, W-tX and S-mY collected the patient’s clinical data; X-yZ reviewed liver biopsies; JJ, Y-mG, Z-fB analysed the data. JJ and R-lW translated and wrote the paper. X-hX and J-bW took charge of the project and amended the paper. All authors read and approved the final version of manuscript.
Funding: This work was supported by the National Key Technology R&D Program (no. 2015ZX09501-004-001-008 and 2015ZX09501004-001-002), the National TCM Industry Science and Technology Program (no. 201507004-04), the National Natural Science Foundation of China (nos. 81373984 and 81403126) and Beijing Natural Science Foundation (no. 7152142).
Competing interests: None declared.
Ethics approval: The ethics committee of Beijing 302 Hospital approved this study (No: 2017056D).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Zimmerman HJ. Hepatotoxicity. The adverse effects of drugs and other chemicals on the liver. 2nd edn Philadelphia (PA: Lippincott, Williams and Wilkins, 1999:430. [Google Scholar]
- 2. O’Dell JR, Zetterman RK, Burnett DA. Centrilobular hepatic fibrosis following acetaminophen-induced hepatic necrosis in an alcoholic. JAMA 1986;255:2636–7. 10.1001/jama.1986.03370190120036 [DOI] [PubMed] [Google Scholar]
- 3. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109(7):950–66. 10.1038/ajg.2014.131 [DOI] [PubMed] [Google Scholar]
- 4. Fromenty B. Drug-induced liver injury in obesity. J Hepatol 2013;58:824–6. 10.1016/j.jhep.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 5. Michaut A, Moreau C, Robin MA, et al. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int 2014;34:e171–e179. 10.1111/liv.12514 [DOI] [PubMed] [Google Scholar]
- 6. Fontana RJ, Hayashi PH, Gu J, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology 2014;147:96–108. 10.1053/j.gastro.2014.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navarro VJ, Khan I, Björnsson E, et al. Liver injury from herbal and dietary supplements. Hepatology 2017;65:363–73. 10.1002/hep.28813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu T, Li X, Zou ZY, et al. The Prevalence and Determinants of Using Traditional Chinese Medicine Among Middle-aged and Older Chinese Adults: Results From the China Health and Retirement Longitudinal Study. J Am Med Dir Assoc 2015;16:1002.e1–1002.e5. 10.1016/j.jamda.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 9. Hsu LM, Huang YS, Tsay SH, et al. Acute hepatitis induced by Chinese hepatoprotective herb, xiao-chai-hu-tang. J Chin Med Assoc 2006;69:86–8. 10.1016/S1726-4901(09)70119-4 [DOI] [PubMed] [Google Scholar]
- 10. Teschke R, Eickhoff A. Herbal hepatotoxicity in traditional and modern medicine: actual key issues and new encouraging steps. Front Pharmacol 2015;6:72 10.3389/fphar.2015.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu Y, Niu M, Chen J, et al. Hepatobiliary and pancreatic: Comparison between Chinese herbal medicine and Western medicine-induced liver injury of 1985 patients. J Gastroenterol Hepatol 2016;31:1476–82. 10.1111/jgh.13323 [DOI] [PubMed] [Google Scholar]
- 12. Jung KA, Min HJ, Yoo SS, et al. Drug-Induced Liver Injury: Twenty Five Cases of Acute Hepatitis Following Ingestion of Polygonum multiflorum Thunb. Gut Liver 2011;5:493–9. 10.5009/gnl.2011.5.4.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015;148:1340–52. 10.1053/j.gastro.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 2011;89:806–15. 10.1038/clpt.2011.58 [DOI] [PubMed] [Google Scholar]
- 15. Chalasani N, Regev A. Drug-Induced Liver Injury in Patients With Preexisting Chronic Liver Disease in Drug Development: How to Identify and Manage? Gastroenterology 2016;151:1046–51. 10.1053/j.gastro.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 16. Teschke R, Danan G. Diagnosis and Management of Drug-Induced Liver Injury (DILI) in Patients with Pre-Existing Liver Disease. Drug Saf 2016;39:729–44. 10.1007/s40264-016-0423-z [DOI] [PubMed] [Google Scholar]
- 17. European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012;57:399–420. 10.1016/j.jhep.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 18. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 19. European Association for the Study of the Liver. EASL 2017 Clinical Practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98. 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 20. Omata M, Kanda T, Wei L, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int 2016;10:702–26. 10.1007/s12072-016-9717-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol 2015;63:971–1004. 10.1016/j.jhep.2015.06.030 [DOI] [PubMed] [Google Scholar]
- 22. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 2009;51:237–67. 10.1016/j.jhep.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 23. Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 1990;11:272–6. [DOI] [PubMed] [Google Scholar]
- 24. Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci 2015;17:14–33. 10.3390/ijms17010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu YC, Mao YM, Chen CW, et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int 2017;11:221–41. 10.1007/s12072-017-9793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang JB, Zhu Y, Bai ZF, et al. Guidelines for the Diagnosis and Management of Herb-Induced Liver Injury. Chin J Integr Med 2018;24:696–706. 10.1007/s11655-018-3000-8 [DOI] [PubMed] [Google Scholar]
- 27. Xiao X, Tang J, Mao Y, et al. Guidance for the clinical evaluation of traditional Chinese medicine-induced liver injury. Acta Pharm Sin B 2018. 10.1016/j.apsb.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kleiner DE, Chalasani NP, Lee WM, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology 2014;59:661–70. 10.1002/hep.26709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olsson S, Pal SN, Dodoo A. Pharmacovigilance in resource-limited countries. Expert Rev Clin Pharmacol 2015;8:449–60. 10.1586/17512433.2015.1053391 [DOI] [PubMed] [Google Scholar]
- 30. Nguyen GC, Sam J, Thuluvath PJ. Hepatitis C is a predictor of acute liver injury among hospitalizations for acetaminophen overdose in the United States: a nationwide analysis. Hepatology 2008;48:1336–41. 10.1002/hep.22536 [DOI] [PubMed] [Google Scholar]
- 31. Robles-Diaz M, Lucena MI, Kaplowitz N, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology 2014;147:109–18. 10.1053/j.gastro.2014.03.050 [DOI] [PubMed] [Google Scholar]
- 32. Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med 2012;7(S3):193–9. 10.1007/s11739-012-0802-0 [DOI] [PubMed] [Google Scholar]
- 33. Eisenbach C, Sieg O, Stremmel W, et al. Diagnostic criteria for acute liver failure due to Wilson disease. World J Gastroenterol 2007;13:1711–4. 10.3748/wjg.v13.i11.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Núñez M. Clinical syndromes and consequences of antiretroviral-related hepatotoxicity. Hepatology 2010;52:1143–55. 10.1002/hep.23716 [DOI] [PubMed] [Google Scholar]
- 35. Teschke R, Danan G. Drug-induced liver injury: Is chronic liver disease a risk factor and a clinical issue? Expert Opin Drug Metab Toxicol 2017;13:425–38. 10.1080/17425255.2017.1252749 [DOI] [PubMed] [Google Scholar]
- 36. Philips CA, Paramaguru R, Joy AK, et al. Clinical outcomes, histopathological patterns, and chemical analysis of Ayurveda and herbal medicine associated with severe liver injury-A single-center experience from southern India. Indian J Gastroenterol 2018;37:9–17. 10.1007/s12664-017-0815-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-023567supp001.pdf (654.4KB, pdf)