Abstract
Objective:
High-frequency pulsed electromagnetic field (PEMF) stimulation is an emerging noninvasive therapy that we have shown increases cerebral blood flow (CBF) and tissue oxygenation in the healthy rat brain. In this work we have tested the effect of PEMF on the brain at high intracranial pressure (ICP). We previously showed that high ICP in rats caused a transition from capillary (CAP) to non-nutritive microvascular shunt (MVS) flow, tissue hypoxia and increased blood brain barrier (BBB) permeability.
Methods:
Using in vivo 2-photon laser scanning microscopy (2PLSM) over the rat parietal cortex, we studied the effects of PEMF on microvascular blood flow velocity, tissue oxygenation (NADH autofluorescence), BBB permeability and neuronal necrosis during 4 hours of elevated ICP to 30 mmHg.
Results:
PEMF significantly dilated arterioles, increased capillary blood flow velocity and reduced MVS/capillary ratio compared to sham treated animals. These effects led to a significant decrease in tissue hypoxia, BBB degradation and neuronal necrosis.
Conclusions:
PEMF attenuates high ICP-induced pathological microcirculatory changes, tissue hypoxia, BBB degradation and neuronal necrosis.
Keywords: Cerebral blood flow, High Intracranial pressure, Microvascular shunts, Pulsed Electromagnetic Field, Rats
Introduction
High intracranial pressure (ICP), is a serious consequence of severe brain injury that often leads to cerebral ischemia, cerebral edema, tissue compression, herniation, restricting blood supply to the entire brain, and finally, brain death. Current treatment paradigms for high ICP are initiated in tiers each focused on reducing ICP to prevent secondary injury without clinically proven neuroprotective strategies. High-frequency pulsed electromagnetic field (PEMF) stimulation is an emerging noninvasive therapy that induces small electrical currents in tissue. It has been shown that PEMF has anti-inflammatory effect in traumatized brain and have been suggested as adjunctive treatment in brain disorders [1]. We recently demonstrated that PEMF exposure in the healthy rat brain induces vasodilation, increases microvascular blood flow velocity and tissue oxygenation [2]. In previous works we have also shown for the first time, in a rat brain, that high ICP induces a transition from low velocity capillary flow to high velocity non-nutritive microvascular shunt flow (MVS) resulting in tissue hypoxia, brain edema, blood brain barrier damage and neuronal death [3-4]. Here, we determined whether PEMF could mitigate pathological consequences caused by non-nutritive MVS flow induced by high ICP.
2. Materials and Methods
The institutional animal care and use committee of the University of New Mexico Health Sciences Center approved the protocol for these studies, which were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Most of the procedures used in this study have been previously described [2, 3].
Experimental Paradigm
Using in vivo 2-photon laser scanning microscopy (2PLSM) over the rat parietal cortex, we studied the effects of PEMF on microvascular red blood cell flow velocity visualized by serum labeled with tetra-methylrhodamine dextran (TMR), tissue oxygenation (NADH autofluorescence), BBB permeability (TMR extravasation) and neuronal necrosis (i.v. Propidium Iodide) during 4 hours of elevated ICP. ICP and arterial pressure, rectal and cranial temperatures, blood gases and electrolytes were monitored. After baseline imaging at normal ICP (10 mmHg), rats were subjected to high ICP (30 mmHg) by raising an artificial cerebrospinal fluid reservoir connected to a catheter in the cisterna magna. At ICP of 30 mmHg, PEMF was applied for 30 min using the SofPulse device and imaging continuously performed for up to 4 hours after the treatment (10 rats). The PEMF signal was a 27.12-MHz carrier modulated by a 3-msec burst repeating at 5 Hz. The signal amplitude was adjusted to provide 6 ± 1 V/m within the rat brain. Controls were treated with sham PEMF (10 rats).
Surgery
Acclimated Sprague–Dawley male rats (Harlan Laboratories, Indianapolis, IN, USA), weighing between 300 and 350 g, were intubated and mechanically ventilated on 2 % isoflurane/30 % oxygen/70 % nitrous oxide. Rectal and temporal muscle temperature thermistors were inserted. Femoral venous and arterial catheters were inserted for injections, arterial pressure monitoring, and blood sampling. A catheter was inserted into the cisterna magna for ICP monitoring and manipulation. For imaging a craniotomy 5 mm in diameter was made over the left parietal cortex, filled with 2 % agarose/saline, and sealed with a cover glass.
Microscopy
An Olympus BX51WI upright microscope and a water-immersion LUMPlan FL/IR 20×/0.50 W objective were used. Excitation (740 nm) was provided by a Prairie View Ultima multiphoton laser scan unit powered by a Millennia Prime 10 W diode laser source pumping a Tsunami Ti: sapphire laser (Spectra-Physics, Mountain View, CA, USA). Blood plasma was labeled by i.v. injection of tetramethylrhodamine isothiocyanate dextran (155 kDa) in physiological saline (5 % wt/ vol). All microvessels in an imaging volume (500 × 500 × 300 μm) were scanned at each study point, measuring the diameter and blood flow velocity in each vessel (3–20 μm Ø). Tetramethylrhodamine fluorescence was band pass filtered at 560–600 nm and NADH autofluorescence at 425–475 nm. Imaging data processing and analysis were carried out using the NIH ImageJ processing package.
Statistical Analyses
Statistical analyses were carried out using Student’s t test or the Kolmogorov – Smirnov test where appropriate. Differences between groups were determined using two-way analysis of variance (ANOVA) for multiple comparisons and post hoc testing using the Mann-Whitney U test. The statistical significance level was set at P < 0.05. Data are presented as mean ± SEM.
3. Results
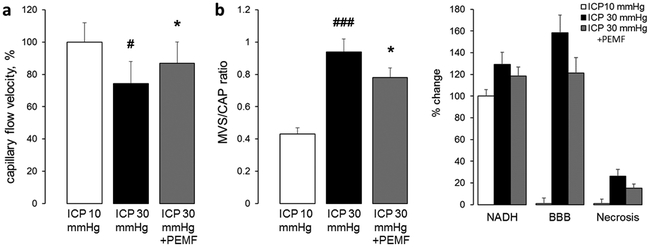
As in our previous studies, increase in ICP to 30 mmHg in sham-stimulated group caused a redistribution of blood flow from normal capillary flow to MVS flow with an increase in the MVS/CAP ratio from 0.43 ± 0.04 to 0.94 ± 0.08, p<.001 from baseline (Fig. 1b). Red blood cells flow velocities in capillaries decreased to 74.2 ± 13.8, p<0.05 from baseline (Fig. 1a). Pathological changes in cerebral microcirculation led to reduction of tissue oxygenation as reflected by increase in NADH autofluorescence to 129.1 ± 11.2%, p<0.01 from baseline; and BBB damage as reflected by increase of perivascular fluorescence due TMR extravasation to 158.2 ± 16.3, p<.001 (Fig. 1c). At the end of monitoring, 4 hours after ICP increase, 26.1 ± 6.2% of neurons died by necrotic mechanism as reflected by propidium iodide fluorescence in neuronal nuclei, p<0.001 (Fig. 1c); i.v. injected propidium iodide become fluorescent after binding to nucleic acids but as a cell membrane impermeable molecule labels only necrotic cells with damaged membranes [5].
Figure 1.
High frequency pulsed electromagnetic field stimulation enhances capillary flow velocity (a); reduces microvascular shunt/capillary flow ratio (b); and attenuates tissue hypoxia, blood brain barrier damage and necrosis of neurons caused by four hours of intracranial hypertension (ICP=30 mmHg). Data are presented as mean ± SEM, n=10 rats per group, #p<0.05, ##p<0.01, ###p<0.001 from a baseline of ICP=10 mmHg, *p<0.05, **p<0.01 from sham-treated group.
PEMF treatment dilated arterioles by 4.5 ± 3.2%, p<0.05 from sham-treated group. The increased blood volume perfused through arterioles elevated blood flow velocities in capillaries to 86.8 ± 13.2%, p<0.05 (Fig. 1a). As a result, MVS/CAP ratio was lower than in sham-treated group (0.78 ± 0.06, p<0.05, Fig. 1b). These were associated with decreased tissue hypoxia as reflected by a lower NADH autofluorescence (118.3 ± 8.4%, p<0.05) and decreased BBB permeability as reflected by reduced dye extravasation (121.1 ± 14.2%, p<0.01c). PEMF reduced neuronal necrosis to 15.2 ± 3.6%, p<0.05 compared to sham treated group (Fig. 1c).
4. Discussion
Our results show that PEMF reduces tissue hypoxia, BBB degradation and neuronal necrosis at high ICP by increasing cerebral microvascular perfusion via reducing MVS flow, increasing flow through capillaries as a result of dilatation of arterioles which we have shown occurs by nitric oxide dependent mechanism [1].
5. Conclusion
PEMF attenuates high ICP-induced pathological microcirculatory changes, tissue hypoxia, BBB degradation and neuronal necrosis and has a potential as an effective supportive therapy.
Acknowledgments
This work was supported by Rio Grande Neurosciences and National Institutes for Health P20GM109089. We thank Anthony Gravagne from the Department of Physics and Astronomy, University of New Mexico, for designing and manufacturing the non-magnetic plastic stereotactic head frame for imaging.
Footnotes
Conflict of Interest Statement We declare that we have no conflict of interest.
6 References
- 1.Rasouli J, Lekhraj R, White NM, Flamm ES, Pilla AA, Strauch B, Casper D. Attenuation of interleukin-1beta by pulsed electromagnetic fields after traumatic brain injury. Neurosci Lett. 2012. June 21;519(1):4–8. [DOI] [PubMed] [Google Scholar]
- 2.Bragin DE, Statom GL, Hagberg S, Nemoto EM. Increases in microvascular perfusion and tissue oxygenation via pulsed electromagnetic fields in the healthy rat brain. J Neurosurg. 2015. May;122(5):1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bragin DE, Bush RC, Müller WS, Nemoto EM. High intracranial pressure effects on cerebral cortical microvascular flow in rats. J Neurotrauma. 2011. May;28(5):775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai X, Bragina O, Zhang T, Yang Y, Rao GR, Bragin DE, Statom G, Nemoto EM. High Intracranial Pressure Induced Injury in the Healthy Rat Brain. Crit Care Med. 2016. August;44(8):e633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fumagalli S, Coles JA, Ejlerskov P, Ortolano F, Bushell TJ, Brewer JM, De Simoni MG, Dever G, Garside P, Maffia P, Carswell HV. In vivo real-time multiphoton imaging of T lymphocytes in the mouse brain after experimental stroke. Stroke. 2011. May;42(5):1429–36. [DOI] [PubMed] [Google Scholar]