Abstract
OBJECTIVES
Both chronic obstructive pulmonary disease (COPD) and diabetes mellitus (DM) are highly prevalent in Trinidad, West Indies. Our objective was to evaluate the prevalence of DM in a cohort of Trinidadian patients with COPD and investigate the possible impact of diabetes on COPD using standard outcome measures, that is, lung function, exacerbations, quality of life and depression questionnaires, as well as mortality.
MATERIALS AND METHODS
This was a cross-sectional follow-up study utilizing a cohort of 105 patients from chest clinics in the three major general hospitals in Trinidad.
RESULTS
Diabetes was diagnosed based on a glycated hemoglobin (HbA1c) level of ≥6.5% (or a prior self-reported history), and for pre-diabetes, of 5.7%–6.4%. Of 105 patients, 40% fulfilled the criteria for diabetes and 40% for pre-diabetes. Of those diagnosed with diabetes, 38% obtained this diagnosis de novo. A history of intravenous corticosteroid use was associated with higher HbA1c levels (p=0.043) upon diagnosis. The percentage of predicted forced vital capacity was negatively related to HbA1c (p=0.033), but those with diabetes also had a greater body mass index (p=0.001). After a 1-year follow-up, mortality was significantly greater among patients with diabetes (p=0.026). Patients with at least one exacerbation in the past year or poorer lung function parameters had worse quality of life (p≤0.040) and depression (p≤0.018) scores. Notably, 31.4% of the total cohort exhibited clinically significant depression scores.
CONCLUSION
This study revealed that a high proportion of COPD patients in tertiary care had diabetes or pre-diabetes.
Keywords: COPD, depression, diabetes mellitus, glycated hemoglobin A1c, mortality
INTRODUCTION
According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease characterized by persistent respiratory symptoms and airflow limitation due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases [1]. It is currently the fourth leading cause of death and the fifth leading cause of disability globally [2]. Prior studies in hospitals and clinics in Trinidad have reported a COPD prevalence of approximately 21% [3,4]. A similar prevalence has been reported for type 2 diabetes mellitus (DM) in this population [5]. The interaction between these two quite common conditions in Trinidad and Tobago, to the best of our knowledge, has not been previously studied.
Although persistent airflow limitation is the defining feature of COPD, it is a complex, heterogeneous, and multi-morbid condition [6], with a typical COPD patient likely to report having four or more comorbidities [7]. In the National Health and Nutrition Examination Survey 2001–2008 [8], DM was identified as the third most influential comorbidity on self-rated health among COPD patients. Furthermore, comorbid diabetes has been found to increase the risk of hospitalization and mortality in patients with COPD in the United States and Australia [9].
European and North American studies have found that DM is more common in COPD patients [10,11] with an odds ratio of up to 2.0 for diabetes in COPD [10]. It is possible that DM may develop among patients with COPD as a result of chronic inflammation, hypoxia, or concomitant corticosteroid treatment or that they may both share a common antecedent in the form of cigarette smoking.
This study aimed to define the prevalence of diabetes in a cohort of Trinidadian subjects with COPD and investigate whether it impacted outcome measures related to COPD, including lung function, exacerbations, quality of life and depression scales, and mortality.
MATERIALS AND METHODS
Study Design and Sample
This was a cross-sectional, follow-up study, and so it utilized an antecedent cohort of patients (n=105) [12,13], but due to attrition, only 58 were recruited of which 2 were excluded (each having a total hemoglobin of <7 g/dL). Thus, 49 new participants were sought via convenience sampling. All of our final 105 participants originated from the chest clinics in the three major general hospitals in Trinidad (Port-of-Spain, Mount Hope, and San Fernando).
Authors declare that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (amended in October 2013). Approvals were obtained from the ethics committee of the Faculty of Medical Sciences, University of the West Indies, St. Augustine. Written informed consent was obtained from all patients who participated in this study.
COPD Diagnosis and Spirometry
All participants had a clinical diagnosis of COPD, which was confirmed using spirometry, and were aged ≥40 years. Patients with a post-bronchodilator ratio of the forced expiratory volume in 1 second (FEV1) to the forced vital capacity (FVC) of ≥0.70 [1], contraindications to spirometry [14], and patients who had known severe anemias, acute blood losses, recent transfusions, or pregnancy [15] were excluded from the study. Also, patients whose total hemoglobin was <7 g/dL were excluded as it would have rendered subsequent testing of the glycated hemoglobin level to be inaccurate.
Demographic and anthropometric data including height and weight were measured and recorded. Patients with a body mass index (BMI) <21 kg/m2 were considered as cachectic [16]. Post-bronchodilator spirometry was performed using a hand-held turbine spirometer (Micro Spirometer Cat No: MS01 Micro Medical Limited, Rochester, Kent, United Kingdom) after administration of 300 μg of aerosolized salbutamol [14]. This was not technically possible in two patients who were unable to complete the FVC maneuver. Predicted FEV1 and FVC were calculated using the Global Lung Initiative 2012 Excel sheet calculator [17]. The percentage of predicted FEV1 values were subsequently used to classify the severity of airflow limitation in our patients according to the GOLD stages [1].
Diabetes Diagnosis
The glycated hemoglobin (HbA1c) level was tested on a finger-prick capillary blood sample using a digital communication analyzer (DCA) Vantage Analyzer (Siemens Healthcare Diagnostics Ltd., Frimley, Camberley, United Kingdom). Patients were classed as having DM if they gave a positive medical history and/or were on treatment for such, or if independently their HbA1c was ≥6.5% in keeping with the American Diabetes Association criteria [15]. According to the same criteria, individuals with glucose levels above normal but not yet in the diabetic range are recognized as having pre-diabetes and being at increased risk for developing DM [15]. Patients with an HbA1c ranging between 5.7% and 6.4% were classed as having pre-diabetes and those with an HbA1c of <5.7% as normal [15]. All individuals who had an HbA1c of ≥5.7% and were not known to be diabetic or on treatment for diabetes were given a referral letter to an appropriate diabetic service.
Questionnaires
Patients were classified as smokers if they smoked a minimum of 100 cigarettes in their lifetime and otherwise as never smokers [18]. Patients were asked about the number of exacerbations in the past year requiring hospitalizations and their inhaled, oral, and intravenous corticosteroid usage in the past 3 to 4 months. Patients who used inhaled corticosteroids were found to have used a combination of long-acting beta2-agonist and corticosteroid inhalers (e.g., formoterol/budesonide or salmeterol/fluticasone propionate inhalers) alone or together with additional corticosteroid inhalers (e.g., fluticasone propionate or beclomethasone dipropionate inhalers). Two questionnaires on the quality of life were administered: St. George’s Respiratory Questionnaire (SGRQ) [19] and COPD Assessment Test (CAT) [20] as well as two on depression: Center for Epidemiologic Studies Depression Scale (CES-D) [21] and Center for Epidemiologic Studies Depression Scale-Revised (CESD-R) [22] after the necessary permissions were obtained. For all questionnaires, increasing scores indicated worsening parameters, and for this analysis, clinically significant depression was defined as a score of ≥16 on the CES-D and CESD-R scales [23, 24]. Of the total studied population (n=105), we were able to collect 1-year mortality data on 89 participants.
Statistical Analysis
Statistical analyses were performed using the IBM Statistical Package for the Social Sciences Statistics Version 20 (IBM SPSS Statistics Corp.; Armonk, NY, USA). Variables were expressed as mean (standard deviation, SD), median (interquartile range, IQR), or number (n, %) as appropriate. The chi-squared or Kruskal-Wallis tests were used to test the differences in discrete variables. Whereas for continuous variables, the t-test or Mann-Whitney U test were used. Spearman’s (Spearman’s rho, rs) or Pearson’s (Pearson’s r) correlations were employed in comparisons of variables as appropriate. Associations were considered significant at the 5% level.
RESULTS
From our study population of 105 COPD patients, 83.8% were male, and the mean (SD) age was 67.4 (11.0) years (Table 1). Patients were primarily of Indo-Trinidadian (52.4%) and Afro-Trinidadian (38.1%) ethnicities, and the median (IQR) BMI was 25.4 (22.1, 28.7) kg/m2. A significant number of the participants never smoked (22.3%). The majority of patients were of the GOLD Stage 2 COPD severity (50.0%) with 80.8% being GOLD Stage 2 or greater.
Table 1.
Demographic and anthropometric data and outcome measures for all subjects and for non-diabetics and diabetics individually
| Variables | Total (n=105) | Non-diabetes (n=63) | Diabetes (n=42) | ap |
|---|---|---|---|---|
| Mean (SD) age, years | 67.4 (11.0) | 66.5 (12.3) | 68.7 (8.7) | 0.314h |
| Male sex (n (%)) | 88 (83.8) | 51 (81.0) | 37 (88.1) | 0.330f |
| bEthnicity (n (%)) | 0.213g | |||
| Afro-Trinidadian | 40 (38.1) | 29 (46.0) | 11 (26.2) | |
| Indo-Trinidadian | 55 (52.4) | 26 (41.3) | 29 (69.0) | |
| Caucasian | 3 (2.9) | 2 (3.2) | 1 (2.4) | |
| Mixed | 7 (6.7) | 6 (9.5) | 1 (2.4) | |
| Mean (SD) height, cm | 164.3 (8.2) | 165.0 (8.5) | 163.3 (7.6) | 0.296h |
| Median (IQR) weight, kg | 69.4 (60.0,77.9) | 66.0 (58.5,75.3) | 72.2 (64.0,83.8) | 0.016i |
| Median (IQR) BMI, kg/m2 | 25.4 (22.1,28.7) | 24.2 (21.2,27.2) | 27.3 (24.1,30.4) | 0.001i |
| Cachexia (BMI <21 kg/m2) (n (%)) | 15 (14.7) | 13 (21.0) | 2 (5.0) | 0.026f |
| Lung function parameters | ||||
| FEV1, L (mean (SD)) | 1.47 (0.61) | 1.50 (0.63) | 1.44 (0.60) | 0.654h |
| c FEV1 % predicted (mean (SD)) | 59.8 (22.0) | 60.3 (22.7) | 59.0 (21.1) | 0.766h |
| FVC, L (mean (SD)) | 2.59 (0.87) | 2.65 (0.85) | 2.50 (0.92) | 0.421h |
| cFVC % predicted (mean (SD)) | 81.7 (23.4) | 83.3 (22.5) | 79.2 (24.7) | 0.385h |
| FEV1/FVC, % (median (IQR)) | 59.9 (47.6,66.3) | 59.8 (45.7,66.3) | 59.9 (51.1,66.1) | 0.661i |
| PEFR, L/min (median (IQR)) | 209 (134,294) | 215 (131,289) | 208 (141,303) | 0.725i |
| GOLD stages (n (%)) | 0.770g | |||
| Stage 1 (FEV1≥80% predicted) | 20 (19.2) | 11 (17.7) | 9 (21.4) | |
| Stage 2 (50% ≥80% predicted) | 52 (50.0) | 32 (51.6) | 20 (47.6) | |
| Stage 3 (30% ≥50% predicted) | 18 (17.3) | 10 (16.1) | 8 (19.0) | |
| Stage 4 (FEV1 <30% predicted) | 14 (13.5) | 9 (14.5) | 5 (11.9) | |
| HbA1c, % (median (IQR)) | 6.1 (5.7,6.7) | 5.8 (5.5,6.0) | 6.8 (6.5,7.6) | <0.001i |
| At least one exacerbation in past year (n (%)) | 33 (31.4) | 19 (30.2) | 14 (33.3) | 0.731f |
| Smoking history (n (%)) | 0.985g | |||
| Never smoked | 23 (22.3) | 13 (21.0) | 10 (24.4) | |
| Quit >1 year ago | 62 (60.2) | 39 (62.9) | 23 (56.1) | |
| Current smoker or quit ≤1 year ago | 18 (17.5) | 10 (16.1) | 8 (19.5) | |
| dSmoking pack-years (median (IQR)) | 37.5 (10.0,62.5) | 35.5 (12.5,60.0) | 40.0 (4.3,69.5) | 0.658i |
| eCorticosteroid use (n (%)) | ||||
| Inhaled | 73 (70.2) | 42 (66.7) | 31 (75.6) | 0.330f |
| Oral | 10 (9.6) | 5 (7.9) | 5 (12.2) | 0.472f |
| Intravenous | 2 (1.9) | 0 (0.0) | 2 (4.9) | 0.077f |
| SGRQ scores (mean (SD)) | ||||
| Symptoms | 44.42 (22.67) | 43.09 (23.40) | 46.42 (21.64) | 0.464h |
| Activity | 60.81 (28.35) | 59.68 (29.60) | 62.52 (26.62) | 0.617h |
| Impacts | 34.40 (20.88) | 33.61 (21.66) | 35.57 (19.84) | 0.640h |
| Total | 44.09 (21.10) | 43.12 (22.14) | 45.55 (19.62) | 0.566h |
| CAT score (mean (SD)) | 16.0 (8.8) | 15.5 (9.4) | 16.7 (7.8) | 0.499h |
| CES-D score (median (IQR)) | 7.5 (2.0,16.0) | 6.5 (2.0,16.3) | 8.0 (3.0,16.5) | 0.426i |
| CESD-R score (median (IQR)) | 7.0 (3.0,19.0) | 7.0 (2.0,20.0) | 9.0 (4.0,18.3) | 0.393i |
| Clinically significant CES-D score (i.e., ≥16) (n (%)) | 27 (26.0) | 16 (25.8) | 11 (26.2) | 0.965f |
| Clinically significant CESD-R score (i.e., ≥16) (n (%)) | 33 (31.4) | 20 (31.7) | 13 (31.0) | 0.932f |
| 1-year mortality (n (%)) | 6 (6.9) | 1 (1.9) | 5 (14.3) | 0.026f |
comparing non-diabetes and diabetes;
based on patient’s perception;
calculated using the Global Lungs Initiative 2012 Excel sheet calculator;
one pack-year is defined as smoking 20 cigarettes daily for 1 year;
in past 3 to 4 months;
Chi-squared test;
Kruskal–Wallis test;
t-test;
Mann-Whitney U test;
BMI: body mass index; CAT: COPD assessment test; SD: standard deviation; CES-D: center for epidemiologic studies depression scale; CESD-R: center for epidemiologic studies depression scale-revised; SGRQ: St. George’s Respiratory Questionnaire
Non-diabetes includes normal and pre-diabetes, and diabetes includes a prior medical history and newly diagnosed diabetes
Figure 1 shows that of 105 patients, 42 had diabetes, of which up to 38% were newly diagnosed, having an HbA1c in the diabetic range. A further 40.0% of the total cohort was pre-diabetic, and 20.0% was normal. As shown in Table 1, subjects with DM had a significantly greater median (IQR) BMI (27.3 (24.1, 30.4)) than non-diabetics (24.2 (21.2, 27.2)) (p=0.001). HbA1c levels increased with body weight (rs=0.222, p=0.025) and BMI (rs=0.296, p=0.003). Cachexia was noted more frequently among non-diabetics (p=0.026). The use of intravenous corticosteroids in the past 3 to 4 months was associated with an increased HbA1c (rs=0.199, p=0.043). Among the patients who used conventional long-acting beta2-agonist and corticosteroid combination inhalers (n=73), those who used additional corticosteroid inhalers (n=4) had increased HbA1c levels (rs=0.261, p=0.007), and diabetes was more prevalent among them (rs=0.248, p=0.011). A negative association was found between the FVC percentage predicted and HbA1c (Pearson’s r=−0.210, p=0.033).
Figure 1.
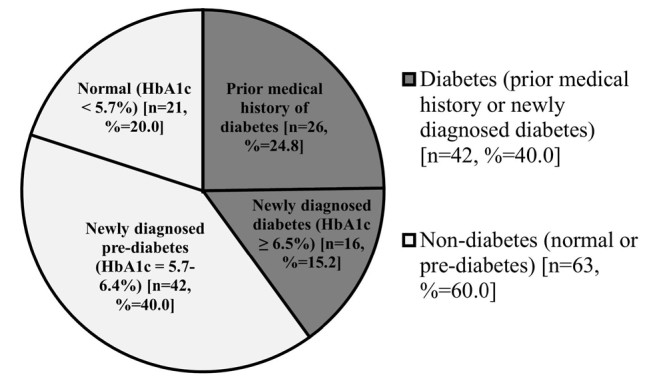
Graph showing the prevalence of diabetes in COPD patients
HbA1c: glycated haemoglobin; COPD: chronic obstructive pulmonary disease
There were no significant differences observed between diabetics and non-diabetics in lung function parameters, GOLD stages, history of COPD exacerbations, smoking history and pack-years of smoking, corticosteroid use, and CAT, SGRQ, CES-D, and CESD-R scores. However, the number of deaths after 1 year among those with diabetes (n=5) was significantly (p=0.026) greater than among non-diabetics (n=1).
Lung Function
FEV1, FVC, and the peak expiratory flow rate decreased with increasing age (p≤0.007). The CES-D and CESD-R scores increased with decreasing lung function parameters (p≤0.015) and increasing GOLD stages (0.242≤rs≤0.267, p≤0.014). There were significant correlations between the SGRQ total and CAT scores, and all lung function parameters (−0.475≤rs≤−0.278, p≤0.004) and GOLD stages (0.362≤rs≤0.430, p<0.001). The individual SGRQ scores also correlated well with all lung function parameters and GOLD stages (p≤0.036).
Exacerbations
The occurrence of at least one exacerbation in the past year was associated with a history of oral corticosteroid use (rs=0.198, p=0.044) along with increased dosages of inhaled corticosteroids per day (rs=0.208, p=0.035). There were also significant associations between patients with at least one exacerbation in the past year and CAT (rs=0.350, p<0.001), SGRQ symptoms (rs=0.435, p<0.001), SGRQ activity (rs=0.201, p=0.040), SGRQ impacts (rs=0.353, p<0.001), SGRQ total (rs=0.338, p<0.001), CES-D (rs=.232, p=0.018), and CESD-R (rs=0.256, p=0.008) scores.
Quality of Life and Depression
All SGRQ and CAT scores were positively associated with inhaled corticosteroid use (0.198≤rs≤0.329, p≤0.044) and the dosage (0.188≤rs≤0.236, p<0.05). All SGRQ and CAT scores were positively (p<.001 for all) related to CES-D (0.554≤rs≤0.705 for SGRQ, rs=0.664 for CAT) and CESD-R (0.629≤rs≤0.792 for SGRQ, rs=0.719 for CAT) scores. As expected, CAT scores correlated well (p<0.001) to the SGRQ total (rs=0.820) and component scores. Notably, CES-D and CESD-R scores correlated well together (rs=0.925, p<0.001). From Table 1, it can be seen that 26.0% and 31.4% of the cohort of patients had clinically significant depression scores as assessed by the CES-D and CESD-R, respectively.
DISCUSSION
The prevalence of diabetes and pre-diabetes in our COPD cohort (n=105) was 40% each, leaving only 20% with normal glucose tolerance as assessed by HbA1c. We found HbA1c to be associated with the use of intravenous corticosteroids, increased BMI, and a decreased FVC percentage predicted. Patients with at least one COPD exacerbation in the past year or those with reduced lung function were more likely to have a worse quality of life and higher depression scores. Overall, 31.4% of our cohort had clinically significant depression scores. Also, after a 1-year follow-up, diabetics in our cohort had a significantly greater mortality than non-diabetics.
Comparison with Existing Literature
The high prevalence of 40% diabetes among COPD patients in our study differs markedly from the 12.7% reported by Mannino et al. [9] in the United States. Mannino et al. [9] dealt with participants from the general community, whereas our study looked at patients in hospital clinics. Also, they included patients with respiratory symptoms, but without spirometric abnormality. In fact, only 12.8% of patients in that study were GOLD Stage 2 or higher, compared to 80.8% in our COPD cohort. In another US study, in which over half of the study population had what was defined as moderate to high complexity disease (complexity of the illness being used as a proxy for COPD GOLD stage severity), diabetes prevalence was found to be comparatively higher at 21.9%–28.8% (commercial and Medicare dataset populations respectively) [10]. In a UK study, Feary et al. [11] analyzed primary care records and found that 12.2% of patients with a diagnosis of COPD also had a diagnosis of DM or were on treatment for such. They also found that COPD patients have a two-fold higher risk of having new-onset DM. This is consistent with our finding of 40% DM among our COPD cohort when compared with that of 20.5% reported previously in the general population of Trinidad and Tobago aged 16–64 years [5].
Additionally, the high prevalence of DM could be attributed to the high mean (SD) age of our cohorts, 67.4 (11.0) years. Hennis et al. [25] observed a higher incidence of DM with increasing age, while the prevalence of DM in citizens of Trinidad and Tobago aged 55–64 years was 27.6%, greater than the mean percentage for the entire Trinidadian population mentioned above [5]. This is possibly due to declining glucose tolerance caused by peripheral insulin resistance [26] along with an increase in visceral fat [27], and it is visceral fat that may be the likely cause of the relatively higher BMI among our diabetics. Interestingly, we found a significantly greater number of cachectic patients among non-diabetics than diabetics. Still, diabetics and pre-diabetics, both of which are closely associated with obesity, comprised a large proportion of our COPD cohort, and this may explain the relatively lower prevalence of cachexia of 14.7% compared with 20%–40% reported in the existing literature [16].
In our study, we found that all patients who used intravenous corticosteroids within the past 3 to 4 months exhibited abnormal HbA1c levels in the diabetic range, that is >6.5%. Whether this association is cause or effect could not be established in this cross-sectional study. Rogliani et al. [28] recently found an association between the use of inhaled corticosteroids and the presence of diabetes in COPD, but that the use of a combination corticosteroid and beta2-agonist inhaler reduced this. In our study, increased HbA1c levels and diabetes prevalence were seen among patients who used additional inhaled corticosteroids along with the combination long-acting beta2-agonist and steroid inhalers.
There was a negative correlation between the HbA1c and FVC percentage predicted, with diabetics having a mean (SD) FVC percentage predicted of 79.2% (24.7%). This suggests a mild restrictive defect being associated with DM and increased HbA1c, consistent with the previous literature [29]. This restrictive disposition may be because of our diabetics’ higher BMI, but low-grade systemic inflammation or hyperglycemia-induced diabetic microangiopathy may also be involved [29].
We found that patients with at least one COPD exacerbation in the past year or those with reduced lung function were more likely to have a worse quality of life and higher depression scores. These results are consistent with the literature [23,30,31], and in fact, a more recent study by Yohannes et al. [24] found that patients with depression experienced more exacerbations. Depression is well known to be more prevalent in COPD, and the prevalence of depression in our COPD cohort was found to be 31.4%, compared with 14% in a community sample from the Trinidadian population [32]. Furthermore, previous studies have found similar prevalences of 21.6%–38% of depression in COPD patients [23,24]. Also, depression has been found to be associated with increased 3-year but not 1-year mortality [33].
In keeping with the findings of prior studies that comorbid DM is associated with a higher risk of death [9] the 1-year mortality among diabetics in this study was significantly higher (p=0.026) than among non-diabetics.
Strengths and Limitations
This study is as far as we know one of the first in the West Indies, a region with a high background prevalence of type 2 diabetes, to objectively evaluate the prevalence of diabetes in a COPD population and to demonstrate not only the doubled risk of diabetes, but also a heightened risk of mortality. Interestingly, up to 38% of our diabetics were diagnosed incidentally, and among those who were non-diabetic, 67% had pre-diabetes. Previous studies on COPD and DM have not reported prevalence rates for pre-diabetes as here reported. Although an American Diabetes Association expert panel report highlighted the higher rates of progression of pre-diabetic individuals (up to 70%) toward diabetes [33], many studies on COPD comorbidities did not focus on its prevalence.
To the best of our knowledge, this study is also one of the first in the West Indies to utilize the CESD-R and furthermore, confirm its strong correlation (p<0.001) to its predecessor, the CES-D.
In this study, a point-of-care HbA1c assay (Siemens DCA Vantage Analyzer) was used, although we note that the American Diabetes Association does not recommend its use for diagnosis [15]. Leca et al. [34] found that point-of-care assays under-evaluated HbA1c levels as compared to the central laboratory, and as such, we may have underestimated the prevalence of DM. Also, we note that of all the point-of-care HbA1c assays available, the Siemens DCA Vantage Analyzer has the best sensitivity and specificity for diagnosis [35]. Lastly, the Global Lung Initiative equations may not have adequately covered Trinidadian ethnic groups [17]. Finally, the presence of multiple comorbidities was not studied, and they might have influenced some of the functional and mortality outcomes in our study population.
In conclusion, we observed a 40% prevalence of diabetes in this COPD cohort. From our findings, whereas the co-existence of DM does not appear to affect the functional measures of COPD significantly including FEV1, exacerbation frequency, quality of life, and depression, there is a notable increase in 1-year mortality. It is hoped that this study highlights the necessity of screening for diabetes in COPD patients as 38% of our diabetic patients were newly diagnosed, and its presence impacts on 1-year mortality. We recommend further studies to confirm these findings and also to explore the influence of DM on COPD prognosis and mortality.
Acknowledgements
We would like to thank Mr Sanjiva Singh for assistance with the HbA1c assays and the staff and patients of the Port-of-Spain, Mount Hope and San Fernando chest clinics.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Faculty of Medical Sciences, The University of the West Indies, St Augustine Campus and the North-West and North-Central Regional Health Authorities, Trinidad and Tobago.
Informed Consent: Written informed consent was obtained from all patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.S.; Design - T.S.; Supervision - T.S., S.M., S.S.; Resource - T.S., Su.Te., G.D.; Materials - T.S., Su.Te., G.D., S.S., S.M.; Data Collection and/or Processing - K.R., B.B., A.A., F.A., Sh.To., R.L., S.B., S.G.; Analysis and/or Interpretation - T.S., K.R., B.B.; Literature Search - S.S., K.R., B.B., A.A., F.A., Sh.To., R.L., S.B., S.G.; Writing - K.R., S.S., T.S., Su.Te., B.B., A.A., F.A., Sh.To., R.L., S.B., S.G., S.M., G.D.; Critical Reviews - T.S., Su.Te., S.S.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Global strategy for the diagnosis, management, and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017. (cited 2017 December 5). Available from: URL: http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf.
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seemungal T, Harrinarine R, Rios M, et al. Obstructive lung disease in acute medical patients. West Indian Med J. 2008;57:7–13. [PubMed] [Google Scholar]
- 4.Thorington P, Rios M, Avila G, et al. Prevalence of chronic obstructive pulmonary disease among stable chronic disease subjects in primary care in Trinidad, West Indies. J Thorac Dis. 2011;3:177–82. doi: 10.3978/j.issn.2072-1439.2011.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health, Government of the Republic of Trinidad and Tobago. Trinidad and Tobago chronic non-communicable disease risk factor survey (Pan American STEPS) 2012. (Cited 2017 October 7). Available from: URL: http://www.who.int/chp/steps/TrinidadAndTobago_2011_STEPS_Report.pdf.
- 6.Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–35. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 8.Putcha N, Puhan MA, Hansel NN, et al. Impact of co-morbidities on self-rated health in self-reported COPD: an analysis of NHANES 2001–2008. COPD. 2013;10:324–32. doi: 10.3109/15412555.2012.744963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32:962–9. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 10.Mapel DW, Dutro MP, Marton JP, et al. Identifying and characterizing COPD patients in US managed care. A retrospective, cross-sectional analysis of administrative claims data. BMC Health Serv Res. 2011;11:43. doi: 10.1186/1472-6963-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feary JR, Rodrigues LC, Smith CJ, et al. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65:956–62. doi: 10.1136/thx.2009.128082. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed S, Mohammed H, Donaldson GC, et al. Exacerbations Are Related To Depression And SGRQ In West Indian Subjects With COPD. In B42. The World Is Not Enough: COPD Is A Global Disease. Am J Respir Crit Care Med. 2014:1–272. [Google Scholar]
- 13.Seemungal TAR, Mohammed S, Mohammed H, et al. A Lower Level Of Education Is Related To Disease Severity And Biomass Exposure In West Indian Subjects With COPD. In A97. COPD Susceptibility: Traffic, Temperature, and Tobacco. What to Target? Am J Respir Crit Care Med. 2014:1–272. [Google Scholar]
- 14.Levy ML, Quanjer PH, Booker R, et al. Diagnostic spirometry in primary care: Proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations. Prim Care Respir J. 2009;18:130–47. doi: 10.4104/pcrj.2009.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8–16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 16.Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31:492–501. doi: 10.1183/09031936.00074807. [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Current Cigarette Smoking Among Adults - United States, 2005–2014. (n.d.). (cited 2017 November 18). Available from: URL: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6444a2.htm.
- 19.Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 20.Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 22.Eaton WW, Muntaner C, Smith C, Tien A, Ybarra M. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R) In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment. 3rd ed. Mahwah, New Jersey: Lawrence Erlbaum Associates Inc; 2004. pp. 363–77. [Google Scholar]
- 23.Hanania NA, Müllerova H, Locantore NW, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study investigators. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183:604–11. doi: 10.1164/rccm.201003-0472OC. [DOI] [PubMed] [Google Scholar]
- 24.Yohannes AM, Müllerová H, Hanania NA, et al. Long-term Course of Depression Trajectories in Patients With COPD: A 3-Year Follow-up Analysis of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints Cohort. Chest. 2016;149:916–26. doi: 10.1016/j.chest.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 25.Hennis A, Wu SY, Nemesure B, et al. Diabetes in a Caribbean population: epidemiological profile and implications. Int J Epidemiol. 2002;31:234–9. doi: 10.1093/ije/31.1.234. [DOI] [PubMed] [Google Scholar]
- 26.Fink RI, Kolterman OG, Griffin J, et al. Mechanisms of insulin resistance in aging. J Clin Invest. 1983;71:1523–35. doi: 10.1172/JCI110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanaya AM, Harris T, Goodpaster BH, et al. Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care. 2004;27:1375–80. doi: 10.2337/diacare.27.6.1375. [DOI] [PubMed] [Google Scholar]
- 28.Rogliani P, Calzetta L, Segreti A, et al. Diabetes mellitus among outpatients with COPD attending a university hospital. Acta Diabetol. 2014;51:933–40. doi: 10.1007/s00592-014-0584-0. [DOI] [PubMed] [Google Scholar]
- 29.Pinto Pereira LM, Seemungal TAR, Teelucksingh S, et al. Restrictive pulmonary deficit is associated with inflammation in sub-optimally controlled obese diabetics. J Thorac Dis. 2013;5:289–97. doi: 10.3978/j.issn.2072-1439.2012.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Critic Care Med. 1998;157:1418–22. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 31.Bandhan T. The prevalence of selected vascular disease risk factors in a community setting in Trinidad. West Indian Med J. 2006;54:78. [Google Scholar]
- 32.Fan VS, Ramsey SD, Giardino ND, et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007;167:2345–53. doi: 10.1001/archinte.167.21.2345. [DOI] [PubMed] [Google Scholar]
- 33.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–9. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 34.Leca V, Ibrahim Z, Lombard-Pontou E, et al. Point-of-Care Measurements of HbA1c: Simplicity Does Not Mean Laxity With Controls. Diabetes Care. 2012;35:e85. doi: 10.2337/dc12-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenters-Westra E, Slingerland RJ. Six of eight hemoglobin A1c point-of-care instruments do not meet the general accepted analytical performance criteria. Clin Chem. 2010;56:44–52. doi: 10.1373/clinchem.2009.130641. [DOI] [PubMed] [Google Scholar]


