Abstract
Angiotensin-converting enzyme inhibitors (ACE-I) are widely used in diseases, such as hypertension, congestive heart failure, and myocardial infarction. Although these drugs are well tolerated, one out of five patients discontinues ACE-I due to drug side effects, mainly chronic cough. However, the pathogenesis of ACE-I-induced cough remains controversial and requires further study. In this review, the mechanisms that are suggested in ACE-I-induced cough pathophysiology will be discussed in detail in light of the current literature.
Keywords: Cough, angiotensin converting enzyme inhibitors, hypertension
INTRODUCTION
Angiotensin-converting enzyme inhibitors (ACE-I) have been used for many years in the treatment of cardiovascular and metabolic diseases. In addition to its antihypertensive effects, reducing effects on mortality and complications of diseases, such as coronary artery disease, heart failure, diabetes, and diabetic nephropathy, have also been observed [1]. This medicine has also become very common in chronic renal failure treatments with proteinuria and post-myocardial infarction [2]. Although they are well-tolerated medicines, almost one-fifth of patients discontinue their treatments due to side effects, especially cough [3]. The incidence of cough associated with ACE-I is reported to be 3.9% to 35% conducted in different populations [4–6]. ACE-I-induced cough may develop within hours after the first dose or even weeks or months later [7]. The cough is predominantly seen in females and non-smokers [4,8–12]. However, the pathogenesis of ACE-I-induced cough remains controversial. The underlying mechanisms of the ACE-I-induced cough are multifactorial.
Angiotensin-Converting Enzyme Inhibition Mechanism
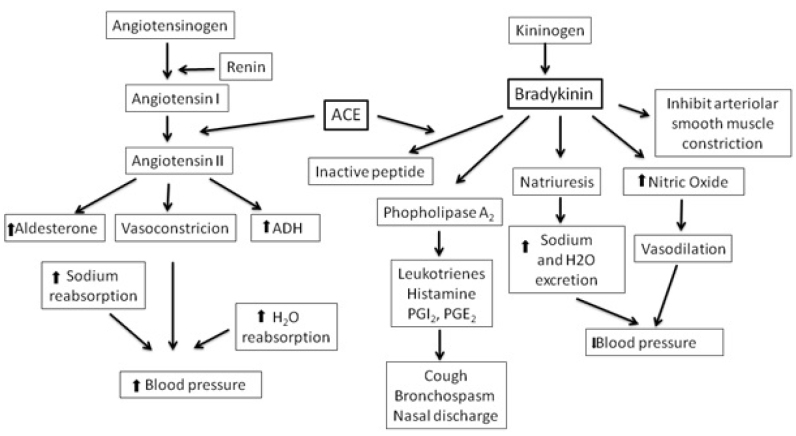
Angiotensin-converting enzyme (ACE) inhibition mechanism is a very important development in hypertension treatment. With ACE inhibition, the renin-angiotensin-aldosterone cascade is blocked. Generally, renin is produced and released in response to a decreased in blood flow to the juxtaglomerular apparatus of the kidney or in response to a decreased in the filtration of the sodium chloride concentration. It makes the degradation of hepatic angiotensinogen to its inactive peptide, angiotensin I. Then, angiotensin I is converted to active angiotensin II by ACE produced by the capillaries in the alveoli. Angiotensin II has many physiological effects, such as increasing the resistance of blood vessels, causing adrenal cortex aldosterone release, and stimulating vasopressin [2,13–15]. ACE-I is a competitive inhibitor of ACE and prevents conversion of angiotensin I to angiotensin II. ACE is also responsible for the degradation of bradykinin. Active bradykinin is produced by its precursor kininogen, and kininogen is decomposed by kallikrein. Bradykinin has a short half-life because it is rapidly degraded by ACE (Figure 1) [15]. Thus, the half-life of bradykinin can be prolonged by ACE-I with ACE inhibition, and its activity and concentration can increase. Angiotensin receptor blockers (ARBs), also known as angiotensin II receptor antagonists, cause vasodilatation, decrease vasopressin secretion, and decrease aldosterone production and secretion by blocking angiotensin II to bind to its receptors [16]. In ACE-I, ARBs are also indicated for hypertension, congestive heart failure (CHF), and diabetic nephropathy. They have no effect on ACE activities and do not cause inhibition of bradykinin degradation. Owing to this reason, some of the side effects of ACE-I and ARBs differ.
Figure 1.
Effect of renin-angiotensin/kallikrein-kinin system on blood pressure regulation [15]
ACE: angiotensin-converting enzyme; ADH: antidiuretic hormone; PG: prostaglandin
Side Effects of ACE-I
ACE-I t have some undesirable side effects. The side effects are often specific to the class of the medicine depending on its mechanism of action not the medicine itself. Owing to this reason, any ACE-I side effect can also occur with the use of other ACE-I. In a cohort study, 19% of patients using ACE-I have been shown to discontinue their medications due to side effects (mostly persisting cough) [3]. Owing to its mechanism of action, hypotension due to ACE-I may occur and usually develop secondary to use with other medications that have a significant salt deprivation or diuretic effect [17]. Hyperkalemia is another common side effect. The incidence of hyperkalemia in patients treated with ACE-I is approximately 3.3% [18,19]. Secondary cough and angioedema are idiosyncratic reactions to ACE-I, and these effects are dose-independent [17]. In the cases of development of upper airway symptoms and cough with the use of this medicine in patients with obstructive sleep apnea, disease severity may increase [7]. In addition to the side effects due to the mechanism of action of ACE-I, specific side effects may occur depending on the drug molecular structure. For example, captopril sulfhydryl-related skin rash, neutropenia, tasting disorders, and nephritic syndrome are some of its side effects. Some of these side effects are idiosyncratic, whereas others are dose-related [20].
Clinical Features of ACE-I-Induced Cough
ACE-I-induced cough only occurs in susceptible individuals independent of the dose of the drug. Therefore, it is an idiosyncratic reaction. It is a non-immune type B hypersensitivity reaction according to the new terminology and is one of the well-defined side effects of ACE-I [21]. This cough is typically dry with a tickling or scratching feeling in the throat. The incidence of cough associated with the drug has been reported to be between 3.9% and 35% among patients using ACE-I [4–6]. On the other hand, ACE-I is responsible for 0%–3% of chronic cough etiology in prospective studies evaluating patients with chronic cough complaints [22–24].
ACE-I-induced cough may occur within hours after first intake of the dose or even weeks or months later. ACE-I may sensitize the cough reflex. Owing to this reason, it may increase the severity of chronic cough due to other causes [25]. Coughs due to these medications can decrease within 1 to 4 weeks after discontinuation, but in some cases, this can take up to 3 months [26].
Pathogenesis of ACE-I-Induced Cough
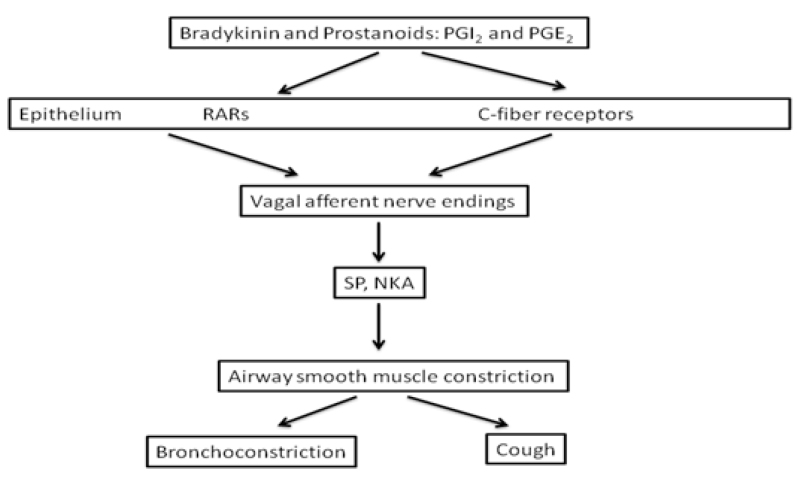
The mechanism of ACE-I-induced cough is still unclear. The possible mediators that play a role in the development of cough are bradykinin and substance P, which are destroyed by ACE. Thus, bradykinin and substance P accumulate in the upper and lower respiratory tracts by inhibition of this enzyme by ACE-I. Bradykinin also stimulates prostaglandins [4,27]. Figure 2 shows the mechanisms of ACE-I-induced pulmonary dysfunction [15]. However, although this is the possible mechanism, the question of why the cough does not occur in all ACE-I users is still debatable. In other studies conducted to shed light on this situation, patients with ACE-I-induced cough with bronchial hyperreactivity (BHR) [28], asthma history [29], CHF [5], increased sensitivity of bradykinin-dependent airway sensory nerve fibers [26], bradykinin receptor gene polymorphism [30,31], increased cough reflex sensitivity [25,32], aminopeptidase P (APP) enzyme deficiency in the breakdown of bradykinin [33], and mechanisms that include ACE insertion/deletion polymorphism have been proposed [34]. The result from all these studies suggests that there cannot be only one mechanism that is responsible for ACE-I-induced cough. A combination of two or more of the above mechanisms may develop into ACE-I-induced cough.
Figure 2.
ACE-I induced pulmonary dysfunction [15]
ACE: angiotensin-converting enzyme; NKA: neurokinin A; PG: prostaglandin; RARs: rapidly adapting stretch receptors; SP: substance P
Relationship between ACE-I-Induced Cough, BHR, and Asthma
The first studies about the mechanism of ACE-I-induced cough investigated the relationship between ACE-I-induced cough, asthma, and BHR. In a retrospective study, eight out of nine patients with ACE-I-induced cough were shown to have BHR, whereas BHR was detected in only one out of nine patients who used ACE-I but did not have a cough. In the present study, the underlying BHR was considered to be related to ACE-I-induced cough, and the authors recommended precaution while using ACE-I in individuals with BHR or asthma [28]. In another study, six patients with ACE-I-induced cough and two patients with uncontrolled asthma were included as study groups, and nine patients who used this medicine without any issues were included as control groups. Patients in the control group underwent pulmonary function tests and bronchial provocation tests (BPTs). In study group patients, cough reflex was measured before and after the intake of ACE-I for 2 weeks. After ACE-I was taken again, a significant decrease in forced expiratory volume in one second (FEV1), a 35% decrease in histamine BPT, and a significant increase in cough reflex were determined. In this study, ACE-I-induced coughing was thought to be a variant of coughing in asthma [35]. However, contrary to these studies, there are also studies suggesting that previous asthma or BHR history does not pose a risk for developing ACE-I-induced cough [29,36,37]. In one of these studies, 3 years of data were collected in 1013 patients using ACE-I and 1017 patients using bezafibrate. The incidence of cough (12.3% vs. 2.7%, p<0.0001) and bronchospasm (5.5% vs. 2.3%, p<0.0001) was higher in patients receiving ACE-I. However, an increase in the incidence of adverse respiratory side effects has not been reported in patients with a history of asthma or bronchospasm (16%) compared with patients without a history of bronchospasm [36].
Whether or not ACE-I itself causes BHR is another research topic. In healthy subjects, the effects of ACE-I on BHR were investigated in cases with ACE-I, and ACE-I has not been shown to be effective in the development of BHR [29,38]. In patients with asthma, the relationship between ACE-I-induced cough and decrease in lung function was also investigated, and there was no significant association [29]. Pulmonary functions were evaluated in a double-blind study in 21 patients using ACE-I (14 asthma and 7 allergic rhinitis). Baseline forced vital capacity (FVC), FEV1, FEV1/FVC ratio, and methacholine BPT values were measured after a drug washout period. Patients were then started on spirapril or enalapril for 3 weeks, and after 3 weeks, measurements of pulmonary function test and methacholine BPT were repeated. There were no significant changes in spirometric measurements or in methacholine BPT before or after treatment. On the other hand, the frequency of coughing and wheezing increased in both groups, but not more than the general population (14% cough and 5% wheezing). However, in the present study, patients were allowed to continue their medication, such us theophylline and inhaled β-agonists, until 36 h before the spirometry and methacholine challenge tests. However, the most important limitation of the study is the lack of an active control group and the comparison to the general population [29]. In a prospective double-blind study by Riska et al. [39], the effects of captopril and calcium channel antagonist verapamil on the pulmonary function of 12 patients with stable asthma were compared. After 4 weeks of treatment with captopril or verapamil, there was no difference in asthma symptoms, peak expiratory flow, or FEV1 between the two treatments. After 3 years, in a research that had been conducted by the same researchers, there was no difference in FEV1 changes before and after captopril or verapamil treatment. Patients receiving 4 weeks of treatment on both branches were allowed to use corticosteroids and bronchodilators, and it may have masked the symptoms that may occur with ACE-I. However, there was no increase in β2 agonist and corticosteroid use rates in both groups. In addition, the incidence of increased cough was similar in both the active drug group and the placebo group [40]. In a study by Sala et al. [37], ACE-I captopril was given for 4 weeks to 16 patients with asthma, and the difference in bronchial response was studied before and after treatment applications. FEV1 values and methacholine BPT values were measured before and after treatment with ACE-I. The incidence of cough was not reported in the study, but there was no significant difference in BHR measured by methacholine BPT before and after treatment. In conclusion, in these studies, FEV1 changes were not found during ACE-I use in diseases with chronic airway obstruction [29]. All these studies did not achieve a clear conclusion that asthma poses a risk for the development of secondary cough in the use of ACE-I. One reason for this, may be speculative, ACE-I-induced cough pathophysiology may be caused by multiple etiologies, rather than by a single mechanism. However, a definite conclusion in this topic is difficult to achieve due to the fact that these studies are data obtained as a result of short-term use of ACE-I, low statistical power analyses, no control of the use of certain medications that may affect pulmonary function, and the potential for these drugs to suppress cough associated with ACE-I.
ACE-I-Induced Cough and Bradykinin Degradation Defect
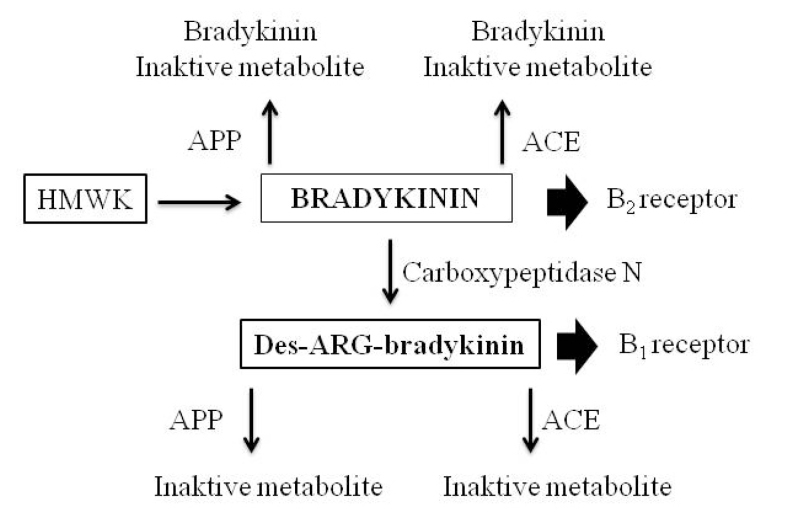
Another possible mechanism by which ACE-I-induced cough may be explained is that it may be associated with a defect in the degradation of bradykinin. It normally has a short half-life due to the rapid degradation of bradykinin. Three metalloproteinases are responsible for the degradation of bradykinin. These are ACE/kininase II, APP, and neutral endopeptidase. ACE is more effective than APP in the degradation of bradykinin, and APP is more effective than neutral endopeptidase [33,41]. ACE-I blocks ACE/kininase II activity, thus increasing the level of bradykinin by reducing its degradation (Figure 3) [33]. However, ACE-I-induced cough occurs in a small proportion of patients receiving this medicine. At this point, other mechanisms come into consideration in the pathogenesis of ACE-I-induced cough. The effect of ACE inhibition on the metabolism of quinine and the role of APP enzyme are thought to be important in understanding the side effects of this medicine [33]. However, more focus was on studies about the degradation of bradykinin and ACE-I-induced angioedema relationship. Bradykinin is metabolized to N-Arg-bradykinin, the metabolite of active bradykinin by ACE/kininase II. In half of the patients with ACE-I-induced angioedema, they have been shown to have an enzymatic deficiency in the breakdown of this active molecule. Therefore, the increase in activity of bradykinin would be much higher when ACE-I is used [33,42]. In another study, there was no significant difference in terms of the carboxypeptidase-N enzyme (kininase I enzyme) when individuals with ACE-I-induced angioedema were compared with healthy controls. Therefore, reduced plasma APP activity has been suggested not to be an increased risk factor for the development of ACE-I-induced angioedema [43]. Overall withdrawal rates because of the adverse events (cough and angioedema) are lower with ARBs than with ACE-I [44,45]. Since their actions on bradykinin are different, these mechanisms are mentioned in the ACE inhibition mechanism section.
Figure 3.
Major pathways of bradykinin metabolism [33]
ACE: angiotensin converting enzyme; APP: amino peptidase-P; HMWK: high molecular weight kininogen
ACE-I-Induced Cough and Gene Polymorphisms
Pharmacogenetics, which is trying to reveal the genetic determinants of response to drugs and other chemicals, is a very fast developing branch of genetics. Local variability in genes or polymorphism may differentiate individual responses to certain drugs by affecting medicine metabolism or receptors. A polymorphism can be in a single nucleotide (single-nucleotide polymorphism, SNP), or it can occur in multiple fragments of this gene (insertion/deletion/duplication/inversion) [33]. ACE and bradykinin β2 receptor (BDKRB2), SNP, and insertion/deletion polymorphisms, which may be responsible for the development of ACE-I secondary cough, have been studied.
The degradation of bradykinin in circulation is much faster than that of ACE DD genotypes. These genotype prophylactic peptides appear to contribute to reducing respiratory concentrations of substance P and bradykinin [46]. The means by which ACE inhibitors affect the respiratory system is thought to be through an increase of substance P, which is released from the vagal and glossopharyngeal sensory nerves in the pharynx and upper airways, and is naturally degraded by ACE [7,47]. In this case, this will increase the cough reflex. However, in a meta-analysis of ACE-I-induced cough pharmacokinetics, no correlation between ACE insertion/deletion polymorphism and secondary cough development was found [48].
After ACE genes, BDKRB2 genes are the second most frequently examined genes by studies on different polymorphisms [48]. The relationship between ACE-I-induced cough and BDKRB2-58T/C polymorphism (rs1799722) was determined in some studies. The majority of the populations in these studies are of East Asian origin [49–53]. Three of these studies with East Asian individuals have been combined with an earlier meta-analysis and found a significant association with ACE-I-induced cough [54]. However, this association has not been shown in a study in Chinese patients with non-insulin-dependent diabetes mellitus [55]. Additionally, in a study with Caucasian Spanish subjects, the association between ACE-I-induced cough and BDKRB2-58T/C was not found, but the association with different SNPs in the BDKRB2 gene has been found [51]. In another study with Spanish subjects, the association between four different SNPs in the BDKRB2 gene and ACE-I-induced cough was shown [51]. In these two large-scale Spanish studies, significant associations with SNPs, including genes, membrane metalloendopeptidase, prostaglandin E receptor 3, and ACE, have been shown. However, none of these have been replicated in other populations [48]. No correlation between SNPs in the bradykinin 1 receptor gene, which is another bradykinin receptor, and ACE-I-induced cough is found [51,52]. In association with ACE-I-induced cough, gene polymorphisms other than the BDKRB2 gene polymorphism have also been identified. In a study of a Korean patient cohort, the relationship between ACE-I-induced cough and neurokinin 2 gene receptor polymorphism was reported [53]. In an another recent study, Hallberg et al. [56] identified nearly genome-wide significant associations in cytoplasmic linker associated protein 1, phosphodiesterase 11A, potassium calcium-activated channel subfamily M regulatory beta subunit 2, protransforming growth factor A, solute carrier family 38 member 6, and matrix metalloproteinase 16. The strongest association was with rs62151109 in cytoplasmic linker associated protein 1. Finally, the authors proposed that ACE-I-induced cough is potentially associated with genes that are independent of bradykinin pathways.
In conclusion, inconsistent results between ACE-I-induced cough and ACE insertion/deletion polymorphisms and BDKRB2 gene polymorphism have been reported. Inconsistency in different association studies can be explained with changes in study design, small relative risk magnitude, etiological heterogeneity, and selection of non-causal polymorphisms [33,57]. We hypothesize that, in addition to the above reasons, an attempt is made to investigate a single causal factor, particularly in the development of ACE-I-induced cough. Although it is speculative, we believe that coughing in these patients is the result of the existence of more than one of the reasons that have been previously investigated and shown as a risk factor. In other words, in patients with asthma (one of the risk factors for ACE-I-induced cough) who do not develop ACE-I-induced cough, none of the other risk factors can be seen. In those who are not asthmatic and develop ACE-I secondary cough, cough may occur because of two or more risk factors other than asthma in the same patient. Further work is needed to support this theory.
ACE-I-Induced Cough and Heart Failure
It is also a matter of curiosity whether there is a relationship between CHF and ACE-I-induced cough. Ravid et al. [5] showed significant differences in cough development between patients using ACE-I (n=164) due to systemic hypertension and patients using ACE-I (n=104) due to CHF. In their study, cough developed in 50 (18.6%) patients receiving ACE-I. Twenty-three (14%) patients with systemic hypertension developed cough in 24.7±17.1 weeks, and 27 (26%) patients with CHF developed cough in 12.3±12 weeks (p<0.001). The authors concluded that the frequency of coughing from ACE-I is higher in patients with CHF than in those with hypertension [5]. However, it is very difficult to say the true incidence of ACE-I-related cough in cases with heart failure since heart failure itself can cause coughing. Moreover, in some patients, ACE-I therapy may resolve cough-associated heart failure. These effects may be due to better alveolar-capillary gas transfer, ventilation/perfusion imbalance, and/or better pulmonary congestion [7,58]. However, there is also a meta-analysis showing that ACE-I-induced cough is less frequent in patients using ACE-I due to heart failure than in patients using ACE-I for systemic hypertension. In a meta-analysis of randomized placebo-controlled trials, 65,054 patients from 22 included studies were analyzed. Placebo-adjusted ACE-I cough was 37% of 13.5% reported cases on ACE-I, whereas 8.5% reported cases on placebo were equivalent to 63% of cases on ACE-I, indicating the other potential factors for cough than ACE-I in a substantial number of cough cases on ACE-I. Placebo-adjusted ACE-I cough had the highest rates of arterial hypertension (85%) and the lowest rates of heart failure (29%). The authors proposed that other causes of cough, particularly in heart failure, should be excluded before ACE-I withdrawal [59].
ACE-I-Induced Cough and Cough Reflex Sensitivity
Increased sensitivity of bradykinin-dependent airway sensory nerve fibers has been suggested as one of the potential mechanisms of ACE-I-induced cough [27]. Experimental stimuli with capsaicin showed increased cough reflex sensitivity in patients with ACE-I-induced cough, which decreased with discontinuation of the drug (25,32). Capsaicin cough reflex sensitivity has also been shown to increase in patients with asthma [60,61].
In conclusion, ACE-I-induced cough mechanisms, such as BHR, asthma history, APP enzyme deficiency in bradykinin degradation, ACE insertion/deletion polymorphism, bradykinin receptor gene polymorphism, CHF, and increased cough reflex sensitivity, have been proposed. However, none of these factors have been shown to be responsible for ACE-I-induced cough alone. The result of all these studies suggests that there cannot be only one mechanism responsible for ACE-I-induced cough. Only combinations of two or more of the above mechanisms may result in ACE-I secondary cough.
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest: The author have no conflicts of interest to declare.
Financial Disclosure: The author declared that this study has received no financial support.
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461–8. [PubMed] [Google Scholar]
- 3.Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- 4.Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy: a review of the literature and pathophysiology. Ann Intern Med. 1992;117:234–42. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 5.Ravid D, Lishner M, Lang R, et al. Angiotensin-converting enzyme inhibitors and cough: a prospective evaluation in hypertension and in congestive heart failure. J Clin Pharmacol. 1994;34:1116–20. doi: 10.1002/j.1552-4604.1994.tb01989.x. [DOI] [PubMed] [Google Scholar]
- 6.Brugts JJ, Arima H, Remme W, et al. The incidence and clinical predictors of ACE-inhibitor induced dry cough by perindopril in 27,492 patients with vascular disease. Int J Cardiol. 2014;176:718–123. doi: 10.1016/j.ijcard.2014.07.108. [DOI] [PubMed] [Google Scholar]
- 7.Sica DA, Brath L. Angiotensin-converting enzyme inhibition-emerging pulmonary issues relating to cough. Congest Heart Fail. 2006;12:223–6. doi: 10.1111/j.1527-5299.2006.05746.x. [DOI] [PubMed] [Google Scholar]
- 8.Os I, Bratland B, Dahlöf B, et al. Female preponderance for lisinopril-induced cough in hypertension. Am J Hypertens. 1994;7:1012–5. doi: 10.1093/ajh/7.11.1012. [DOI] [PubMed] [Google Scholar]
- 9.Coulter DM, Edwards IR. Cough associated with captopril and enalapril. Br Med J (Clin Res Ed) 1987;294:1521–3. doi: 10.1136/bmj.294.6586.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strocchi E, Malini PL, Valtancoli G, et al. Cough during treatment with angiotensin converting enzyme inhibitors: analysis of predisposing factors. Drug Invest. 1992;4:69–72. doi: 10.1007/BF03258383. [DOI] [Google Scholar]
- 11.Gibson GR. Enalapril-induced cough. Arch Intern Med. 1989;149:2701–3. doi: 10.1001/archinte.149.12.2701. [DOI] [PubMed] [Google Scholar]
- 12.Alharbi FF, Kholod AAV, Souverein PC, et al. The impact of age and sex on the reporting of cough and angioedema with renin-angiotensinsystem inhibitors: a case/noncase study in VigiBase. Fundam Clin Pharmacol. 2017;31:676–84. doi: 10.1111/fcp.12313. [DOI] [PubMed] [Google Scholar]
- 13.Smith RE, Ashiya M. Antihypertensive therapies. Nat Rev Drug Discov. 2007;6:597–8. doi: 10.1038/nrd2354. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 15.Packard KA, Wurdeman RL, Arouni AJ. ACE inhibitor-induced bronchial reactivity in patients with respiratory dysfunction. Ann Pharmacother. 2002;36:1058–67. doi: 10.1345/aph.1A332. [DOI] [PubMed] [Google Scholar]
- 16.Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg. 2004;130:1416–9. doi: 10.1001/archotol.130.12.1416. [DOI] [PubMed] [Google Scholar]
- 17.Izzo JL, Jr, Weir MR. Angiotensin-converting enzyme inhibitors. J Clin Hypertens (Greenwich) 2011;13:667–75. doi: 10.1111/j.1751-7176.2011.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai AS, Swedberg K, McMurray JJ, et al. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol. 2007;50:1959–66. doi: 10.1016/j.jacc.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 19.Bezalel S, Mahlab-Guri K, Asher I, et al. Angiotensin Converting Enzyme Inhibitor Induced Angioedema. Am J Med. 2015;128:120–5. doi: 10.1016/j.amjmed.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Jackson B, Maher D, Matthews PG, et al. Lack of cross sensitivity between captopril and enalapril. Aust N Z J Med. 1988;18:21–7. doi: 10.1111/j.1445-5994.1988.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 21.Wedi B. Definitions and mechanisms of drug hypersensitivity. Expert Rev Clin Pharmacol. 2010;3:539–51. doi: 10.1586/ecp.10.32. [DOI] [PubMed] [Google Scholar]
- 22.Mello CJ, Irwin RS, Curley FJ. The predictive values of thecharacter, timing, and complications of chronic cough in diagnosing its cause. Arch Intern Med. 1996;156:997–1003. doi: 10.1001/archinte.1996.00440090103010. [DOI] [PubMed] [Google Scholar]
- 23.Irwin RS, Curley FJ, French CL. Chronic cough: the spectrum and frequency of causes, key components of the diagnostic evaluation, and outline of specific therapy. Am Rev Respir Dis. 1990;141:640–7. doi: 10.1164/ajrccm/141.3.640. [DOI] [PubMed] [Google Scholar]
- 24.Smyrnios NA, Irwin RS, Curley FJ. Chronic cough with a history of excessive sputum production: the spectrum and frequency of causes and key components of the diagnostic evaluation, and outcome of specific therapy. Chest. 1995;108:991–7. doi: 10.1378/chest.108.4.991. [DOI] [PubMed] [Google Scholar]
- 25.Morice AH, Lowry R, Brown MJ, et al. Angiotensin-converting enzyme and the cough reflex. Lancet. 1987;330:1116–8. doi: 10.1016/S0140-6736(87)91547-9. [DOI] [PubMed] [Google Scholar]
- 26.Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:169–73. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 27.Fox AJ, Lalloo UG, Belvisi MG, et al. Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat Med. 1996;2:814–7. doi: 10.1038/nm0796-814. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman J, Casanova JE, Riendl P, et al. Bronchial hyperreactivity and cough due to angiotensin-converting enzyme inhibitors. Chest. 1989;95:544–8. doi: 10.1378/chest.95.3.544. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman J, Schmitt S, Barnard J, et al. Angiotensin-converting enzyme inhibitors in patients with bronchial responsiveness and asthma. Chest. 1992;101:922–5. doi: 10.1378/chest.101.4.922. [DOI] [PubMed] [Google Scholar]
- 30.Mukae S, Aoki S, Itoh S, et al. Bradykinin B(2) receptor gene polymorphism is associated with angiotensin-converting enzyme inhibitor-related cough. Hypertension. 2000;36:127–31. doi: 10.1161/01.HYP.36.1.127. [DOI] [PubMed] [Google Scholar]
- 31.Ignjatovic T, Tan F, Brovkovych V, et al. Novel mode of action of angiotensin I converting enzyme inhibitors: direct activation of bradykinin B1 receptor. J Biol Chem. 2002;277:16847–52. doi: 10.1074/jbc.M200355200. [DOI] [PubMed] [Google Scholar]
- 32.O’Connell F, Thomas VE, Pride NB, et al. Capsaicin cough sensitivity decreases with successful treatment of chronic cough. Am J Respir Crit Care Med. 1994;150:374–80. doi: 10.1164/ajrccm.150.2.8049818. [DOI] [PubMed] [Google Scholar]
- 33.Nikpoor B, Qing Ling D, Rouleau GA. Acute adverse reactions associated with angiotensinconverting enzyme inhibitors: genetic factors and therapeutic implications. Expert Opin Pharmacother. 2005;6:1851–6. doi: 10.1517/14656566.6.11.1851. [DOI] [PubMed] [Google Scholar]
- 34.Lee YJ, Tsai JC. Angiotensin-converting enzyme gene insertion/deletion, not bradykinin B2 receptor -58T/C gene polymorphism, associated with angiotensin-converting enzyme inhibitor-related cough in Chinese female patients with non-insulin-dependent diabetes mellitus. Metabolism. 2001;50:1346–50. doi: 10.1053/meta.2001.27212. [DOI] [PubMed] [Google Scholar]
- 35.Bucknall CE, Neilly JB, Carter R, et al. Bronchial hyperreactivity in patients who cough after receiving angiotensin converting enzyme inhibitors. Br Med J (Clin Res Ed) 1988;296:86–8. doi: 10.1136/bmj.296.6615.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood R. Bronchospasm and cough as adverse reactions to the ACE inhibitors captopril, enalapril and lisinopril. A controlled retrospective cohort study. Br J Clin Pharmacol. 1995;39:265–70. doi: 10.1111/j.1365-2125.1995.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sala H, Abad J, Juanmiquel L, et al. Captopril and bronchial reactivity. Postgrad Med J. 1986;62:76–7. [PubMed] [Google Scholar]
- 38.Dicpinigaitis PV, Dobkin JB. Effect of angiotensin-converting enzyme inhibition on bronchial responsiveness. J Clin Pharmacol. 1996;36:361–4. doi: 10.1002/j.1552-4604.1996.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 39.Riska H, Stenius-Aarniala B, Sovijarvi AR. Comparison of the effects of an angiotensin converting enzyme inhibitor and a calcium channel blocker on blood pressure and respiratory function in patients with hypertension and asthma. J Cardiovasc Pharmacol. 1987;10:79–81. doi: 10.1097/00005344-198710100-00030. [DOI] [PubMed] [Google Scholar]
- 40.Riska H, Sovijärvi AR, Ahonen A, et al. Effects of captopril on blood pressure and respiratory function compared to verapamil in patients with hypertension and asthma. J Cardiovasc Pharmacol. 1990;15:57–61. doi: 10.1097/00005344-199001000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Moreau ME, Garbacki N, Molinaro G, et al. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.SRJ05001X. [DOI] [PubMed] [Google Scholar]
- 42.Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232–7. doi: 10.1124/jpet.102.038067. [DOI] [PubMed] [Google Scholar]
- 43.Adam A, Cugno M, Molinaro G, et al. Aminopeptidase P in individuals with a history of angio-oedema on ACE inhibitors. Lancet. 2002;359:2088–9. doi: 10.1016/S0140-6736(02)08914-6. [DOI] [PubMed] [Google Scholar]
- 44.Mavrakanas TA, Lipman MN. Angiotensin-Converting Enzyme Inhibitors vs. Angiotensin Receptor Blockers for the Treatment of Hypertension in Adults With Type 2 Diabetes: Why We Favour Angiotensin Receptor Blockers. Can J Diabetes. 2018;42:118–23. doi: 10.1016/j.jcjd.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-Converting Enzyme Inhibitors in Hypertension: To Use or Not to Use? J Am Coll Cardiol. 2018;71:1474–82. doi: 10.1016/j.jacc.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 46.Brown NJ, Blais C, Gandhi SK, et al. ACE insertion/deletion genotype affects bradykinin metabolism. J Cardiovasc Pharmacol. 1998;32:373–7. doi: 10.1097/00005344-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Tomaki M, Ichinose M, Miura M, et al. Angiotensin converting enzyme (ACE) inhibitorinduced cough and substance P. Thorax. 1996;51:199–201. doi: 10.1136/thx.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahmoudpour SH, Leusink M, van der Putten L, et al. Pharmacogenomics. 2013;14:249–60. doi: 10.2217/pgs.12.206. [DOI] [PubMed] [Google Scholar]
- 49.Mukae S, Aoki S, Itoh S, et al. Bradykinin B2 receptor gene polymorphism is associated with angiotensin-converting enzyme inhibitor-related cough. Hypertension. 2000;36:127–31. doi: 10.1161/01.HYP.36.1.127. [DOI] [PubMed] [Google Scholar]
- 50.Mukae S, Itoh S, Aoki S, et al. Association of polymorphisms of the renin-angiotensin system and bradykinin B2 receptor with ACE-inhibitor-related cough. J Hum Hypertens. 2002;16:857–63. doi: 10.1038/sj.jhh.1001486. [DOI] [PubMed] [Google Scholar]
- 51.Grilo A, Sáez-Rosas MP, Santos-Morano J, et al. Identification of genetic factors associated with susceptibility to angiotensin-converting enzyme inhibitors-induced cough. Pharmacogenet Genomics. 2011;21:10–7. doi: 10.1097/FPC.0b013e328341041c. [DOI] [PubMed] [Google Scholar]
- 52.Mas S, Gasso P, Alvarez S, et al. Pharmacogenetic predictors of angiotensin-converting enzyme inhibitor-induced cough: the role of ACE, ABO, and BDKRB2 genes. Pharmacogenet Genomics. 2011;21:531–8. doi: 10.1097/FPC.0b013e328348c6db. [DOI] [PubMed] [Google Scholar]
- 53.Kim TB, Oh SY, Park HK, et al. Polymorphisms in the neurokinin-2 receptor gene are associated with angiotensin-converting enzyme inhibitor-induced cough. J Clin Pharm Ther. 2009;34:457–64. doi: 10.1111/j.1365-2710.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- 54.Nishio K, Kashiki S, Tachibana H, et al. Angiotensin-converting enzyme and bradykinin gene polymorphisms and cough: a meta-analysis. World J Cardiol. 2011;3:329–6. doi: 10.4330/wjc.v3.i10.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee YJ, Tsai JC. Angiotensin-converting enzyme gene insertion/deletion, not bradykinin B2 receptor -58T/C gene polymorphism, associated with angiotensin-converting enzyme inhibitor-related cough in Chinese female patients with non-insulin-dependent diabetes mellitus. Metabolism. 2001;50:1346–50. doi: 10.1053/meta.2001.27212. [DOI] [PubMed] [Google Scholar]
- 56.Hallberg P, Persson M, Axelsson T, et al. Genetic variants associated with angiotensin-converting enzyme inhibitor-induced cough: a genome-wide association study in a Swedish population. Pharmacogenomics. 2017;18:201–13. doi: 10.2217/pgs-2016-0184. [DOI] [PubMed] [Google Scholar]
- 57.Tabor HK, Risch NJ, Myers RM. Candidate-gene approaches for studying complex genetic traits: practical considerations. Nat Rev Genet. 2002;3:391–7. doi: 10.1038/nrg796. [DOI] [PubMed] [Google Scholar]
- 58.Agusti A, Bonet S, Arnau JM, et al. Adverse effects of ACE inhibitors in patients with chronic heart failure and/or ventricular dysfunction: meta-analysis of randomised clinical trials. Drug Saf. 2003;26:895–908. doi: 10.2165/00002018-200326120-00004. [DOI] [PubMed] [Google Scholar]
- 59.Vukadinović D, Vukadinović AN, Lavall D, et al. Rate of Cough During Treatment With Angiotensin-Converting Enzyme Inhibitors: A Meta-Analysis of Randomized Placebo-Controlled Trials. Clin Pharmacol Ther. 2018 Jan 13; doi: 10.1002/cpt.1018. doi: 10.1002/cpt.1018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 60.Nakajima T, Nishimura Y, Nishiuma T, et al. Cough sensitivity in pure cough variant asthma elicited using continuous capsaicin inhalation. Allergology Int. 2006;55:149–55. doi: 10.2332/allergolint.55.149. [DOI] [PubMed] [Google Scholar]
- 61.Jesenak M, Babusikova E, Petrikova M, et al. Cough reflex sensitivity in various phenotypes of childhood asthma. J Physiol Pharmacol. 2009;60:61–5. [PubMed] [Google Scholar]