Introduction
Over the past few decades, scleral lenses have re-emerged and become increasingly utilized in medically necessary contact lens fittings. Advancements in oxygen permeable materials, complex lathing and manufacturing technologies, as well as innovative optical designs have made them a premier treatment for a wide range of therapeutic indications. However, scleral lenses possess a unique relationship with the ocular surface, as compared to other rigid gas permeable (GP) lenses, by vaulting over the cornea and limbus, landing on the conjunctival tissue overlying the sclera. They maintain apposition to the ocular surface through sub-atmospheric suction forces, and trap a reservoir of fluid between the scleral lens and the cornea and perilimbal conjunctiva. Since the majority of corneal oxygen supply originates exogenously, many researchers hypothesize that a thick tear reservoir poses the risk of inducing corneal hypoxia, predominantly centrally1,2,3, although this view is not universal.4 In addition, these large diameter lenses settle onto the conjunctival tissue, creating a suction effect due to the lower pressure under the lens compared to atmospheric pressure. This may create stagnation of the tears and lack of exchange under the lens, potentially instigating inflammatory complications due to the accumulation of metabolic waste products.1,5,6
Another scleral lens challenge is a phenomenon known as “midday fogging” (MDF). This accumulation of debris in the post-lens tear reservoir (TR) causes a significant reduction in visual performance in many individuals, necessitating removal of the scleral lens and re-application after instillation of fresh solution.7,8 MDF affects 20–33% of scleral lens wearers, and in one study cohort, limited consecutive scleral lens wear time to an average of just 4.45 hours per day before lens removal and re-application was needed.9,10,11 It is likely that there are different components of debris, including lipids, proteins, cell fragments, and other tear film components, that are present in variable amounts in each individual that experiences MDF.12
Due to the suction and settling of the scleral lens, there is slight to no movement with this lens modality. This is believed to cause a reduction, or in some cases a lack of tear exchange entirely, especially if the peripheral curves are closely aligned to the conjunctiva in every quadrant. Subjective measures of the sodium fluorescein (NaFl) pattern under the scleral lens have shown little to no change in observed fluorescence within the TR over an 8-hour period.13 Most previous studies have relied on a qualitative assessment of fluorescein rather than an objective measure of a fluorescence signal, which may not indicate the true level of fluorescein entering the TR. Although the effect of tear exchange on the success of scleral lens wear is still not well determined, it is believed that if tear exchange is completely stagnant, metabolic byproducts may accumulate in the TR.5,6
Historically, tear exchange estimates have been calculated by fluorophotometry.5,14–17 This technique is widely used for investigation of fluid exchange in the various structures of the eye, both anterior and posterior segment, and has been further modified to be utilized in measurements of tear flow under contact lenses.14,18–21 Using this method, researchers are able to determine the fluorescein concentration in the pre-corneal TR without having to collect tear specimens.14,16 This technique has classically been used to measure tear exchange rates in corneal GP wear, as well as with hydrogel and silicon-hydrogel soft lenses. Exchange rates for corneal GP lenses range from 10–20% per blink, while soft lenses are as low as 1–2% per blink.22–25 High molecular weight FITC Dextran is typically utilized when working with quantitative fluorophotometry because it is compatible with tear components, and does not penetrate the corneal epithelium.26 Essential to this study, fluorophotometry systems are able to measure fluorescent intensity of the TR (via fluorescein instillation) as well as the ocular surface tissues (natural fluorescence). By continuously measuring the TF fluorescent intensity, we can determine the inflow of fresh tears into the TF, which will dilute the TR and cause a reduction in measured fluorescent intensity.
Minimal data regarding tear inflow, outflow or exchange with scleral lenses exists, although studies have shown a reduction in TR thickness (due to settling) of approximately 100 um over a period of 8 hours,27 indicating a net movement of fluid out of the scleral lens system during wear. A study back in 1970 demonstrated the use of fluorophotometry to measure tear exchange with poly-methyl methacrylate (PMMA) scleral lenses that were modified with fenestrations and channels to improve tear oxygenation. This study reported exchange coefficients to represent levels of tear exchange, and overall found a relatively broad variability of exchange (i.e. one subject fully exchanged fluid in 5 minutes, and another only 30% in an hour).28 In modern high-Dk scleral lenses, fenestrations and channels are rarely incorporated due to the fabrication complications and their propensity for bubble formation at the sites of modification.29 A more recent study by Paugh et al. looked at tear exchange with non-fenestrated, gas permeable scleral lenses, showing an average exchange rate in of 0.57±0.6% per minute over the first 30 minutes of wear, and 0.42±0.5% per minute 30–60 minutes post application.30
The purpose of this pilot study was to evaluate tear inflow in a scleral lens system using fluorophotometry, and indirectly assess the exchange of the TR in habitual scleral lens wearers who experience MDF.
Methods
Primary Tear Exchange Study
Subjects were recruited from the University Eye Institute’s Cornea and Contact Lens Service. Inclusion criteria included an eye examination within the last 2 years, and habitual scleral lens wear; approximately 50% of these patients were selected based on a report of MDF, and the other half did not have MDF. Exclusion criteria included active anterior segment infection or known hypersensitivity to solutions used in the study. To reduce additional confounding factors, subjects were asked to discontinue any topical ophthalmic medications for 24 hours prior to their appointment. This study was carried out in accordance with the University of Houston Committee for Protection of Human Subjects and the experiment adhered to the tenets of the Declaration of Helsinki.
Thirty-three subjects were included in the study and assigned to one of the following groups: Group 1 was composed of habitual scleral lens wearers with reported MDF; Group 2 was composed of habitual wearers without report of MDF; Group 3 was composed of a control group of non-habitual scleral lens wearers with absent anterior segment pathology.
Groups 1 and 2 consisted of habitual scleral lens wearers who have been wearing their lenses routinely for a minimum of 6 months. Group 1 (11 subjects) consisted of subjects that reported at least one interruption in their daily scleral lens wear due to MDF (they had to remove and refresh the saline in these lenses at least every 8 hours during continuous wear on an average day). The second group (12 subjects) were subjects that reported uninterrupted scleral lens wear (>8 hours continuous wear time), representing more successful lens wearers. All lenses had spherical landing zone curvatures. Group 3 consisted of 10 subjects without the presence of any corneal pathology, who were recruited to serve as a control group, allowing the comparison of data between regular and irregular corneas. These neophyte lens wearers were fitted in spherical landing zone diagnostic scleral lenses for this study.
At visit 1, subjects from Groups 1 and 2 reported to the study center wearing their habitual scleral lenses, at which time their visual history and acuity were recorded, and anterior segment evaluation was performed to establish eligibility for the study. Their contact lens fit was assessed with biomicroscopy, and the subjects completed the validated Texas Eye Research and Technology Center (TERTC) dry eye questionnaire to evaluate ocular dryness and lens comfort.31 These subjects had previously been fit by experienced scleral lenses fitters at the University Eye Institute, and all exhibited clearance over the cornea and limbus, with no compression of the conjunctival blood vessels. There were no specific criteria for levels of central clearance, but the range was 50–690μm with an average of 305μm. Individuals in Group 3 (normal, non-habitual scleral lens wearers) were fitted in a diagnostic scleral lens (either CustomStable, manufactured by Valley Contax, Springfield, OR, or Zenlens, manufactured by Alden Optical, Lancaster, NY) at visit 1. The goal fit at this visit was to vault the cornea and limbus (with approximately 200–400μm central clearance prior to settling) and land gently without compression of the conjunctiva blood vessels.
Anterior segment ocular coherence tomography (OCT) imaging (Visante, Carl Zeiss, Germany) was performed to visualize the fit of the lenses and quantify the TR depth, or sagittal depth for all subjects. Control subjects were fitted with diagnostic scleral lenses prior to the day of experimental testing, and were tested using the same protocol as Groups 1 and 2 at the experimental visit.
Tear fluorescein intensity concentration was quantified with the Fluorotron Fluorophotometer (Fluorotron Master FS2, Mountainview, CA). The fluorophotometer measures the natural fluorescence over a 2mm area along the optical path of the eye. In this study, the instrument detects the fluorescent intensity of the cornea + scleral lens combination as well as the fluorescein that is applied to the saline that becomes the TR within the scleral lens chamber. The measurement included the natural fluorescence of the cornea with no scleral lens in place to obtain baseline data for each patient. After baseline fluorophotometry measurements, the concave portion of the lens was filled with non-preserved saline solution (Unisol, Alcon Laboratories, Fort Worth, TX) and mixed with 2μl of 5% high molecular weight fluorescein (FITC Dextran, molecule weight 70KDa, Sigma-Greenway Pharmacy), then applied to the subject’s eye. Using the fluorophotometer, measurements were made to assess the concentration of fluorescein (ng/ml) beneath the lens. Figure 1 illustrates an example of a fluorophotometry scan prior and after fluorescein instillation beneath the lens.
Figure 1:
Fluorophotometer (Fluorotron) scans displaying before (left) and after (right) lens application with fluorescein. (Y-axis represents the concentration of fluoroscein in ng/ml and the x-axis is axial ocular depth).
For each tested eye, 18 fluorescein concentration measurements were obtained over a 4 hour period, with more frequent measurements taken during the first hour of wear as the lens settled: measurements were taken every 5 minutes for 60 minutes, then every 15 minutes for 60 minutes, followed by every 30 minutes until a 4-hour wear period had been reached.
Development of Experimental Eye Model
There are limited publications on the use of fluorophotometry to measure tear exchange, and most of these studies have focused on soft lenses and small diameter corneal GP lenses, rather than the scleral lens that contains a large TR and thus a thick fluorescent tear film once FITC Dextran is added. We observed a high variability in the data collected here; therefore, we designed a model eye after the completion of the human experiments to improve the understanding of the variation observed, and aid in the interpretation of the data. Model eye data were collected using this system to measure fluorescein concentration in systems of known tear reservoir thicknesses (Values: 340μm, 500μm), eye rotational orientations, and fluorescein concentrations. Scleral lenses were secured to the model eyes with adhesive (Nailene ultra quick nail adhesive, Irvine, CA) to prevent fluid loss. The lenses were designed with a 1.5mm diameter hole, 2mm from the edge of the lens at 90° to facilitate pipetting of non-preserved saline and specified FITCDextran concentrations into the post-lens tear reservoir once the lens was secured onto the model eye.
Each of the two simulator eye-lens combinations underwent a series of measurements with the fluorophotometer. Using a pipette, 2 μL of 5% FITC-Dextran was instilled under the lens, with the remaining portion consisting of saline. To obtain measurements off axis, the model eye was rotated slightly left and right to orient the measurement axis with a specific mark on the lens 2mm from the geometric center of the lens.
Figure 2 depicts the set-up of the simulator system for primary gaze. By collecting this control data, which inherently is measured without the added variables of tear exchange and other biologic factors, we were able to establish the precision and repeatability of the instrument at measuring the concentration of fluorescein in a scleral lens system.
Figure 2:
Simulator eye with attached scleral lens and fluorescein in lens bowl, setup on the fluorophotometer in central orientation. Rubr eye model was designed and manufactured by University of Houston College of Optometry Technical Services. During the actual measurement process, a red light lamp is used to see, and room lights are turned off as fluorophotometry must be done in the dark without any fluorescent lighting in order to avoid measurement errors.
Data Analysis and Statistics
Historically, the peak value of the fluorescein concentration curve is used as the single concentration value at each time point, values are plotted over time, and the slope of the best fit line represents the tear turnover value.32 While this method works well for soft lenses and corneal GP lenses that have a relatively negligible post lens tear reservoir, the scleral lens may have anywhere from 200–600μm of central corneal clearance, leading to variability in tear lens volume. Therefore, a custom MATLAB algorithm was written to automate the quantification of fluorescein under the central portion of the lens.
This MATLAB algorithm operated on only the subset of data associated with the cornea and scleral lens. The remainder of the dataset (portions associated with the anterior chamber, crystalline lens and deeper) were discarded. The extent of the original dataset associated with the cornea and scleral lens was determined through manual examination of each dataset. For each subject, and at each time point, a trapezoidal integration of the area under the curve was performed on the data associated with the cornea and SL. In Figure 2b, this integration of the area under the curve extends from ~14mm to ~21.5mm on the X axis, and includes all area under the curve, all the way to a value of 0 on the Y axis. From this value, we subtracted the portion of the area that fell below the lowest Y value in this region. In Figure 2b, this is the area that extends from 14mm to 21.5mm on the X axis, and from 0 to 2 on the Y axis. After processing, the resulting value was used as a measure of fluorescein concentration, rather than a single point value.
Tear inflow rate was calculated as the percentage decrease per minute of fluorescein concentration measured over time. From this, the concentration of FITC was plotted over time to transform into a tear decay plot, with the slope representing the inflow rate. Statistical analysis for the primary tear study was done using the Mann-Whitney test for non-parametric unpaired data. Tear inflow rates were compared between groups 1 and 2; each group was also compared to the normal non-habitual controls (Group 3). The Spearman rank test was used to analyze the correlation of measurements in the model control eyes.
Results
Simulator Experiment Results
In the eye with a 340μm TR depth, there were no differences found when the central tilted measurements were compared to each other (p=0.26), or the inferior tilted measurements compared to each other, although the inferior measurements were especially quite variable (p=0.17). There was a significant difference in fluorescein intensity when the control eye was moved off axis inferiorly (p=0.01) (Figure 3). In the model eye with the larger TR depth, 500μm, there was similar disagreement in the measurements when the lens was translated inferiorly (p=0.03). Also similarly to the 340μm TF depth model, there was no difference in fluorescein intensity during the various tilt measurements in the central zone (p=0.78) or in the inferior zone (p=0.48). There was variability in the fluorescein intensity values in both the 340um and 500um TF depths; however, there was no significant difference between the average fluorescein intensity measurements when using the two models (p=0.31). Figure 4 displays data from one of the experimental eye models over the two-hour period, with average concentration measurements from all six positions of gaze.
Figure 3:
Mean concentration of FITC-dextran (ng/ml) in the central zone (left) and the inferior zone (right), using the model eye with low concentration of fluorescein (2μl) with two sagittal depths (340 and 500μm). Measurements were taken every 15 minutes over a two-hour period. There is variability observed in the concentration measurements with both lens designs, despite being in a closed system lacking tear exchange.
Figure 4:
Summary of simulator data for 2 μL concentration for one of the simulator eye models. The average fluorescein concentration is plotted for all positions of gaze, and the error bars represent the standard deviation. Each color represents a different position of gaze. Note the variation, even though this was measured in a closed system. Greater concentrations of fluorescein are seen in the inferior zones when compared to the central orientation.
Primary Tear Exchange Experiment Results
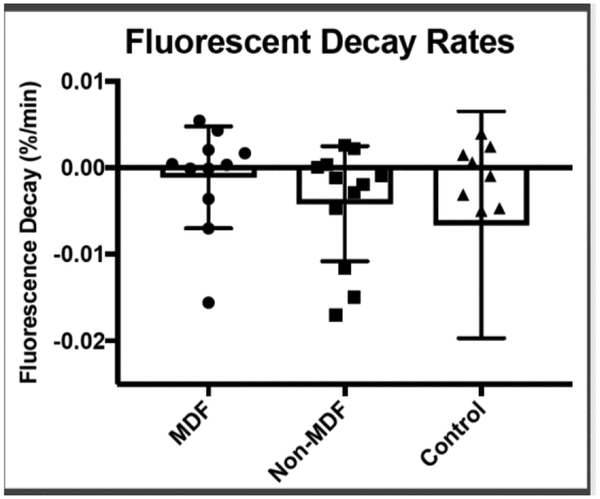
There was no significant difference in fluorescein intensity during scleral lens wear between the three groups, indicating that the rate of turnover is less than the variability seen in the measurement technique, with no groups showing evidence of large levels of tear outflow or inflow. Table 1 summarizes mean results of the parameters studied for each subject group. The study revealed no statistical difference in tear fluorescein intensity change (inflow rate) in the MDF group (mean 0.11±0.59%/min) when compared to the non-MDF group (mean: 0.42±0.67%/min) (p-0.26), and although there was a high amount of variability, there was a trend toward a slower inflow rate in the MDF group. The neophyte group, who exhibited no anterior segment pathology, had a greater mean tear inflow rate of 0.65±1.3%/min, with high amounts of variability (Figure 5).
Table 1.
shows the mean post-lens tear reservoir depth, dry eye score, and tear exchange rate for each group of subjects. For the TERTC dry eye questionnaire grading system, scores of <17 indicated normal, 17–32 dry eye suspect, and >32 dry eye syndrome.
| Summary of study results for each group | |||
|---|---|---|---|
| Control | |||
| Post-lens tear reservoir depth (μm) | 283 | 326 | 210 |
| Dry Eye Score | 29.5 | 30.4 | 23.5 |
| Exchange rate (%/min) | 0.111 | 0.417 | 0.659 |
Figure 5:
Mean tear inflow rates for MDF, Non-MDF, and neophyte groups. While subjects who experienced midday fogging had lower tear inflow rates on average, due to high variation in rates, there was no statistically significant difference. Higher variability in the control group may be a result of the lenses not being adequately fit, since trial lenses were used for the purpose of this study, rather than the habitual lenses worn by the adapted subjects.
As examples, subjects 6 and 34, who were both classified as interrupted wearers due to MDF (Group 1), are shown in Figure 6 to demonstrate the variability observed in this study. One subject showed a steady decline in fluorescence over time, while the other remained relatively stable over the entire 4 hours.
Figure 6:
Subjects 6 and 34 were both categorized as interrupted wearers due to MDF. Subject 6 exhibited a relatively flat decay curve, while Subject 34 shows a steep decline before leveling off after approximately 1 hour. This illustrates the high variability between subjects within the same group.
There were no significant difference between the tear film reservoir thickness in the MDF (mean: 283 μm) and the non-MDF (mean: 326 μm) groups (p=0.53). Table 2 shows the complete dataset for each subject.
Table 2.
shows demographic and study information for individual subjects in each group.
| Subject | Age | Gender | Sag (μm) |
Group | Lens Type | Diameter (mm) |
Lens thickness (μm) |
|---|---|---|---|---|---|---|---|
| 001 | 25 | M | 140 | Uninterrupted | Digiform | 18 | 300 |
| 003 | 30 | F | 530 | Uninterrupted | MSD | 15.8 | 340 |
| 004 | 45 | F | 690 | Uninterrupted | Jupiter | 18 | 330 |
| 007 | 30 | F | 310 | Uninterrupted | Jupiter | 18.2 | 400 |
| 009 | 43 | M | 220 | Uninterrupted | Digiform | 18 | 250 |
| 010 | 25 | F | 170 | Uninterrupted | Jupiter | 18.2 | 420 |
| 013 | 29 | M | 550 | Uninterrupted | Jupiter | 16.6 | 430 |
| 016 | 30 | M | 290 | Uninterrupted | Digiform | 18 | 280 |
| 022 | 31 | M | 50 | Uninterrupted | Digiform | 18 | 340 |
| 030 | 30 | F | 450 | Uninterrupted | Zen Lens | 17 | 400 |
| 031 | 31 | F | 200 | Uninterrupted | Custom Stable |
16.8 | 380 |
| 033 | 20 | M | 320 | Uninterrupted | Zen Lens | 17 | 400 |
| 005 | 41 | M | 230 | Interrupted | Jupiter | 18.8 | 370 |
| 006 | 33 | M | 430 | Interrupted | MSD | 15.8 | 400 |
| 008 | 27 | F | 300 | Interrupted | Jupiter | 18 | 410 |
| 011 | 47 | M | 480 | Interrupted | Digiform | 18 | 220 |
| 014 | 37 | F | 70 | Interrupted | Digiform | 18 | 180 |
| 017 | 30 | M | 230 | Interrupted | Custom Stable |
16.7 | 370 |
| 034 | 69 | M | 170 | Interrupted | Jupiter Reverse |
18.2 | 400 |
| 035 | 31 | M | 420 | Interrupted | Zen Lens | 16 | 420 |
| 036 | 25 | M | 270 | Interrupted | Zen Lens | 16 | 400 |
| 037 | 50 | M | 250 | Interrupted | Zen Lens | 17 | 370 |
| 038 | 29 | F | 270 | Interrupted | OneFit | 14.9 | 300 |
| 015 | 32 | M | 260 | Control | Custom Stable |
16.8 | 400 |
| 018 | 26 | F | 230 | Control | Zen Lens | 16 | 400 |
| 019 | 24 | F | 350 | Control | Custom Stable |
16.8 | 400 |
| 020 | 24 | F | 210 | Control | Custom Stable |
16.8 | 400 |
| 021 | 24 | F | 120 | Control | Custom Stable |
16.8 | 400 |
| 025 | 25 | M | 250 | Control | Zen Lens | 16 | 400 |
| 026 | 25 | M | 150 | Control | Zen Lens | 16 | 400 |
| 027 | 25 | F | 100 | Control | Zen Lens | 16 | 400 |
| 028 | 24 | F | 160 | Control | Zen Lens | 16 | 400 |
| 029 | 25 | M | 390 | Control | Zen Lens | 17 | 400 |
Post hoc power analysis showed that this study was powered at 21.8%. Calculations were done to determine sample size needed to show a significant difference between groups (powered at 80%), and we estimate that approximately 58 subjects would be needed in each group to show a difference in tear inflow rate between the groups.
Discussion
Historically, tear exchange with contact lenses has been considered necessary in order to provide adequate oxygen to the cornea, and prevent toxic metabolic products from accumulating under the lens.23,33,34 In contrast, it has been proposed that excessive tear exchange, or tear inflow, may be the culprit behind MDF by drawing debris and metabolic byproducts under the lens.35,36
Results of the model eye experiments indicated that the concentration of fluorescein under the lens, as well as misalignment, may lead to data outliers and high variability. When we analyzed the repeatability of the control eye measurements, we found that the repeatability was similar in the two model eyes with different tear reservoir depths, and that the variability is high and relies on careful alignment for accuracy. These data indicate that moving the eye off-axis, especially inferior, can reduce the reliability of the fluorescein intensity measurements.
Here we showed that a small amount of tear inflow (<1%/min) occurred underneath a scleral lens in each study group. Individuals with MDF did show a trend towards less tear inflow than those without, however it was not shown to be significant. More work is needed to determine if there is a clinically significant difference in tear inflow and outflow patterns in MDF. Ultimately, this data indicates that tear exchange does not provide a complete explanation for MDF. Our clinical recommendation based on this study and considering that there is still much unknown about the necessity of tear exchange with scleral lenses, is to consider each patient and their unique ocular surface health requirements individually.35,37
Fluorophotometry to measure tear exchange:
Fluorophotometry is considered the gold standard for measuring tear exchange during contact lens wear, but it is not without its limitations.5,16,21,38 Using this technology on scleral lens-wearing eyes presented some unique challenges for data collection and interpretation. Historically, the term tear exchange (tear turnover, tear flow, tear pumping and tear mixing) has been used to imply movement of tears from under the lens, both in and out.39–42 This terminology of “tear exchange” implies a bidirectional circulation of tears, but the fluorophotometer essentially measures the fluorescein concentration, or intensity, from beneath the lens over time, providing little knowledge of the replenishment of tears from the ocular surface outside the lens perimeter. The fluorophotometer indirectly estimates tear inflow rate, by measuring the average rate of fluorescein intensity change over time. Negative slope values of fluorescein intensity over time graphs indicate an overall loss in fluorescein concentration, and thus a presumed inflow of tear into the system. Positive values occur when there is a loss of tears, but not fluorescein particles (the loss of tears is based on the well-known behavior of the scleral lens to settle and have a reduced TF volume over time27).
To date there are minimal publications that report measuring the rate of tear exchange, tear inflow, or outflow beneath a scleral lens. A recent publication by Paugh et al. measured tear exchange under a scleral lens utilizing fluorophotometry during the lens settling period over the first 30 minutes of wear compared to the 30–60 minute time period.30 They found that tear exchange was 0.57±0.6%/min initially, then 0.42±0.5%/min up to the 60 minute mark, but no significant difference in the two rates (p=0.515).30 This study compares well to the present study showing an overall mean tear inflow rate of 0.4±0.9%/min, also with a large amount of variation between subjects.
While substantial published data is available concerning fluorophotometry with corneal rigid lenses and soft lenses, little is known about the application of this technique with scleral lenses.5 This study aimed to explore the normal variation in the data collected in a scleral lens system by designing simulator eyes with absent tear exchange. The control experiment revealed considerable disparities in the quantified fluorescence of various regions of the TR. Measurements off-axis inferiorly varied significantly from those measured in the central region of the eye. Results of this control experiment indicate that flow of fluorescein under the lens, as well as patient misalignment and the inherent variability of the instrumentation may lead to data outliers. This may be of little consequence given the large volume of measurements that help to account for these variations, but highlights a unique difference in scleral lenses from other lens designs—mainly that there is a highly variable range of tear layer depths in each subject, as well as between subjects. Future studies in scleral lens tear turnover should consider improved fluorophotometer protocols involving optimal patient alignment and a standardized measurement zone in order to yield improved accuracy in measurements and minimize variation. Extreme care should be taken to align the subject properly during this type of experiment. More work should be done to determine the repeatability of the instrument with different tear depths, although this control data suggests that deeper TR may yield more reliable fluorescein concentration readings.
Another limitation of the study was that subjective data in the form of a dry eye survey was used to assess dry eye status and used as an inclusion criterion, instead of objective clinical findings. Since MDF is a tear related phenomenon, future studies may benefit from including both subjective and objective data to assess the ocular surface of subjects. In addition, the present pilot study included subjects wearing their habitual lenses, without standardized diameter, thickness, power, and other parameter variables. Future work with fluorophotometry should attempt to control these variables to improve comparisons between groups.
Midday Fogging Management
As of yet, there are no peer-reviewed, large clinical studies that provide evidence-based treatment and management of MDF. Most literature gives anecdotal management options based on individual clinical experiences and preferences. Decreasing the sagittal depth of the lens is a common troubleshooting method that sometimes eliminates, or at least minimizes patient fogging.9,11,43 Many practitioners find that some patients benefit from instilling several drops of a viscous non-preserved artificial tear in the lens reservoir, in addition to non-preserved saline.10,34,44 The present study suggests that tear exchange is not on its own a single cause of MDF. Our clinical experience and intuition suggests that the MDF conditions is multifactorial, and therefore it is not likely that there will be one universal solution to alleviate its occurrence.
Conclusion
The reemergence of scleral lenses has revolutionized the treatment and management options available for corneal and ocular surface disease. There is no doubt that this lens modality has facilitated improved vision, comfort, enhanced clinical outcomes, and quality of life to a population of patients who have failed with previous treatment strategies. Nevertheless, as with all new advancements, unique complications and limitations exist. MDF is among these limitations, and while progress has been made to overcome this limitation, its existence still appears to be multifactorial in nature.
This study sought to evaluate tear inflow into a scleral lens system and indirectly assess the turnover of the TR beneath a scleral lens, specifically in habitual scleral lens wearers who did and did not experience MDF to analyze the effect of scleral lens tear film turnover on the incidence of MDF. Results revealed no statistical difference in tear fluorescein change in the MDF group (mean 0.111%/min) when compared to the non-MDF group (mean: 0.417%/min), with a high variability of the turnover rates.
Fluorophotometry has long been the gold standard for quantifying tear exchange, but may require a different protocol when applied to measuring tear inflow, or turnover, with scleral lenses due to the unique relationship with the cornea and variable post-lens tear layer. Further research should aim to address the causes of variability and improve measurement techniques with fluorophotometry for the scleral lens system. This study showed that measurements can vary considerably and are sensitive to patient positioning. The interactions of a scleral lens with the cornea and tear layer are considerably different than those observed with other lens modalities, implying the need for an evolved, more complete understanding of the effects of scleral lens on the ocular surface.
Acknowledgements:
This study was supported in part by National Institute of Health/National Eye Institute Grant P30 EY007551. A special thanks to Dr. Julia Benoit for guidance in statistical analysis and Chris Kuether with Technical Services for assistance designing the model-eye.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Michaud L, van der Worp E, Brazeau D, Warde R, Giasson CJ. Predicting estimates of oxygen transmissibility for scleral lenses. Contact Lens Anterior Eye. 2012;35(6):266–271. doi: 10.1016/j.clae.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Florkey L, Fink BA, Ph D, Mitchell GL, Hill RM. Tear Exchange and Oxygen Reservoir Effects in Silicone Hydrogel Systems. 2003;29:90–92. doi: 10.1097/01.ICL.0000042380.42931.01. [DOI] [PubMed] [Google Scholar]
- 3.Fatt I Gas-to-Gas oxygen permeability measurements on RGP and silicone rubber lens materials. Int Contact Lens Clin. 1991;18(9):192–199. doi: 10.1016/0892-8967(91)90007-M. [DOI] [Google Scholar]
- 4.Bergmanson JPG, Ezekiel DF, Van Der Worp E. Scleral Contact Lenses and Hypoxia. Theory versus Practice. Contact Lens Ant Eye. 2015;38:145–147. [DOI] [PubMed] [Google Scholar]
- 5.Muntz A, Subbaraman LN, Sorbara L, Jones L. Tear exchange and contact lenses: a review. J Optom. 2015;8(1):2–11. doi: 10.1016/j.optom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eiden B Scleral lenses: We don’t know what we don’t know. Contact Lens Spectr. 2016;31(October 2016):14, 15. [Google Scholar]
- 7.Rathi VM, Mandathara PS, Vaddavalli PK, Srikanth D, Sangwan VS. Fluid filled scleral contact lens in pediatric patients: challenges and outcome. Cont Lens Anterior Eye. 2012;35(4):189–192. doi: 10.1016/j.clae.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Pecego M, Barnett M, Mannis MJ, Durbin-Johnson B. Jupiter Scleral Lenses: The UC Davis Eye Center Experience. Eye Contact Lens Sci Clin Pract. 2012;38(3):179–182. doi: 10.1097/ICL.0b013e31824daa5e. [DOI] [PubMed] [Google Scholar]
- 9.Caroline P, Andre M. Cloudy Vision with Sclerals. Contact Lens Spectr. 2012;27(June 2012):56. [Google Scholar]
- 10.Walker MK, Bergmanson JP, Miller WL, Marsack JD, Johnson L a. Complications and fitting challenges associated with scleral contact lenses: A review. Contact Lens Anterior Eye. 2015. doi: 10.1016/j.clae.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Ne L, Wl M, Am M, Polizzi C, E VDW, Jpg B. Midday Visual Fogging in Scleral Lens Wearers – Does Fit Matter. Vol 54; 2012. [Google Scholar]
- 12.Hemmati RT, Walker MK, Bergmanson J. Scleral Lens tear film reservoir analysis: protein and lipid concentrations in midday fogging; Poster presented at GSLS; January 2014, Las Vegas, Nevada [Google Scholar]
- 13.Morrison S, Walker M, Caroline P, Kinoshita B, Lampa M, Andre M et al. Tear Exchange Beneath Scleral Lenses?Posterpresented at: GSLS;January 2014, Las Vegas, Nevada. [Google Scholar]
- 14.Mishima S, Gasset A, Klyce SD, Baum JL. Determination of Tear Volume and Tear Flow. 1966;5(3):264–276. [PubMed] [Google Scholar]
- 15.Xu KP, Tsubota K. Correlation of tear clearance rate and fluorophotometric assessment of tear turnover. Br J Ophthalmol. 1995;79(11):1042–1045. doi: 10.1136/bjo.79.11.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eter N, Gobbels M. A New Technique for tear film fluorophotometry. Br J Ophthalmol. 2002;86(6):616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunha-Vaz J History of fluorophotometry. Grafe’s Arch Clinc Exp Ophthalmol. 1985;222:168. [Google Scholar]
- 18.Smith AT, Jones DP, Sturrock GD, Wright P. An improved objective slit-lamp fluorophotometer using tungsten-halogen lamp excitation and synchronous detection. 1977:722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munnerlyn CR, Gray JR, Hennings DR. Design considerations for a fluorophotometer for ocular research. Graefe’s Arch Clin Exp Ophthalmol. 1985;222(4–5):209–211. doi: 10.1007/BF02133676. [DOI] [PubMed] [Google Scholar]
- 20.Maus TL, Brubaker RF. Measurement of Aqueous Humor Flow by Fluorophotometry in the Presence of a Dilated Pupil. IOVS. 1999;40(2):542–546. [PubMed] [Google Scholar]
- 21.Pearce EI, Keenan BP, McRory C. An improved fluorophotometric method for tear turnover assessment. Optom Vis Sci. 2001;78(1):30–36. [DOI] [PubMed] [Google Scholar]
- 22.Paugh JR, Stapleton F, Keay L, Ho A. Tear exchange under hydrogel contact lenses: Methodological considerations. Investig Ophthalmol Vis Sci. 2001;42(12):2813–2820. [PubMed] [Google Scholar]
- 23.Quinn T GP Versus Soft Lenses. Contact Lens Spectr. 2012;27(March 2012):34–39. [Google Scholar]
- 24.McNamara NA, Polse KA, Bonnano J. Fluorophotometry in Contact Lens Research: The Next Step. Optom Vis Sci. 1998;75(5):316–322. doi: 10.1097/00006324-199805000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Berger RE, Corrsin S. A surface tension gradient mechanism for driving the precorneal tear film after a blink. J Biomech. 1974;7(3):225–238. doi: 10.1016/0021-9290(74)90013-X. [DOI] [PubMed] [Google Scholar]
- 26.Sorbara L, Simpson T, Vaccari S, Jones L, Fonn D. Tear turnover rate is reduced in patients with symptomatic dry eye. Cont Lens Anterior Eye. 2004;27(1):15–20. doi: 10.1016/j.clae.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Kauffman MJ, Gilmartin CA, Bennett ES, Bassi CJ. A Comparison of the short-term settling of three scleral lens designs. Optom Vis Sci. 2014;91(12):1462–1466. doi: 10.1097/OPX.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 28.Marice D, Ruben M. Fluid exchange under scleral contact lenses in relation to wearing time. Br J Ophthalmol. 1970;54(486). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rathi VM, Mandathara PS, Taneja M, Dumpati S, Sangwan VS. Scleral lens for keratoconus: Technology update. Clin Ophthalmol. 2015;9:2013–2018. doi: 10.2147/OPTH.S52483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paugh JR, Chen E, Heinrich C, et al. Silicone Hydrogel and Rigid Gas-Permeable Scleral Lens Tear Exchange. Eye Contact Lens. 2018;44(2):1. doi: 10.1097/ICL.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 31.Narayanan S, Miller W, Prager T, et al. The Diagnosis and Characteristics of Dry Eye. Eye Contact Lens. 2005;31(3):96–104. [DOI] [PubMed] [Google Scholar]
- 32.McKinney A, Miller W, Leach N, Polizzi C, van der Worp E, Bergmanson J. The Cause of Midday Visual Fogging in Scleral Gas Permeable Lens Wearers. Invest Ophthalmol Vis Sci. 2013:ARVO E-Abstract 5483. [Google Scholar]
- 33.Sonsino J, Schornack M. Troubleshooting Scleral Lenses. Contact Lens Spectr. 2013;28(May 2013):26–33. [Google Scholar]
- 34.Nichols JJ, Bennett ES, Starcher L. Education Highlights from the 2015 GSLS. Contact Lens Spectr. 2015;30(April 2015):22–30. [Google Scholar]
- 35.Hill G, Michaud L, Jedlicka J. Addressing scleral lens clinical concerns. Optom Times. 2016:2016–2018. [Google Scholar]
- 36.Jedlicka J Solving Scleral Lens Complications. Contact Lens Spectr. 2012;27(October 2012):40–44. [Google Scholar]
- 37.Walker M, Bergmanson JP, Marsack JD, Miller W, Johnson L. Complications and Fitting Challenges Assocaited with Scleral Contact Lenses: A Review. Contact Lens Anterior Eye. 2016;39(2):88–96. [DOI] [PubMed] [Google Scholar]
- 38.Jones DP. Ophthalmic fluorophotometry: an improved slit lamp fluorophotometer. 1979;(May):365–370. [DOI] [PubMed] [Google Scholar]
- 39.Polse KA. Tear flow under hydrogel contact lenses. Invest Ophthalmol Vis Sci. 1979;18:409–413. [PubMed] [Google Scholar]
- 40.Kok J, Boets E, van Best J, Kijlstra A. Fluorophotometric assessment of tear turnover under rigid contact lenses. Cornea. 1992;11:515–517. [DOI] [PubMed] [Google Scholar]
- 41.Wagner L, Polse K, Madnell R. Tear pumping and edema with soft contact lenses. Invest Ophthalmol Vis Sci. 1980;19:1397–1400. [PubMed] [Google Scholar]
- 42.McNamara N, Polse K, Brand R, Graham A, Chan J, McKenney C. Tear Mixing Under a Soft Contact Lens: Effects of Lens Diameter. Am J Ophthalmol. 1999;127(6):659–665. [DOI] [PubMed] [Google Scholar]
- 43.Walker M Scleral lenses, clearing the fog. ISITE online J. 2014;December:2014. [Google Scholar]
- 44.Top Taylor-Westt S. 10 complications of scleral lenses. Optician. 2014:34–39. [Google Scholar]